Abstract
Influenza virus infections are associated with a significant number of illnesses and deaths on an annual basis. Many of the deaths are due to complications from secondary bacterial invaders, including Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pyogenes. The β-hemolytic bacteria S. pyogenes colonizes both skin and respiratory surfaces, and frequently presents clinically as strep throat or impetigo. However, when these bacteria gain access to normally sterile sites, they can cause deadly diseases including sepsis, necrotizing fasciitis, and pneumonia. We previously developed a model of influenza virus:S. pyogenes super-infection, which we used to demonstrate that vaccination against influenza virus can limit deaths associated with a secondary bacterial infection, but this protection was not complete. In the current study, we evaluated the efficacy of a vaccine that targets the M protein of S. pyogenes to determine whether immunity toward the bacteria alone would allow the host to survive an influenza virus:S. pyogenes super-infection. Our data demonstrate that vaccination against the M protein induces IgG antibodies, in particular those of the IgG1 and IgG2a isotypes, and that these antibodies can interact with macrophages. Ultimately, this vaccine-induced immunity eliminated death within our influenza virus:S. pyogenes super-infection model, despite the fact that all M protein-vaccinated mice showed signs of illness following influenza virus inoculation. These findings identify immunity against bacteria as an important component of protection against influenza virus:bacteria super-infection.
1. Introduction
Streptococcus pyogenes is a β-hemolytic, Gram-positive pathogen that causes significant morbidity and mortality on an annual basis [1]. These bacteria have the ability to colonize both the skin and the respiratory tract, and cause non-invasive diseases such as pharyngitis, impetigo, and scarlet fever [2;3]. However, when these bacteria gain access to sterile sites, invasive diseases like necrotizing fasciitis, sepsis, toxic shock syndrome, and pneumonia can develop. Post-infection sequelae, including acute post-streptococcal glomerulonephritis, and acute rheumatic fever can also occur [4]. Worldwide, S. pyogenes is associated with approximately 500,000 deaths per year [1], with up to 1,850 deaths in the US from invasive diseases alone [5]. While, it has recently been estimated that only 11–12% of invasive S. pyogenes infections exhibit lower respiratory tract complications [6], there is a surprising 38% case fatality rate associated with S. pyogenes pneumonia [7].
It is well-accepted that influenza viruses have the ability to predispose infected hosts toward secondary bacterial complications [8], and secondary bacterial infections contributed to the excess mortality observed during past influenza pandemics [9]. Of note, during the 1918 pandemic, as many as 90% of the 40–50 million estimated deaths were due to complications associated with secondary bacterial infections [10]. These secondary bacterial infections contribute to pneumonia and influenza-related deaths during seasonal influenza epidemics as well, with the excess mortality varying from season to season [11–14]. Influenza viruses and S. pyogenes share a common seasonality [15–17], and vaccines against S. pyogenes are not currently available [18]. As such, we rely on antibiotics [19], anti-viral agents [20], and vaccines against influenza virus [21] to limit the impact of influenza virus:S. pyogenes super-infections. Of these, antibiotics have demonstrated an ability to prevent infection with S. pyogenes when given prophylactically, but this practice is typically limited to defined populations like military recruits [22].
We recently developed a model of influenza virus:S. pyogenes super-infection in mice, using an H3N2 influenza virus and a serotype M3 isolate of S. pyogenes [23]. We have been evaluating the effects of vaccination against either influenza virus or S. pyogenes to determine the contribution of immunity against these pathogens toward preventing mortality after super-infection. Our previous work demonstrated that vaccination against influenza virus could limit progression toward a secondary bacterial infection with S. pyogenes, but that the protection was not complete. These results corroborate the findings of other lab-based [15] and clinical studies [24–26] that demonstrate that vaccination against influenza viruses can limit, but not eliminate, complications from secondary bacterial infections. To date, the benefits of vaccine-induced anti-bacterial immunity toward protection within models of influenza virus:bacteria super-infection have not been completely defined.
In the current study, we tested the hypothesis that vaccine-induced immunity against the bacteria S. pyogenes can prevent death after influenza virus:S. pyogenes super-infection. In order to test this, we used a vaccine that induces immunity toward the M protein of 6 different S. pyogenes serotypes, including the M3 serotype used in our studies [27]. This M protein vaccine has been used in both animal studies [28] and human clinical trials [29] to demonstrate safety and efficacy, but it has not been tested in an animal model of poly-microbial infection. We show that mice vaccinated against the M protein of S. pyogenes are protected after super-infection, and that this protection is evident despite significant illness associated with influenza virus inoculation. These findings are discussed in the context of host immune responses that can be targeted to prevent deadly influenza virus:S. pyogenes super-infections.
2. Methods and Material
2.1. Mice
Adult (6–8-week-old) female BALB/cJ mice were obtained from Harlan Laboratories (Indianapolis, IN). All animal experiments were performed following the guidelines established and approved by the Animal Care and Use committee at the University of South Dakota (Vermillion, SD).
2.2. Vaccination with S. pyogenes M-protein antigens
Groups of mice were vaccinated intramuscularly (i.m.) at 21 to 28 day intervals with 30 μg of a recombinant hexavalent S. pyogenes M protein vaccine in a 100 μL volume [28;29]. The vaccine was delivered in 2 mg/mL Alum (General Chemical, Berkeley Heights, NJ) as an adjuvant, and control mice received Alum alone.
2.3. Serum antibody titers
Sera collected from the orbital plexus of anesthetized mice were analyzed by ELISA, as described previously [28]. For these ELISAs, 96-well plates (Becton Dickinson and Company, Franklin Lakes, NJ) were coated with 5 μg/mL of the indicated M peptides (M1, M3, M5, M6, M19, and M24) diluted in 0.1 M sodium carbonate (pH 9.8), and IgG (H+L) was detected in serum samples. ELISA plates were washed using PBS containing 0.05% Tween 20 (PBS-T), and two-fold serial dilutions of individual mouse sera were incubated for 2 hours at 37°C. Plates were washed with PBS-T, and HRP-conjugated goat anti-mouse IgG (H+L) (Sigma, St. Louis, MO) was added to each well. After washing, HRP was detected using One-Step-TMB Turbo substrate (Thermo Scientific, Rockford, IL), the OD was measured at 450 nm using a Biotek EL808 plate reader (Biotek, Winooski, VT), and end-point titers were calculated. End-point titers are presented as the reciprocal serum dilution corresponding with the last well demonstrating an OD450 of 0.1 in the titration curve. Serum samples that did not result in an OD of 0.1 at the starting dilution of 1:100 were assigned a titer of 50 for the purpose of graphing and statistics. Vaccination was repeated to achieve an IgG (H+L) antibody titer of 1:3200 against the M3 serotype, and 5 separate inoculations with the vaccine were required.
When IgG1 and IgG2a antibodies were detected, the ELISA used was similar to the one described previously [30], with the exception that the antigen used for coating was the hexavalent M protein vaccine [27] diluted to 5 μg/mL in 0.1 M sodium carbonate (pH 9.8). For this ELISA, alkaline phosphatase-conjugated goat anti-mouse IgG1 and IgG2a antibodies (Southern Biotech, Birmingham, AL) were used along with 1 mg/mL para-Nitrophenyl phosphate substrate (Sigma), to exploit the AP:pNPP enzyme:substrate reaction. One hour after addition of pNPP substrate, OD was measured at 405 nm, and mid-point titers are presented as the reciprocal serum dilution corresponding to 50% maximal binding calculated for each individual serum sample using its respective titration curve.
2.4. Nanoparticle Synthesis, Amine Quantitation, and Protein Conjugation
Fluorescein-encapsulated silica nanoparticles were synthesized according to previously described methods with minor alterations [31]. Briefly, Fluorescein isothiocyanate (FITC) isomer I and (3-aminopropyl)triethoxysilane (APTES) were stirred in absolute ethanol, producing FITC-APTES conjugate. Concurrently, cyclohexane, Triton X-100, n-hexanol, and water were combined to create a water-in-oil emulsion. The FITC-APTES conjugate solution, tetraethyl orthosilicate, and 14.5 M NH4OH were added to this micro-emulsion. After stirring, 3-(trihydroxysilyl)propyl methylphosphonate, monosodium salt, (THPMP) was added, and mixed for 24 hours at RT. Ethanol was then added to disrupt the micro-emulsions, and nanoparticles were isolated by centrifugation. Nanoparticles were washed three times in ethanol and air-dried. Nanoparticle size was measured by TEM, and the presence of surface amines was confirmed by a qualitative ninhydrin test [32]. Further quantitation of surface amino-groups was performed by adapting methods that have been described previously to analyze proteins [33]. The concentration of amino-groups present was recalculated into their molar amount per 1 mg of nanoparticles.
Protein-nanoparticle conjugates were prepared using known coupling methods [34] incorporating a stock solution of 1 mg/mL succinic anhydride in N,N-dimethylformamide, and a qualitative ninhydrin test of the resulting nanoparticles confirmed the lack of amine functionality. The resulting carboxylic acid groups were activated by reaction with 2 mg 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and 1 mg N-hydroxysulfosuccinimide sodium salt (sulfo-NHS) (both solutions are 1 mg/mL in 0.1 M, pH 6.0 2-(N-morpholino)ethanesulfonic acid (MES) buffer). The nanoparticle solution was diluted by 1 mL with MES and centrifuged. The precipitated nanoparticles were washed with 0.1 M, pH 7.4 phosphate buffered saline (PBS) and resuspended in PBS. Approximately 1 mg of hexavalent M protein (3 mg/mL in PBS) was shaken with the nanoparticle solution for 5 hours. The protein-nanoparticle conjugates were centrifuged, and washed with PBS.
2.5. Nanoparticle-coupled cellular uptake assay
FITC-labeled nanoparticles conjugated with the hexavalent M protein vaccine were resuspended in 0.5 mL PBS containing 0.2% BSA (Sigma, St. Louis, MO), and incubated with sera for 60 minutes at 37°C. One million J774A.1 BALB/c murine macrophage cells (American Type Culture Collection, Manassas, VA) were added, and the nanoparticle:serum mixtures were incubated with these cells for 60 minutes at 37°C. Cells were washed, and uptake was quantitated using an Accuri C6 flow cytometer (Accuri Cytometers Ltd., Ann Arbor, MI), with CFlow Plus software (Accuri).
2.6. Confocal slide preparation and imaging
Cells were fixed on slides and cover slips were added prior to imaging with an Olympus Fluoview 1000 Laser Scanning Confocal Microscope (Olympus America, Inc., Center Valley, PA). Samples were scanned using a 60× 1.4 numerical aperture oil-immersion objective and 488-nm argon laser, and images were collected using FluoView software (Olympus) for 3-D reconstruction.
2.7. Influenza virus:S. pyogenes super-infection model
The influenza virus:S. pyogenes super-infection model used for this study was similar to the one described previously by our group [23]. The influenza virus expressing hemagglutinin (HA) and neuraminidase (NA) from A/Hong Kong/1/68-H3N2, designated HK68, was kindly provided by Jonathan A. McCullers (St. Jude Children’s Research Hospital, Memphis, TN) [30;35]. Mice in both vaccine groups were first challenged intranasally (i.n.) with a sub-lethal dose of HK68 virus (0.1 LD50, 104.75 TCID50) on day 0. On day 7 after influenza virus inoculation, mice were randomly divided into groups that received the S. pyogenes isolate MGAS315 (serotype M3) at either the previously calculated 0.1 LD50 (106 CFU) or subsequent log dilutions (105, 104, 103 CFU) of bacteria. Dilutions of either virus or bacteria were delivered in PBS via the i.n. route (100 μL). Morbidity (weight loss) and mortality (survival) were observed during the days after influenza virus inoculation.
2.8. Statistical analyses
Figures were created using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA), and data were analyzed using Statmost programs (Dataxiom Software Inc. Los Angeles). Values were accepted as significant if P<0.05.
3. Results
3.1. Vaccination using hexavalent M vaccine induces antibodies toward S. pyogenes M serotype variants
Mice vaccinated with the hexavalent M protein vaccine (Figure 1) showed increased IgG (H+L) antibody titers against the M3 and M24 peptides, with optimal seropositivity (100% of titers being 1:100 or above), and the highest mean titers observed. Antibody titers against the M5 (39% seropositive), M6 (36% seropositive), M19 (64% seropositive), and M1 (75% seropositive) peptides were suboptimal in both the percent seropositivity and the mean titers. Despite this, antibody titers against the M5 peptide were the only ones that failed to achieve a significant increase (P>0.05) in vaccinated animals, when compared to unvaccinated mice. From this, we conclude that the hexavalent M protein vaccine was immunogenic in our mouse model, with 100% of vaccinated mice being seropositive toward the M3 protein expressed by MGAS315 [23]. To further evaluate the antibody-mediated immune response induced by vaccination, we measured the amount of serum IgG1 and IgG2a antibody isotypes against the hexavalent vaccine (Figure 2). Similar to results from total IgG ELISAs, antibodies of both the IgG1 and IgG2a isotypes were below the detectable limit in sera obtained from unvaccinated mice, while sera from vaccinated mice had significantly increased levels of hexavalent protein-reactive antibodies for both isotypes (P<0.05).
Figure 1.
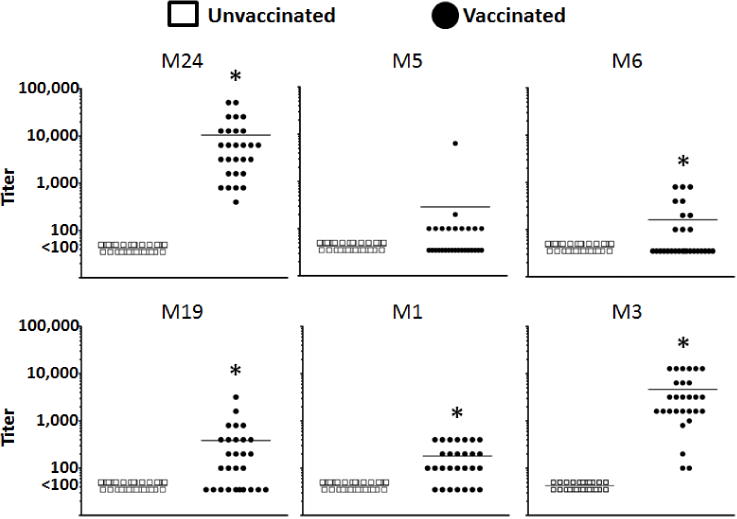
Serum IgG antibody titers after vaccination with the hexavalent M protein vaccine. Mice (n=28 per group) were vaccinated with either the hexavalent M vaccine or alum, as a control (unvaccinated). Each data point represents the end-point titer of an individual serum sample against the designated M peptides (M24, M5, M6, M19, M1, or M3), individually. The mean titers of unvaccinated and vaccinated sera are represented as a single bar. *Indicates a significant difference between the vaccinated and unvaccinated sera (P<0.05) using a paired, two-tailed Student’s t-test.
Figure 2.
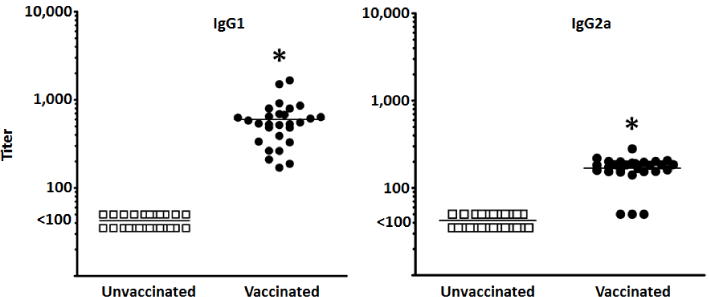
Serum IgG1 and IgG2a antibody titers after vaccination with the hexavalent M protein vaccine. Mice (n=28 per group) were either vaccinated with the hexavalent M vaccine or alum, as a control (unvaccinated). Each data point represents the 50% maximal binding titer of an individual serum sample against the hexavalent M protein. The mean titers of unvaccinated and vaccinated sera are represented as a single bar. *Indicates a significant difference between the vaccinated and unvaccinated sera (P<0.05) using a paired, two-tailed Student’s t-test.
3.2. Antibodies against the S. pyogenes M protein enhance nanoparticle uptake by murine macrophages
The hexavalent vaccine used in this study was specifically designed to induce antibodies against opsonic epitopes from selected M proteins [18;36]. Detection of antibodies by ELISA demonstrated the specificity of these antibodies against individual M peptide antigens via their Fab region. However, to demonstrate that the Fc portions of these antibodies have the ability to enhance host effector responses, we developed an assay to measure uptake by macrophages. To achieve this, we coupled the hexavalent M protein construct to SiO2 nanoparticles labeled with the fluorescent dye FITC via sulfo-NHS-activated carboxyl groups on the nanoparticles (Figure 3). These nanoparticles were 75–100 nm, as determined with TEM (Figure 3A).
Figure 3.
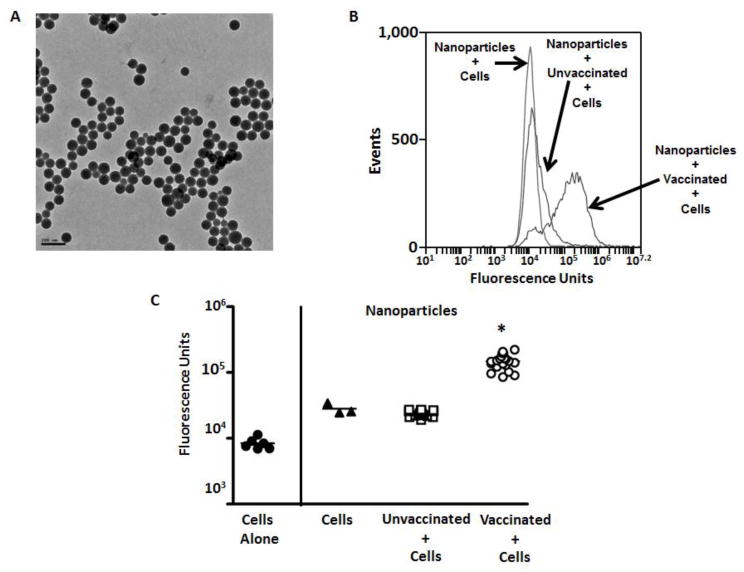
Serum-mediated uptake of FITC-labeled SiO2 nanoparticles that were linked to the hexavalent M protein. TEM images of the nanoparticles (A) are presented, with the bar indicating 0.2 μm. Uptake of fluorescent nanoparticles was quantitated using flow cytometry, and results for pooled sera (B) and individual sera (C) are presented. *Indicates a significant difference between the sera from vaccinated and unvaccinated animals (P<0.05) using one way ANOVA and post hoc Tukey’s multiple comparison test.
Uptake of nanoparticles by a murine macrophage cell line (J774A.1) was quantitated using flow cytometry. Using both pooled (Figure 3B) and individual serum samples (Figure 3C), we found that fluorescent intensity was significantly increased in the presence of sera from vaccinated mice compared to sera from unvaccinated animals (P<0.05). As expected, fluorescence in the group that included nanoparticles pre-incubated with sera from unvaccinated mice demonstrated a mean intensity that was similar to that observed with cells incubated with nanoparticles alone. These data showed that vaccine-induced antibodies against the hexavalent M protein vaccine, which includes the M3 expressed by MGAS315, can bind to protein-conjugated nanoparticles and promote interactions with murine macrophages. Efforts to detect similar uptake using individual M3 peptides were not successful (data not shown), which we attributed to reduced availability of epitopes on peptide constructs bound to SiO2 nanoparticles. In order to determine if the nanoparticles were internalized by macrophages, we used confocal microscopy (Figure 4, Supplemental Figure 1, and Supplemental Figure 2), which showed internalization in the presence of sera from vaccinated mice.
Figure 4.
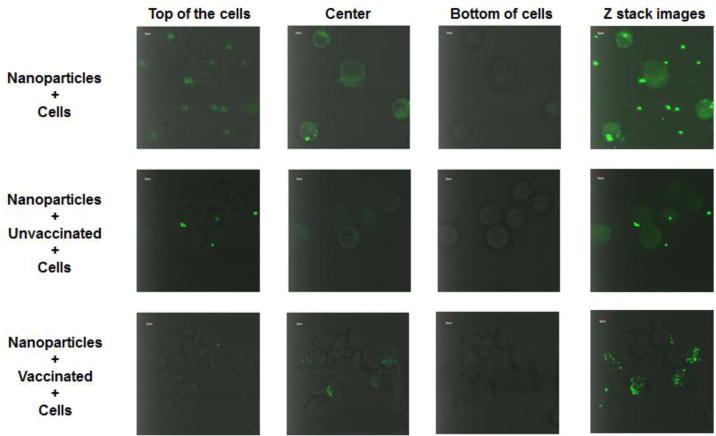
Serum-mediated uptake of FITC-labeled SiO2 nanoparticles that were linked to the hexavalent M protein, visualized using confocal microscopy. Confocal microscopy was used to visualize the uptake of fluorescent nanoparticles by J774A.1 macrophages. Images were taken from the top, center, and bottom of the cells. The entire stack of images in the Z plane are also presented.
3.3. Vaccination against the S. pyogenes M protein prevents illness and eliminates mortality during an influenza virus:S. pyogenes super-infection
Previous work by our group [23;37] and others [15] demonstrated that vaccination against influenza virus could decrease, but not eliminate, death in models of influenza virus:bacteria super-infection. Specifically, despite the absence of weight loss following inoculation with influenza virus, overall survival was only 67–75% after inoculation of bacteria, depending on whether a formalin-inactivated or live, attenuated form of the vaccine was used. In the current study, we evaluated the impact of anti-M protein immunity toward preventing morbidity (Figure 5) and mortality (Figure 6) after super-infection. To do so, mice were inoculated with a sub-lethal dose of influenza virus (0.1 LD50) seven days prior to secondary infection with one of four sub-lethal doses of S. pyogenes (106–103 CFU).
Figure 5.
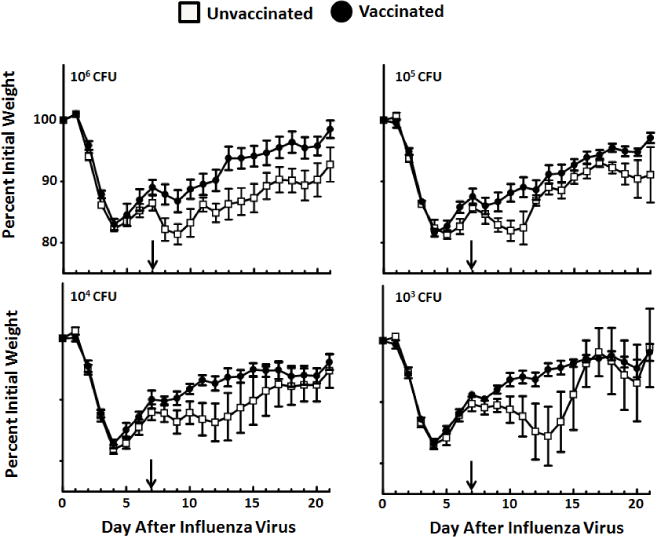
Body weight after influenza virus:S. pyogenes super-infection. Mice from each vaccine group (n = 28 for vaccinated and n = 28 for unvaccinated) were randomly separated for challenge with S. pyogenes at four different sub-lethal doses (103–106 CFU). Each challenge dose included 7 mice per vaccine group. The arrow within the figure represents the day of MGAS315 infection (day 7 after influenza virus challenge). Weights are calculated as percent initial body weight for each individual mouse, with the mean ± SEM presented for each day after influenza virus infection.
Figure 6.
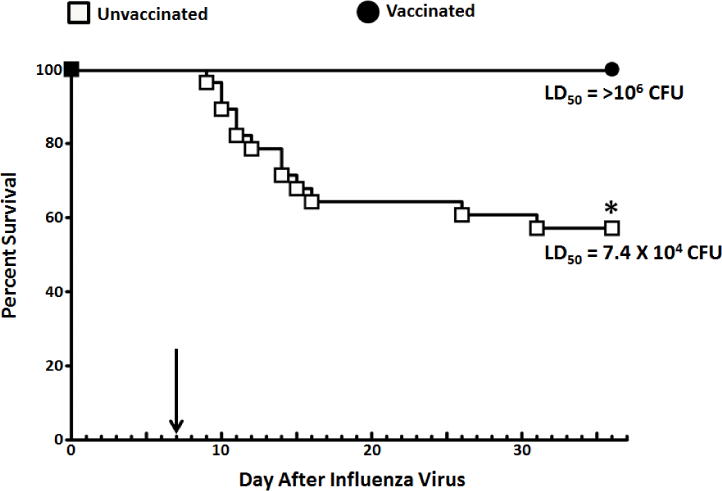
Survival after influenza virus:S. pyogenes super-infection. Mice from each vaccine group (n = 28 for vaccinated and n = 28 for unvaccinated) were randomly separated for challenge with S. pyogenes at four different doses (103–106 CFU). For each challenge dose, n = 7 per vaccine group. This dose response was used to calculate an LD50 in both unvaccinated and vaccinated animals after super-infection. The arrow within the figure represents the day of MGAS315 inoculation (Day 7 after influenza virus inoculation). *Indicates a significant difference between vaccinated and unvaccinated mice (P <0.05) using a Log Rank chi-squared test on the Kaplan Meier survival data.
Mice in both the unvaccinated and vaccinated groups lost weight after influenza virus challenge, as expected [23]. Upon inoculation with MGAS315 at day 7, unvaccinated animals experienced a second period of weight loss that varied based on the dose of S. pyogenes delivered. These data demonstrate that weight loss was challenge dose-dependent in unvaccinated mice. Vaccinated mice lost weight, but only among mice receiving the two highest doses of MGAS315 (105 and 106 CFU). The LD50 for unvaccinated mice was 7.4 × 104 CFU S. pyogenes, which was associated with 58% survival across all four challenge doses. In contrast, all the mice vaccinated against the M protein of S. pyogenes survived, resulting in an LD50 that was greater than the highest dose of bacteria delivered (106 CFU S. pyogenes).
4. Discussion
In this study, we tested the hypothesis that vaccine-induced immunity against S. pyogenes infection will prevent death in an influenza virus:S. pyogenes super-infection model. Our data show that a strong IgG antibody response against the streptococcal M protein, including antibodies of the IgG1 and IgG2a isotypes, fully protected against death. Importantly, the antibodies induced by vaccination also interacted with macrophages to promote uptake of hexavalent M protein-coated nanoparticles. Our findings are important for future consideration of anti-bacterial vaccination as a method toward limiting severe illnesses and deaths during influenza virus epidemics and pandemics, and provide evidence that a vaccine targeting the M protein of S. pyogenes would impact the incidence of diseases associated with this pathogen.
Vaccines to prevent erysipelas and scarlet fever can be traced back to the early 1900’s [38;39]. Early studies by Rebecca Lancefield demonstrated immunogenic differences between S. pyogenes strains [40], in particular those associated with expression of the M protein (encoded by the emm gene) [41]. To date, over 120 M serotypes have been identified [42], although the majority of invasive diseases are associated with a more limited repertoire of M serotypes [43]. The M protein is a key antigen expressed by S. pyogenes and it has the ability to promote resistance to phagocytosis [44;45] while also being the antigen toward which antibody-mediated protective immunity is directed [46–49]. It is worth noting that during the influenza virus pandemic of 1918, whole-cell vaccines against bacteria were used to limit complications with secondary bacterial pneumonia, and there is evidence that deaths were prevented using this approach [50]. In fact, the manuscript by Chien et al. [50] cites a report from 1919 where a vaccine specifically designed to target hemolytic streptococci showed 100% protection against death in 144 vaccinated individuals that were infected with influenza virus [51]. Using more modern approaches, we show that a recombinant polypeptide vaccine against the M protein can provide complete protection against mortality in a model of influenza virus:S. pyogenes super-infection.
Our influenza virus:S. pyogenes super-infection model yields high mortality [23], which allows for a stringent test of vaccine-induced protection in the context of S. pyogenes infection. Here we report that vaccine-induced antibodies against the S. pyogenes M protein protect against an otherwise fatal influenza virus:S. pyogenes super-infection. Our data showed that IgG antibodies were induced by this vaccine, including those of the IgG1 and IgG2a isotypes, and that these antibodies are able to interact with murine macrophages, possibly using Fc receptors [52]. Variability in the antibody response against the individual M peptides has been reported previously in BALB/c mice [28] after vaccination with the hexavalent M protein construct used in this study. Since the vaccine administered was protein-based, and delivered to BALB/cJ mice with Alum as an adjuvant, a bias toward a Th2-like, IgG1 antibody response could be expected [53]. However, our data also show a significant increase in IgG2a antibodies after vaccination, indicating that Th1-like immunity could be induced by this vaccine as well [54]. The importance of host:Fc interactions in the pathogenesis of S. pyogenes can be seen in the evolution of methods for masking the Fc portion of host antibodies by variants of this bacterial species. These include expression of proteins that either bind the Fc portion of antibodies, like Protein H [55] and Protein I [56], or degrade the Fc region directly, like EndoS [57] and IdeS [58;59]. Interestingly, a recent study demonstrated that antibody interaction with S. pyogenes could dictate the distribution of bacteria during an invasive S. pyogenes infection [60].
The protection that we observed using the hexavalent M protein vaccine was superior to the vaccine-induced protection that we previously reported in animals that were vaccinated against influenza virus [23]. This finding was surprising because all of the S. pyogenes M protein-vaccinated mice were susceptible to influenza virus infection prior to inoculation with bacteria. These findings indicate that this vaccine-induced immunity against S. pyogenes M protein can overcome influenza virus-mediated defects in host immune responses [8], ultimately protecting against secondary bacterial complications. To date, the literature describing clearance of S. pyogenes indicates that interactions with antibodies, complement proteins, and phagocytic cells (neutrophils and macrophages) are associated with protective immunity [49;61–63]. However, at the current time, immune correlates of protection against S. pyogenes are not well-defined [64]. This is in contrast to the influenza virus vaccine field where an antibody titer of 1:40, detected using the hemagglutination inhibiton assay, is an accepted correlate of protection [9;65]. These vaccine-induced correlates of protection are typically defined using multiple human vaccine studies [66;67], which have not been performed for these M protein vaccines [18]. Future evaluation of the protective response in our model will focus on understanding the specific antibody:host interactions that can result in protection, even within a virus-weakened host. The results from our ex vivo uptake assay provide evidence that Fc receptor interactions with murine macrophages may play a role [68;69], and future studies will evaluate the contribution of these cells toward the protection observed in vivo.
In addition, future work will consider the contributions of neutrophils [70;71] and complement components [72], toward protection, as well as the potential breadth of vaccine-induced immunity against distinct M peptides, which will require further optimization of the nanoparticle uptake assay. It is worth noting that when vaccines against influenza virus [23] and S. pyogenes M proteins (data presented here) were delivered individually, the influenza vaccine impacted body weight after influenza virus challenge, while the benefits of the hexavalent M protein vaccine were seen in survival after inoculation with S. pyogenes. Based on these findings, one would expect that vaccinating against both pathogens would yield optimal immunity as evidenced by lack of morbidity (weight loss) and mortality throughout the course of a super-infection. This situation is relevant to the clinical application of vaccines against S. pyogenes M protein, and can also be explored in future laboratory and clinical studies.
Historically, many labs studying super-infections evaluate the influenza virus-mediated effects on host immunity that predispose toward death, using either S. pneumoniae [73;74] or Staphylococcus aureus [75–78] as secondary invaders [8]. The information obtained from these published super-infection models can be used to identify host responses toward influenza viruses that may allow for increased susceptibility to super-infection with S. pyogenes. These can include influenza virus-associated effects on epithelial cells [79;80], macrophages [81–84], and/or neutrophils [85;86], which combine to enhance bacterial pathogenesis. However, since S. pyogenes establishes distinct clinical diseases, and has unique pathogenic properties [87;88], future work is needed to consider this bacteria individually for its contribution to secondary infections [89;90].
Our results show that antibodies induced in mice after vaccination against S. pyogenes M protein can recognize the M protein vaccine linked to nanoparticles and induce uptake of these nanoparticles by a murine macrophage cell line (J774A.1). These experiments were designed to use M protein vaccine-coated nanoparticles as a replacement for viable streptococci in the classic bactericidal test of Lancefield [48]. Since the macrophage is only one cellular component associated with protection of S. pyogenes [91], there is more work required to define the immune mechanisms associated with vaccine-induced protection in our model. However, our previous evaluation of vaccine-induced correlates of protective immunity against influenza viruses has indicated contributions of Fc receptors, macrophages [92], and distinct IgG isotypes [30] toward influenza virus clearance. Applying this knowledge of immune function to S. pyogenes M protein:antibody interactions, we hypothesize that the clearance observed in our model is optimized by Fab recognition of the M protein epitopes [27] in conjunction with Fc binding to Fc receptors expressed by host effector cells [93]. This situation would allow the antibodies induced by vaccination with an M protein vaccine to link the infecting S. pyogenes isolate to host phagocytic cells, resulting in bacterial clearance despite defects in host immunity associated with antecedent influenza virus infection. It is worth noting that the study by Ely et al. [51] reported 100% protection against hemolytic streptococcus in influenza virus-infected humans that had been vaccinated against these bacteria. Similarly, more than 90 years later, we present a benefit associated with vaccine-induced anti-bacterial immunity toward preventing mortality in a model of influenza virus:bacteria super-infections.
Supplementary Material
Supplemental Figure 1. Serum-mediated uptake of FITC-labeled SiO2 nanoparticles that were linked to the hexavalent M protein, visualized using confocal microscopy. Confocal microscopy was used to visualize the uptake of fluorescent nanoparticles by J774A.1 macrophages. Videos represent an entire Z series for each set of cells, as represented in Figure 4.
Supplemental Figure 2. Serum-mediated uptake of FITC-labeled SiO2 nanoparticles conjugated to hexavalent M proteins. Fluorescent images are shown, with multiple fields.
Acknowledgments
The authors would like to thank Jonathan A. McCullers (University of Tennessee Health Science Center, Memphis, TN) for providing the influenza viruses used in these studies, and James D. Hoefelmeyer (University of South Dakota, Vermillion, SD) for assistance with the TEM images. This work was supported by Start-up funds provided by the Division of Basic Biomedical Sciences at USD (VCH), the USD Foundation (VCH), a New Faculty Development Award through the Office of Research and Sponsored Programs at USD (VCH), the Sanford School of Medicine Medical Student Summer Research program (BAJ), the U.Discover program at USD (MJS, TB, and JS), an Inside TRACK award from the Office of Research and Sponsored Programs (ORSP) at USD (VCH), an Interdisciplinary Research Award through the ORSP at USD (VCH and GS), an NSF Grant (CHE 0840507) for acquisition of the TEM instrument, an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) (P20GM103443) (VCH and MSC), and by the US Public Health Service, NIH grant AI-010085 from the National Institute of Allergy and Infectious Diseases (JBD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
J. Dale is the inventor of certain technologies related to the development of group A streptococcal vaccines. The University of Tennessee Research Foundation has licensed the technology to Vaxent, LLC, of which J. Dale is a member and Chief Scientific Officer. All other authors declare that they have no financial conflict of interest to disclose.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005 Nov;5(11):685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Tanz RR, Shulman ST. Chronic pharyngeal carriage of group A streptococci. Pediatr Infect Dis J. 2007 Feb;26(2):175–6. doi: 10.1097/01.inf.0000255328.19808.be. [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ. Group A streptococcal infections of the skin: molecular advances but limited therapeutic progress. Curr Opin Infect Dis. 2006 Apr;19(2):132–8. doi: 10.1097/01.qco.0000216623.82950.11. [DOI] [PubMed] [Google Scholar]
- 4.Martin JM, Green M. Group A streptococcus. Semin Pediatr Infect Dis. 2006 Jul;17(3):140–8. doi: 10.1053/j.spid.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007 Oct 1;45(7):853–62. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 6.Olsen RJ, Ashraf M, Gonulal VE, Ayeras AA, Cantu C, Shea PR, et al. Lower respiratory tract infection in cynomolgus macaques (Macaca fascicularis) infected with group A Streptococcus. Microb Pathog. 2010 Jul 30; doi: 10.1016/j.micpath.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Muller MP, Low DE, Green KA, Simor AE, Loeb M, Gregson D, et al. Clinical and epidemiologic features of group a streptococcal pneumonia in Ontario, Canada. Arch Intern Med. 2003 Feb 24;163(4):467–72. doi: 10.1001/archinte.163.4.467. [DOI] [PubMed] [Google Scholar]
- 8.McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014 Apr;12(4):252–62. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 9.McCullers JA, Huber VC. Correlates of vaccine protection from influenza and its complications. Hum Vaccin Immunother. 2012 Jan 1;8(1) doi: 10.4161/hv.8.1.18214. [DOI] [PubMed] [Google Scholar]
- 10.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008 Oct 1;198(7):962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunes B, Viboud C, Machado A, Ringholz C, Rebelo-de-Andrade H, Nogueira P, et al. Excess mortality associated with influenza epidemics in portugal, 1980 to 2004. PLoS One. 2011;6(6):e20661. doi: 10.1371/journal.pone.0020661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen J, Mazick A, Glismann S, Molbak K. Excess mortality related to seasonal influenza and extreme temperatures in Denmark, 1994–2010. BMC Infect Dis. 2011;11:350. doi: 10.1186/1471-2334-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gran JM, Iversen B, Hungnes O, Aalen OO. Estimating influenza-related excess mortality and reproduction numbers for seasonal influenza in Norway, 1975–2004. Epidemiol Infect. 2010 Mar;25:1–10. doi: 10.1017/S0950268810000671. [DOI] [PubMed] [Google Scholar]
- 14.Lee VJ, Yap J, Ong JB, Chan KP, Lin RT, Chan SP, et al. Influenza excess mortality from 1950–2000 in tropical Singapore. PLoS One. 2009;4(12):e8096. doi: 10.1371/journal.pone.0008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto S, Kawabata S, Fujitaka H, Uehira T, Okuno Y, Hamada S. Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine. 2004 Jul 29;22(21–22):2887–93. doi: 10.1016/j.vaccine.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Lamagni TL, Neal S, Keshishian C, Alhaddad N, George R, Duckworth G, et al. Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Emerg Infect Dis. 2008 Feb;14(2):202–9. doi: 10.3201/eid1402.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaber J, Saeed S, Ihekweazu C, Efstratiou A, McCarthy N, Moore O. Group A streptococcal infections during the seasonal influenza outbreak 2010/11 in South East England. Euro Surveill. 2011;16(5) [PubMed] [Google Scholar]
- 18.Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53–63. doi: 10.1007/978-0-387-73960-1_5. [DOI] [PubMed] [Google Scholar]
- 19.Barrozo CP, Russell KL, Smith TC, Hawksworth AW, Ryan MA, Gray GC. National Department of Defense surveillance data for antibiotic resistance and emm gene types of clinical group A streptococcal isolates from eight basic training military sites. J Clin Microbiol. 2003 Oct;41(10):4808–11. doi: 10.1128/JCM.41.10.4808-4811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullers JA. Preparing for the next influenza pandemic. Pediatr Infect Dis J. 2008 Oct;27(10 Suppl):S57–S59. doi: 10.1097/INF.0b013e3181684d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SE, Eick A, Bloom MS, Brundage JF. Influenza immunization and subsequent diagnoses of group A streptococcus-illnesses among U.S. Army trainees, 2002–2006. Vaccine. 2008 Jun 25;26(27–28):3383–6. doi: 10.1016/j.vaccine.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999 May;5(3):379–85. doi: 10.3201/eid0503.990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaussee MS, Sandbulte HR, Schuneman MJ, DePaula FP, Addengast LA, Schlenker EH, et al. Inactivated and live, attenuated influenza vaccines protect mice against influenza:Streptococcus pyogenes super-infections. Vaccine. 2011 Mar 23; doi: 10.1016/j.vaccine.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3–26. doi: 10.1093/epirev/mxj003. [DOI] [PubMed] [Google Scholar]
- 25.Ozgur SK, Beyazova U, Kemaloglu YK, Maral I, Sahin F, Camurdan AD, et al. Effectiveness of inactivated influenza vaccine for prevention of otitis media in children. Pediatr Infect Dis J. 2006 May;25(5):401–4. doi: 10.1097/01.inf.0000217370.83948.51. [DOI] [PubMed] [Google Scholar]
- 26.Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995 Oct;149(10):1113–7. doi: 10.1001/archpedi.1995.02170230067009. [DOI] [PubMed] [Google Scholar]
- 27.Dale JB. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine. 1999 Jan;17(2):193–200. doi: 10.1016/s0264-410x(98)00150-9. [DOI] [PubMed] [Google Scholar]
- 28.Hall MA, Stroop SD, Hu MC, Walls MA, Reddish MA, Burt DS, et al. Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections. Infect Immun. 2004 May;72(5):2507–12. doi: 10.1128/IAI.72.5.2507-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotloff KL, Corretti M, Palmer K, Campbell JD, Reddish MA, Hu MC, et al. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA. 2004 Aug 11;292(6):709–15. doi: 10.1001/jama.292.6.709. [DOI] [PubMed] [Google Scholar]
- 30.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006 Sep;13(9):981–90. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santra S, Yang H, Dutta D, Stanley JT, Holloway PH, Tan W, et al. TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem Commun (Camb) 2004 Dec 21;(24):2810–1. doi: 10.1039/b411916a. [DOI] [PubMed] [Google Scholar]
- 32.Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford; New York: 2000. [Google Scholar]
- 33.Friedman M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem. 2004 Feb 11;52(3):385–406. doi: 10.1021/jf030490p. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zhao W, O’Donoghue MB, Tan W. Fluorescent nanoparticles for multiplexed bacteria monitoring. Bioconjug Chem. 2007 Mar;18(2):297–301. doi: 10.1021/bc060255n. [DOI] [PubMed] [Google Scholar]
- 35.Huber VC, Thomas PG, McCullers JA. A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine. 2009 Feb 18;27(8):1192–200. doi: 10.1016/j.vaccine.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beachey EH, Bronze M, Dale JB, Kraus W, Poirier T, Sargent S. Protective and autoimmune epitopes of streptococcal M proteins. Vaccine. 1988 Apr;6(2):192–6. doi: 10.1016/s0264-410x(88)80027-6. [DOI] [PubMed] [Google Scholar]
- 37.Huber VC, Peltola V, Iverson AR, McCullers JA. Contribution of Vaccine-Induced Immunity toward either the HA or the NA Component of Influenza Viruses Limits Secondary Bacterial Complications. J Virol. 2010 Apr;84(8):4105–8. doi: 10.1128/JVI.02621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biwer ET. Section of Municipal Health Officers: Some preliminary note on the use of streptococcus vaccine as a means of prophylaxis against scarlet fever. J Am Public Health Assoc. 1911 Sep;1(9):643–5. doi: 10.2105/ajph.1.9.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver GH, Boughton TH. The injections of heterologous streptococci, killed by galactose, in erysipelas and in scarlet fever. J Infect Dis. 1908;5(5):608–15. [Google Scholar]
- 40.Hirst GK, Lancefield RC. Antigenic properties of the type-specific substance derived from Group A hemolytic streptococci. J Exp Med. 1939 Feb 28;69(3):425–45. doi: 10.1084/jem.69.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lancefield RC. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–13. [PubMed] [Google Scholar]
- 42.Cole JN, Barnett TC, Nizet V, Walker MJ. Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol. 2011;9(10):724–36. doi: 10.1038/nrmicro2648. [DOI] [PubMed] [Google Scholar]
- 43.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009 Oct;9(10):611–6. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 44.Dale JB, Washburn RG, Marques MB, Wessels MR. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996 May;64(5):1495–501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacks-Weis J, Kim Y, Cleary PP. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J Immunol. 1982 Apr;128(4):1897–902. [PubMed] [Google Scholar]
- 46.Lancefield RC, Perlmann GE. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancefield RC. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959 Aug 1;110(2):271–92. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancefield RC. The antigenic complex of streptococcus haemolyticus: I. Demonstration of a type-specific substance in extracts of Streptococcus hemolyticus. J Exp Med. 1928 Jan 1;47(1):91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones KF, Fischetti VA. The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis of group A M6 streptococci. J Exp Med. 1988 Mar 1;167(3):1114–23. doi: 10.1084/jem.167.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chien YW, Klugman KP, Morens DM. Efficacy of Whole-Cell Killed Bacterial Vaccines in Preventing Pneumonia and Death during the 1918 Influenza Pandemic. J Infect Dis. 2010 Dec 1;202(11):1639–48. doi: 10.1086/657144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ely CF, Lloyd BJ, Hitchcock CD, Nickson DH. Influenza as seen at the Puget Sound Navy Yard. JAMA. 1919;72:24–8. [Google Scholar]
- 52.Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, et al. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A. 2010 Nov 9;107(45):19396–401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bungener L, Geeraedts F, Ter VW, Medema J, Wilschut J, Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008 May 2;26(19):2350–9. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 54.Buchanan JM, Vogel LA, Van CV, Metzger DW. Interleukin 12 alters the isotype- restricted antibody response of mice to hen eggwhite lysozyme. Int Immunol. 1995 Sep;7(9):1519–28. doi: 10.1093/intimm/7.9.1519. [DOI] [PubMed] [Google Scholar]
- 55.Akesson P, Cooney J, Kishimoto F, Bjorck L. Protein H–a novel IgG binding bacterial protein. Mol Immunol. 1990 Jun;27(6):523–31. doi: 10.1016/0161-5890(90)90071-7. [DOI] [PubMed] [Google Scholar]
- 56.Medina E, Molinari G, Rohde M, Haase B, Chhatwal GS, Guzman CA. Fc-mediated nonspecific binding between fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J Immunol. 1999 Sep 15;163(6):3396–402. [PubMed] [Google Scholar]
- 57.Collin M, Olsen A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001 Jun 15;20(12):3046–55. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su YF, Chuang WJ, Wang SM, Chen WY, Chiang-Ni C, Lin YS, et al. The deficient cleavage of M protein-bound IgG by IdeS: Insight into the escape of Streptococcus pyogenes from antibody-mediated immunity. Mol Immunol. 2011 Sep 16;49:134–42. doi: 10.1016/j.molimm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 59.von Pawel-Rammingen U, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002 Apr 2;21(7):1607–15. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nordenfelt P, Waldemarson S, Linder A, Morgelin M, Karlsson C, Malmstrom J, et al. Antibody orientation at bacterial surfaces is related to invasive infection. J Exp Med. 2012 Dec 17;209(13):2367–81. doi: 10.1084/jem.20120325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allison AC. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 62.Flores AE, Johnson DR, Kaplan EL, Wannamaker LW. Factors influencing antibody responses to streptococcal M proteins in humans. J Infect Dis. 1983 Jan;147(1):1–15. doi: 10.1093/infdis/147.1.1. [DOI] [PubMed] [Google Scholar]
- 63.Horstmann RD, Sievertsen HJ, Knobloch J, Fischetti VA. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1657–61. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreland NJ, Waddington CS, Williamson DA, Sriskandan S, Smeesters PR, Proft T, et al. Working towards a Group A Streptococcal vaccine: Report of a collaborative Trans- Tasman workshop. Vaccine. 2014 May 14; doi: 10.1016/j.vaccine.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 65.Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013 Mar 15;207(6):974–81. doi: 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008 Aug 1;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 67.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006 Jun 12;24(24):5093–107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 68.Verma A, Ngundi MM, Meade BD, De PR, Elkins KL, Burns DL. Analysis of the Fc gamma receptor-dependent component of neutralization measured by anthrax toxin neutralization assays. Clin Vaccine Immunol. 2009 Oct;16(10):1405–12. doi: 10.1128/CVI.00194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Unkeless JC, Kaplan G, Plutner H, Cohn ZA. Fc-receptor variants of a mouse macrophage cell line. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1400–4. doi: 10.1073/pnas.76.3.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartshorn KL, Crouch E, White MR, Colamussi ML, Kakkanatt A, Tauber B, et al. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol. 1998 Jun;274(6 Pt 1):L958–L969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 71.Courtney H, Ofek I, Simpson WA, Beachey EH. Characterization of lipoteichoic acid binding to polymorphonuclear leukocytes of human blood. Infect Immun. 1981 May;32(2):625–31. doi: 10.1128/iai.32.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnsson E, Berggard K, Kotarsky H, Hellwage J, Zipfel PF, Sjobring U, et al. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J Immunol. 1998 Nov 1;161(9):4894–901. [PubMed] [Google Scholar]
- 73.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006 Jul;19(3):571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008 May;14(5):558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 75.Metzger DW, Sun K. Immune dysfunction and bacterial coinfections following influenza. J Immunol. 2013 Sep 1;191(5):2047–52. doi: 10.4049/jimmunol.1301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011 Mar 15;203(6):880–8. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson KM, Choi SM, McHugh KJ, Mandalapu S, Enelow RI, Kolls JK, et al. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1beta production in mice. J Immunol. 2013 Nov 15;191(10):5153–9. doi: 10.4049/jimmunol.1301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, et al. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J Infect Dis. 2014 Mar;209(6):865–75. doi: 10.1093/infdis/jit527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003 Mar 15;187(6):1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 80.Herold S, Steinmueller M, von WW, Cakarova L, Pinto R, Pleschka S, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008 Dec 22;205(13):3065–77. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sawyer WD. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–56. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- 82.Warshauer D, Goldstein E, Akers T, Lippert W, Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–77. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- 83.Rodgers BC, Mims CA. Role of macrophage activation and interferon in the resistance of alveolar macrophages from infected mice to influenza virus. Infect Immun. 1982 Jun;36(3):1154–9. doi: 10.1128/iai.36.3.1154-1159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lowy RJ, Dimitrov DS. Characterization of influenza virus-induced death of J774.1 macrophages. Exp Cell Res. 1997 Aug 1;234(2):249–58. doi: 10.1006/excr.1997.3602. [DOI] [PubMed] [Google Scholar]
- 85.Ruutu P, Vaheri A, Kosunen TU. Depression of human neutrophil motility by influenza virus in vitro. Scand J Immunol. 1977;6(9):897–906. doi: 10.1111/j.1365-3083.1977.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 86.Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999 Apr 1;93(7):2395–403. [PubMed] [Google Scholar]
- 87.Lynskey NN, Lawrenson RA, Sriskandan S. New understandings in Streptococcus pyogenes. Curr Opin Infect Dis. 2011 Jun;24(3):196–202. doi: 10.1097/QCO.0b013e3283458f7e. [DOI] [PubMed] [Google Scholar]
- 88.Pfoh E, Wessels MR, Goldmann D, Lee GM. Burden and economic cost of group A streptococcal pharyngitis. Pediatrics. 2008 Feb;121(2):229–34. doi: 10.1542/peds.2007-0484. [DOI] [PubMed] [Google Scholar]
- 89.Okamoto S, Kawabata S, Terao Y, Fujitaka H, Okuno Y, Hamada S. The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection. Infect Immun. 2004 Oct;72(10):6068–75. doi: 10.1128/IAI.72.10.6068-6075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okamoto S, Kawabata S, Nakagawa I, Okuno Y, Goto T, Sano K, et al. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol. 2003 Apr;77(7):4104–12. doi: 10.1128/JVI.77.7.4104-4112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldmann O, Rohde M, Chhatwal GS, Medina E. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004 May;72(5):2956–63. doi: 10.1128/IAI.72.5.2956-2963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001 Jun 15;166(12):7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 93.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998 Jun;76(6):231–48. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Serum-mediated uptake of FITC-labeled SiO2 nanoparticles that were linked to the hexavalent M protein, visualized using confocal microscopy. Confocal microscopy was used to visualize the uptake of fluorescent nanoparticles by J774A.1 macrophages. Videos represent an entire Z series for each set of cells, as represented in Figure 4.
Supplemental Figure 2. Serum-mediated uptake of FITC-labeled SiO2 nanoparticles conjugated to hexavalent M proteins. Fluorescent images are shown, with multiple fields.


