Abstract
Intracellular pH (pHi) regulation is essential for cell function. Notably, several unique sperm ion transporters and enzymes whose elimination causes infertility are either pHi dependent or somehow related to pHi regulation. Amongst them are: CatSper, a Ca2+ channel; Slo3, a K+ channel; the sperm-specific Na+/H+ exchanger and the soluble adenylyl cyclase. It is thus clear that pHi regulation is of the utmost importance for sperm physiology. This review briefly summarizes the key components involved in pHi regulation, their characteristics and participation in fundamental sperm functions such as motility, maturation and the acrosome reaction.
Keywords: sperm, intracellular pH, sperm motility, sperm chemotaxis
INTRODUCTION
Maintaining intracellular pH (cytoplasmic pH or pHi), within physiological boundaries is fundamental for cell function. Most cellular processes are influenced and operate within a restricted pHi range. Changes in pHi affect the ionization state of weak acids and bases which are present in most proteins and many biomolecules [1]. Therefore, as metabolic activity acidifies the cytosol and cells can be exposed to a varying external pH (pHe), lack of pHi regulation can have severe functional consequences. In general, cells finely tune their pHi employing several dynamic mechanisms whose balance in terms of production, elimination, transport and buffering of H+ determine its value at a certain time. Cells slow down or speed up the activity of a wide range of plasma membrane ion transporters that move acids and/or bases [2]. As anticipated, HCO3− and H+ transporters are amongst the key players in pHi regulation.
Cells can exhibit drastic changes in transit from a dormant to an active state, a condition which often entails a sudden pH change. Spermatozoa (sperm) are a clear example as they are exposed dramatic changes in the ionic composition of their surroundings during their journey towards fertilizing the egg. Mammalian sperm encounter an acidic media with a low HCO3− concentration in the epididymis, which keeps them quiescent, while in the female uterus and oviduct the luminal fluid contains a high HCO3− concentration and becomes increasingly alkaline, a condition required for fertilization (reviewed in [3]). It is worth noting that several unique sperm ion transporters and enzymes, whose elimination cause infertility, are either pHi dependent or somehow related to pHi regulation. Amongst them are: CatSper, a Ca2+ channel [4]; Slo3, a K+ channel [5]; the sperm specific Na+/H+ exchanger [6] and the soluble adenylyl cyclase [7], [8] (Fig. 1). Thus, pHi regulation is of the utmost importance for sperm. Numerous strategies have evolved in the last 30 years to measure pHi. Their advantages and limitations are being continuously revised. These techniques involve determinations in cell populations or in single cells and include: 1) 31P nuclear magnetic resonance (NMR), 2) weak acid-base distribution, and 3) optical absorbance and fluorescence techniques [9], [10]. In the last years pHi is being studied in sperm mostly using fluorescent probes. Here we will attempt to give a summarized critical panorama of the field with focus on the main molecular players of pHi regulation in sperm and on the functions where they participate.
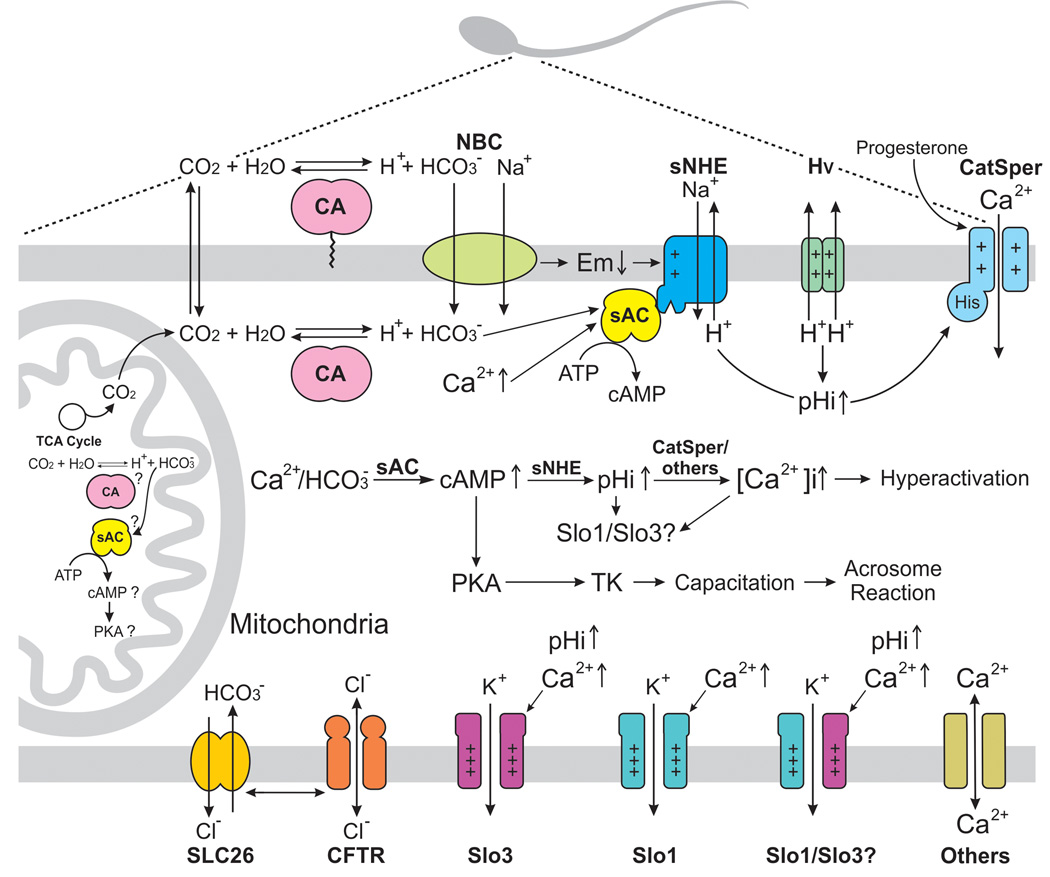
Figure 1. Model of mammalian sperm pHi regulation during capacitation/hyperactivation.
The CO2 / HCO3− are equilibrated inside the female tract by membrane associated carbonic anhydrases (CAs). The influx of HCO3 into the sperm is mediated by Na+/HCO3 cotransporters (NBC) and Cl/HCO3 exchangers (SLC26). Probably the cytoplasmic CAs also contribute to the HCO3 increase by conversion of cytosolic CO2. Specifically, SLC26A3 and A6 isoforms appear to have a physical interaction with CFTR, which may permeate HCO3 besides Cl. Aside, HCO3 influx hyperpolarizes mouse spermatozoa in a [Na+]e-dependent manner via NBC. HCO3activates (along with Ca2+) a soluble adenylyl cyclase (sAC) leading to a cAMP increase, which activates a PKA (which may also depend on [Ca2+]i) and also may bind to the cyclic nucleotide binding domain of the sperm specific Na+/H+ exchanger (sNHE) inducing a pHi increase in mouse. sNHE may also be stimulated by the hyperpolarization that occurs upon sperm capacitation. Unlike mouse, a voltage-dependent H+ channel (Hv) may be involved in human sperm pHi increase. In both species, the pHi increase (among other factors) activates a sperm specific Ca2+ permeable channel known as CatSper, which allows an intracellular Ca2+ increase essential for hyperactivation. We include an addition Ca2+ transporter to indicate that other systems participate in [Ca2+]i regulation. On the other hand, K+ channels Slo3 and Slo1 (or their heterotetramer) are activated by intracellular alkalinization and/or by Ca2+ (human sperm), contributing to the hyperpolarization that occurs during capacitation. CO2 is also generated inside mitochondria through the tricarboxylic acid cycle (TCA) and it may serve as substrate for intracellular or intramitochondrial CAs. The HCO3 may also activate intramitochondrial sAC which in turn would trigger protein phosphorylation. Although mammalian sperm possess many mitochondria inside the midpiece, for didactic purposes only one mitochondrion is shown here.
Key molecules involved in sperm pHi regulation
The main ion transporters in charge of pHi regulation can be arranged into two groups: 1) membrane H+ transporters and 2) HCO3− transporters. The first group includes Na+/H+ exchangers of the solute carrier 9 family (SLC9) [11] and H+ channels [12], [13]. The second group is constituted by the HCO3− transporters of the SLC4 [14], [15], SLC26 families [16]. and the cystic fibrosis transmembrane conductance regulator (CFTR) is able to conduct HCO3− [17]–[21]. As carbonic anhydrases (CAs) catalyze CO2/HCO3- equilibrium, they are also considered.
Soluble Adenylyl Cyclase
The discovery of soluble adenylyl cyclase (sAC) has allowed a better understanding of how cells locally produce the fundamental second messenger cAMP. Additionally, as HCO3− directly regulates sAC, this enzyme is able to translate CO2/HCO3−/pH changes into cAMP levels. Furthermore, the sperm specific Na+/H+ exchanger described below has a cAMP binding site and forms a stable complex with sAC thus it is intimately related to cAMP metabolism (reviewed in [22]).
Unlike transmembrane ACs (tmACs), sAC is insensitive to forskolin (independent of G-protein) and activated by HCO3− [23] and Ca2+ [24]. In 1999, the primary structure of sAC from rat was determined, which lacks transmembrane segments [25]. Curiously, the catalytic domains of sAC are more similar to those of cyanobacteria ACs than to those found in eukaryotic tmACs. As anticipated, the AC activity of cyanobacteria is also up-regulated by HCO3− [26].
A careful study of rat and mouse testis mRNA revealed that there are two alternative splicing products, which independently encode sACt and sACfl [27]. It has been reported that sAC is essential for sperm function since sAC-null male mice are infertile due to a severe defect in sperm motility [7], [8]. Although sAC gene is preferentially expressed in testis, sACt is also found in somatic cells. It is localized in cytoplasm and/or intracellular organelles such as mitochondria and considered to play a role to monitor CO2/ HCO3−/pH [28].
Na+/H+ exchangers
Na+/H+ exchangers (NHEs), also known as Na+/H+ antiporters (NHA), are integral membrane proteins that catalyze the exchange of Na+ for H+ across lipid bilayers and are ubiquitously distributed in almost all living organisms. They contribute to the basic homeostatic mechanisms that control pHi, cellular volume and intra-organelle pH [29]. In mammals, the SLC9 gene family encodes 13 evolutionarily conserved NHEs divided into three subgroups (SLC9A, SLC9B and SLC9C) [30]. The subfamily SLC9A encompasses nine isoforms (NHE1-9), the SLC9B subgroup consists of two isoforms (NHA1 and NHA2) and the SLC9C subgroup consists of a sperm-specific NHE (sNHE) and a putative NHE (NHE11). It has been reported that mammalian spermatozoa express four isoforms of NHEs, NHE1 (SLC9A1), NHE5 (SLC9A5) [31], [32], sNHE (SLC9C1) [6] and the testis-specific NHE (NHA1/SLC9B1) [33].
In general, NHEs are composed of 12 transmembrane segments which catalyze the ion exchange and a cytoplasmic carboxyl terminal regulatory domain which interacts with several proteins including cytoskeleton-associated components, kinases and Ca2+ binding proteins [34]. In contrast, the sNHE is constituted by 14 putative transmembrane segments and a long cytoplasmic carboxyl terminus [6]. The sNHE lacks the first two transmembrane segments found in the other NHEs; instead it has 4 subsequent extra transmembrane segments, which are structurally similar to the voltage sensor domain found in voltage-gated ion channels [6]. This is of particular interest given that sea urchin spermatozoa have a voltage-dependent NHE in the flagellar plasma membrane [35], [36]. In addition, the sNHE has a putative cyclic nucleotide binding consensus domain at the carboxyl terminus [6].
Although NHE1 was found in human and rat spermatozoa and NHE5 in rat spermatozoa [31], [32], the physiological roles of these NHEs are unknown. On the other hand, sNHE was demonstrated to be essential for sperm function since its elimination causes infertility in male mice due to a severe defect on sperm motility [6]. Intracellular alkalinization by NH4Cl partially recovered sperm motility of sNHE-null mice. In contrast, addition of membrane-permeable cAMP analogues almost fully recovered the sperm motility of the mutant sperm [6]. A careful study of sperm from sNHE-null mice revealed that their sAC activity was highly diminished [37]. Notably, immunoblot analysis indicated that sACfl is missing in this mutant although sACt is apparently not affected. Further experiments suggested that sNHE is physically associated with sAC and both proteins reciprocally stabilize their protein expression in HEK293 cells [37]. In addition, proteomic analysis of sea urchin sAC-associated proteins revealed that sNHE is one of the major sAC-associated proteins [38]. These experimental results suggest that sAC and sNHE form a functional complex. Nevertheless, the localization of sAC reported so far, the midpiece of sperm flagellum [8], does not correspond to the localization of sNHE, the principal piece of sperm flagellum [6]. This discrepancy could be explained by the specificity of the antibody used for sAC immunostaining, which detects preferentially sACt [28]. If this is the case, sACt and sACfl would be differentially localized in mammalian spermatozoa.
The fact that sNHE possesses a predicted cyclic nucleotide binding domain (CNBD) together with the physical interaction between sNHE and sAC suggests that cAMP regulates the activity of sNHE. However, some proteins found in prokaryotes and eukaryotes have a structurally almost identical domain to the CNBD that does not interact with cyclic nucleotides (cyclic nucleotide binding homology domains, CNBHD) [39], [40]. Therefore, biochemical experiments are required to examine if sNHE indeed has a functional CNBD. Interestingly, human sNHE lacks an arginine residue present in most CNBDs, which plays a critical role for the interaction with the phosphate group of a cyclic nucleotide [40]. Mouse and sea urchin sperm conserve this arginine residing in each predicted CNBD of sNHE, suggesting some functional differences between human sNHE and those of mouse and sea urchin. Further investigations are required to clarify these issues.
The testis-specific NHE identified by Liu et al. in 2010 [33] is now classified into a new family of NHE, NHA1 (SLC9B1). NHA1 has 12 transmembrane segments as other NHEs, but lacks the long cytoplasmatic C-terminal. Immunofluorescence demonstrated that NHA1 is localized in the principal piece of sperm flagellum in mouse spermatozoa. Furthermore, anti-NHEA1 antibody reduced sperm motility and the rate of in vitro fertilization. Therefore, NHA1 is proposed to regulate sperm motility. In order to define the physiological function of NHA1 in spermatozoa, it is desirable to analyze sperm function of NHA-1 knockout animals.
Voltage gated H+ channel
In 2006, two groups independently identified a voltage-gated H+ channel (Hv channel) [41], [42], which is composed of 4 transmembrane segments structurally similar to the voltage sensor domain commonly found in other voltage-gated ion channels. This channel exists as a homodimer with two H+ pores, since each subunit forms a H+ permeable pore. The Hv channel is activated by membrane potential depolarization and an outward H+ gradient across the plasma membrane. In addition, unsaturated fatty acids such as arachidonic acid enhance the Hv channel activity while Zn2+ potently inhibits it [13].
Whole-cell patch-clamp recordings from human sperm revealed a relatively large voltage-dependent H+ conductance [12]. Western blotting and immunostaining confirmed that sperm possess Hv channels in the principal piece of the flagellum. The sperm Hv channel conserves all the biophysical properties displayed in somatic cells or heterologous systems, including being potently inhibited by Zn2+. This last property is particularly important for sperm pHi regulation since it is known that human seminal fluid contains a millimolar range of Zn2+, which should completely block the H+ conductance of the Hv channel, and it may account for the action of Zn2+ as a decapacitation factor [43]. In addition, sperm Hv channels are activated by the endocannabinoid anandamide, which may explain part of its positive effects on mammalian reproduction [12].
Although human sperm display large H+ currents (100 pA/pF at +100 mV), those of mouse sperm are quite small (<10 pA/pF) [12]. These results indicate that human and mouse sperm regulate their pHi differently.
Carbonic anhydrases
Besides the H+ carriers and the HCO3− transporters, the cells also depend on carbonic anhydrases (CAs) to properly regulate their pHi. CAs are ubiquitous metalloenzymes (depending on the isoform, they require Zn2+, Fe2+, Co2+ or Cd2+ as cofactor) present in the three life domains (bacteria, archaea and eukarya). The principal function of CAs is to catalyze the reversible reaction of carbon dioxide hydration to bicarbonate and proton (CO2 + H2O ↔ HCO3− + H+). CAs are encoded by five gene families without apparent evolutionary relationship: α, β, γ, δ and δ. The sixteen isoforms of αCAs (ACI-ACXV) are the only CAs present in mammals showing distinct subcellular and tissue distribution, kinetic properties and sensitivity to inhibitors [44]. Despite the importance of CAs in the regulation of pHi in all living organisms, so far little information is available about their presence in mammalian sperm and even less is known about their function in the physiology of these cells (see Table 1).
Table 1.
Molecules involved in the regulation of pHi in mammalian sperm
| Molecule | Species/Localization | Technique | Function | Reference |
|---|---|---|---|---|
| CAI | H | ELISA | - | [139] |
| CAII | H | ELISA | - | [139] |
| H, M | Northern blot | - | [141] | |
| M | Western Blot | - | ||
| H/PA; R/A | IC | - | [140] | |
| H, R | Western Blot | - | ||
| CAIV | M/A, ES, PA, MP* | SEM | - | |
| M | RT-PCR Western Blot |
Motility | [46] | |
| M | ||||
| M/A, ES, PA, MP, | IC | |||
| PP* | ||||
| CAXII | M | PCR | - | [142] |
| IC | - | |||
| CAXIII | H, M | PCR | - | [143] |
| H | IH | - | ||
| sNHE | M | Northern Blot | Motility | [6] |
| M | Western Blot | |||
| M/PP | IC | |||
| NHA1 Hv |
M/PP | IC | Motility | [33] |
| H/PP | Patch Clamp ISH | Motility | [12] | |
| H | Western Blot | |||
| IC | ||||
| H/PP | ||||
| NBC | M | - | Capacitation | [60] |
| SLC26A 3 |
M | RT-PCR | Capacitation | [21] |
| M | Western Blot | |||
| M/MP | IC | |||
| SLC26A 6 |
M | RT-PCR | Capacitation | [21] |
| M | Western Blot | |||
| M/MP | IC | |||
Only in corpus and cauda spermatozoa.
Species: H, Human; M, Mouse; R, Rat. Cellular localization: A, Acrosome; ES, Equatorial segment; P, Postacrosomal region; MP, Midpiece; PP, Principal piece. Technique: ISH, In situ hibridization; IC, Immunocytochemistry; IH, Immunohistochemistry; SEM, Scanning electron microscopy.
The presence of different isoforms of αCAs in mammalian spermatozoa suggests a possible role of these metalloenzymes throughout the development of male gametes or during the physiological events that occur prior to fertilization. In fact this seems to be the case at least for motility as a HCO3− intracellular increase, as well as a H+ decrease (both ions produced by the CAs' reaction) are fundamental to this process in mammalian sperm [45]. However, the available studies that suggest the direct participation of CAs in the balance of these ions during motility are still scarce. It has been shown that mouse and human spermatozoa increase their flagellar beat frequency when exposed to CO2 and this effect is inhibited by ethoxyzolamide (EZA; a specific CAs inhibitor) [46], [47]. It was also found that CAIV−/− mouse sperm display a decrease in the total activity of CAs as well as a decrease in their response to CO2 [46].
It is worth mentioning that in marine organisms the participation of CAs in sperm motility has also been studied. In flatfish spermatozoa a 29 kDa CA isoform (probably CAII, considering its aminoacid sequence) was detected in high amounts. Application of EZA strongly affected sperm motility [48]. On the other hand, in spermatozoa from a certain type of squid, the production of H+ by a plasma membrane-anchored CA (along with an intracellular increase of Ca2+) is crucial in the chemotactic signaling pathway of these cells [49].
In many cell types certain CAs isoforms can form complexes with HCO3− transporters. In recent years this association has been called the bicarbonate transport metabolon, a complex of enzymes spatially close to each other that catalyze a series of reactions in a metabolic pathway; in this way the product of one enzyme is the substrate of the next, which allows the reaction to proceed efficiently [50].
Bicarbonate (HCO3−) transporters
HCO3− transporters constitute one of the main families of transmembrane proteins involved in the pHi regulation of mammalian cells. As mentioned previously, this family includes HCO3−transporters SLC4, SLC26, and CFTR [3]. The SLC4 transporter family includes the products of ten genes (SLC4A1-5 and SLC4A7-11). Nine SLC4 family members are integral membrane proteins that carry HCO3− (or carbonate) and at least one monoatomic ion (typically Na+ or Cl−), across the plasma membrane [51]. The physiological function of eight family members is already defined and they fall into three major phylogenetic subfamilies: 1) the Cl−/HCO3− anion exchangers AE1-3 (SLC4A1-3), 2) the electrogenic Na+/HCO3− cotransporters NBCe1 (SLC4A4) and NBCe2 (SLC4A5) and 3) the electroneutral Na+/HCO3− cotransporters NBCn1 (SLC4A7), NDCBE (SLC4A8) and NBCn2 (SLC4A10). The function of the two remaining SLC4 members, AE4 (SLC4A9) and BTR1 (SLC4A11) is still not fully understood [51].
On the other hand, the mammalian SLC26 gene family comprises 11 genes (SLC26A1-A11) that encodes multifunctional anion exchangers and anion channels transporting a broad range of substrates including HCO3−, Cl−, OH−, I−, SO42−, HCOO− and C2O42−. Among SLC26A family, HCO3− permeable members represent the second major subfamily of HCO3− transporters: SLCA3-A4, SLCA6-A9 and SLCA11 [52].
The CFTR is the only member of the ABC family of transporters that acts as an ion channel; it has been shown to be permeable to HCO3− [53]. CFTR is found commonly in the apical membranes of epithelial cells and its mutations give rise to cystic fibrosis (CF), one of the most common genetic diseases in some human groups.
Although so far the available information about the molecular entities, the distribution and function of HCO3− transporters in mammalian sperm physiology is very limited, in the last years a few studies have improved our knowledge on this subject. AE2 (SLC4A2) is localized in the equatorial segment of mammalian sperm head [54], [55]. On the other hand, mutations in human SLC26A3 produce congenital chloride diarrhea (CLD) and patients with CLD are subfertile, suggesting this transporter is important for sperm physiology [56]. In fact, SLC26A8, SLC26A3, as well as SLC26A6 and CFTR, have been identified in the midpiece of mouse/guinea pig spermatozoa [18], [21], [57]–[59]. These transporters physically interact and participate in the pHi increase that takes place during capacitation [21]. Results from another study strongly suggest that an electrogenic Na+/HCO3− cotransporter may also participate in the regulation of capacitation in mouse sperm [60]. In that report it was shown that HCO3− induces a Na+-dependent hyperpolarization of the mouse sperm plasma membrane and this leads to a pHi increase which is sensitive to DIDS (an anion exchanger inhibitor). In the same species it was demonstrated that a Na+-driven Cl−/HCO3− exchange is involved in the pHi regulation, possibly during capacitation [61].
In human spermatozoa the identification of HCO3− transporters remains unexplored.
pHi dependent ion channels , CatSper and Slo3
For over three decades it was known that progesterone and a component of the zona pellucida (ZP3) induce the acrosome reaction (AR, see below) with a strict requirement for an intracellular Ca2+ rise. It was later established that the Ca2+ increase triggered by these biomolecules during the AR is influenced by pHi. Interestingly, artificial intracellular alkalinization of mouse sperm produced a Ca2+ increase [62] and addition of HCO3− increased the beat frequency of sperm [63]. Although it was not completely clear how pHi and Ca2+ changes influence each other to regulate sperm functions, it was proposed that there were channels sensitive to membrane potential and pHi. This notion was later confirmed and the importance of pHi regulation in sperm was strongly highlighted with the discovery of two pHi and voltage sensitive ion channels, CatSper and Slo3, that are exclusively expressed in spermatozoa and whose absence produces male infertility. CatSper was identified in mouse sperm as a putative Ca2+ channel in 2001 [4], [64]. It was then shown that the activity of CatSper is required for hyperactivated motility and fertility although sperm from CatSper null mice can undergo the AR and fertilize zona free eggs [4], [64], [65]. After different approaches and strong efforts, whole-cell patch-clamp recordings in mature sperm became a reality in 2006 [66], such technique thus far has not detected other Ca2+ currents but those mediated by CatSper [67]. The characterization of CatSper has proven difficult due to the high promiscuity among its agonists and the lack of specific antagonists [68] and because heterologous expression has not been successful. However, it is clear that alkalinization increases CatSper activity and that progesterone activates it directly; this is true for human sperm. The ligands for CatSper channels from other species remain to be established.
In 1998 a pHi and voltage sensitive K+ channel (Slo3) highly expressed in mouse testis was described [69]. It was later reported that spermatozoa from Slo3 null mice exhibit several defects, including altered morphology, reduced motility and decreased AR efficiency [5], [70]. Recently, a K+ channel was also recorded in human sperm by two different groups; one claimed that the molecular identity of this channel is Slo3 [71] but the other presented evidence that this current resembled more closely the Ca2+-activated K+ channel, Slo1 [72]. New results from our lab in human sperm revealed that there is a plasma membrane hyperpolarization associated to the capacitation process (see below) and that more than one type of K+ channel may be involved, possibly Slo1 and Slo3 [73]. Regardless of the molecular identity, these sperm specific channels may contribute to the changes on membrane potential, intracellular Ca2+ and pHi, parameters that control not only the AR but other sperm functions. Further investigation is required to understand how and if these channels interact with each other forming a macro protein complex, a possibility that may explain recent findings of antagonist cross sensitivity displayed by CatSper and Slo3 and/or the molecular identity of the K+ current in human sperm [74].
Sperm functions where pHi regulation is critical
Initiation of motility
In most species, spermatozoa are stored immotile in the testis (or epididymis in the case of mammals). In general, it is believed that this quiescent sperm state is maintained by acidification of sperm pHi since the sperm dynein ATPases, motor proteins that generate the flagellar beat, are highly pH dependent [75], [76]. The pH-motility regulation is relatively well studied in sea urchin sperm.
Sea urchin sperm remain immotile in the gonads due to a low pHi maintained by a high CO2 tension and initiate flagellar beating upon spawning [77]. When sperm are released into seawater (pH 8.0), a rapid H+ efflux occurs [78], elevating pHi from 7.2 to 7.6 [78]. This pHi increase activates axonemal dynein ATPases, which are highly active at alkaline pHi (7.5 – 8.0) [75]. As the mitochondrion is the only ATP source in sea urchin sperm and dynein ATPases are the main consumers, motility and respiration are tightly linked and regulated by pHi [79], [80]. Both sperm pHi and motility depend on external ionic composition; for example low pH (5.0), high [K+] (200 mM) or Na+-free seawater lower sperm pHi to 5.7 – 6.6 [79] and prevent its motility. Notably, pHi alkalinization by NH4Cl addition restores sperm motility under these conditions [78], establishing the direct involvement of pHi as regulator of motility.
The pHi dependence on external pH and Na+, and the inhibition of motility by high external K+ can be explained by the participation of the voltage-sensitive NHE present in the sea urchin sperm flagellum, which interestingly, can be activated by a membrane hyperpolarization [35], [36]. Since sNHE (SLC9C1) possesses a voltage sensor domain [6] and peptide fragments of sNHE were detected in a proteomic analysis of sAC-associated proteins of sea urchin sperm [38], it is more likely that sNHE is the NHE that elevates pHi upon motility initiation in this species. Removal of tracer elements such as Zn2+ also lowers pHi retarding sperm motility initiation [81], which suggests that Zn2+ is involved, directly or indirectly, in the regulation of sNHE activity.
Sea urchin sperm chemotaxis
Sperm chemotaxis has been extensively studied in sea urchin spermatozoa. In this marine invertebrates, sperm are attracted toward the egg by sperm-activating peptides (SAPs) which are diffusible peptides released from the external coat of the egg (Review at [82]) SAPs stimulate sea urchin sperm motility and respiration in slightly acidified seawater (pH 6.2 – 6.8) [83], by increasing pHi [84], [85]. This property has been successfully used to screen for SAPs, and to date has led to the identification of hundreds of SAPs from numerous sea urchin species [82].
Currently, it is believed that mainly SAPs guide sperm toward the egg modulating their swimming behavior and, as a result, increasing the fertilization probability (review in [86]). Resact and speract are two of the best characterized SAPs that are chemotactic for two sea urchin species, A. punctulata [87] and L. pictus, respectively [88]. Both trigger a chemotactic response in a Ca2+ dependent manner [87]–[89]. In particular, Ca2+ influx into the flagellum is critical to shape the flagellar beat and the sperm swimming trajectory [90]–[94]. This Ca2+ influx is comprised of [Ca2+]i oscillations mounted on a sustained increase that initiate in the sperm flagella and travel toward the head [95]. Each oscillation transiently increases the flagellar asymmetry and causes the sperm to turn [90]. An orchestrated sequence of turns interspersed with periods of straighter swimming allows sperm to swim towards the chemoattractant source [87]–[89] and allows the sperm to locate the egg (Fig. 2, inset).
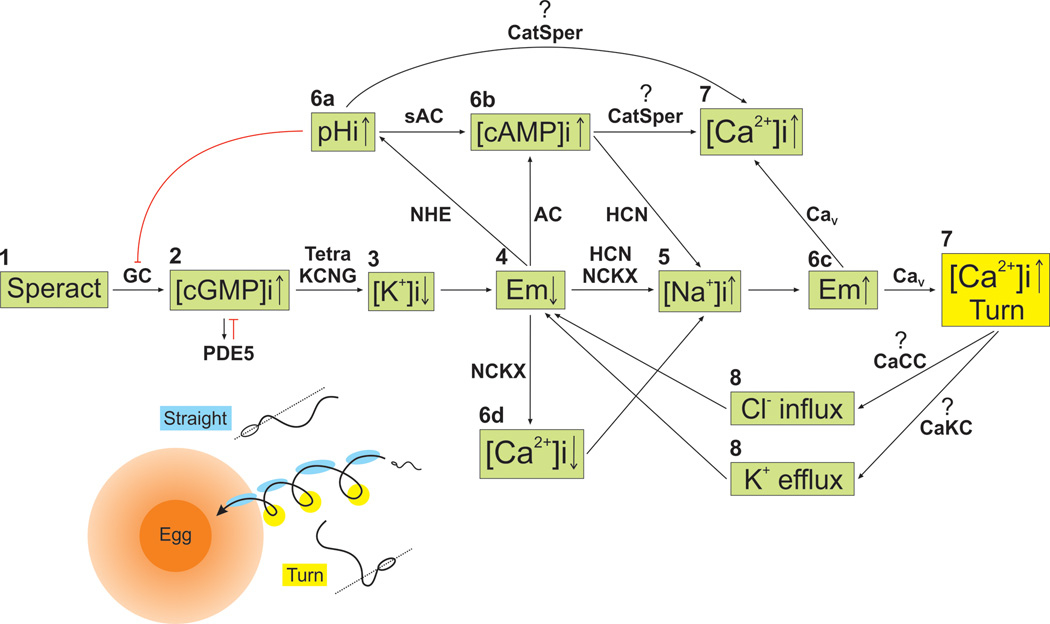
Figure 2. The speract signaling cascade in sea urchin sperm.
After binding to its receptor (1), speract stimulates a membrane guanylyl cyclase (GC), which elevates cGMP (2) that activates tetrameric cGMP-regulated K+ channels (tetraKCNG), causing a membrane potential (Em) hyperpolarization (4) due to K+ efflux (3). This Em change may activate a Na+/H+ exchanger (sNHE) (5a), remove inactivation from voltage-activated Ca2+ channels (CaV), enhance hyperpolarization-activated and cyclic nucleotide-gated channels (HCN), and facilitate Ca2+ extrusion (5d) by K+-dependent Na+/Ca2+ exchangers (NCKX). sNHE activation increases pHi (5a). HCN opening causes Na+ influx (5c) and contributes to depolarize Em activating CaVs, which increase [Ca2+]i (6) and enhance asymmetric flagellar bending allowing sperm to turn. Possibly, the rise of [Ca2+]i also opens Ca2+-regulated K+ channels (CaKC) and Ca2+-regulated Cl channels (CaCC), which then contribute to again hyperpolarize Em (7), removing inactivation from CaV channels and opening HCN channels. During this period sperm swim in a straighter trajectory whose regulation is ill defined but critical for chemotaxis. This succession of events occurs cyclically, generating orchestrated transient [Ca2+]i increases (6) that produce a sequence of turns until one or more of the second messengers in the pathway are downregulated. The pHi (5a) and cAMP increases (5b) stimulate an undetermined Ca2+ influx pathway that contributes to the sustained [Ca2+]i increase (6), and possibly to the depolarization that accompanies the speract response. It is now worth exploring if CatSper participates in this process. Inset: A sperm drifting circular swimming trajectory in a chemoattractant gradient (orange shadow surrounding the egg) is stimulated periodically due to changes in the rate of chemoattractant capture. When swimming towards the egg in an ascending gradient, the onset of [Ca2+]i fluctuations is suppressed until the cell detects an ascending to descending gradient inversion. After a ∼200 ms delay, the spermatozoon undergoes a transient [Ca2+]i increase just before reaching the gradient minima (yellow circles). The rate of change in [Ca2+]i (d[Ca2+]i/dt) controls sperm trajectory. As a result, sperm turn (yellow) when they are swimming away from the egg and swim in a straighter trajectory (blue) while coming closer to the chemoattractant source (i. e., the egg jelly).
However, [Ca2+]i changes are the result of a complex signaling cascade shared, with some differences, by the sea urchin species thus far studied [89], [96]. In the signaling cascade of SAPs (Fig. 2), the cGMP-induced K+ efflux through a TetraKCNG/CNGK channel [97], [98] is an initial and fundamental step. This K+ efflux leads to hyperpolarization of the sperm membrane potential (Em) and modulates the function of several ion transporters and enzymes as shown in Fig. 2. Indeed, when this K+ efflux is inhibited by high K+ seawater, all the sperm responses to SAPs, except for an enhanced accumulation of cGMP, are completely inhibited [99].
One important consequence of this Em hyperpolarization is the pHi increase. This alkalinization, as motility initiation, is pHe dependent and inhibited by high [K+]e and by the absence of [Na+]e [85], [100], thus the Em-sensitive NHE (probably sNHE) is likely involved in the speract response. Our time-resolved kinetic measurements of speract induced pHi and [Ca2+]i changes using fast mixing device [101] and caged compounds (cGMP and speract) [102] revealed that the pHi increase induced by speract and cGMP occurs with a short delay (50 – 200 ms) always preceding the [Ca2+]i increase, supporting the idea that Em hyperpolarization activates the sNHE. However, the physiological relevance of the pHi increase induced by SAPs on sperm chemotaxis is under debate.
At present, it is considered that the rate of [Ca2+]i change (d [Ca2+]i / d t) determines the sea urchin sperm flagellar beating mode and swimming pattern [93]. It has been proposed that voltage-gated Ca2+ channels (Cav channels) are involved in the speract response [90], [103]. Both high voltage-activated (Cav1 and Cav2 families) and low voltage-activated (Cav3 family) transcripts were detected in sea urchin testis; specifically Cav1.2 was localized in the sperm flagellum by immunofluorescence [104]. At a first glance, if only typical Cav channels were involved in sea urchin sperm chemotaxis, the change in pHi induced by SAPs might not have a great impact on [Ca2+]i the regulation. Actually, Solzin et al. (2004) [105] showed that the chemotactic behavior of A. punctulata sperm to resact was not altered in the presence of imidazol (10 mM), which was proposed to function as a pHi buffer. In addition, the authors did not detect an increase in sperm pHi by uncaging cGMP. However, we observed the opposite result, namely, an increase in pHi induced by uncaging cGMP in A. punctulata as well as S. purpuratus sperm [96]. It must be noted also that pHi influences speract-receptor interaction [106], the activity of both GC and sAC [107], [108] and the activity of PDE5 [109]. Therefore, pHi could modulate sea urchin sperm swimming in several ways.
On the other hand, in recent years CatSper, the sperm specific Ca2+ channel activated by alkaline pHi, has been shown to be essential for mouse and human fertility [110], [111] reviewed in [4]. This channel is present in the S. purpuratus genome [113] and sperm from this species display a pHi dependent [Ca2+]i increase [114]. Therefore, it is worth exploring if CatSper participates in the speract cascade and if it is regulated by the pHi increase as in other species. The participation of pHi in chemotaxis could still turn out to be important. Consequently, further investigation is required, in particular single cell fast measurements, to reveal when and where the speract-induced pHi and [Ca2+]i increases occur within the flagellum and how they are related. Furthermore, the lack of specific sNHE inhibitors has hampered examining the role of pHi in motility regulation and generally in sperm physiology, thus identifying such compounds is imperative. Since sNHE is essential for mouse fertilization, specific sNHE inhibitors might also function as contraceptives.
Capacitation and Hyperactivation
Although mammalian spermatozoa acquire the capacity of flagellar motility in the epididymis [115], they remain quiescent in its acidic lumen which results form the V-ATPase activity found in its apical plasma membrane [116], [117]. Upon ejaculation, sperm are exposed to the seminal fluid (pH around 7.5) which ensures sperm motility [116]. However, ejaculated mammalian spermatozoa are unable to fertilize the egg until a process of maturation, named capacitation, takes place. Under physiological condition, spermatozoa complete capacitation in the female reproductive tract, but it is possible to reproduce this process in vitro in an appropriate medium, containing HCO3−, Ca2+ and albumin. Soon after ejaculation, sperm swim progressively with symmetric, low amplitude and high frequency flagellar beat. In contrast, spermatozoa recovered from the ampulla of the oviduct, the site of fertilization, show vigorous motility with asymmetric, high amplitude and low frequency flagellar beat, termed hyperactivation [118]. Hyperactivation is essential for spermatozoa to achieve fertilization in vivo since it is necessary for their migration towards the ampulla in the oviduct [119]. This type of motility is also necessary the penetrate ZP during in vitro fertilization [4]. In this sense, hyperactivation can be considered as part of capacitation, though capacitation is more usually defined as a preparation for sperm to undergo the AR. It has been established that Ca2+ is fundamental for hyperactivation and CatSper is an essential channel in this process. However, it is still not clear what the physiological signals that induce hyperactivation are [118], but it is known that pHe and HCO3−changes are crucial signals. In the rhesus monkey, the pH and HCO3− concentration in the lumen of the oviduct during the follicular phase are 7.2 and 35 mM, respectively, and they increase to 7.6 and 90 mM during ovulation [120]. Therefore, HCO3− and H+ transporters as well as CAs are considered crucial players for both capacitation and hyperactivation, as illustrated in Fig. 1. Since in vitro capacitation requires relatively long periods (≥ 30 min in mouse and several hours in human), the metabolic state, such as mitochondrial CO2 could have an important role in this process, although currently there is no experimental evidence.
The acrosome reaction
The acrosome is a secretory vesicle localized in the apical region of the sperm head in most species, except for some such as teleosts. When sperm receive an appropriate stimulus, this vesicle undergoes exocytosis and releases its acrosomal contents including hydrolytic enzymes; this process is called the acrosome reaction (AR). The AR is a unique single exocytotic event since the outer acrosomal membrane and the plasma membrane fuse at multiple sites and are released from the cell as hybrid vesicles. As a consequence, only the inner acrosomal membrane becomes a new plasma membrane. It is believed that only the sperm that has undergone the AR can penetrate the egg coat and complete sperm-egg fusion [121]. It is known that Ca2+ is one of the key elements needed for AR since its discovery in marine invertebrates [122], though pHi is also crucial to achieve this reaction [123], [124].
In mammals, a pHi rise is fundamental for the ZP-induced AR [124]. This pHi change is inhibited by pertussis toxin, suggesting the involvement of a G-protein (Gi). However, how this pHi increase is achieved remains unknown. On the other hand, recent studies using genetically manipulated mice, which express enhanced green fluorescence protein (EGFP) in the acrosome, revealed that most fertilizing spermatozoa had undergone AR before their contact with ZP [125], questioning if ZP is the physiological inducer of the AR. As mentioned previously, CatSper, Slo3 and the sAC-sNHE complex have been described as sperm-specific important players related to pHi in mammalian spermatozoa. Particularly, CatSper is proposed to participate in the AR besides its role in the hyperactivated sperm motility [126]–[128]. Differences between in vitro and in vivo fertilization have been accumulating. Indeed, many proteins proposed to be involved in the AR are not essential for in vivo fertilization since their elimination using gene-targeted male mice does not result in infertility [129]. Therefore, although technically difficult, understanding the physiological process of mammalian internal fertilization requires examining it in vivo.
In contrast to mammalian AR, it is clear that in the sea urchin, the physiological inducer of the AR is a fucose sulfate polymer (FSP), a major component of egg jelly coat [130]. When FSP binds to its receptor, spermatozoa undergo exocytosis of the acrosomal vesicle and expose the internal face of the acrosomal membrane (now the membrane of the acrosomal tubule). Subsequently, polymerization of actin takes place in the subacrosomal space leading to the formation of the 1 µm long acrosomal tubule. This process depends on the finely coordinated ionic flux changes evoked by FSP (reviewed in [45], [131], [132].
FSP binding to its receptor, suREJ1, evokes Em changes and increases in [Ca2+]i, pHi, [Na+]i, cAMP, IP3 and NAADP (reviewed in [45]. External Ca2+ is necessary for the [Ca2+]i increase and the internal alkalinization (0.1 – 0.2 pH units), which are prerequisites for the AR to occur [122], [123], [133]–[135]. It is thought that at least two different Ca2+ channels, acting sequentially, mediate the Ca2+ entry necessary to induce the AR. The first one is probably a CaV channel considering its sensitivity to verapamil and dihydropyridines [123], [136] and the second, which opens 5 s later, is a pHi-dependent channel activated by an alkalinization. Interestingly, this channel is inhibited by low [Na+]e, high [K+]e or TEA (a K+-channel blocker) [136], conditions that also inhibit the voltage-sensitive NHE [100], probably sNHE. These latter findings suggest the involvement of sNHE in the AR. However, inhibition of the initial Ca2+ entry nearly completely blocks the pHi increase triggered by FSP, implying the participation of a Ca2+-dependent H+ extrusion mechanism [123]. As sNHE itself seems to be Ca2+ independent [84], [85], the pHi increase associated to the AR may involve: a) a Ca2+-dependent K+ channel, which could mediate the sNHE activation through Em changes; b) cAMP produced by sAC, which possibly activates sNHE; and c) another type of NHEs.
Besides modulating the activity of the second Ca2+ channel, the pHi increase promotes actin polymerization leading to the formation of the acrosomal tubule [137]. On the other hand, as the pHi increase depends on pHe, the AR is highly pHe dependent, being prevented at pHe 7.3 – 7.5 and completely induced near physiological pHe 7.6 – 8.0 [138], which suggests the possible involvement of a Hv channel since the H+ gradient across the plasma membrane, as well as Em depolarization activates this channel. Although the importance of pHi in the sea urchin AR has long been known, the molecular mechanism is not fully understood and further investigation in necessary to elicit the details.
PERSPECTIVES
The regulation of pHi is fundamental for sperm function. Now we know that certain sperm-specific proteins essential for fertilization are involved in pHi regulation or regulated by pHi. However, many questions regarding the physiological function of these proteins are still unanswered. Currently it is clear that there are differences between in vitro fertilization and what occurs in the lumen of the oviduct that must be considered to fully understand this fundamental process. In fact, the pHi of the oviductal lumen dramatically elevates upon ovulation due to HCO3− production in the epithelial cells lining it. The pHi and ionic changes in the oviduct must be considered to understand the physiological sperm responses. In this sense, we still need to learn a lot about sperm pHi regulation by CO2, HCO3−, H+, CAs and HCO3− and H+ transporters, as well as their modulators, to understand how these fundamental cells operate and achieve fertilization.
Highlights.
-
➢
Sperm intracellular pH regulation is crucial for fertilization
-
➢
Intracellular pH dependent sperm ion channels
-
➢
Importance of sperm specific Na+/H+ exchanger in motility and capacitation
-
➢
sperm specific Na+/H+ exchanger interacts with soluble adenylyl cyclase
-
➢
carbonic anhydrase/ HCO3 transporter metabolon in sperm function
Acknowledgements
The authors thank Jose Luis De la Vega, Juan García-Rincón, and Shirley Ainsworth, for technical assistance. We thank Juan Manuel Hurtado, Roberto Rodríguez, Alma Valle and Arturo Ocádiz for computer services. This work was supported by National Institute of Health (NIH) Grants R01 HD038082-07A1 (to Pablo Visconti subcontract to AD), Consejo Nacional de Ciencia y Tecnología (CONACyT-Mexico) (2012/177138 to TN and 128566 to AD, TN and CT); Dirección General de Asuntos del Personal Académico/ Universidad Nacional Autónoma de México (DGAPA/ UNAM) (IN225406 to AD, IN203513 to TN and IN202212-3 to CT); The Alexander von Humboldt Foundation (to CT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boron WF. Regulation of intracellular pH. Adv. Physiol. Educ. 2004 Dec;28(1–4):160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- 2.Ruffin VA, Salameh AI, Boron WF, Parker MD. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014 Jan;5:43. doi: 10.3389/fphys.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Wang DK, Chen LM. The physiology of bicarbonate transporters in mammalian reproduction. Biol. Reprod. 2012 Apr;86(4):99. doi: 10.1095/biolreprod.111.096826. [DOI] [PubMed] [Google Scholar]
- 4.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001 Oct;413(6856):603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010 Mar;584(5):1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003 Dec;5(12):1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 7.Esposito G, Jaiswal BS, Xie F, a M, Krajnc-Franken M, a a Robben TJ, Strik AM, Kuil C, a Philipsen RL, van Duin M, Conti M, a Gossen J, Jaiswal BS. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. U. S. A. 2004 Mar;101(9):2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell. 2005 Aug;9(2):249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koty k. Intracellular pH and its measurement. Boca Raton, FL: CRC Press; 1989. p. 189. [Google Scholar]
- 10.Loiselle FB, Casey JR. Measurement of Intracellular pH. Methods Mol. Biol. 2010 Jan;637:311–331. doi: 10.1007/978-1-60761-700-6_17. [DOI] [PubMed] [Google Scholar]
- 11.Orlowski J, Grinstein S. Na+/H+ exchangers. Compr. Physiol. 2011 Oct;1(4):2083–2100. doi: 10.1002/cphy.c110020. [DOI] [PubMed] [Google Scholar]
- 12.V Lishko P, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010 Feb;140(3):327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 13.DeCoursey TE. Voltage-gated proton channels: molecular biology physiology, and pathophysiology of the H(V) family. Physiol. Rev. 2013 Apr;93(2):599–652. doi: 10.1152/physrev.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J. Exp. Biol. 2009 Jun;212(Pt 11):1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J. Exp. Biol. 2009 Jun;212(11):1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sindić A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr. Opin. Nephrol. Hypertens. 2007 Sep;16(Pt 5):484–490. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- 17.Figueiras-Fierro D, Acevedo JJ, Martínez-López P, Escoffier J, V Sepúlveda F, Balderas E, Orta G, Visconti PE, Darszon A. Electrophysiological evidence for the presence of cystic fibrosis transmembrane conductance regulator (CFTR) in mouse sperm. J. Cell. Physiol. 2013 Mar;228(3):590–601. doi: 10.1002/jcp.24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-González EO, Treviño CL, Castellano LE, de la Vega-Beltrán JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J. Biol. Chem. 2007 Aug;282(33):24397–24406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
- 19.Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H. Functional coupling of apical Cl-/HCO3- exchange with CFTR in stimulated HCO3-secretion by guinea pig interlobular pancreatic duct. Am. J. Physiol. Gastrointest. Liver Physiol. 2009 Jun;296(6):G1307–G1317. doi: 10.1152/ajpgi.90697.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi Liu G, Zhu H, Ma ZG, Wang XF, Chen ZH, Zhou SC, Dong HS, Zhang XH, Chung YW, Yuan YY, Yang WX, Chan HC. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl. Acad. Sci. U. S. A. 2007 Jun;104(23):9816–9821. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chávez JC, Hernández-González EO, Wertheimer E, Visconti PE, Darszon A, Treviño CL. Participation of the Cl-/HCO(3)- exchangers SLC26A3 and SLC26A6, the Cl- channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol. Reprod. 2012 Jan;86(1):1–14. doi: 10.1095/biolreprod.111.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quill TA, Wang D, Garbers DL. Insights into sperm cell motility signaling through sNHE and the CatSpers. Mol. Cell. Endocrinol. 2006 May;250(1–2):84–92. doi: 10.1016/j.mce.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J. Biol. Chem. 1985 Aug;260(17):9699–9705. [PubMed] [Google Scholar]
- 24.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. U. S A. 2003 Sep;100(19):10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. U. S A. 1999 Jan;96(1):79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000 Jul;289(5479):625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J. Biol. Chem. 2001 Aug;276(34):31698–31708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- 28.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003 Jan;17(1):82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 29.Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J. Physiol. 2005 Aug;567(Pt 1):3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014 Jan;466(1):61–76. doi: 10.1007/s00424-013-1408-8. [DOI] [PubMed] [Google Scholar]
- 31.Garcia MA, Meizel S. Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol. Reprod. Dev. 1999 Feb;52(2):189–195. doi: 10.1002/(SICI)1098-2795(199902)52:2<189::AID-MRD10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Woo AL, James PF, Lingrel JB. Roles of the Na,K-ATPase alpha4 isoform and the Na+/H+ exchanger in sperm motility. Mol. Reprod. Dev. 2002 Jul;62(3):348–356. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Huang JC, Zuo WL, Lu CL, Chen M, Sen Zhang X, Li YC, Cai H, Zhou WL, Hu ZY, Gao F, Liu YX. A novel testis-specific Na+/H+ exchanger is involved in sperm motility and fertility. Front. Biosci. (Elite Ed) 2010 Jan;2(3):566–581. doi: 10.2741/e115. [DOI] [PubMed] [Google Scholar]
- 34.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004 Feb;447(5):549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee HC. A membrane potential-sensitive Na+-H+ exchange system in flagella isolated from sea urchin spermatozoa. J. Biol. Chem. 1984 Dec;259(24):15315–15319. [PubMed] [Google Scholar]
- 36.Lee HC. The voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa flagellar membrane vesicles studied with an entrapped pH probe. J. Biol. Chem. 1985 Sep;260(19):10794–10799. [PubMed] [Google Scholar]
- 37.Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, Moe OW, Garbers DL. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc. Natl. Acad. Sci. U. S. A. 2007 May;104(22):9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomura M, Vacquier VD. Proteins associated with soluble adenylyl cyclase in sea urchin sperm flagella. Cell Motil. Cytoskeleton. 2006 Sep;63(9):582–590. doi: 10.1002/cm.20147. [DOI] [PubMed] [Google Scholar]
- 39.Brelidze TI, Carlson AE, Sankaran B, Zagotta WN. Structure of the carboxy-terminal region of a KCNH channel. Nature. 2012 Jan;481(7382):530–533. doi: 10.1038/nature10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannan N, Wu J, Anand GS, Yooseph S, Neuwald AF, Venter JC, Taylor SS. Evolution of allostery in the cyclic nucleotide binding module. Genome Biol. 2007 Jan;8(12):R264. doi: 10.1186/gb-2007-8-12-r264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki M, Takagi M, Y Okamura. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006 Apr;312(5773):589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 42.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006 Apr;440(7088):1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aonuma S, Okabe M, Kawaguchi M, Y Kishi. Zinc effects on mouse spermatozoa and in-vitro fertilization. J. Reprod. Fertil. 1981 Nov;63(2):463–466. doi: 10.1530/jrf.0.0630463. [DOI] [PubMed] [Google Scholar]
- 44.Supuran CT. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010 Jun;20(12):3467–3474. doi: 10.1016/j.bmcl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development maturation, and function of spermatozoa. Physiol. Rev. 2011 Oct;91(4):1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- 46.Wandernoth PM, Raubuch M, Mannowetz N, Becker HM, Deitmer JW, Sly WS, Wennemuth G. Role of carbonic anhydrase IV in the bicarbonate-mediated activation of murine and human sperm. PLoS One. 2010 Jan;5(11):e15061. doi: 10.1371/journal.pone.0015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannowetz N, Wandernoth PM, Wennemuth G. Glucose is a pH-dependent motor for sperm beat frequency during early activation. PLoS One. 2012 Jan;7(7):e41030. doi: 10.1371/journal.pone.0041030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inaba K, Dréanno C, Cosson J. Control of flatfish sperm motility by CO2 and carbonic anhydrase. Cell Motil. Cytoskeleton. 2003 Jul;55(3):174–187. doi: 10.1002/cm.10119. [DOI] [PubMed] [Google Scholar]
- 49.Hirohashi N, Alvarez L, Shiba K, Fujiwara E, Iwata Y, Mohri T, Inaba K, Chiba K, Ochi H, Supuran CT, Kotzur N, Kakiuchi Y, Kaupp UB, Baba SA. Sperm from sneaker male squids exhibit chemotactic swarming to CO2. Curr. Biol. 2013 May;23(9):775–781. doi: 10.1016/j.cub.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 50.Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3--related species. Biochim. Biophys. Acta. 2010 Feb;1804(2):410–421. doi: 10.1016/j.bbapap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3) transporters. Mol. Aspects Med. 2013;34(2–3):159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol. Aspects Med. 2013;34(2–3):494–515. doi: 10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cant N, Pollock N, Ford RC. CFTR structure and cystic fibrosis. Int. J. Biochem. Cell Biol. 2014 Feb; doi: 10.1016/j.biocel.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Holappa K, Mustonen M, Parvinen M, Vihko P, Rajaniemi H, Kellokumpu S. Primary structure of a sperm cell anion exchanger and its messenger ribonucleic acid expression during spermatogenesis. Biol. Reprod. 1999 Oct;61(4):981–986. doi: 10.1095/biolreprod61.4.981. [DOI] [PubMed] [Google Scholar]
- 55.Parkkila S, Rajaniemi H, Kellokumpu S. Polarized expression of a band 3-related protein in mammalian sperm cells. Biol. Reprod. 1993 Aug;49(2):326–331. doi: 10.1095/biolreprod49.2.326. [DOI] [PubMed] [Google Scholar]
- 56.Höglund P, Hihnala S, Kujala M, Tiitinen A, Dunkel L, Holmberg C. Disruption of the SLC26A3-mediated anion transport is associated with male subfertility. Fertil. Steril. 2006 Jan;85(1):232–235. doi: 10.1016/j.fertnstert.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 57.Rode B, Dirami T, Bakouh N, Rizk-Rabin M, Norez C, Lhuillier P, Lorès P, Jollivet M, Melin P, Zvetkova I, Bienvenu T, Becq F, Planelles G, Edelman A, Gacon G, Touré A. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: a potential role during sperm capacitation. Hum. Mol. Genet. 2012 Mar;21(6):1287–1298. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- 58.Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, L, Tsang L, Chung YW, Höglund P, Chan HC, Shi QX. Cl- is required for HCO3- entry necessary for sperm capacitation in guinea pig: involvement of a Cl-/HCO3- exchanger (SLC26A3) and CFTR. Biol. Reprod. 2009 Jan;80(1):115–123. doi: 10.1095/biolreprod.108.068528. [DOI] [PubMed] [Google Scholar]
- 59.Chan HC, Sun X. SLC26 anion exchangers in uterine epithelial cells and spermatozoa: clues from the past and hints to the future. Cell Biol. Int. 2014 Jan;38(1):1–7. doi: 10.1002/cbin.10183. [DOI] [PubMed] [Google Scholar]
- 60.Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J. Biol. Chem. 2003 Feb;278(9):7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- 61.Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na(+)-, Cl(-)-, and HCO3(-)-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 1996 Feb;173(2):510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- 62.Santi CM, Santos T, Hernández-Cruz A, Darszon A. Properties of a novel pH-dependent Ca2+ permeation pathway present in male germ cells with possible roles in spermatogenesis and mature sperm function. J. Gen. Physiol. 1998 Jul;112(1):33–53. doi: 10.1085/jgp.112.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlson AE, Hille B, Babcock DF. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol. 2007 Dec;312(1):183–192. doi: 10.1016/j.ydbio.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc. Natl. Acad. Sci. U. S. A. 2001 Oct;98(22):12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. U. S. A. 2003 Dec;100(25):14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439(7077):737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 67.V Lishko P, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012 Oct;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barratt CL, Publicover SJ. Sperm are promiscuous and CatSper is to blame…. EMBO J. 2012 Apr;31(7):1624–1626. doi: 10.1038/emboj.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH- sensitive K+ channel from mammalian spermatocytes. J. Biol. Chem. 1998 Feb;273(6):3509–3516. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 70.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. U. S. A. 2011 Apr;108(14):5879–5884. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenker C, Zhou Y, Müller A, Echeverry FA, Trötschel C, Poetsch A, Xia X-M, Bönigk W, Lingle CJ, Kaupp UB, Strünker T. The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife. 2014 Jan;3:e01438. doi: 10.7554/eLife.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mannowetz N, Naidoo NM, Choo SAS, Smith JF, V Lishko P. Slo1 is the principal potassium channel of human spermatozoa. Elife. 2013 Jan;2:e01009. doi: 10.7554/eLife.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.López-González I, Torres-Rodríguez P, Sánchez-Carranza O, Solís-López A, Santi CM, Darszon A, Treviño CL. Membrane Hyperpolarization during Human Sperm Capacitation. Mol. Hum. Reprod. 2014 Apr; [Google Scholar]
- 74.Mansell SA, Publicover SJ, Barratt CLR, Wilson SM. Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol. Hum. Reprod. 2014 May;20(5):392–408. doi: 10.1093/molehr/gau003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christen R, Schackmann RW, Shapiro BM. Metabolism of sea urchin sperm. Interrelationships between intracellular pH, ATPase activity, and mitochondrial respiration. J. Biol. Chem. 1983 May;258(9):5392–5399. [PubMed] [Google Scholar]
- 76.Giroux-Widemann V, Jouannet P, Pignot-Paintrand I, Feneux D. Effects of pH on the reactivation of human spermatozoa demembranated with Triton X-100. Mol. Reprod. Dev. 1991 Jun;29(2):157–162. doi: 10.1002/mrd.1080290211. [DOI] [PubMed] [Google Scholar]
- 77.Johnson CH, Clapper DL, Winkler MM, Lee HC, Epel D. A volatile inhibitor immobilizes sea urchin sperm in semen by depressing the intracellular pH. Dev. Biol. 1983 Aug;98(2):493–501. doi: 10.1016/0012-1606(83)90378-0. [DOI] [PubMed] [Google Scholar]
- 78.Lee HC, Johnson C, Epel D, Hall M, Station HM. Changes in internal pH associated with initiation of motility and acrosome reaction of sea urchin sperm. Dev. Biol. 1983 Jan;45(1):31–45. doi: 10.1016/0012-1606(83)90004-0. [DOI] [PubMed] [Google Scholar]
- 79.Christen R, Schackmann RW, Shapiro BM. Elevation of the intracellular pH activates respiration motility of sperm of the sea urchin, Strongylocentrotus purpuratus. J. Biol. Chem. 1982 Dec;257(24):14881–14890. [PubMed] [Google Scholar]
- 80.Shapiro BM, Tombes RM. A biochemical pathway for a cellular behaviour: pHi phosphorylcreatine shuttles, and sperm motility. Bioessays. 1985 Sep;3(3):100–103. doi: 10.1002/bies.950030303. [DOI] [PubMed] [Google Scholar]
- 81.Clapper DL, Davis JA, Lamothe PJ, Patton C, Epel D. Involvement of zinc in the regulation of pHi motility, and acrosome reactions in sea urchin sperm. J. Cell Biol. 1985 Jun;100(6):1817–1824. doi: 10.1083/jcb.100.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suzuki N. Structure, function and biosynthesis of sperm-activating peptides and fucose sulfate glycoconjugate in the extracellular coat of sea urchin eggs. Zoolog. Sci. 1995 Feb;12(1):13–27. doi: 10.2108/zsj.12.13. [DOI] [PubMed] [Google Scholar]
- 83.Ohtake H. Respiratory behaviour of sea-urchin spermatozoa. II. Sperm-activating substance obtained from jelly coat of sea-urchin eggs. J. Exp. Zool. 1976 Dec;198(3):313–322. doi: 10.1002/jez.1401980304. [DOI] [PubMed] [Google Scholar]
- 84.Hansbrough JR, Garbers DL. Sodium-dependent activation of sea urchin spermatozoa by speract and monensin. J. Biol. Chem. 1981 Mar;256(5):2235–2241. [PubMed] [Google Scholar]
- 85.Repaske DR, Garbers DLRRDL. A hydrogen ion flux mediates stimulation of respiratory activity by speract in sea urchin spermatozoa. J. Biol. Chem. 1983 May;258(10):6025–6029. [PubMed] [Google Scholar]
- 86.Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int. J. Dev. Biol. 2008 Jan;52(5–6):595–606. doi: 10.1387/ijdb.072550ad. [DOI] [PubMed] [Google Scholar]
- 87.Ward GE, Brokaw CJ, Garbers DL, Vacquier VD. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J. Cell Biol. 1985 Dec;101(6):2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guerrero A, Nishigaki T, Carneiro J, Tatsu Y, Wood CD, Darszon A. Tuning sperm chemotaxis by calcium burst timing. Dev. Biol. 2010 Aug;344(1):52–65. doi: 10.1016/j.ydbio.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 89.Kaupp UB, Kashikar ND, Weyand I. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 2008 Jan;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- 90.Wood CD, Nishigaki T, Furuta T, Baba SA, Darszon A. Real-time analysis of the role of Ca2+ in flagellar movement and motility in single sea urchin sperm. J. Cell Biol. 2005 Jun;169(5):725–731. doi: 10.1083/jcb.200411001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood CD, Nishigaki T, Tatsu Y, Yumoto N, Baba SA, Whitaker M, Darszon A. Altering the speract-induced ion permeability changes that generate flagellar Ca2+ spikes regulates their kinetics and sea urchin sperm motility. Dev. Biol. 2007 Jun;306(2):525–537. doi: 10.1016/j.ydbio.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 92.Böhmer M, Van Q, Weyand I, Hagen V, Beyermann M, Matsumoto M, Hoshi M, Hildebrand E, Kaupp UB. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 2005 Aug;24(15):2741–2752. doi: 10.1038/sj.emboj.7600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alvarez L, Dai L, Friedrich BM, Kashikar ND, Gregor I, Pascal R, Kaupp UB. The rate of change in Ca(2+) concentration controls sperm chemotaxis. J. Cell Biol. 2012 Mar;196(5):653–663. doi: 10.1083/jcb.201106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kashikar ND, Alvarez L, Seifert R, Gregor I, Jäckle O, Beyermann M, Krause E, Kaupp UB. Temporal sampling resetting, and adaptation orchestrate gradient sensing in sperm. J. Cell Biol. 2012 Sep;198(6):1075–1091. doi: 10.1083/jcb.201204024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood CD, Darszon A, Whitaker M. Speract induces calcium oscillations in the sperm tail. J. Cell Biol. 2003 Apr;161(1):89–101. doi: 10.1083/jcb.200212053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int. J. Dev. Biol. 2008 Jan;52(5–6):595–606. doi: 10.1387/ijdb.072550ad. [DOI] [PubMed] [Google Scholar]
- 97.Galindo BE, de la Vega-Beltrán JL, Labarca P, Vacquier VD, Darszon A. Sp-tetraKCNG: A novel cyclic nucleotide gated K(+) channel. Biochem. Biophys. Res.j Commun. 2007 Mar;354(3):668–675. doi: 10.1016/j.bbrc.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 98.Bönigk W, Loogen A, Seifert R, Kashikar N, Klemm C, Krause E, Hagen V, Kremmer E, Strünker T, Kaupp UB. An atypical CNG channel activated by a single cGMP molecule controls sperm chemotaxis. Sci. Signal. 2009 Jan;2(94):ra68. doi: 10.1126/scisignal.2000516. [DOI] [PubMed] [Google Scholar]
- 99.Harumi T, Hoshino K, Suzuki N. Effects of Sperm-Activating Peptide I on Hemicentrotus pulcherrimus Spermatozoa in High Potassium Sea Water. Dev. Growth Differ. 1992 Apr;34(2):163–172. doi: 10.1111/j.1440-169X.1992.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 100.Lee HC, Garbers DL. Modulation of the voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J. Biol. Chem. 1986 Dec;261(34):16026–16032. [PubMed] [Google Scholar]
- 101.Nishigaki T, Zamudio FZ, Possani LD, Darszon A. Time-resolved sperm responses to an egg peptide measured by stopped-flow fluorometry. Biochem. Biophys. Res. Commun. 2001 Jun;284(2):531–535. doi: 10.1006/bbrc.2001.5000. [DOI] [PubMed] [Google Scholar]
- 102.Nishigaki T, Wood CD, Tatsu Y, Yumoto N, Furuta T, Elias D, Shiba K, Baba SA, Darszon A. A sea urchin egg jelly peptide induces a cGMP-mediated decrease in sperm intracellular Ca2+ before its increase. Dev. Biol. 2004 Aug;272(2):376–388. doi: 10.1016/j.ydbio.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 103.Strünker T, Weyand I, Bönigk W, Van Q, Loogen A, Brown JE, Kashikar N, Hagen V, Krause E, Kaupp UB. A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nat. Cell Biol. 2006 Oct;8(10):1149–1154. doi: 10.1038/ncb1473. [DOI] [PubMed] [Google Scholar]
- 104.Granados-Gonzalez G, Mendoza-Lujambio I, Rodriguez E, Galindo BE, Beltrán C, Darszon A. Identification of voltage-dependent Ca2+ channels in sea urchin sperm. FEBS Lett. 2005 Dec;579(29):6667–6672. doi: 10.1016/j.febslet.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 105.ISolzin J, Helbig A, Van Q, Brown JE, Hildebrand E, Weyand I, Kaupp UB. Revisiting the role of H+ in chemotactic signaling of sperm. J. Gen. Physiol. 2004 Aug;124(2):115–124. doi: 10.1085/jgp.200409030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nishigaki T, Darszon A. Real-time measurements of the interactions between fluorescent speract and its sperm receptor. Dev. Biol. 2000 Jul;223(1):17–26. doi: 10.1006/dbio.2000.9734. [DOI] [PubMed] [Google Scholar]
- 107.Garbers DL. Molecular basis of fertilization. Annu. Rev. Biochem. 1989 Jan;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- 108.Nomura M, Beltrán C, Darszon A, Vacquier VD. A soluble adenylyl cyclase from sea urchin spermatozoa. Gene. 2005 Jul;353(2):231–238. doi: 10.1016/j.gene.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 109.Su Y-H, Vacquier VD. Cyclic GMP-specific phosphodiesterase-5 regulates motility of sea urchin spermatozoa. Mol. Biol. Cell. 2006 Jan;17(1):114–121. doi: 10.1091/mbc.E05-08-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ren D, Navarro B, Jackson AC, Hsu S, Shi Q, Clapham DE, Perez G, Tilly JL. A sperm ion channel required for sperm motility and male fertility. Nature. 2001 Oct;413(6856):603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, V Lishko P. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc. Natl. Acad. Sci. U. S. A. 2013 Apr;110(17):6823–6828. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nishigaki T, González-Cota AL, Orta G. CatSper in Male Infertility. In: Weiss N, Koschak A, editors. Pathologies of Calcium Channels. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. pp. 713–728. [Google Scholar]
- 113.Cai X, Clapham DE. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperbeta. PLoS One. 2008 Jan;3(10):e3569. doi: 10.1371/journal.pone.0003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zapata O, Darszon A, Guerrero A, García L, Rodríguez E. Acrosome reaction inactivation in sea urchin sperm. Biochim. Biophys. Acta. 1998 Mar;1401(3):329–338. doi: 10.1016/s0167-4889(97)00127-4. [DOI] [PubMed] [Google Scholar]
- 115.Mohri H, Yanagimachi R. Characteristics of motor apparatus in testicular, epididymal and ejaculated spermatozoa. A study using demembranated sperm models. Exp. Cell Res. 1980 May;127(1):191–196. doi: 10.1016/0014-4827(80)90426-7. [DOI] [PubMed] [Google Scholar]
- 116.Carr DW, Usselman MC, Acott TS. Effects of pH lactate, and viscoelastic drag on sperm motility: a species comparison. Biol. Reprod. 1985 Oct;33(3):588–595. doi: 10.1095/biolreprod33.3.588. [DOI] [PubMed] [Google Scholar]
- 117.Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat. Med. 1996 Apr;2(4):470–472. doi: 10.1038/nm0496-470. [DOI] [PubMed] [Google Scholar]
- 118.Suarez SS. Control of hyperactivation in sperm. Hum. Reprod. Update. 2008;14(6):647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 119.Ho K, Wolff CA, Suarez SS. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev. 2009 Jan;21(2):345–350. doi: 10.1071/rd08183. [DOI] [PubMed] [Google Scholar]
- 120.Maas DH, Storey BT, Mastroianni L. Hydrogen ion and carbon dioxide content of the oviductal fluid of the rhesus monkey (Macaca mulatta) Fertil. Steril. 1977 Sep;28(9):981–985. [PubMed] [Google Scholar]
- 121.Yanagimachi R. Mammalian fertilization. In: Knobile E, Neill JD, editors. The physiology of reproduction. 1994th ed. New York: Raven; 1994. pp. 189–317. [Google Scholar]
- 122.Dan JC. Studies on the acrosome. III. Effect of calcium deficiency. Biol. Bull. 1954 Dec;107(3):335–349. [Google Scholar]
- 123.Schackmann RW, Eddy EM, Shapiro BM. The acrosome reaction of Strongylocentrotus purpuratus sperm. Ion requirements and movements. Dev. Biol. 1978 Aug;65(2):483–495. doi: 10.1016/0012-1606(78)90043-x. [DOI] [PubMed] [Google Scholar]
- 124.Arnoult C, Y Zeng, Florman HM. ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. J. Cell Biol. 1996 Aug;134(3):637–645. doi: 10.1083/jcb.134.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin M, Fujiwara E, Y Kakiuchi, Okabe M, Y Satouh, Baba SA, Chiba K, Hirohashi N. “Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization,”. 2011 doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol. Reprod. 2009 Jun;80(6):1092–1098. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011 Mar;471(7338):382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 128.V Lishko P, Botchkina IL, Y Kirichok. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011 Mar;471(7338):387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 129.Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm’s journey to and interaction with the oocyte. J. Clin. Invest. 2010 Apr;120(4):984–994. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vilela-Silva AC, Hirohashi N, Mourão PA. The structure of sulfated polysaccharides ensures a carbohydrate-based mechanism for species recognition during sea urchin fertilization. Int. J. Dev. Biol. 2008 Jan;52(5–6):551–559. doi: 10.1387/ijdb.072531av. [DOI] [PubMed] [Google Scholar]
- 131.Santella L, Vasilev F, Chun JT. Fertilization in echinoderms. Biochem. Biophys. Res. Commun. 2012 Aug;425(3):588–594. doi: 10.1016/j.bbrc.2012.07.159. [DOI] [PubMed] [Google Scholar]
- 132.Vacquier VD. The quest for the sea urchin egg receptor for sperm. Biochem. Biophys. Res. Commun. 2012 Aug;425(3):583–587. doi: 10.1016/j.bbrc.2012.07.132. [DOI] [PubMed] [Google Scholar]
- 133.Schackmann RW, Christen R, Shapiro BM. Membrane potential depolarization and increased intracellular pH accompany the acrosome reaction of sea urchin sperm. Proc. Natl. Acad. Sci. U. S. A. 1981 Oct;78(10):6066–6070. doi: 10.1073/pnas.78.10.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.García-Soto J, Darszon A. High pH-induced acrosome reaction and Ca2+ uptake in sea urchin sperm suspended in Na+-free seawater. Dev. Biol. 1985 Aug;110(2):338–345. doi: 10.1016/0012-1606(85)90093-4. [DOI] [PubMed] [Google Scholar]
- 135.Trimmer JS, Schackmann RW, Vacquier VD. Monoclonal antibodies increase intracellular Ca2+ in sea urchin spermatozoa. Proc. Natl. Acad. Sci. U. S. A. 1986 Dec;83(23):9055–9059. doi: 10.1073/pnas.83.23.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guerrero A, Darszon A. Evidence for the activation of two different Ca2+ channels during the egg jelly-induced acrosome reaction of sea urchin sperm. J. Biol. Chem. 1989 Nov;264(33):19593–19599. [PubMed] [Google Scholar]
- 137.G Tilney L, Kiehart DP, Sardet C, Tilney M. Polymerization of actin. IV. Role of Ca++ and H+ in the assembly of actin and in membrane fusion in the acrosomal reaction of echinoderm sperm. J. Cell Biol. 1978 May;77(2):536–550. doi: 10.1083/jcb.77.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schackmann RW, Shapiro BM. A partial sequence of ionic changes associated with the acrosome reaction of Strongylocentrotus purpuratus. Dev. Biol. 1981 Jan;81(1):145–154. doi: 10.1016/0012-1606(81)90357-2. [DOI] [PubMed] [Google Scholar]
- 139.Ali Akbar S, Nicolaides KH, Brown PR. Carbonic anhydrase isoenzymes CAI and CAIIin semen, decidua, chorionic villi and various fetal tissues. Early Hum. Dev. 1998 Jul;51(3):205–211. doi: 10.1016/s0378-3782(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 140.Parkkila S, Kaunisto K, Kellokumpu S, Rajaniemi H. A high activity carbonic anhydrase isoenzyme (CA II) is present in mammalian spermatozoa. Histochemistry. 1991 Jan;95(5):477–482. doi: 10.1007/BF00315743. [DOI] [PubMed] [Google Scholar]
- 141.Mezquita P, Mezquita C, Mezquita J. Novel transcripts of carbonic anhydrase II in mouse and human testis. Mol. Hum. Reprod. 1999 Mar;5(3):199–205. doi: 10.1093/molehr/5.3.199. [DOI] [PubMed] [Google Scholar]
- 142.Halmi P, Lehtonen J, Waheed A, Sly WS, Parkkila S. Expression of hypoxia-inducible, membrane-bound carbonic anhydrase isozyme XII in mouse tissues. Anat. Rec. A. Discov. Mol. Cell. EBiol. 2004 Mar;277(1):171–177. doi: 10.1002/ar.a.20001. [DOI] [PubMed] [Google Scholar]
- 143.Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, Parkkila A-K, Saarnio J, Kivelä AJ, Waheed A, Sly WS, Parkkila S. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J. Biol. 2004 Jan;279(4):2719–2727. doi: 10.1074/jbc.M308984200. [DOI] [PubMed] [Google Scholar]