Based on a musculoskeletal model, Neptune et al. (2004) proposed that a substantial cost for human walking occurs early in the single support phase. Their model (originally described by Neptune et al., 2001) performed a large burst of net musculotendon work beginning immediately after double support and ending at mid-stance, as the body center of mass (COM) moved upward. It accounted for “nearly 80% of the net musculotendon work produced over the entire gait cycle,” leading them to conclude that it is a major contributor to the metabolic cost of human walking, with the purpose of raising the COM (Neptune et al., 2004). We were surprised by these claims, because they appear at odds with the literature. We have therefore performed a quantitative comparison of their model (“Model”) with published experimental data (“Human”), resulting in quite different conclusions. The issue is important not only for scientific understanding of the biomechanical determinants of gait, but also for the implications regarding clinical issues including design of surgical procedures (Zajac et al., 2003).
Under question are two portions of a stride where positive work is performed on the COM (“COM work,” also traditionally referred to as “external work”). The first interval is referred to as “Region 2” by Neptune et al. (2004) and corresponds approximately to 15 – 30% of a stride, with heel-strike defined as 0%. The second interval, “Region 4,” refers to the positive work performed late in stance for push-off, from about 48% of a stride until end of stance, about 61%. The Model performs about 15 J of net musculotendon work during Region 2, and about 7.6 J during Region 4. All comparisons will focus on these two Regions.
We compare three types of normal Human walking data with the Model. We reproduce Human joint power data (Figure 2 of Neptune et al., 2001), COM work data (Figure 4 of Neptune et al., 2004), and vertical ground reaction force (GRF) data (from Figure 1 of Neptune et al., 2004), all previously published together with Model results. Also reproduced are an archival set of joint power data (Table 4.34(b) of Winter, 1991) and our own COM work data (Figure 3 of Kuo et al., 2005). These are compared against corresponding quantities and the net musculontendon work from two variants of the same Model (Neptune et al., 2001; Neptune et al., 2004), both based on normal gait. We extracted the data by re-digitizing the appropriate figures, converting units as necessary, and then computing respective quantities. Inconsistencies are noted only when they far exceed the limited accuracy of our estimates and the differences to be expected from distinct data sets. Numerical quantities and details are reported in the Electronic Supplementary Material.
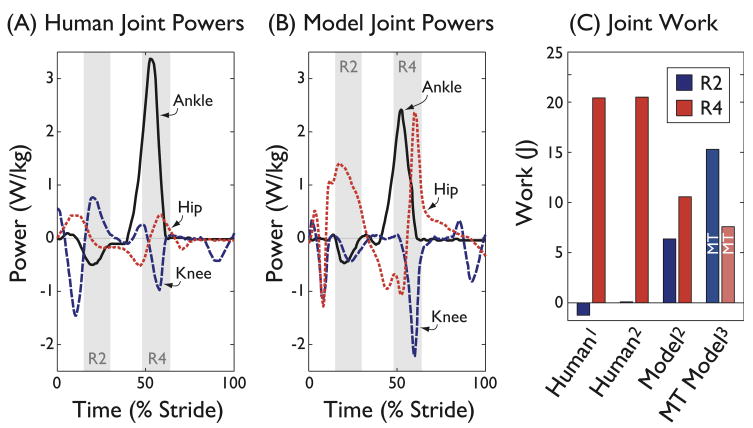
Human data do not support the Model (Neptune et al., 2004). Human joint powers show far less work in Region 2 than the Model (Figure 1). Human net joint work in Region 2 is no more than 0.1 J (Neptune et al., 2004), with only the knee contributing substantial positive work. In contrast, the Model’s knee performs negative work during Region 2, and the hip positive work, both opposite in sign to Human data. These account for relatively low correlation coefficients between Model and Human (r = 0.38 for knee, r = 0.44 for hip; Figure 1B).
Figure 1.
Joint powers from Human and Model. (A) Joint powers for ankle, knee, and hip, from Human data2, plotted vs. time for one stride beginning with heel-strike. Regions 2 and 4 are indicated by shaded areas (R2 and R4). (B) Model joint powers3 (Neptune et al., 2004). Comparison between Human and Model joint powers yielded correlation coefficients r = 0.99 for ankle, r = 0.38 for knee, and r = 0.44 for hip. (C) Net work per stride by Region, comparing two Human data sets against Model’s joint work and net musculotendon (MT) work. Superscripts indicate the following references: 1Winter, 1991; 2Neptune et al., 2001; 3Neptune et al., 2004 (net musculotendon work).
Comparisons of Region 4 also reveal large discrepancies. Both joint work (at the ankle) and COM work (Figure 2) show that Humans perform at least twice as much work (about 20 J) as the Model during push-off. Comparing the Model’s net musculotendon work against Human, the Model performs more than 100 times the Human joint work during Region 2 (Figure 1C), and about one-third during Region 4. In Humans, Region 4 joint work is at least 150 times greater than Region 2 (Neptune et al., 2001).
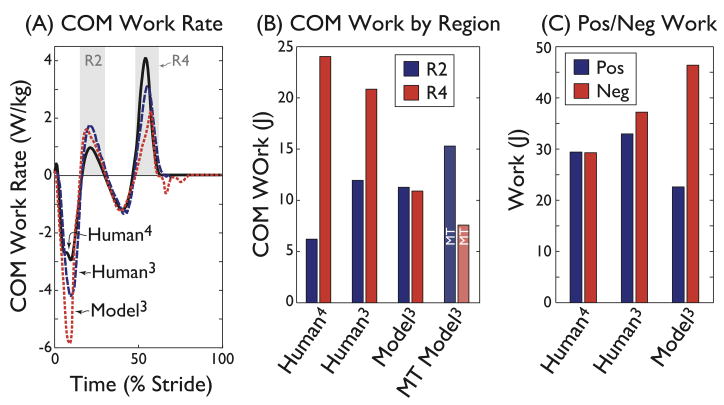
Figure 2.
Work performed on body center of mass (COM) by one leg. (A) COM work rate vs. time, comparing two Human data sets against Model. Superscripts indicate the following references: 3Neptune et al., 2004; 4Kuo et al., 2005. Regions 2 and 4 are shown as shaded areas. (B) Summed COM work for both regions, as well as the net musculotendon (MT) work of Model. Both Human data sets show greater work during Region 4 than Region 2. Model’s COM work and MT work do not agree with each other or with Human. (C) Magnitudes of positive and negative COM work per stride, integrated from COM work rate. Positive and negative work should be equal in magnitude for steady walking. Model performs more than twice as much negative work as positive work, indicating computational error.
Other comparisons fare no better. For example, the vertical GRFs (Figure 3) show considerable differences, with peaks more than one body weight greater in Model than Human. The Model also produces 24% lower average forces during Region 2 than Human, and 41% higher average forces during Region 4 (Figure 3B). Both, however, agree that forces average to less than body weight during the former, and greater than body weight during the latter, which would suggest less muscular effort during Region 2 than Region 4.
Figure 3.
Vertical ground reaction forces (GRFs), comparing Human and Model. (A) Forces plotted vs. time for Human (Neptune et al., 2004; solid line) and Model (dotted line), with Regions 2 and 4 indicated by shaded areas. Assuming symmetric gait, forces for both legs are shown (thicker lines) for the double support phase beginning at 50% of stride. (B) Average ground reaction forces for Regions 2 and 4 (indicated by horizontal lines in A), and for one full stride. Both Human and Model (3Neptune et al., 2004) produce lower forces during Region 2 than Region 4. Model produces much higher peak forces than Human during Region 4. Assuming symmetry, Model’s average force over a stride is about 10% greater than body weight.
There also appear to be several other inconsistencies in the Model. There are cases where one result from the Model does not agree with another, and where the Model either violates physical principles or is not producing steady gait. These are described in the Electronic Supplementary Material.
We have outlined a number of reasons for disagreeing with the conclusions of Neptune et al. (2001, 2004). Much of the Model’s Region 2 positive joint work occurs at the hip, opposite to the negative work of Human. The Model’s knee work in Region 2 is similarly opposite to Human. The Model also severely underestimates Region 4 work, with net musculotendon work less than one-third the joint work of Human. Similarly large discrepancies are observed in COM work and vertical GRFs. The net joint work of 0.1 J seen in Human data suggests that Region 2 accounts for less than 1% of the work over a stride. It is difficult to see how this could translate into 80% of the net musculotendon work of a stride.
Aside from the mismatch with Human data, we also find the interpretation of Region 2 work—to raise the COM—to be overly subjective. The task of raising the COM would be expected to induce a vertical ground reaction force of at least body weight. But average force during Region 2 is less than for any other region, and considerably less than body weight (0.7 in Model, 0.9 in Human; Figure 3). It seems non-objective to assign the region of lowest force the purpose of raising the COM, especially considering that the Model’s knee actually performs negative work over that interval (−3 J vs. +2 J in Human; see Figure 1). We accept that the Human knee performs about 2 J of positive work in Region 2 (after Winter, 1991), but this would only raise the COM by less than 3 mm, and only if the negative work of the ankle and hip were ignored. In any case, several studies show that it is far more energetically costly to walk with a level COM path, indicating a high cost for not raising the COM (Gordon et al., 2009; Kuo, 2007; Massaad et al., 2007; Ortega and Farley, 2005). In interpreting these results, it seems more straightforward to observe that Region 2 work occurs while the stance leg extends, and to avoid assigning it a purpose at odds with experiment.
It is hardly expected that a model will perfectly reproduce joint powers, COM work, and ground reaction forces. These are however the most readily observable quantities for testing musculoskeletal models, and conclusions from a model should not be applied to humans if they are greatly contradicted by such data. Musculoskeletal models have great potential to provide information that cannot easily be observed empirically. Unfortunately, this also poses the problem of how they can be tested by independent experiment. Predictions of muscle force should be subjected to experimental testing, and not be treated as fact. In no model is accuracy guaranteed, and so Human data must serve as the arbiter.
Supplementary Material
Acknowledgments
This work was supported in part by NIH HD055706.
Footnotes
Conflict of Interest Statement
The authors have no financial or personal relationships that could inappropriately influence this work.
References
- Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. Journal of Experimental Biology. 2002;205:3717–27. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- Gard SA, Miff SC, Kuo AD. Comparison of kinematic and kinetic methods for computing the vertical motion of the body center of mass during walking. Hum Mov Sci. 2004;22:597–610. doi: 10.1016/j.humov.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Gordon KE, Ferris DP, Kuo AD. Metabolic and mechanical energy costs of reducing vertical center of mass movement during gait. Arch Phys Med Rehabil. 2009;90:136–44. doi: 10.1016/j.apmr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Kuo AD. The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective. Human Movement Science. 2007;26:617–656. doi: 10.1016/j.humov.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exercise and Sport Sciences Reviews. 2005;33:88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Massaad F, Lejeune TM, Detrembleur C. The up and down bobbing of human walking: a compromise between muscle work and efficiency. J Physiol. 2007;582:789–99. doi: 10.1113/jphysiol.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle mechanical work requirements during normal walking: the energetic cost of raising the body’s center-of-mass is significant. J Biomech. 2004;37:817–25. doi: 10.1016/j.jbiomech.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004b;19:194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Ortega JD, Farley CT. Minimizing center of mass vertical movement increases metabolic cost in walking. J Appl Physiol. 2005;99:2099–107. doi: 10.1152/japplphysiol.00103.2005. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Neptune RR. Differences in muscle function during walking and running at the same speed. J Biomech. 2006;39:2005–13. doi: 10.1016/j.jbiomech.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. Waterloo, Ontario: Waterloo Biomechanics; 1991. [Google Scholar]
- Zajac FE, Neptune RR, Kautz SA. Biomechanics and muscle coordination of human walking: part II: lessons from dynamical simulations and clinical implications. Gait Posture. 2003;17:1–17. doi: 10.1016/s0966-6362(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Zmitrewicz RJ, Neptune RR, Sasaki K. Mechanical energetic contributions from individual muscles and elastic prosthetic feet during symmetric unilateral transtibial amputee walking: a theoretical study. J Biomech. 2007;40:1824–31. doi: 10.1016/j.jbiomech.2006.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.