Abstract
Autologous cardiac progenitor cells (CPCs) isolated as cardiospheres (CSps) represent a promising candidate for cardiac regenerative therapy. A better understanding of the origin and mechanisms underlying human CSps formation and maturation is undoubtedly required to enhance their cardiomyogenic potential. Epithelial-to-mesenchymal transition (EMT) is a key morphogenetic process that is implicated in the acquisition of stem cell-like properties in different adult tissues, and it is activated in the epicardium after ischemic injury to the heart. We investigated whether EMT is involved in the formation and differentiation of human CSps, revealing that an up-regulation of the expression of EMT-related genes accompanies CSps formation that is relative to primary explant-derived cells and CSp-derived cells grown in a monolayer. EMT and CSps formation is enhanced in the presence of transforming growth factor β1 (TGFβ1) and drastically blocked by the type I TGFβ-receptor inhibitor SB431452, indicating that TGFβ-dependent EMT is essential for the formation of these niche-like 3D-multicellular clusters. Since TGFβ is activated in the myocardium in response to injury, our data suggest that CSps formation mimics an adaptive mechanism that could potentially be enhanced to increase in vivo or ex vivo regenerative potential of adult CPCs.
Introduction
Cardiovascular disease remains the leading cause of mortality and morbidity in Western countries. The vast majority of clinically relevant cardiovascular disease results from the death of cardiac cells that are replaced by noncontractile fibrotic tissue, thus leading to pathological ventricular remodeling and heart failure [1,2]. Therefore, an intense effort during the last decade has been focused on identifying endogenous cardiac progenitor cells (CPCs) that can be expanded ex vivo and reintroduced as an autologous regenerative therapy [3,4]. A promising candidate population of resident CPCs can be readily obtained from cells that spontaneously migrate out of primary cardiac explants (explant-derived cells [EDCs]) and form cardiospheres (CSps) which recreate in vitro a niche-like microtissue [5]. CSp-derived cells (CDCs) can be expanded in monolayers [6] and retain the ability to form secondary cardiospheres (IICSps) when cultured under appropriate conditions. These cells can contribute in vivo to all the 3 main cell lineages of the heart (endothelial, smooth muscle cells, and cardiomyocytes), [7] and can provide a sufficient number of adult autologous CPCs for clinical applications. CDCs have been successfully employed for a randomized phase I clinical trial (CADUCEUS), showing the safety of these cells and an unprecedented increase in viable myocardium, which is consistent with therapeutic regeneration [8]. Furthermore, in a recent head-to-head comparative study, CDCs injected into infarcted mice hearts resulted in superior improvement of cardiac function, the highest cell engraftment and myogenic differentiation rates, and the least-abnormal cardiac remodeling 3 weeks after treatment, compared with other populations of human adult stem cells of diverse origin and to a sorted c-kit+ subpopulation [9]. In addition, preclinical studies conducted on both small [10] and large animal models [11] show that CSps are superior to CDCs in improving hemodynamics and regional function, and in attenuating ventricular remodeling [12], thus paving the way for a future clinical trial (RECONSTRUCT, Study NCT01496209). The regenerative capability of CSps is reportedly linked to their 3D niche-like structure, which favours the maintenance of “stemness” features, while conferring higher resistance to oxidative stress, thus enhancing in vivo engraftment [10]. Despite these advances, modulation of the balance between differentiation and paracrine signalling of CSps and CDCs [13] as reparative mechanisms remains unclear and needs to be resolved in order to interpret the emerging clinical data. In particular, the signals that govern CSps formation and cardiogenic, as distinct from a fibrogenic, differentiation remain unclear, especially when cells are recovered from or transplanted into the inhospitable ischemic environment of an infarcted heart [14].
Here, we analyzed the role of epithelial-to-mesenchymal transition (EMT) and its reverse process, mesenchymal-to-epithelial transition (MET), during generation of CSps from human auricular biopsies by means of gene expression analysis at different culture stages and in vitro treatments with transforming growth factor β (TGFβ) [15,16], which is a key EMT inducer, and its antagonist SB431452 [17]. EMT and MET are well known to play pivotal roles in embryogenesis [18], with 4 distinct waves of EMT occurring at different stages of heart morphogenesis [19]. EMT has also been associated with the acquisition of stem cell properties in both adult tissues and cancer [19–21] and also with cardiac postischemic remodeling [14]. We found that EMT controlled by TGFβ signaling is essential for the formation of CSps. Moreover, the gene profiles of CSps formation in vitro resembles the in vivo response of epicardial cells to ischemic cardiac injury, which also involves EMT, suggesting that TGFβ and EMT during CSps formation recapitulate aspects of the subepicardial niche and support the proliferation of adult CPCs.
Materials and Methods
Cell cultures
Human auricolar biopsies (10 patients) were cultivated as explants, and CPCs were isolated with the CSp protocol, as previously described [5,22]. Briefly, EDCs were collected weekly up to 3 times from each explant, and seeded on poly-D-lysine (BD-Biosciences) coated wells (9,000 cells/cm2) to obtain CSps. CSps were then collected by gentle pipetting and expanded on fibronectin (BD-Biosciences) coating as CDCs [6]; IICSps were obtained by replating CDCs in CSp-forming conditions.
In vitro treatments
EDCs or CDCs from 4 different patients were treated with TGFβ (2.5–5 ng/mL) (PeproTech, Inc.) or its inhibitor SB431542 (1, 10, or 25 μg/mL) (Sigma-Aldrich) for 1, 2, 3, 4, or 7 days. Preformed CSps (3 days after EDCs seeding) from 3 different patients were treated 3 or 4 days with TGFβ (5 ng/mL) or its inhibitor SB431542 (10 μg/mL). Proliferation was measured by WST-8 assay (Alexis Bioch.) according to the manufacturer's instructions. For each condition, we plated 2,000 cells/well in triplicate for 4 time points in 96-multiwell plates. CSps were imaged, then counted, and measured at different time points using ImageJ software. Five random fields per well acquired at 4×magnification were used for a quantitative analysis. Cells were collected after 7 days of treatments for RNA extraction.
Immunostainings
Immunostainings were performed on EDCs, EDCs plated to obtain CSps in presence of 10 μM SB431542 or 5 nM TGFβ, CSps, and CDCs. All samples were fixed with 4% paraformaldehyde (Sigma-Aldrich) at 4°C for 10 min, washed thrice with phosphate-buffered saline (PBS) and twice with washing buffer: 1% bovine serum albumin (Sigma-Aldrich), 0.02% Triton (Panreac Química S.L.U.) in PBS with Ca+2/Mg+2, and 50 mM Glycine (Sigma-Aldrich). Nonspecific antibody binding sites were blocked with 10% goat serum (Sigma-Aldrich) before incubation with primary antibodies: Snai1, α smooth muscle actin, Wilms tumor 1 (Abcam), Notch3, NOTCH1 (Santa Cruz Biotechnology, Inc.), β-catenin, and E-cadherin (BD-Biosciences). After washing 4 times in washing buffer, slides were incubated with the appropriate secondary antibody (Alexa 488- or 568-conjugated antibody IgG; Life Technologies Corporation), then counterstained with Hoechst 33342 (5 μg/mL in PBS; Life Technologies Corporation), and mounted with Vectashield medium (Vector Laboratories). Fluorescence micrographs were acquired using an Olympus FV-1000 spectral confocal microscope with a 20×and 40×(oil immersion) objectives and Olympus Fluoview software (Olympus).
RNA isolation and real-time quantitative PCR
EDCs (n=7), CSps (n=9), CDCs (n=4), IICSps (n=4), EDCs, or CDCs plated to form CSps/IICSps in the presence of TGFβ (n=7) or SB431542 (n=6) were collected, and RNA was extracted using the column-based purification kit (Genaid). 500 ng of RNA were retro-transcribed using the QuantiTect Reverse Transcription Kit (Qiagen). The obtained cDNA was used to load 2 lanes of the Taqman Customed MicroArray Cards (Applied-Life Technologies). Each card allows the analysis of 48 genes for 4 samples in duplicate. The preloaded taqman probes have been selected from the Applied Biosystem database and include genes involved in cellular reprogramming of induced-pluripotent stem cells (iPSC) [23,24], induced-cardiomyocyte generation [25,26], EMT [18], MET, endothelial-to-mesenchymal transition (EndMT), hypoxia [27], migration, stemness, and markers of cardiac, and vascular and mesenchymal cells (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). TaqMan Univ PCR MasterMix no UNG was used on an ABI PRISM 7900 HT sequence detector (Applied Biosystems). Quantification analysis was performed using the ΔΔCq method, with GAPDH as the reference gene (selected as the best and most stable among 3 analyzed), and EDCs or untreated CSps as calibrator.
Statistical analysis
Data are presented as mean value±standard error of the mean. Two-sided Student's t-test was used to evaluate the statistical significance in gene expression, CSps number and diameter, and metabolic activity among the different culture stages. Significance threshold was set at P≤0.05.
Results
EMT is involved in CSps formation
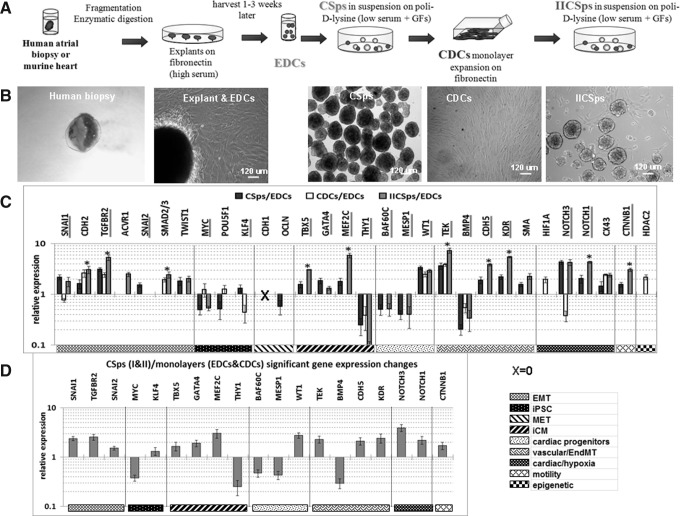
Quantitative gene expression analyses conducted on human adult EDCs, CSps, CDCs, and IICSps (Fig. 1A, B) revealed several key trends (Fig.1C, D). First, the EMT associated genes [18] SNAI1, TGFBR2, and SNAI2 were significantly up-regulated in 3D structures (CSps/IICSps) compared with the cells in monolayers (EDCs/CDCs) (Fig. 1D). CDH2, SMAD2/3, and TGFBR2 expression increased at all culture stages compared with EDCs, even in CDCs (Fig. 1C), suggesting the possible occurrence of intermediate states of EMT [28]. Another mediator of EMT that is directly activated by both HIF1α and the BMP/TGF pathway [27,29] is NOTCH1, which sustains CPC self-renewal [30]. NOTCH1 was up-regulated in CSps/IICSps compared with EDCs/CDCs. NOTCH3 was similarly up-regulated in 3D cultures and down-regulated in CDCs, suggesting that NOTCH3 and NOTCH1 might act in a similar manner (NOTCH3 controls self-renewal and hypoxia survival in human stem/progenitor cells isolated as mammospheres [31]). Second, we investigated stemness genes used in reprogramming to obtain iPSC [23,24] that might reflect a spontaneous loss or acquisition of a stem state by EDCs at the CSp stage. We observed that MYC was down-regulated in 3D structures compared with monolayers, and POU5F1 was down-regulated in CSps versus EDCs/CDCs, which is consistent with their role in promoting MET through the down-regulation of TGFβ/TGFβR and SNAI1, respectively [32]. An exception is KLF4, which can potentiate the TGFβ pathway [33], and was up-regulated in CSps and down-regulated in CDCs compared with EDCs. BMP7, a MET inducer and a TGFβ antagonist [34], was never detectable; CDH1 was significantly down-regulated at all culture stages compared with EDCs, in accordance with previous studies [10], while CTNNB1 was significantly up-regulated in 3D structures versus monolayers, consistently with a greater mobility. Third, mesenchymal markers varied according to cell type. ACTA2 was significantly up-regulated in I/IICSps, and GJA1 [35] was up-regulated in all culture stages compared with EDCs. In contrast, THY1, as a marker of cardiac fibroblasts [25], was significantly down-regulated at all culture stages, in particular CSps and IICSps. Markers of embryonic mesendoderm, including uncommitted cardiac progenitors, were down-regulated in I/IICSps compared with monolayers, including MESP1 [36,37] and SMARCD3 (encoding the BAF60c subunit of the Swi/Snf-like chromatin remodeling complex) [38]. However, later markers of CPCs, such as GATA4, TBX5, and MEF2C (which were recently reported as being capable of inducing cardiac fibroblast transdifferentiation into cardiomyocyte-like cells [25,26]), were up-regulated in 3D structures compared with monolayers, suggesting that CSps comprise atypical cardiac mesoderm [39]. Further, TBX5 and MEF2C were up-regulated in IICSps compared with CSps. Endothelial progenitor markers (KDR, CDH5, and TEK) were similarly up-regulated at all culture stages compared with EDC, and more so in IICSps. BMP4, mostly involved in EndMT [40] and late stages of cardiomyocyte differentiation, was down-regulated in 3D structures. Lastly, investigating epicardial markers, we found WT1 to be up-regulated in all 3D structures. Hypoxia in the CSp core [41] might induce WT1, a crucial mediator of epicardial EMT, similar to WT1 activation in vivo by ischemia in the adult epicardium [42–44].
FIG. 1.
EMT is involved in CSps formation. (A) Schematic representation of cell culture procedures. (B) Representative images of a human auricular biopsy, a cardiac explant surrounded by migrating EDCs, CSps, CDCs, and IICSps. (C) Significantly modulated (P<0.05) gene expression levels in CSps (n=9), CDCs (n=4), and IICSps (n=4), normalized to EDCs (n=7), assessed by Q-RT-PCR (ΔΔCt method, GAPDH as reference gene for all experiments), and presented on a logarithmic scale. *P<0.05 IICSps versus CSps. (D) Significantly modulated (P<0.05) gene expression levels between 3D structures (CSps and IICSps, n=13) and monolayers (EDCs and CDCs, n=11) assessed by Q-RT-PCR and presented on a logarithmic scale. Scale bars=120μm. EDCs, explant-derived cells; CDCs, cardiosphere-derived cells; IICSps, secondary cardiospheres; EMT, epithelial-to-mesenchymal transition; iPSC, induced pluripotent stem cells; MET, mesenchymal-to-epithelial transition; iCM, induced cardiomyocyte; EndMT, endothelial-to-mesenchymal transition; BAF60c, SMARCD3.
Inhibition of TGFβ pathway blocks CSps formation
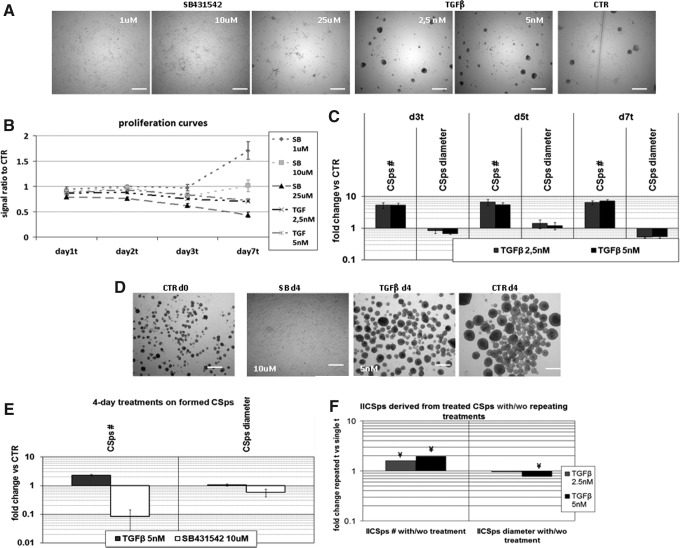
To establish a functional relationship between EMT and CSps formation or differentiation, cultures were treated with TGFβ, a potent promoter of EMT in numerous normal and pathological contexts [15,16]. TGFβ reproducibly enhanced primary or IICSps formation (Fig. 2A, C), whereas the divergent TGFβ superfamily member BMP4 and Activin had no effect (data not shown). TGFβ caused CSps to form 2 days earlier than control and increased the overall yield, producing more compact structures (Fig.2A, C). Cell proliferation, measured indirectly in terms of metabolic activity of viable cells [45], was moderately reduced (Fig. 2B), consistently with previous studies showing that the proliferation rate is lower in CSps culture compared with adherent cells [10]. These effects were not observed in IICSps derived from previously TGFβ-treated primary CSps in the absence of TGFβ; only repeated treatments with the growth factor produced significantly higher numbers of IICSps compared with controls (Fig. 2F).
FIG. 2.
SB431542 inhibits and TGFβ enhance CSps formation. (A) Representative images of EDCs plated to form CSps with or without (CTR) 7 day treatments with SB431542 (1, 10 or 25μM) or TGFβ (2.5 or 5 nM). (B) Proliferation curve by WST-8 assay of different TGFβ and SB431542 treatments, normalized to standard culture conditions (n=4). (C) CSps yield and size in TGFβ-treated samples versus controls (n=4). No CSps were detected in the presence of SB431542. (D) Representative images of preformed CSps at day 0 and after 4 days of TGFβ or SB431542 treatments. (E) CSps number and diameter in TGFβ 5 nM or SB431542 10 μM treatments for 4 days on preformed CSps (n=3). (F) IICSps derived from CSps in the presence of TGFβ do not maintain memory of the treatment. Bars show the average fold change in the number and diameter of IICSps (from a cell line of already treated primary CSps) obtained by repeating the TGFβ treatment, normalized to IICSps obtained without the second TGFβ treatment. Scale bar=280 μm. SB, SB431542; d, days; t, treatment; TGFβ, transforming growth factor β; ¥, p<0.05 versus untreated.
We repeated the experiments with SB431542, which blocks the type I receptors (Alk4/5/7) for TGFβ and Activin [17,46]. TGFBR1 mediates most TGFβ1 functions, including EMT [47–49]. No I/IICSps were obtained in the presence of this inhibitor, neither from EDCs nor from CDCs, (Fig. 2A, C), while it induced the spreading and dissolving of previously formed CSps after 3 (Supplementary Fig. S1) or 4 days (Fig. 2D, E).
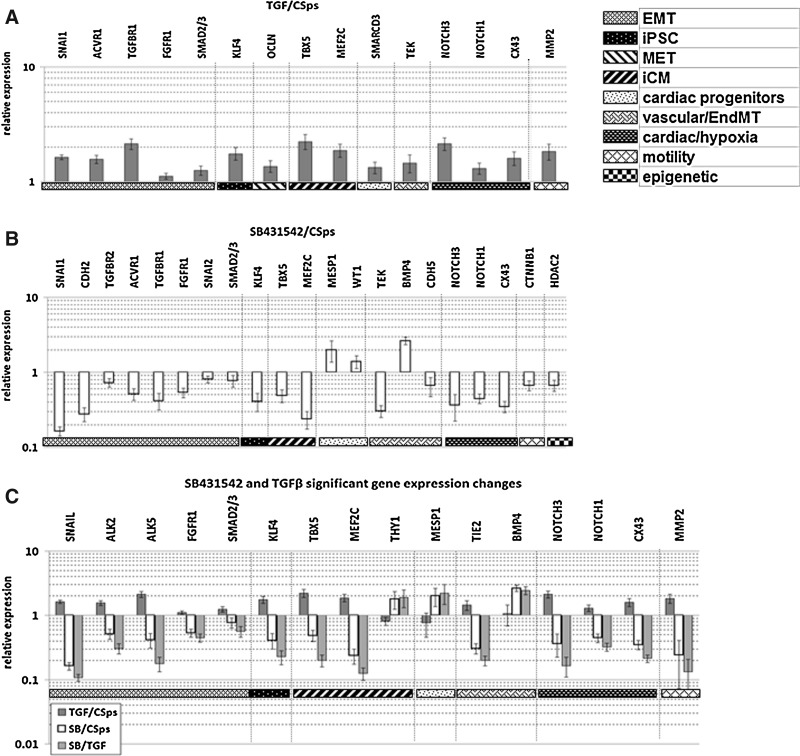
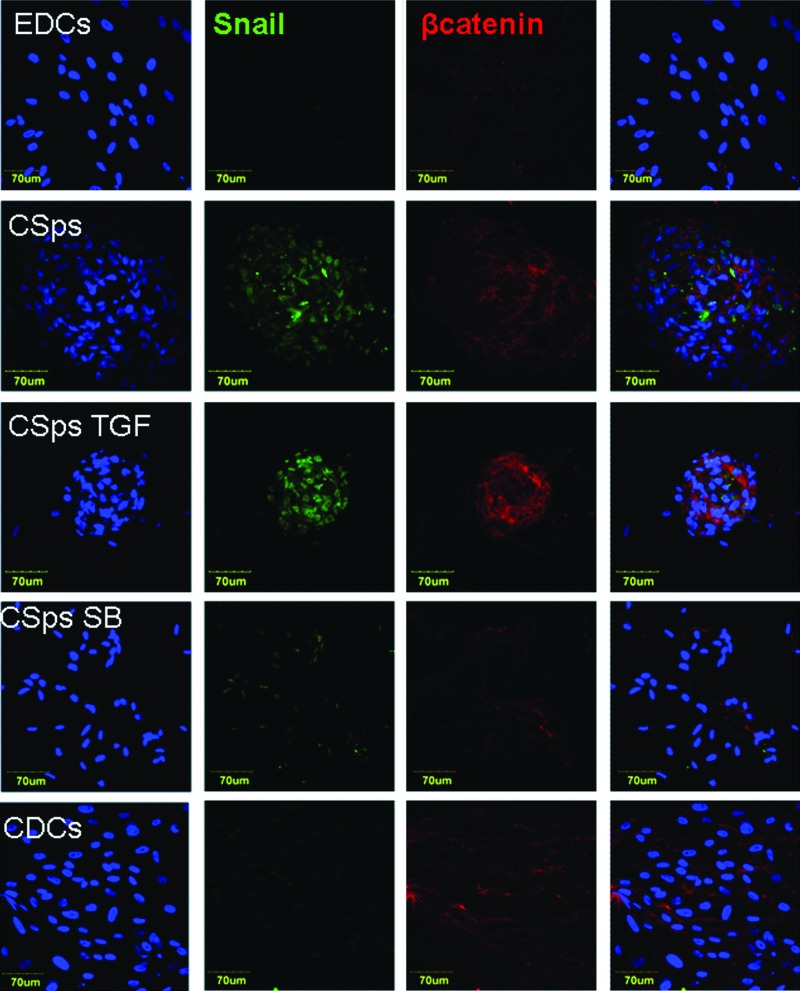
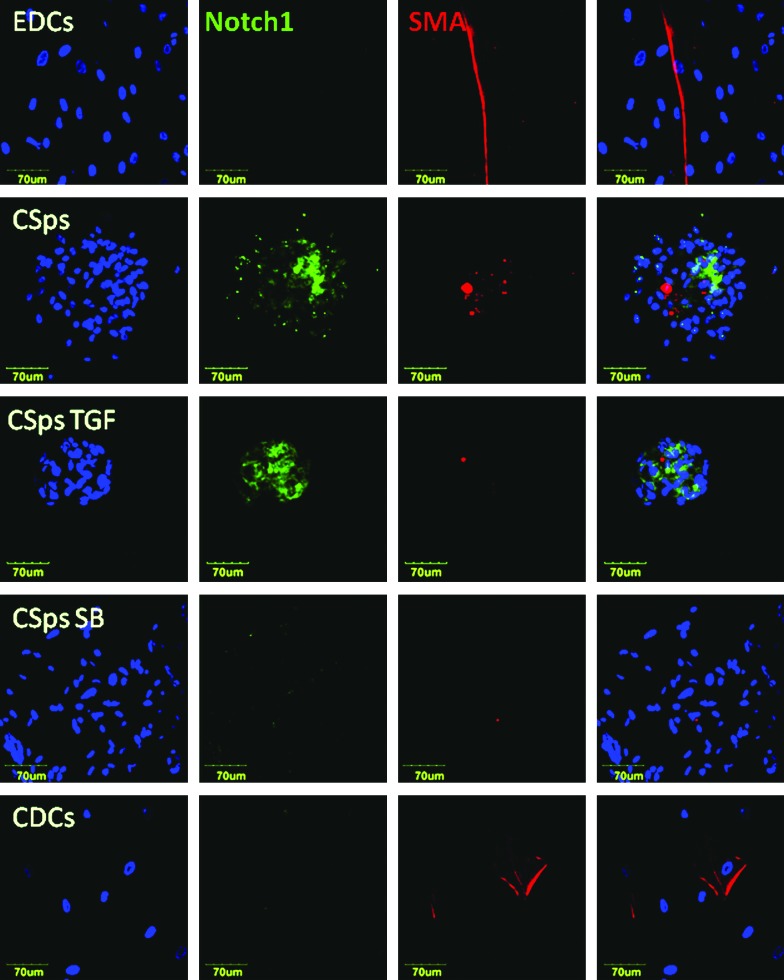
Treatment of EDCs/CDCs with SB431542 or TGFβ elicited opposite effects on the analyzed genes (Fig. 3). In particular, genes characteristically up-regulated in 3D culture (CSps/IICSps), including SNAI1, ACVR1, TGFBR1, FGFRI, SMAD2/3, KLF4, TBX5, MEF2C, NOTCH3, NOTCH1, GJA1, and TEK, were significantly up-regulated in the presence of TGFβ and down-regulated by its inhibitor. On the other hand, BMP4, MESP1, and THY1 (down-regulated in 3D structures compared with monolayers) were significantly up-regulated in the presence of SB431542, and unchanged or slightly down-regulated in the presence of TGFβ compared with control CSps. Immunostainings confirmed the differential regulation at the protein level. Comparing confocal images, acquired with the same settings, brighter and more widespread fluorescence signals were related to SNAI1 (Fig. 4); NOTCH1 (Fig. 5) and Notch3 (Supplementary Fig. S2) in 3D structures were detectable compared with monolayers (EDCs, CDCs, and SB-treated EDCs). Similarly, WT1 (Supplementary Fig. S3) also appeared more abundant in CSps and TGFβ/SB-treated CSps, than in EDCs and CDCs. Taken together, these results are consistent with the idea that TGFβ-dependent EMT promotes and controls CSps formation.
FIG. 3.
SB431542 treatment down-regulates, while TGFβ further up-regulates the expression of genes that are up-regulated in 3D structures versus monolayers. (A) Significantly modulated (P<0.05) gene expression levels in CSps/IICSps obtained in presence of TGFβ (n=7) versus untreated controls (n=13) assessed by Q-RT-PCR and presented on a logarithmic scale. (B) Significantly modulated (P<0.05) gene expression levels in EDCs/CDCs plated to form CSps/IICSps, respectively, in the presence of SB431542 10 μM for 7 days (n=6) versus untreated CSps/IICSps as control (n=13). (C) Normalized gene expression levels that were significantly modulated both in TGFβ (n=7) and SB431542 (n=6) treated samples versus untreated CSps (n=13). SB, SB431542; SB/TGF, SB431542 treated CSps versus TGFβ treated CSps, both normalized versus untreated CSps.
FIG. 4.
SNAI1 expression and CTNNB1 localization at different culture stages. Immunofluorescence staining of EDCs, CSps, and EDCs plated to form CSps in the presence of TGFβ (5 nM) or SB431542 (10 μM), and CDCs for SNAI1 (SNAIL, green), CTNNB1 (βcatenin, red), and Hoechst (blue). Confocal images were acquired with a 40×oil immersion objective, with the same settings and from the same set of stainings. For CSps, 1 representative slice has been selected out of a stack of 10–15 slices (1.5 μm step). Color images available online at www.liebertpub.com/scd
FIG. 5.
NOTCH1 is up-regulated in 3D structures. Immunofluorescence staining of EDCs, CSps, and EDCs plated to form CSps in the presence of TGFβ (5 nM) or SB431542 (10 μM), and CDCs for NOTCH1 (green), ACTA2 (SMA, red), and Hoechst (blue). Confocal images were acquired with a 40×oil immersion objective, with the same settings and from the same set of stainings. For CSps, 1 representative slice has been selected out of a stack of 10–15 slices (1.5 μm step). Color images available online at www.liebertpub.com/scd
Discussion
CSps are a promising candidate for autologous cardiac cell therapy. The aim of this study was to gain an insight into both the origin and the mechanism underlying the generation of these niche-like 3D multicellular clusters. Profiling the morphological and gene expression changes that occur during formation of the 3D structures (CSps/IICSps), relative to monolayers (EDCs/CDCs), suggested that EMT underlies the formation of CSps and the subsequent differentiation of later-stage cardiogenic progenitors. Thus, CSps and IICSps formation involved the up-regulation of markers of EMT (e.g., SNAI1, TGFBR2, TGFBR1, and TWIST) and cardiac progenitors (e.g., TBX5, MEF2C, CDH5, KDR, NOTCH1, and CTNNB1) (Fig. 1). Interestingly, some of the same genes also changed significantly in CDCs versus EDCs, but in the opposite way compared with 3D structures (SNAI1, KLF4, and NOTCH3), indicating divergence of lineage specification between 3D and monolayer cultures. In some cases, however, gene profiles in CDCs were more similar to CSps than to EDCs (e.g., ↑TGFBR2, ↓THY1, ↑TEK, and ↓BMP4), suggesting that CDCs represent an intermediate stage that retains memory of passing through the CSp stage, which seems to function as a selective/inductive stage [50]). Together, these data indicate that the formation of the 3D structures involves EMT and supports the differentiation of cardiac progenitors.
EMT is a critical morphogenetic process for heart development [19]. In particular, cells from the epicardial layer of the developing heart delaminate during early gestation and migrate into the underlying myocardium to form fibroblasts, certain cells of the coronary vasculature, and possibly a minor proportion of cardiomyocytes [51,52]. Recent studies suggest that this process might be recapitulated in the adult heart after myocardial infarction, such that epicardial-derived progenitor cells, or EPDCs, may provide a source of cells for myocardial regeneration [53]. A key finding of our study is that WT-1, a zinc-finger transcription factor that marks the activated epicardium [52], is significantly up-regulated on CSps formation. WT-1 binds to promoters of SNAI1 and CDH1 to induce or inhibit their activity, respectively, and promotes EMT [54]. Together with the EMT gene profile, the significant up-regulation of WT1 on CSps formation suggests a re-activation of the developmental and injury-induced epicardial program that might constitute an in vitro recapitulation of the formation of cardiogenic progenitors during development and in the adult [53,55–57].
Mechanistically, we provide evidence that TGFβ directs EMT during CSps formation. Accordingly, genes that characterize 3D structures (relative to monolayer cultures) were up-regulated by TGFβ and down-regulated by SB431542. Significantly, TGFβ enhanced CSps yield, whereas SB431542 drastically inhibited it while inducing the spreading of pre-existing CSps. The significant up-regulation of NOTCH in the 3D structures is in agreement with previous studies showing the integration of TGFβ and NOTCH signaling during EMT [29,58,59], and is possibly related to the stem-cell promoting properties of TGFβ in other adult tissues [20,21].
In conclusion, our study implicates TGFβ-dependent EMT as an essential pathway for CSps formation and the acquisition of cardiogenic properties by progenitor cells, mimicking the niche aspects proposed for the subepicardium after injury in the adult heart. Significantly, these studies define a transcriptomic signature for human CSps and their subsequent in vitro culture stages that will aid future studies to enhance their cardiogenic potential.
Supplementary Material
Acknowledgments
This project was funded by the Italian MIUR and Pasteur Institute, Cenci-Bolognetti Foundation. E.F. was supported by a Pasteur Institute, Cenci–Bolognetti Foundation fellowship. The authors acknowledge Dr. Pilar Ruiz-Lozano and Dr. Ramón Díaz-Trelles for their helpful suggestions and critical discussions.
Authors Disclosure Statement
No competing financial interests exist.
References
- 1.Dobaczewski M. Gonzalez-Quesada C. Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeffer MA. Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Gaetani R. Barile L. Forte E. Chimenti I. Ionta V. Di Consiglio A. Miraldi F. Frati G. Messina E. Giacomello A. New perspectives to repair a broken heart. Cardiovasc Hematol Agents Med Chem. 2009;7:91–107. doi: 10.2174/187152509787847128. [DOI] [PubMed] [Google Scholar]
- 4.Forte E. Chimenti I. Barile L. Gaetani R. Angelini F. Ionta V. Messina E. Giacomello A. Cardiac cell therapy: the next (re)generation. Stem Cell Rev. 2011;7:1018–1030. doi: 10.1007/s12015-011-9252-8. [DOI] [PubMed] [Google Scholar]
- 5.Messina E. De Angelis L. Frati G. Morrone S. Chimenti S. Fiordaliso F. Salio M. Battaglia M. Latronico MV, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 6.Smith RR. Barile L. Cho HC. Leppo MK. Hare JM. Messina E. Giacomello A. Abraham MR. Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 7.Davis DR. Ruckdeschel Smith R. Marban E. Human cardiospheres are a source of stem cells with cardiomyogenic potential. Stem Cells. 2010;28:903–904. doi: 10.1002/stem.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makkar RR. Smith RR. Cheng K. Malliaras K. Thomson LE. Berman D. Czer LS. Marban L. Mendizabal A, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li TS. Cheng K. Malliaras K. Smith RR. Zhang Y. Sun B. Matsushita N. Blusztajn A. Terrovitis J, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li TS. Cheng K. Lee ST. Matsushita S. Davis D. Malliaras K. Zhang Y. Matsushita N, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee ST. White AJ. Matsushita S. Malliaras K. Steenbergen C. Zhang Y. Li TS. Terrovitis J, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 12.Shen D.Cheng K.Marban E.2012Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction J Cell Mol Med [Epub ahead of print]DOI: 10.1111/j.1582-4934.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chimenti I. Smith RR. Li TS. Gerstenblith G. Messina E. Giacomello A. Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudoulas KD. Hatzopoulos AK. Cardiac repair and regeneration: the Rubik's cube of cell therapy for heart disease. Dis Model Mech. 2009;2:344–358. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zavadil J. Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP. Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 17.Laping NJ. Grygielko E. Mathur A. Butter S. Bomberger J. Tweed C. Martin W. Fornwald J. Lehr R, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R. Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua KN. Poon KL. Lim J. Sim WJ. Huang RY. Thiery JP. Target cell movement in tumor and cardiovascular diseases based on the epithelial-mesenchymal transition concept. Adv Drug Deliv Rev. 2011;63:558–567. doi: 10.1016/j.addr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Mani SA. Guo W. Liao MJ. Eaton EN. Ayyanan A. Zhou AY. Brooks M. Reinhard F. Zhang CC, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caja L. Bertran E. Campbell J. Fausto N. Fabregat I. The transforming growth factor-beta (TGF-beta) mediates acquisition of a mesenchymal stem cell-like phenotype in human liver cells. J Cell Physiol. 2011;226:1214–1223. doi: 10.1002/jcp.22439. [DOI] [PubMed] [Google Scholar]
- 22.Chimenti I. Gaetani R. Barile L. Forte E. Ionta V. Angelini F. Frati G. Messina E. Giacomello A. Isolation and expansion of adult cardiac stem/progenitor cells in the form of cardiospheres from human cardiac biopsies and murine hearts. Methods Mol Biol. 2012;879:327–338. doi: 10.1007/978-1-61779-815-3_19. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 25.Ieda M. Fu JD. Delgado-Olguin P. Vedantham V. Hayashi Y. Bruneau BG. Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian L. Huang Y. Spencer CI. Foley A. Vedantham V. Liu L. Conway SJ. Fu JD. Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustafsson MV. Zheng X. Pereira T. Gradin K. Jin S. Lundkvist J. Ruas JL. Poellinger L. Lendahl U. Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Jordan NV. Johnson GL. Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle. 2011;10:2865–2873. doi: 10.4161/cc.10.17.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluppel M. Wrana JL. Turning it up a Notch: cross-talk between TGF beta and Notch signaling. Bioessays. 2005;27:115–118. doi: 10.1002/bies.20187. [DOI] [PubMed] [Google Scholar]
- 30.Nemir M. Pedrazzini T. Functional role of Notch signaling in the developing and postnatal heart. J Mol Cell Cardiol. 2008;45:495–504. doi: 10.1016/j.yjmcc.2008.02.273. [DOI] [PubMed] [Google Scholar]
- 31.Sansone P. Storci G. Giovannini C. Pandolfi S. Pianetti S. Taffurelli M. Santini D. Ceccarelli C. Chieco P. Bonafe M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- 32.Li R. Liang J. Ni S. Zhou T. Qing X. Li H. He W. Chen J. Li F, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R. Han M. Zheng B. Li YJ. Shu YN. Wen JK. Kruppel-like factor 4 interacts with p300 to activate mitofusin 2 gene expression induced by all-trans retinoic acid in VSMCs. Acta Pharmacol Sin. 2010;31:1293–1302. doi: 10.1038/aps.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisberg M. Shah AA. Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 35.Asazuma-Nakamura Y. Dai P. Harada Y. Jiang Y. Hamaoka K. Takamatsu T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res. 2009;315:1190–1199. doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Saga Y. Kitajima S. Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- 37.Willems E. Lanier M. Forte E. Lo F. Cashman J. Mercola M. A chemical biology approach to myocardial regeneration. J Cardiovasc Transl Res. 2011;4:340–350. doi: 10.1007/s12265-011-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lickert H. Takeuchi JK. Von Both I. Walls JR. McAuliffe F. Adamson SL. Henkelman RM. Wrana JL. Rossant J. Bruneau BG. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–112. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- 39.Mercola M. Ruiz-Lozano P. Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medici D. Shore EM. Lounev VY. Kaplan FS. Kalluri R. Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelm JM. Ehler E. Nielsen LK. Schlatter S. Perriard JC. Fussenegger M. Design of artificial myocardial microtissues. Tissue Eng. 2004;10:201–214. doi: 10.1089/107632704322791853. [DOI] [PubMed] [Google Scholar]
- 42.Limana F. Capogrossi MC. Germani A. The epicardium in cardiac repair: from the stem cell view. Pharmacol Ther. 2011;129:82–96. doi: 10.1016/j.pharmthera.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Wagner KD. Wagner N. Bondke A. Nafz B. Flemming B. Theres H. Scholz H. The Wilms' tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 2002;16:1117–1119. doi: 10.1096/fj.01-0986fje. [DOI] [PubMed] [Google Scholar]
- 44.Wagner KD. Wagner N. Wellmann S. Schley G. Bondke A. Theres H. Scholz H. Oxygen-regulated expression of the Wilms' tumor suppressor Wt1 involves hypoxia-inducible factor-1 (HIF-1) FASEB J. 2003;17:1364–1366. doi: 10.1096/fj.02-1065fje. [DOI] [PubMed] [Google Scholar]
- 45.Kanemura Y. Mori H. Kobayashi S. Islam O. Kodama E. Yamamoto A. Nakanishi Y. Arita N. Yamasaki M, et al. Evaluation of in vitro proliferative activity of human fetal neural stem/progenitor cells using indirect measurements of viable cells based on cellular metabolic activity. J Neurosci Res. 2002;69:869–879. doi: 10.1002/jnr.10377. [DOI] [PubMed] [Google Scholar]
- 46.Inman GJ. Nicolas FJ. Callahan JF. Harling JD. Gaster LM. Reith AD. Laping NJ. Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 47.Bax NA. van Oorschot AA. Maas S. Braun J. van Tuyn J. de Vries AA. Groot AC. Goumans MJ. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFbeta-signaling and WT1. Basic Res Cardiol. 2011;106:829–847. doi: 10.1007/s00395-011-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willis BC. Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 49.Halder SK. Beauchamp RD. Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altomare C. Barile L. Marangoni S. Rocchetti M. Alemanni M. Mostacciuolo G. Giacomello A. Messina E. Zaza A. Caffeine-induced Ca(2+) signaling as an index of cardiac progenitor cells differentiation. Basic Res Cardiol. 2010;105:737–749. doi: 10.1007/s00395-010-0111-6. [DOI] [PubMed] [Google Scholar]
- 51.Cai CL. Martin JC. Sun Y. Cui L. Wang L. Ouyang K. Yang L. Bu L. Liang X, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou B. Ma Q. Rajagopal S. Wu SM. Domian I. Rivera-Feliciano J. Jiang D. von Gise A. Ikeda S, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limana F. Bertolami C. Mangoni A. Di Carlo A. Avitabile D. Mocini D. Iannelli P. De Mori R. Marchetti C, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol. 2010;48:609–618. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Estrada OM. Lettice LA. Essafi A. Guadix JA. Slight J. Velecela V. Hall E. Reichmann J. Devenney PS, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limana F. Zacheo A. Mocini D. Mangoni A. Borsellino G. Diamantini A. De Mori R. Battistini L. Vigna E, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 56.Russell JL. Goetsch SC. Gaiano NR. Hill JA. Olson EN. Schneider JW. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res. 2011;108:51–59. doi: 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Meglio F. Castaldo C. Nurzynska D. Romano V. Miraglia R. Bancone C. Langella G. Vosa C. Montagnani S. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol. 2010;49:719–727. doi: 10.1016/j.yjmcc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Blokzijl A. Dahlqvist C. Reissmann E. Falk A. Moliner A. Lendahl U. Ibanez CF. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163:723–728. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zavadil J. Cermak L. Soto-Nieves N. Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.