Abstract
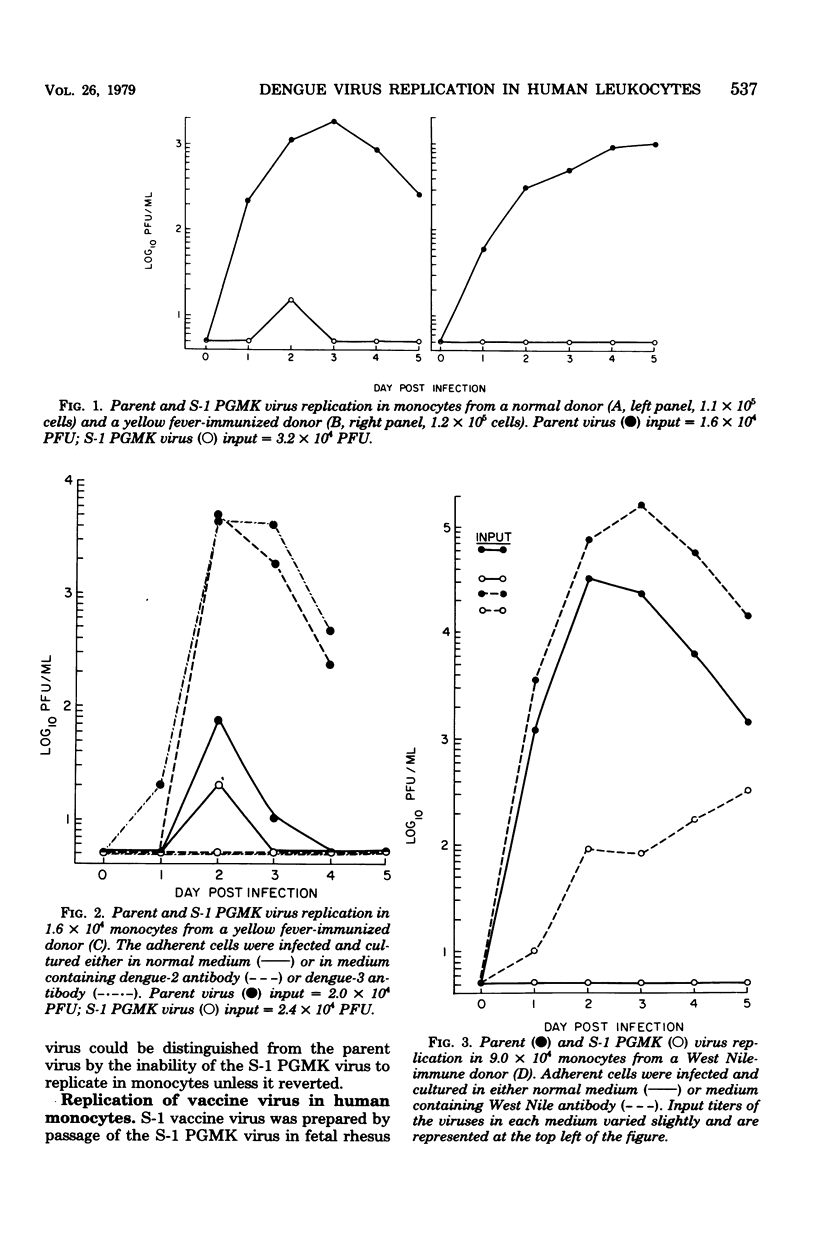
Three passage levels of dengue-2 virus strain PR-159, obtained during the course of deriving the attenuated S-1 vaccine, were tested for their ability to replicate in subpopulations of human peripheral blood leukocytes: (i) 6th primary African green monkey kidney (PGMK) cell passage (parent virus); (ii) 19th PGMK cell passage of a small-plaque-forming clone derived from the parent virus (S-1 PGMK virus); and (iii) virus derived by four additional passages of the S-1 PGMK virus in diploid fetal rhesus lung cells (S-1 vaccine virus). Replication of these PR-159 viruses and another strain of dengue-2 virus adapted to Raji cells (16681-Raji virus) was measured in adherent and nonadherent mononuclear cells. All viruses except the S-1 PGMK virus replicated in monocytes. Occasional replication of the S-1 PGMK virus was associated with reversion to parent virus. The addition to the monocyte cultures of low concentrations of homologous dengue-2 antibody or non-neutralizing heterologous antibody increased the yield of the parent virus as much as 400-fold. This phenomenon of immune enhancement usually enabled the S-1 PGMK virus to replicate slowly in monocytes, but the progeny virus produced large plaques similar to the parent virus. Replication of the S-1 vaccine virus in cultured monocytes did not result in the appearance of large plaques. We could not recover S-1 vaccine virus from monocytes harvested from infected volunteers in the same manner that monocytes from natural human infections yield wild virus. The three passage levels of PR-159 virus were tested for replication in lymphocytes in comparison with the 16681-Raji virus. Only the 16681-Raji virus replicated in human lymphocytes cultured with or without enhancing antibody.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Eckels K. H., Brandt W. E., Harrison V. R., McCown J. M., Russell P. K. Isolation of a temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect Immun. 1976 Nov;14(5):1221–1227. doi: 10.1128/iai.14.5.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Chow J. S., Marchette N. J. Immunological enhancement of dengue virus replication. Nat New Biol. 1973 May 2;243(122):24–26. [PubMed] [Google Scholar]
- Halstead S. B., Marchette N. J., Sung Chow J. S., Lolekha S. Dengue virus replication enhancement in peripheral blood leukocytes from immune human beings. Proc Soc Exp Biol Med. 1976 Jan;151(1):136–139. doi: 10.3181/00379727-151-39160. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977 Feb 24;265(5596):739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison V. R., Eckels K. H., Sagartz J. W., Russell P. K. Virulence and immunogenicity of a temperature-sensitive dengue-2 virus in lower primates. Infect Immun. 1977 Oct;18(1):151–156. doi: 10.1128/iai.18.1.151-156.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Beall J. L. Chromatographic separations of alphavirus strains by hydroxylapatite. J Clin Microbiol. 1977 Sep;6(3):238–243. doi: 10.1128/jcm.6.3.238-243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Gorelkin L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J Infect Dis. 1975 Dec;132(6):667–676. doi: 10.1093/infdis/132.6.667. [DOI] [PubMed] [Google Scholar]
- Marchette N. J., Halstead S. B., Chow J. S. Replication of dengue viruses in cultures of peripheral blood leukocytes from dengue-immune rhesus monkeys. J Infect Dis. 1976 Mar;133(3):274–282. doi: 10.1093/infdis/133.3.274. [DOI] [PubMed] [Google Scholar]
- Marchette N. J., Halstead S. B. Phytohemagglutinin enhancement of dengue-2 virus replication in nonimmune rhesus monkey peripheral blood leukocytes. Infect Immun. 1978 Jan;19(1):40–45. doi: 10.1128/iai.19.1.40-45.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J. S., Diwan A. R., Falkler W. A., Jr, Yang H. Y., Halstead S. B. Dengue carrier culture and antigen production in human lymphoblastoid lines. Intervirology. 1975;5(3-4):137–149. doi: 10.1159/000149891. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Brandt W. E., Russell P. K., Dixon F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol. 1976 Sep;117(3):953–961. [PubMed] [Google Scholar]



