Abstract
The purpose of this study was to compare: a new five-layered poly (L–lactide–co–caprolactone) (PLC) membrane and small intestinal submucosa (SIS) as a control in rat urinary bladder wall regeneration. The five-layered poly (L–lactide–co–caprolactone) membrane was prepared by an electrospinning process. Adipose tissue was harvested from five 8-week old male Wistar rats. Adipose derived stem cells (ADSCs) were seeded in a density of 3×106 cells/cm2 onto PLC membrane and SIS scaffolds, and cultured for 5-7 days in the stem cell culture medium. Twenty male Wistar rats were randomly divided into five equal groups. Augmentation cystoplasty was performed in a previously created dome defect. Groups: (I) PLC+ 3×106ADSCs; (II) SIS+ 3×106ADSCs; (III) PLC; (IV) SIS; (V) control. Cystography was performed after three months. The reconstructed urinary bladders were evaluated in H&E and Masson's trichrome staining. Regeneration of all components of the normal urinary bladder wall was observed in bladders augmented with cell-seeded SIS matrices. The urinary bladders augmented with SIS matrices without cells showed fibrosis and graft contraction. Bladder augmentation with the PLC membrane led to numerous undesirable events including: bladder wall perforation, fistula or diverticula formation, and incorporation of the reconstructed wall into the bladder lumen. The new five-layered poly (L–lactide–co–caprolactone) membrane possesses poorer potential for regenerating the urinary bladder wall compared with SIS scaffold.
Introduction
The small intestine is commonly used for urinary tract reconstruction, either for bladder augmentation, replacement, or urinary diversion. Unfortunately, these techniques are associated with numerous complications [1]. Many natural and synthetic biomaterials such as plastic mold, gelatin sponge, Japanese paper, preserved dog bladder, lyophilized human dura, bovine pericardium, small intestinal submucosa, bladder acellular matrix, or composites of collagen and polyglycolic acid were used for human urinary bladder regeneration with a wide range of outcomes [2]. However, the ideal biomaterial for urinary bladder reconstruction has not been found thus far. The scaffolds used in tissue engineering should mimic the ability of the extracellular matrix (ECM) to regulate cell functions such as cell division, differentiation, and apoptosis [3]. The biomaterial intended for urinary bladder reconstruction should be not only biocompatible and biodegradable but also waterproof, flexible, elastic and able to provide good mechanical strength [4].
Decellularized extracellular matrices such as small intestinal submucosa (SIS) or bladder acellular matrix (BAM) have been widely used for urinary bladder reconstruction in various models [5]–[8]. However, acellular matrices have some disadvantages including immunogenicity, lot- to- lot variability, and inadequate biomechanical properties [9]. Consequently, there is a growing interest in developing new synthetic biomaterials for urinary bladder regeneration [10], [11]. Aliphatic polyesters such as poly(glycolide) (PGA), poly(lactide) (PLA), poly(ε-caprolactone) (PCL), and their copolymers have been widely used in tissue engineering with favorable results [12]–[16]. Several in vitro studies revealed the significant potential of PLC membranes for tissue engineering in urology [17], [18]. This data has encouraged us to produce the PLC membrane for urinary bladder wall regeneration. The electrospinning process is a promising way for providing nanofibrous scaffolds, which closely mimic the natural extracellular matrix structure [19]. Matrices made of electrospun nanofibers when compared to natural collagen matrices have certain advantages: they are non-allergenic, do not involve risk of transferring diseases (e.g. prions), and are easy to prepare, handle, and store. Urine is extremely cytotoxic for stem cells [20], [21]. Therefore, the separation of stem cells from the toxic environment of urine seems to be crucial for urinary bladder tissue engineering. In this experiment, we evaluate poly (L–lactide–co–caprolactone), a new nanofibrous and durable membrane, designed to isolate cells from urine in tissue engineered urinary bladders.
Materials and Methods
Synthesis of five-layered poly (L-lactide-co-caprolactone) membrane via an electrospinning technique
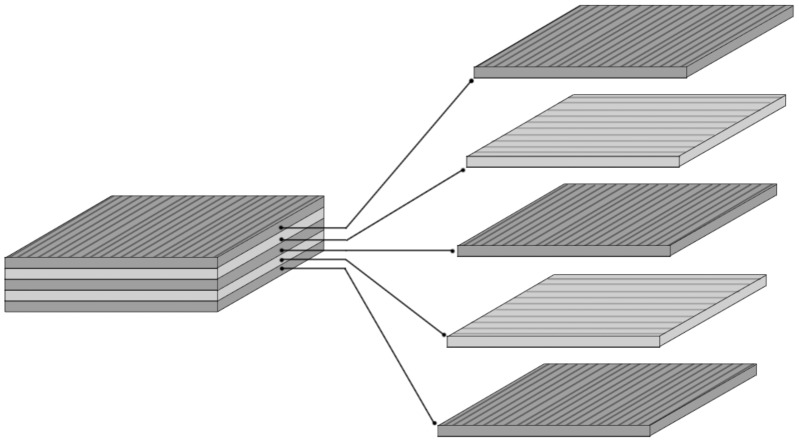
Poly (L–lactide–co–caprolactone) (PLC) (Purac, Netherlands), chloroform (CHCl3) (POCh, Poland), N,N-dimethylformamide (DMF)(POCh, Poland), sodium bicarbonate (NaHCO3) (POCh, Poland) were used as supplied, without further purification. Nanomaterials were prepared using an electrospinning process described by Li et al [22]. Briefly, PLC was dissolved in a mixture of CHCl3 and DMF (mass proportion 16∶1) to form 9% solution and left overnight. Electrospinning was conducted in a custom-made polycarbonate chamber of approximate volume of 1 m3. The electrospinning setup consisted of custom-made voltage power supply (HVPS) with adjustable output voltage. The electrospun solution was transferred from a metering syringe pump (New Era Pumping Systems, Great Britain) through a hydraulic system to a nozzle made of blunt needle (outer volume 0.4 mm) connected to HVPS output. Each element of the setup has a commercial market counterpart. Electrically grounded aluminum foil attached to a fast rotating polypropylene mandrel served as a target. After collection of each consecutive layer of directed nanofibrous material, the foil was detached from the mandrel, positioned orthogonally to a previous direction, and attached back to the mandrel. Material of a plywood-like structure was formed after 5 repetitions of the fiber collection process (Fig. 1). Electrospinning parameters were as follows: voltage- 20 kV, polymer solution flow- 800 µl/h, spinneret to collector distance- 20 cm, mandrel speed- 4100 rpm, mandrel diameter- 60 mm.
Figure 1. Scheme of five-layered electrospun membrane structure.
Modification of five-layered poly (L-lactide-co-caprolactone) membrane
The surface of nanomaterial was modified by treatment with a 0.5 M solution of sodium bicarbonate dissolved in deionized water. Filter paper was soaked with a treatment solution and placed on top of the membrane for 30 min. The results were measured using a goniometric method by measuring contact angle of 2 µl droplet of water placed on a membrane before and after modification (OCA 20, DataPhysics Instruments GmbH, Filderstad, Germany). ImageJ software (NIST, USA) was used to evaluate data.
Poly (L-lactide-co-caprolactone) membrane nanofiber thickness and pore size
The ImageJ software was used to assess fiber diameters. For each sample of nanofiber material, three SEM images were acquired and 33 measurements were taken from each image. The measurement was made perpendicular to the fiber's axis. Scaffold porosity (ε) was calculated by the method given by Hu [23] using density of the scaffold (ρscafflold) and density of neat PLC (ρPLC): ε = 1- (ρscafflold/ρPLC). Characteristic 3-dimensional pore diameter (d3D) was calculated by the method given by Tomadakis and Robertson [24] on the basis of scaffold porosity (ε) and mean fiber diameter (ω): d3D = − ω/ln(ε)
Analysis of the mechanical properties of five-layered poly (L-lactide-co-caprolactone) membrane
The tensile tests were conducted on a load frame of a servohydraulic material testing machine (MTS 242.01 actuator, Eden Prairie, USA). The specimen (25 mm length, 12.5 mm width) was mounted into flat grips with gauge base 12.5 mm. During the test, the specimen was longitudinally extracted at a rate of 0.3 mm/s to failure. The grip travel and specimen load were continuously measured over the test procedure duration with a precision force transducer (Interface, model 1500, measuring range 125 N, resolution 0.0625 N) and MTS system linear variable differential transformer (measuring range 100 mm, resolution 0.01 mm). Stress/strain curve for the specimen was generated and the ultimate tensile strength, maximum strain and elastic modulus were determined. The strength (MPa) was calculated by dividing the value of failure load by initial cross-sectional area of the specimen. The maximum strain (the strain value corresponding to the ultimate stress) was calculated as the elongation of the specimen divided by the initial gauge length (mm/mm). The elastic modulus (MPa) was defined as the slope of the linear regions of the stress/strain curve.
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health [25]. The protocol was approved by the Nicolaus Copernicus University Ethics Committee (no. 4/2012).
Adipose Derived Stem Cell Isolation and Culture
Adipose tissue was harvested from the retroperitoneal space from five syngeneic 8-week male Wistar rats. The animals were euthanized with an overdose of ketamine 75 mg/kg (Biowet, Poland). ADSCs were isolated according to the method described by Safford et al [26]. Briefly, adipose tissue (1 g) was digested in collagenase type I solution (1 mg/ml, Sigma- Aldrich, Germany) for 30 min at 37°C with shaking. The reaction was stopped by adding Dulbecco's Modified Eagle's Medium (DMEM; PAA, Austria), supplemented with 10% fetal bovine serum (FBS; PAA, Austria) and antibiotics (PAA, Austria). The cell suspension was filtered by a 100 µm cell strainer (Becton Dickinson, USA) and centrifuged at 350×g for 5 min. Viable cells were counted via trypan blue staining and seeded on 25 cm2 flask in a density of 15×103 cells/cm2. ADSCs were cultured in a medium consisting of DMEM supplemented with 10% FBS, fibroblast growth factor (FGF, 10 ng/ml; Sigma- Aldrich, Germany), penicillin/streptomycin (100 U/100 µg/ml) and amphotericin B (100 µg/ml) (PAA, Austria) at 37°C, 5%CO2 and 95% humidity until 3rd passage.
Adipose Derived Stem Cells Characterization
To confirm the ADSCs phenotype, cells were subjected to antigen analysis by flow cytometry. Detached cells from the 3rd passage were washed and re-suspended with phosphate buffered saline (PBS). Approximately 1×106 cells were incubated with monoclonal primary antibodies conjugated with PE or FITC against CD34 (Santa Cruz Biotechnology, Inc, USA; 20 µl/sample), CD44 (Millipore, USA; 10 µl/sample), CD45 (BD, Pharmingen, USA; 0.06 µg/sample) and CD90 (Millipore, USA; 10 µl/sample) for 30 min. Expression level of each surface marker was quantified using an EPICS XL flow cytometer (Beckman Coulter, USA).
The differentiation of ADSCs into adipogenic, osteogenic and chondrogenic lineages was induced by culture in appropriate differentiation media, according to manufacturer's instruction (Invitrogen, USA). Negative control cells were maintained in DMEM/Ham's F-12 supplemented with 10% FBS and antibiotics. Adipogenesis was measured by the accumulation of neutral lipids in fat vacuoles, stained with Oil-Red-O (Sigma- Aldrich, Germany). Osteogenesis was confirmed using Alizarin Red staining (Millipore, USA). Chondrogenic differentiation was evaluated by anti-collagen type II immunocytochemical staining (anti-collagen II clone 6B3, Millipore, USA, 1∶100, 16 h, 4°C).
Biomaterial Cytotoxicity Assay
Biomaterial cytotoxicity was determined using extract toxicity assay according to ISO-10993 norm. A piece of biomaterial (∼6 cm2) was extracted in DMEM supplemented with 10% FBS and antibiotics (1 ml) at 37°C for 120 h. The extract was filtered and stored at 4°C for up to one week. Extract cytotoxicity was determined using the xCELLigence system (Real-Time Cell Analyzer Dual Plate, RTCA DP, Roche Applied Science, Germany). The xCELLigence system is a unique, cellular impedance- based systems that allows the real- time monitoring of cell growth. When adherent cells attach and spread on the sensor surface of an electrode, increases in impedance are recorded. The changes in impedance are expressed as cell index (CI). For this purpose, ADSCs were seeded on E-Plates (4×103 cells/well) and cultured until reaching a log- phase growth in DMEM supplemented with 10% FBS and antibiotics. Then the medium was changed for a fresh one (negative control) or medium supplemented with 25%, 50% and 75% biomaterial extract (PLC25, PLC50, PLC75, SIS25, SIS50, SIS75 respectively). The experiment was continued until ADSCs achieved the plateau growth phase.
Analysis of Adipose Derived Stem Cells Growth on the poly (L-lactide-co-caprolactone) membrane
ADSCs were seeded on the 1 cm2 of poly (L-lactide-co-caprolactone) electrospun membrane or on small intestinal submucosa (SIS) (Surgisis, Biodesign, USA) mounted on cell crowns in a density of 3×106 cells/cm2, and cultured for 7 or 14 days. ADSCs growth on PLC and SIS scaffolds was assessed by scanning electron microscopy. For this purpose, the specimens were fixed in 2% paraformaldehyde and 2,5% glutaraldehyde in phosphate buffer for 2 h, post-fixed in 1% OsO4 and dehydrated with grades series of ethyl alcohol followed by acetone. Next the specimens were critically-dried and coated with gold particles before observation in scanning electron microscope (JEOL JSM-6390LV, Japan).
Graft preparation
To prepare the grafts for bladder augmentation, the ADSCs were seeded on PLC or SIS scaffolds and cultured for 5-7 days.
Augmentation cystoplasty
Twenty syngeneic male Wistar rats weighing between 250 and 300 g were randomly divided into five equal groups. Sixteen rats, anesthetized with sodium pentobarbital (15 mg/kg, i.p., Biowet, Poland) and lidocaine (20 mg/kg, i.m., Polfa, Poland), underwent hemicystectomy and bladder augmentation with approximately 1 cm2 of graft. The anastomosis line was marked by 8.0 monofilament non-absorbable marker sutures to identify the graft borders. In the first group, bladders were reconstructed using PLC membrane seeded with 3×106 ADSCs. In the second group, bladders were augmented using SIS seeded with 3×106 ADSCs. In the third and fourth groups, bladders were reconstructed with unseeded PLC and SIS membranes, respectively. In order to achieve a good analgesic effect, the lidocaine was additionally injected (20 mg/kg, i.m.) after the procedure. The fifth group (control) was left intact. Cystography, morphological and histological studies were performed after three months follow-up.
Cystography
Under general anesthesia with sodium pentobarbital (15 mg/kg, i.p.) and lidocaine (20 mg/kg, i.m.), the animal was placed in supine position with knees flexed. A small, flexible catheter (3 Fr, Galmed, Poland) was gently inserted into the rat's urethra and the urinary bladder was injected with radiocontrast (0.5 ml of 20% iopromide, Bayer Pharma AG, Germany). The cystograms were performed by fluoroscopy (Actube Dental 5D2, exposition 60 kV and 6mAm). The animals were euthanized with an overdose of ketamine (75 mg/kg).
Histological staining
The bladder samples were fixed in 10% buffered formaldehyde, using routine procedure of tissue processing, and embedded in paraffin. Cross-sections of whole bladders were made. The 4 µm thick paraffin sections were stained with hematoxylin and eosin. The connective tissue components and muscle layer were stained according to Masson staining. The smooth muscle abundance was assessed using the ImageJ program according to the method described previously [5]. The analysis was repeated for nine areas from each of the specimens.
Statistical analysis
Statistical differences between groups were determined by one-way ANOVA followed by Tamhane' post hoc multiple-comparison test (IBM SPSS Statistics, Predictive Solutions, Poland). Statistically significant differences were defined as having p<0.05.
Results
Synthesis and characterization of five-layered poly (L-lactide-co-caprolactone) membrane
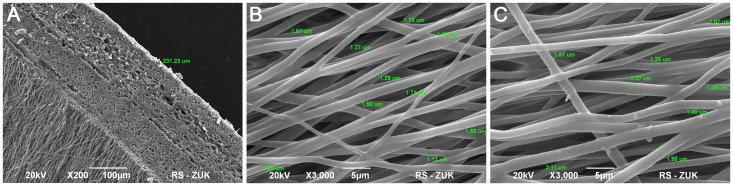
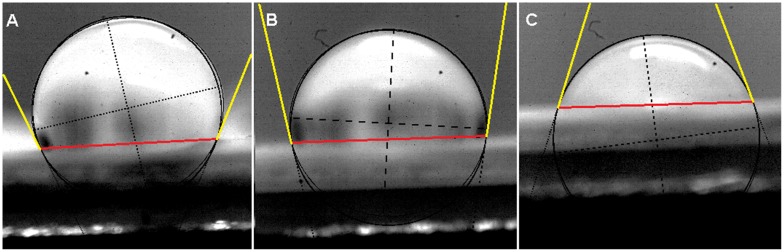
The electrospun five-layered poly (L-lactide-co-caprolactone) membrane thickness was approximately 230 µm (Fig. 2A). The membrane had a micro-porous structure (Fig. 2B). The mean 3D pore diameter was 2.69±0.62 µm. The fiber diameter ranged between 740 nm and 2.27 µm (mean 1.39±0.32 µm). The NaHCO3 treatment did not significantly change the thickness of PLC fibers (mean 1.51±0.36 µm) and 3D pore diameter (mean 2,91±0. µm)(p>0.05) (Fig. 2C). The contact angle of PLC membrane before modification was 110 degrees. Sodium bicarbonate (NaHCO3) treatment led to a decrease of the contact angle of the PLC membrane by 25°C (Fig. 3). The mechanical properties of the PLC membrane are given in Table 1.
Figure 2. Ultrastructural analysis of the electrospun poly (lactydo-co-caprolactone) (PLC) membrane: thickness of five- layered PLC membrane (A), the surface of unmodified PLC membrane (B) the surface of PLC membrane modified with sodium bicarbonate (C).
Scanning Electron Microscopy, bar 5 µm, 100 µm.
Figure 3. Contact angle measurements: untreated poly (lactydo-co-caprolactone) (PLC) membrane, 110° (A), NaHCO3 treated PLC membrane: unmodified side- 97° (C) and modified side- 72° (B).
Contact angle is the angle between the baseline of the drop (marked in red) and the tangent at the drop boundary (marked in yellow).
Table 1. Tensile properties of the five-layered poly (lactydo-co-caprolactone) (PLC) membrane and bladder specimens.
| Material | Ultimate tensile strain (mm/mm) | Ultimate tensile strength (MPa) | Elastic modulus R1 (MPa) | Elastic modulus R2 (MPa) |
| PLC | 4.35 | 11.4 | 16.0 | 2.25 |
| Rat bladder (27) | 2.03 | 0.72 | - | 0.76 |
| Pig bladder (27) | 1.66 | 0.32 | - | 0.26 |
| Human bladder (27) | 0.69 | 0.27 | - | 0.25 |
The elastic range 1 (R1) was demonstrated up to 24% of strain and the elastic range 2 (R2) between 70 % and 429 %.
Analysis of Phenotype and Multipotent Character of Adipose Derived Stem Cells
Flow cytometry confirmed the ADSCs phenotype. ADSCs derived from the third passage were positive for the CD44+ (50% of cells) and CD90+ (87% of cells) markers and negative for typical endothelial and hematopoietic markers CD34+ (16% of cells) and CD45+ (13% of cells). ADSCs were able to differentiate into adipocytes, osteoblasts and chondrocytes after cultivation in respective media (Fig. 4). Controls showed negative results.
Figure 4. Differentiation potential of adipose derived stem cells: a positive anti-collagen II staining after chondrogenic induction, bar 200 µm (A), Alizarin Red staining after osteogenic induction, bar 500 µm (B), Oil Red O staining after adipogenic induction, bar 20 µm (C).
Analysis of Biomaterial Cytotoxicity in vitro
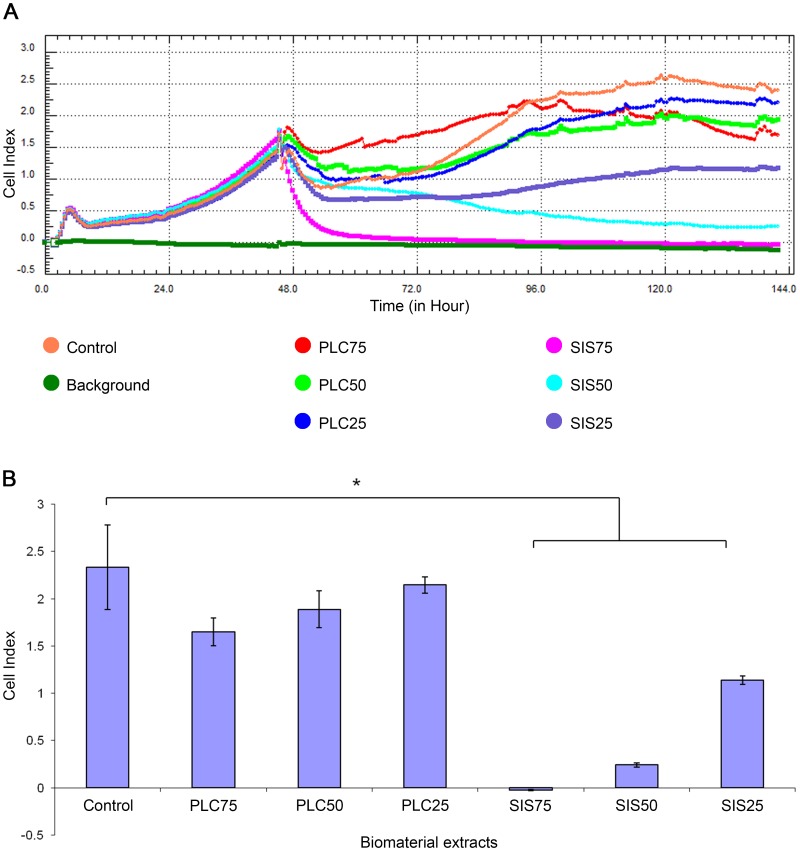
Real- time cell analysis of the cytotoxicity of the PLC and SIS extracts is presented in Fig. 5A. There was no significant decrease in cell indexes following 96 hours of incubation of ADSCs with PLC25, PLC50 and PLC75 extracts compared to the negative control (p>0.05). However, there was significant reduction in cell indexes following incubation of ADSCs with SIS25, SIS50 and SIS75 extracts compared to negative control (p<0.05) (Fig. 5B).
Figure 5. Analysis of poly (lactydo-co-caprolactone) (PLC) and small intestinal submucosa (SIS) cytotoxicity using Real Time Cell Analyzer (RTCA).
Adipose derived stem cells were treated with 75%, 50% and 25% extracts of PLC (PLC75, PLC50, PLC25 respectively) and SIS (SIS75, SIS50, SIS25 respectively). The results are presented as: cell growth curves (A), mean cell index ± standard deviation after 96 hours of cell incubation with extracts. The statistical significance is shown as * p<0.05 (B)
Analysis of Growth of Adipose Derived Stem Cells on Five- layered Poly (L-lactide-co-caprolactone) Membrane and Small Intestinal Submucosa
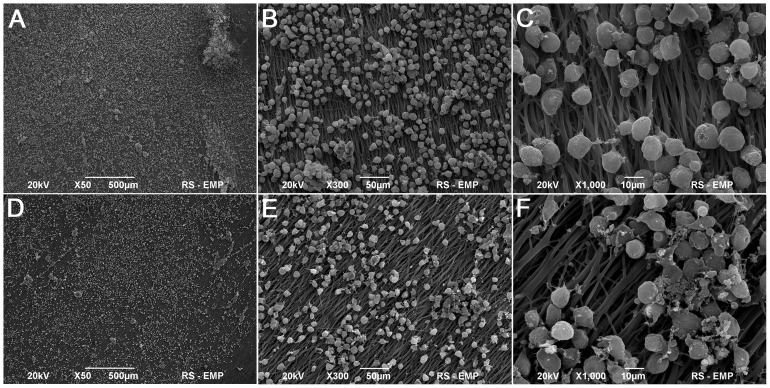
ADSCs evenly covered the surfaces of both SIS and PLC membranes (Fig. 6, 7). After 7 days of culturing, the morphology of ADSCs cultured on SIS was normal but only single cells had a flattened shape and elongated cellular processes (Fig. 7A–C). The number of ADSCs adhering to the SIS surface increased after 14 days of culture (Fig. 7D–E). In contrast, a significant number of ADSCs cultured on PLC membrane attached to the biomaterial surface after only 7 days of culture (Fig. 6A–C). The ADSCs had the normal morphology of living cells. The cell divisions were observed. After 14 days of culture, the cells formed a dense layer adhering well to the PLC membrane (Fig. 6D–E).
Figure 6. Poly (lactydo-co-caprolactone) membrane seeded with adipose derived stem cells (ADSCs), 7th (A,B,C) and 14th day of culture (D,E,F), Scanning Electron Microscopy, bar 500 µm, 50 µm, 10 µm.
Figure 7. Small intestinal submucosa seeded with adipose derived stem cells (ADSCs), 7th (A,B,C) and 14th day of culture (D,E,F), Scanning Electron Microscopy, bar 500 µm, 50 µm, 10 µm.
Analysis of Urinary Bladder Augmentation
Twelve from sixteen rats survived the 3 months observation time. The highest mortality rate (n = 50%) was observed in PLC groups. Surprisingly, deaths were observed between 2nd and 3rd months of observation. The cause of death was urinary bladder perforation, which occurred within the implanted biomaterial. We found that in many cases the biomaterial implant had twisted, formed a coil and detached from the augmented urinary bladder.
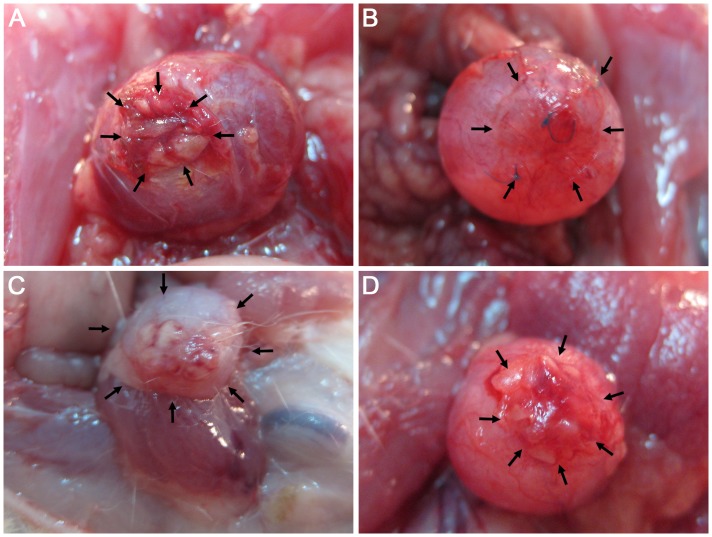
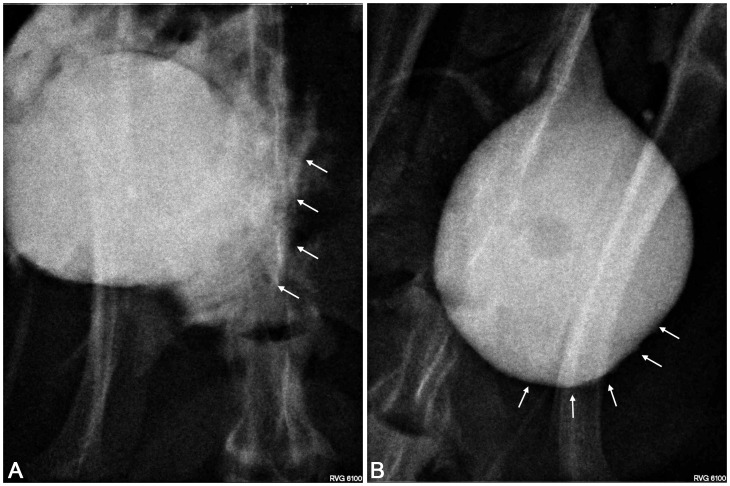
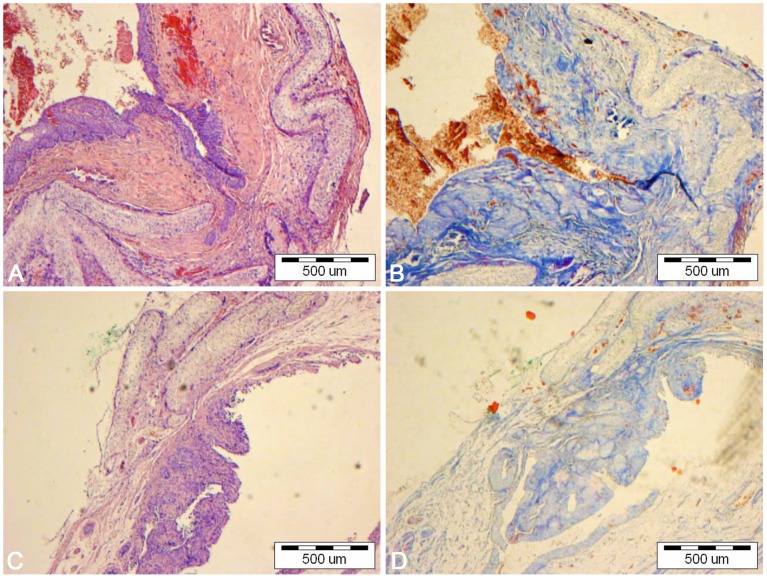
Urinary bladder augmentation with the PLC membrane (1st and 3rd groups) led to numerous undesirable events including: perforation, fistula or diverticula formation, and incorporation of the reconstructed wall into the urinary bladder lumen (Fig. 8AC; Fig. 9A). Histological analysis of reconstructed urinary bladders revealed no integration of PLC with bladder tissues (Fig. 10).
Figure 8. Macroscopic analysis of reconstructed bladders.
Bladder augmented with adipose derived stem cells (ADSCs) seeded poly (lactydo-co-caprolactone) (PLC) (A), ADSCs seeded small intestinal submucosa (SIS) (B), unseeded PLC (C) and unseeded SIS (D). The arrows point out the reconstructed area.
Figure 9. Cystography.
Urinary bladders augmented with poly (lactydo-co-caprolactone) membrane (A) and small intestinal submucosa seeded with adipose derived stem cells (B). The arrows point out the reconstructed area.
Figure 10. Bladders augmented with poly (lactydo-co-caprolactone) membrane seeded (A,B) and unseeded (C,D) with adipose derived stem cells.
Light microscope, H&E (A,C) and Trichrome Masson staining (B,D), bar = 500 µm.
Bladders augmented with SIS seeded with ADSCs (2nd group) mimicked native bladders in gross examination (Fig. 8B). Cystography showed proper, regular shape of reconstructed bladders (Fig. 9B). Regeneration of all components of the normal urinary bladder wall was observed. However, smooth muscle fibers were less abundant in the reconstructed wall compared with native bladder wall (26.1±5.4% vs. 55.6±9.3%, p<0.05) (Fig. 11AB, Fig. 11EF, Fig. 12). In the bladders augmented with SIS matrices without cells (4th group), fibrosis and graft contraction occurred (Fig. 8D; Fig. 11CD).
Figure 11. Bladders augmented with small intestinal submucosa seeded (A,B) and unseeded (C,D) with adipose derived stem cells.
Native bladders in control group (E,F). Light microscope, H&E (A,C,E) and Trichrome Masson staining (B,D,F), bar = 200 µm.
Figure 12. Smooth muscle content in bladders augmented with: poly (lactydo-co-caprolactone) (PLC), PLC seeded with adipose derived stem cells (ADSCs), small intestinal submucosa (SIS), SIS seeded with ADSCs and native bladder wall (control), respectively.
Values are expressed as mean± standard deviation. The statistical significance is shown as * p<0.05.
Discussion
In the present study, the five- layered PLC nanofibrous membrane was produced with the use of a fast-rotating mandrel as a target. Such a type of target ensures a high degree of nanofibers orientation in a specified axis. In order to obtain a membrane of increased strength, a set of 5 orthogonally directed layers of nanofibers was collected. Such a membrane is supposed to have a reinforced structure preventing stretching in all directions. The ultimate tensile strain of PLC membrane was higher compared to rat (2.03 mm/mm, 0.72 MPa) and human (0,69 mm/mm, 0.27 MPa) urinary bladders. Similarly, the elastic modulus of PLC membrane was higher compared to human (0.25 MPa) and rat urinary bladder (0.76 MPa) [27]. These results indicate that the newly synthesized material is elastic and resistant to mechanical treatment. The material was designed to isolate stem cells from the toxic environment of urine. This was achieved because of the high hydrophobicity of the material (both comonomers: L-lactide and ε-caprolactone as well as their copolymer are hydrophobic) and very small air bubbles retained in its structure. However, highly hydrophobic surfaces are not colonized by cells and they cannot sustain cell proliferation. In order to decrease the material's surface hydrophobicity we selected chemical modification of nanofibers [28]–[30]. Other methods are also applied [31], among them are e. g. plasma treatment [32], adhesive protein treatment [33)], silk fibroin modification [34], arginine-glycine-aspartate (RGD) treatment, protein immobilization [35], or surface mineralization with apatite [36]. Our selection was based on the fact that only the surface of the nanofibrous membrane was to be modified while the bulk of the material was left unmodified in order to retain its waterproof properties. A too drastic decrease of hydrophobicity could lead to the loss of isolative properties of the bulk material against the toxic environment of urine, and consequently cause death of stem cells seeded on the nanomaterial surface. The surface of modified electrospun PLC membrane showed improved ADSCs adhesive properties compared with commercially available collagen matrix (SIS). ADSCs adhered to the electrospun PLC membrane and formed a dense layer. Just as in the case of SIS, the PLC membrane pore size was not large enough for cell migration into the scaffold. Such migration was only possible during a subsequent nanomaterial in vivo degradation. Further modifications of the porosity of the biomaterial are required. The biomaterial could be degraded in vitro before implantation. Another option is synthesis of a biomaterial comprised of layers of different porosity and hydrophobicity. The layer with larger pore sizes and decreased hydrophobicity would allow for directed cell adhesion and migration into the scaffold, while the layer with smaller pore sizes and increased hydrophobicity would isolate the cells from the toxic environment of urine.
We found that the PLC extract did not have a cytotoxic effect on ADSCs in all tested concentrations, as opposed to commercially available SIS extract, which was also confirmed by other authors [37]. In this study, SIS was used as a control because it is the reference material for urinary bladder tissue engineering. To exclude the variables arising from SIS self- preparation we used the commercially available and clinically applicable porcine SIS (Surgisis, Biodesign, USA).
Our in vitro analyses showed that the new PLC membrane has better in vitro properties compared to SIS, therefore, the use of new PLC membrane in bladder tissue engineering was rational.
Unfortunately, contrary to our expectations the electrospun PLC membrane did not provide an appropriate environment for urinary bladder wall regeneration. There were many differences in regeneration effects between PLC (1st and 3rd) and SIS (2nd and 4th) groups. The use of adipose derived stem cells did not affect the results of bladder augmentation with PLC membrane but enhanced the smooth muscle regeneration in bladders reconstructed with SIS. Numerous side effects such as: bladder wall perforation, fistula or diverticula formation, or incorporation of the reconstructed wall into the bladder lumen were observed in both PLC groups (1st and 3rd). The PLC membrane did not integrate with surrounding bladder tissues. Three months after surgery, PLC membrane degradation was observed, however no signs of remodeling occurred.
Probably, the numerous complications observed in this study result from the multi-layer construction of the membrane. We speculate that a membrane synthesized from non-oriented nanofibrous structure with only a thin layer of directed nanofibers on both surfaces would avoid complications, however it requires future investigation.
In conclusion, the poly L-lactide-co-caprolactone five-layered membrane is not suitable for urinary bladder wall regeneration.
Funding Statement
The authors have no support or funding to report.
References
- 1. Baka-Ostrowska M (2011) Bladder augmentation and continent urinary diversion in boys with posterior urethral valves. Cent Eur J Urol 64: 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pokrywczynska M, Adamowicz J, Sharma A, Drewa T (2014) Human urinary bladder regeneration through tissue engineering-an analysis of 131 clinical cases. Exp Biol Med 239: 264–71. [DOI] [PubMed] [Google Scholar]
- 3. Adamowicz J, Kowalczyk T, Drewa T (2013) Tissue engineering of urinary bladder – current state of art and future perspectives. Cent Eur J Urol 66: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pokrywczynska M, Jundziłł A, Adamowicz J, Drewa T (2013) Tissue engineering-experimental method of urinary bladder regeneration. Postepy Hig Med Dosw 67: 790–9. [DOI] [PubMed] [Google Scholar]
- 5. Pokrywczynska M, Jundzill A, Bodnar M, Adamowicz J, Tworkiewicz J, et al. (2013) Do mesenchymal stem cells modulate the milieu of reconstructed bladder wall? Arch Immunol Ther Exp 61: 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma AK, Bury MI, Marks AJ, Fuller NJ, Meisner JW, et al. (2011) A nonhuman primate model for urinary bladder regeneration using autologous sources of bone marrow-derived mesenchymal stem cells. Stem Cells 29: 241–50. [DOI] [PubMed] [Google Scholar]
- 7. Landman J, Olweny E, Sundaram CP, Andreoni C, Collyer WC, et al. (2004) Laparoscopic mid sagittal hemicystectomy and bladder reconstruction with small intestinal submucosa and reimplantation of ureter into small intestinal submucosa: 1-year followup. J Urol 171: 2450–5. [DOI] [PubMed] [Google Scholar]
- 8. Paterson RF, Lifshitz DA, Beck SD, Siqueira TM Jr, Cheng L, et al. (2002) Multilayered small intestinal submucosa is inferior to autologous bowel for laparoscopic bladder augmentation. J Urol 168: 2253–7. [DOI] [PubMed] [Google Scholar]
- 9. Kropp BP, Cheng EY, Lin HK, Zhang Y (2004) Reliable and reproducible bladder regeneration using unseeded distal small intestinal submucosa. J Urol 172: 1710–3. [DOI] [PubMed] [Google Scholar]
- 10. Shakhssalim N, Rasouli J, Moghadasali R, Aghdas FS, Naji M (2013) Bladder smooth muscle cells interaction and proliferation on PCL/PLLA electrospun nanofibrous scaffold. Int J Artif Organ 36: 113–20. [DOI] [PubMed] [Google Scholar]
- 11.Maya H, Vincent M, Nötzli S, Madduri S, Tullio S, et al.. (2013) Increased porosity of electrospun hybrid scaffolds improved bladder tissue regeneration. J Biomed Mater Res A DOI 10.1002/jbm.a.34889. [DOI] [PubMed]
- 12. Fernández J, Etxeberria A, Sarasua JR (2012) Synthesis, structure and properties of poly(L-lactide-co-ε-caprolactone) statistical copolymers. J Mech Behav Biomed Mater 9: 100–12. [DOI] [PubMed] [Google Scholar]
- 13. Jeong SI, Kim BS, Lee YM, Ihn KJ, Kim SH, et al. (2004) Morphology of elastic poly(L-lactide-co-epsilon-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaffolds. Biomacromolecules 5: 1303–9. [DOI] [PubMed] [Google Scholar]
- 14. Knight TA, Payne RG (2013) Characterization of a PGA-based scaffold for use in a tissue-engineered neo-urinary conduit. Methods Mol Biol 1001: 179–88. [DOI] [PubMed] [Google Scholar]
- 15. Andrychowski J, Frontczak-Baniewicz M, Sulejczak D, Kowalczyk T, Chmielewski T, et al. (2013) Nanofiber nets in prevention of cicatrisation in spinal procedures. Experimental study. Folia Neuropathol 51: 147–57. [DOI] [PubMed] [Google Scholar]
- 16. Bretcanu O, Misra SK, Yunos DM, Boccaccini AR, Roy I, et al. (2009) Electrospun nanofibrous biodegradable polyester coatings on Bioglass-based glass-ceramics for tissue engineering. Materials Chemistry and Physics 118: 420–426. [Google Scholar]
- 17. Sartoneva R, Haimi S, Miettinen S, Mannerström B, Haaparanta AM, et al. (2011) Comparison of a poly-L-lactide-co-ε-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J R Soc Interface 8: 671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sartoneva R, Haaparanta AM, Lahdes-Vasama T, Mannerström B, Kellomäki M, et al. (2012) Characterizing and optimizing poly-L-lactide co-ε-caprolactone membranes for urothelial tissue engineering. J R Soc Interface 9: 3444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Bai Y, Liao G, Peng E, Wu B, et al. (2013) Electrospun Poly(L-lactide)/Poly(ε-caprolactone) Blend Nanofibrous Scaffold: Characterization and Biocompatibility with Human Adipose-Derived Stem Cells. PLoS One 8: e71265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adamowicz J, Kloskowski T, Tworkiewicz J, Pokrywczynska M, Drewa T (2012) Urine is a highly cytotoxic agent: does it influence stem cell therapies in urology? Transplant Proc 44: 1439–41. [DOI] [PubMed] [Google Scholar]
- 21. Davis NF, Callanan A, McGuire BB, Flood HD, McGloughlin TM (2011) Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology 77: 1007.e1–7. [DOI] [PubMed] [Google Scholar]
- 22. Li WJ, Cooper JA, Mauck RL, Tuan RS (2006) Fabrication And Characterization Of Six Electrospun Poly(α,ω-Hydroxy Ester)-Based Fibrous Scaffolds For Tissue Engineering Applications. Acta Biomaterialia 4: 377–385. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Grainger DW, Winn SR, Hollinger JO (2002) Fabrication of poly(alpha-hydroxy acid) foam scaffolds using multiple solvent systems, J. Biomed.Mater. Res. A 59: , 563–572. [DOI] [PubMed] [Google Scholar]
- 24. Tomadakis MM, Robertson TJ, (2003) Pore size distribution, survival probability, and relaxation time in random and ordered arrays of fibers, J. Chem. Phys. 119: , 1741–1749. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council (2011) Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press.
- 26. Safford KM, Hicok KC, Safford SD (2002) Neurogenic differentiation of murine and human adipose derived stromal cells. Biochem Biophys Res Commun 294: 371–379. [DOI] [PubMed] [Google Scholar]
- 27. Dahms SE, Piechota HJ, Dahiya R, Lue TF, Tanagho EA (1998) Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. Br J Urol 82: 411–9. [DOI] [PubMed] [Google Scholar]
- 28. Chen F, Lee CN, Teoh SH (2007) Nanofibrous modification on ultra-thin poly (e-caprolactone) membrane via electrospinning. Materials Science and Engineering: C 27: 325–332. [Google Scholar]
- 29. Park JS, Kim JM, Jun LS, Geun LS, Young-Keun J (2007) Surface hydrolysis of fibrous poly (ε-caprolactone) scaffolds for enhanced osteoblast adhesion and proliferation. Macromol Res 15: 424–429. [Google Scholar]
- 30. Ang LP, Cheng ZY, Beuerman RW, Teoh SH, Zhu X, et al. (2006) The development of a serum-free derived bioengineered conjunctival epithelial equivalent using an ultrathin poly (ε-caprolactone) membrane substrate. Invest Ophthalmol & Vis Sci 47: 105–112. [DOI] [PubMed] [Google Scholar]
- 31. Goddard JM, Hotchkiss JH (2007) Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci 32: 698–725. [Google Scholar]
- 32. Yamaguchi M, Shinbo T, Kanamori T, Wang PC, Niwa M, et al. (2004) Hirakawa K, Kamiya M. Surface modification of poly (L-lactic acid) affects initial cell attachment, cell morphology, and cell growth. J Artif Organs 7: 187–193. [DOI] [PubMed] [Google Scholar]
- 33. Zhu Y, Leong MF, Ong WF, Chan-Park MB, Chian KS (2007) Esophageal epithelium regeneration on fibronectin grafted poly (L-lactide-co-caprolactone)(PLLC) nanofiber scaffold. Biomaterials 28: 861–868. [DOI] [PubMed] [Google Scholar]
- 34. Cai K, Yao K, Cui Y, Yang Z, Li X, et al. (2002) Influence of different surface modification treatments on poly (D, L-lactic acid) with silk fibroin and their effects on the culture of osteoblast in vitro. Biomaterials 23: 1603–1611. [DOI] [PubMed] [Google Scholar]
- 35. Yoon JJ, Song SH, Lee DS, Park TG (2004) Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials 25: 5613–5620. [DOI] [PubMed] [Google Scholar]
- 36. Yu HS, Jang JH, Kim TI, Lee HH, Kim HW (2009) Apatite-mineralized polycaprolactone nanofibrous web as a bone tissue regeneration substrate. J Biomed Mater Res Part A 88: 747–754. [DOI] [PubMed] [Google Scholar]
- 37. Feil G, Christ-Adler M, Maurer S, Corvin S, Rennekampff HO, et al. (2006) Investigations of urothelial cells seeded on commercially available small intestine submucosa. Eur Urol. 50: , 1330–7. [DOI] [PubMed] [Google Scholar]