Abstract
Background
Frailty is a state of vulnerability to stressor events. There is uncertainty about the beneficial effects of exercise interventions for older people with frailty. The Home-based Older People’s Exercise (HOPE) programme is a 12 week exercise intervention for older people with frailty designed to improve mobility and function.
Methods
We tested feasibility of the HOPE programme in a two arm, assessor blind pilot RCT. Eligibility criteria included living at home and receiving case manager care, being housebound or attending day centres in Bradford, UK. Intervention participants received the HOPE programme; control participants received usual care. Objectives were to gather process, resource, management and scientific data to inform the design of a definitive trial. Primary outcome was mobility, measured using the timed-up-and-go test (TUGT). Secondary outcomes were activities of daily living, health related quality of life and depression. Participants were stratified by baseline TUGT score. Randomisation was by the University of Leeds Clinical Trials Research Unit.
Results
84 participants were recruited. 45 were randomised to intervention and 39 to control. 40 intervention participants and 30 control participants were included in the intention-to-treat analysis. There was a non-significant trend towards a clinically important improved outcome in the intervention group (mean adjusted between group difference in TUGT 28.6s, 95% CI −8.5, 65.9s). There were no differences in secondary outcomes.
Conclusion
The HOPE trial has provided preliminary evidence that the deterioration in mobility experienced by older people with frailty may be reduced through a 12 week exercise intervention. The pilot trial has provided the necessary data to design a future definitive RCT.
Background
Frailty is a state of vulnerability to stressor events (1) as a consequence of cumulative decline in multiple physiological systems. A quarter to a half of people over 85 years have frailty and are at significantly increased risk of falls, disability, care home admission and death (2, 3). Reducing the prevalence or severity of frailty could have considerable benefits for older people, their families and society. It is important to consider whether interventions to improve outcomes are effective across the spectrum of frailty, and trials that incorporate valid and reliable measures of frailty are required (1).
Exercise has physiological effects on the brain, endocrine and immune systems, and skeletal muscle (4-8) and may be beneficial for older people with frailty. A recent systematic review concluded that the Otago Exercise Programme (OEP), a home-based falls prevention intervention, was effective at reducing falls and mortality (9, 10) in older people. However, evidence from falls prevention studies is not necessarily generalisable across the frailty spectrum as falls prevention tends to be targeted at older people who are living independently or have few restrictions in activities of daily living (ADL). This group of older people is unlikely to have significant frailty. Additionally, the majority of falls prevention interventions include an aerobic component which may not be appropriate for the most frail, considering the low energy expenditure and fatiguability that characterise frailty.
A 2012 systematic review reported preliminary evidence that home-based exercise interventions for older people with frailty may slow the progression to disability, but there was considerable uncertainty regarding important outcomes including quality of life and admission to long-term care (11). Conclusions were limited by the small number (n=6) of randomised controlled trials (RCTs) of variable methodological quality. Notably, none of the trials used validated measures to record baseline frailty of participants.
The MRC framework for developing and evaluating complex health interventions stresses the importance of pilot work prior to a definitive trial (12). The Home-based Older People’s Exercise (HOPE) programme is an exercise intervention for older people with frailty, designed to improve mobility and function. We report feasibility testing of the HOPE programme in a pilot RCT that incorporates a valid and reliable baseline frailty measure.
Objectives
A successful pilot RCT provides important process, resource, management and scientific data (13). The objectives of the HOPE trial were to gather this data to inform the design of a definitive trial, particularly to test for a preliminary estimate of effectiveness.
Methodology
A detailed description of the trial methodology has been provided elsewhere (14) and an overview is outlined here. Trial methods were informed by international guidelines on designing RCTs of interventions to prevent functional decline in older people with frailty (15).
Design
A two arm, assessor blind pilot RCT comparing the effectiveness of the HOPE programme with usual care.
Eligibility criteria and recruitment methods
A particular challenge of frailty research is recruiting an appropriate study population. A key objective for this pilot trial was to investigate methods of recruiting older people with frailty. Our approach was to use eligibility criteria to exclude the robust and then measure frailty in those who were recruited (15).
Our eligiblity criteria were; people living at home and under the care of a case manager or community matron; the housebound (identified through Read code searching of general practitioner (GP) registers of National Institute for Health Research (NIHR) ‘Research Ready’ GP practices); attending a day centre or respite care; residence in assisted living sites; at discharge from intermediate care hospitals; and following attendance at elderly medicine outpatient clinics in Bradford, UK. People were excluded if they were: unable to stand and walk independently; currently participating in an alternative exercise programme; registered blind. Those who had poorly controlled angina; had another household member already in the trial; had severe dementia or were receiving palliative care, were also excluded.
Verbal assent was first sought by the member of the health or social services team coordinating individual care. Potential participants identified from GP registers were contacted by letter from the practice and responses were mailed directly to the study team. Those expressing an interest were visited at home by trained clinical researchers who undertook detailed screening, explained the study in full to eligible candidates and provided written information leaflets. Informed consent was obtained prior to baseline assessment.
Description of the intervention
The HOPE programme is a 12 week progressive exercise intervention that is presented to participants in an exercise manual and delivered by community-based physiotherapists. For this trial, the intervention was delivered by Bradford Teaching Hospitals NHS Foundation Trust community physiotherapists. The manual contains five sections; 1) information, 2) safety tips, 3) good posture, 4) exercises and 5) staying on track. The core constituents of the HOPE programme are strengthening exercises for the muscle groups required for basic mobility skills like getting out of bed, standing up from a chair, walking a short distance and getting off the toilet (16). Maintenance of these basic mobility skills is critical for older people with frailty because impairment increases risk of immobility, causing further loss of muscle mass, activity limitation and potential dependence on others for care. The exercises require no special equipment and can be performed without professional supervision.
To account for the spectrum of frailty, the HOPE programme is graded into three levels. Participants are stratified to the appropriate level using their baseline performance on the timed-up-and-go test (TUGT). This measures, in seconds, the time taken to stand up from a standard chair, walk a distance of 3 metres, turn, walk back to the chair and sit down.
The TUGT was developed as a basic mobility test for older people (17) and has good accuracy for identifying frailty (18). The original TUGT validation study identified that those who complete the test in 30 seconds or more tend to require assistance with climbing stairs and leaving the house. Therefore, participants completing the TUGT in ≥30 seconds are stratified to HOPE programme level 1, which contains very simple chair based exercises. Those who complete the test in 20-29 seconds demonstrate greater variability in mobility, balance and functional ability and are stratified to level 2, which is the intermediate level. Those who complete the test in less than 20 seconds tend to be independently mobile, able to get in and out of a chair without assistance and climb stairs and are stratified to level 3.
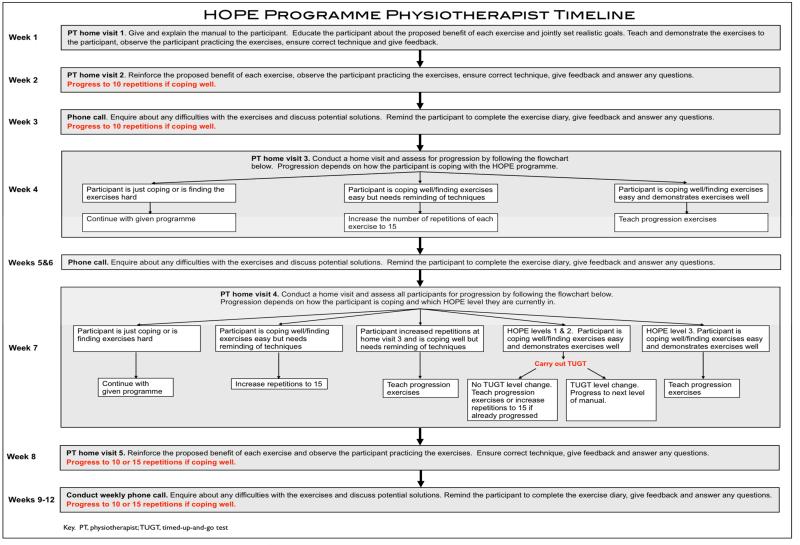
The HOPE Programme physiotherapist timeline is provided in figure 1. Physiotherapists receive intervention training in a two hour workshop. At the beginning of the intervention participants are requested to perform five repetitions of each exercise in the routine. This progresses to 10 and then 15 repetitions as performance improves. The exercise routine takes less than 15 minutes to complete, and participants are requested to complete the routine 3 times a day on 5 days of the week.
Figure 1.
HOPE programme physiotherapist timeline
Participants receive weekly support from physiotherapists through five face-to-face home visits and seven telephone calls. If participants are coping well with the exercises they are encouraged to progress within the programme. Progression is by increasing repetitions, introducing new exercises or advancing to the next level of the HOPE programme.
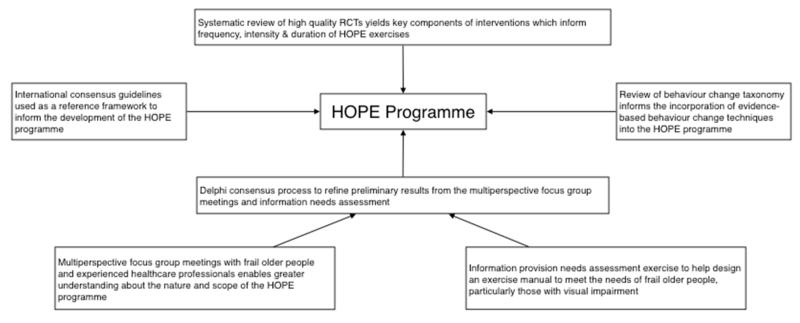
A summary of the HOPE programme development process, including behavior change theory underpinning the intervention, has been described previously (14). A schematic representation summarising the evidence synthesis process to develop the HOPE programme is provided in web appendix 1.
Description of usual care
The control group continued to receive usual care from the primary healthcare team and, other than baseline and follow-up assessments, had no contact with the research team.
Baseline assessment
Baseline assessment was conducted by the researcher in the participant’s home and included age, sex, cognitive assessment (Mini-Mental State Examination, MMSE), co-morbidity index (19) and Edmonton Frail Scale (EFS). The EFS is a valid and reliable measure of frailty that samples 10 domains, including cognitive impairment, functional ability and mobility, measured using the TUGT (20). A score of >8 identifies people who are frail with a maximum score of 17 representing the highest level of frailty.
Outcome measures
The primary outcome was mobility, measured using the TUGT (17). The TUGT demonstrates good agreement with measures of functional ability and is considered sensitive to mobility changes (17, 21, 22). An improvement of 1.4 seconds has been identified as the minimum clinically important difference (MCID) (23).
Secondary outcomes were
Self-reported modified Barthel Index of ADL (24). The Barthel Index assesses functional status on a 20 point scale; higher scores indicate greater independence.
EuroQol Group 5-Dimension Self-Report Questionnaire (EQ-5D) (25) The EQ-5D is a standardised measure of health utility reported as a summary index score (0 for dead, 1 for perfect health and negative values for states worse than death).
Geriatric Depression Scale – Short Form 15 (GDS) (26). The GDS is a screen for the presence and severity of depression in older people; a score of 0-4 indicates no depression, 5-10 is suggestive of mild depression and 11-15 is suggestive of severe depression.
All outcomes were collected at baseline and at 14 weeks post-randomisation.
Recruitment and follow-up rates were calculated to investigate study feasibility. Completion of data items was recorded to investigate acceptability of the outcome measures. Intervention completion rates and intervention adherence, measured using daily self-completed adherence diaries, were calculated to help determine acceptability of the intervention. Total adherence was defined as the percentage of days that the exercises were completed three times a day for five days over the course of the 12 week intervention. Partial adherence was defined as the percentage of days that the exercises were completed at least once a day for five days a week over the course of the 12 week intervention. Diary completion was defined as the percentage of the adherence diary that was completed. Participating therapists recorded total time for home visits, including travel time. Outcome assessors recorded episodes of unblinding at follow-up visits.
Sample size
To inform the design of a future definitive study (13), a recruitment target of 100 participants (50 per group) was set. Formal sample size calculations are not appropriate in pilot trials (13).
Randomisation
Participants were stratified by the baseline TUGT (levels 1-3, see above) and underwent randomisation using restricted blocks of random size with an allocation ratio of 1:1. Generation and storage of the HOPE trial randomisation sequence and individual participant randomisation was by the University of Leeds Clinical Trials Research Unit (CTRU), ensuring allocation concealment.
Analysis plan
Baseline differences between the control and intervention groups were compared. Histograms were plotted to assess the distribution of data for the primary and secondary outcome measures and assessed for normailty by visual inspection. Skewed data were log transformed to yield lognormal distributions.
All outcome measures were summarised and 95% confidence intervals constructed for the difference in outcomes between control and intervention groups. Change scores were calculated by subtracting follow-up values from baseline values. Those unable to complete the TUGT at follow-up were assigned a score of 300s, as this was the maximum time recorded for completion of the test in the original validation study (17). As analysing change does not control for baseline imbalances because of regression to the mean (27), analysis of covariance (ANCOVA) tests were used for continuous outcomes, with adjustment for baseline values. Both adjusted and unadjusted values were tested to detect which had the smaller variance. Risk ratios with 95% confidence intervals were used for binary outcomes. Missing data were addressed by listwise exclusion (28). The final intention-to-treat analysis included all randomised participants for whom the follow-up assessment of the primary outcome measure was available.
Sources of funding
This work was supported by a Dunhill Medical Trust and Royal College of Physicians Joint Research Fellowship awarded to the lead author (AC).
Results
Trial recruitment
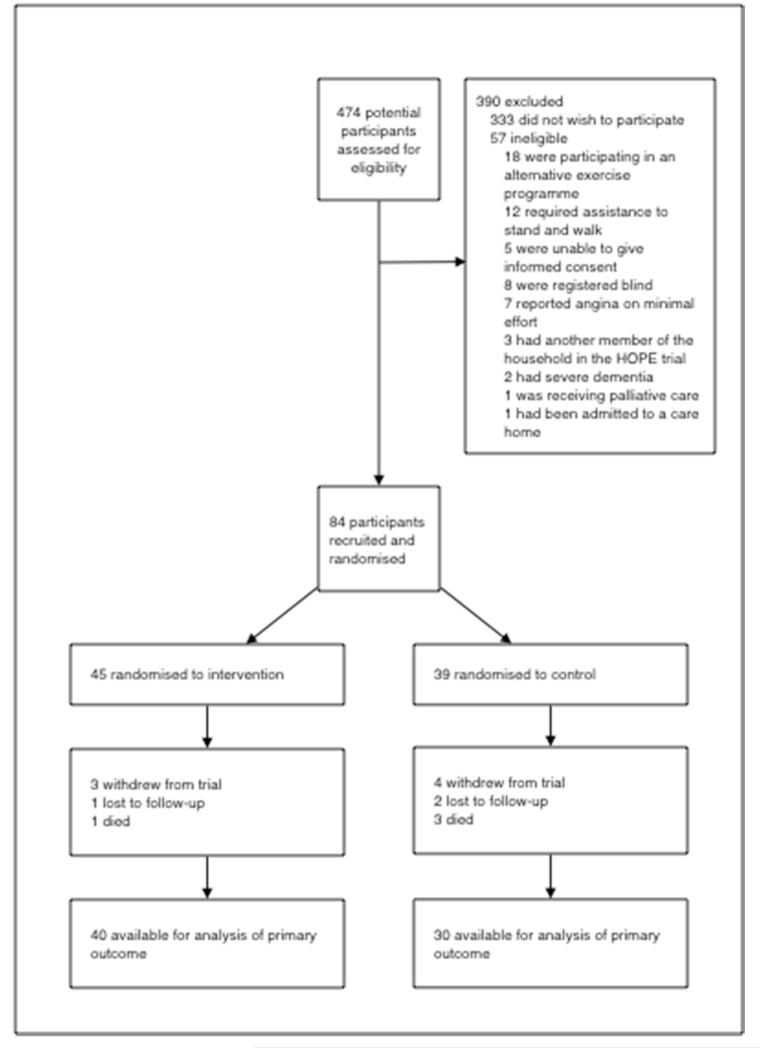
Between July 2010 and November 2011 474 potential participants were contacted to assess for eligibility and 84 (18%) were recruited. 45 were randomised to intervention and 39 to control. Seven participants withdrew from the trial, three were lost to follow-up and four participants died. Follow-up information is therefore available for 70 participants (83% of those randomised; 40 intervention participants and 30 control participants) (CONSORT diagram, figure 2). Web table 1 summarises recruitment rates from different sources.
Figure 2.
CONSORT diagram showing flow of participants through the HOPE trial
Baseline characteristics
The mean age of participants was 79 years (standard deviation, SD, 9.2 years) and 71% were female. No important differences in baseline characteristics were identified (table 1). The mean baseline EFS was 8.1 (SD 2.6). The overall mean baseline TUGT for all the participants was 51.0 seconds (SD 63.6 seconds).
Table 1.
Baseline characteristics.
| Characteristic | Intervention Group (n = 45) | Control Group (n = 39) |
|---|---|---|
|
| ||
| Age - years, mean (SD) | 79.4 (7.9) | 78.0 (10.5) |
|
| ||
| Sex, no. (%) | ||
| female | 33 (73%) | 27 (69%) |
| male | 12 (27%) | 12 (31%) |
|
| ||
| Ethnicity, no. (%) | ||
| Caucasian | 38 (84%) | 33 (85%) |
| Asian | 7 (16%) | 6 (15%) |
|
| ||
| Living circumstances, no. (%) | ||
| living alone | 24 (53%) | 17 (44%) |
| living with spouse/partner | 14 (31%) | 11 (28%) |
| living with family | 7 (16%) | 11 (28%) |
|
| ||
| Mobility aid used *, no. (%) | ||
| independent | 20 (45%) | 20 (51%) |
| walking stick(s) | 15 (35%) | 10 (26%) |
| Zimmer frame | 6 (15%) | 6 (15%) |
| 3/4-wheeled walker | 2 (5%) | 3 (8%) |
|
| ||
| Charlson comorbidity index, mean (SD) | 2.4 (1.9) | 2.8 (2.1) |
|
| ||
| TUGT (seconds), mean (SD) | 50.9 (62.0) | 51.2 (66.2) |
|
| ||
| Stratification level, no. (%) | ||
| Level 1 (≥ 30 seconds on TUGT) | 21 (47%) | 19 (49%) |
| Level 2 (20-29 seconds on TUGT) | 11 (24%) | 9 (23%) |
| Level 3 (0-19 seconds on TUGT) | 13 (29%) | 11 (28%) |
|
| ||
| Edmonton frail scale, mean (SD) | 7.8 (2.4) | 8.3 (2.7) |
|
| ||
| Barthel index, mean (SD) | 15.8 (3.6) | 15.6 (3.9) |
|
| ||
| Mini-mental state examination, mean (SD) | 25.7 (4.7) | 24.4 (4.7) |
|
| ||
| Geriatric depression scale, mean (SD) | 3.6 (2.6) | 4.8 (2.9) |
- numbers do not add up to totals due to missing data
Key: SD, standard deviation; TUGT, timed-up-and-go test
Intervention completion rates and participant adherence
28 participants in the intervention group (70%) completed the 12 week programme. Seven participants (18%) dropped out from the intervention, four participants were considered by the physiotherapist to have rehabilitation needs that were not provided by the HOPE programme and one relative withdrew a participant with moderate dementia, as they were non-compliant. Adherence diaries were returned by 27 of the 28 participants (96%) who completed the 12 week intervention. Of the adherence diaries returned, mean diary completion was 64%, mean total adherence was 46% and mean partial or total adherence was 67%.
Completion of data items
There were no missing data at baseline or follow up for MMSE, Charlson co-morbidity index, TUGT, Barthel index or EFS. There was one missing data item for baseline EQ-5D but none for follow-up EQ-5D. There were two missing data items for baseline GDS and three for follow-up GDS.
Therapy resources required
Therapy records were returned for 34 participants (85%). The mean number of completed home visits was 3.7 (SD 1.6) and the mean number of completed telephone calls was 3.5 (SD 2.8). The mean recorded time for a completed home visit (including travel time) was 39 minutes (SD 9.5 minutes) and the mean time for a completed telephone call was 4 minutes (SD 1.2 minutes).
Outcomes
Unadjusted and adjusted between group differences in primary and secondary outcomes are summarised in table 2. Although mobility had, on average, deteriorated in both groups at follow-up (intervention group mean change in TUGT −10.4s (95%CI −34.7, 13.9s), control group mean change in TUGT −39.1s (95% CI −67.2, −11.0s) there was a non-significant trend towards a clinically important improved outcome in the intervention group (mean adjusted between group difference in TUGT 28.6s, 95% CI −8.5, 65.9s).
Table 2.
Unadjusted and adjusted mean between-group differences in the primary and secondary outcomes. To control for baseline imbalances, data were adjusted using analysis of covariance (ANCOVA) tests. Data are presented for the 70 participants for whom both baseline and follow-up data were available.
| Outcome measure | Intervention group (mean (SD)), n=40 | Control group (mean (SD)), n=30 | Unadjusted between-group differences (mean (95% CI)) | Adjusted between-group differences (mean (95% CI)) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | |||
| Timed-up-and-go test (seconds) | 52.0 (62.4) | 62.4 (77.7) | −10.4 (64.0) | 57.9 (74.1) | 97.0 (116.7) | −39.1 (90.6) | 28.7 (−8.2, 65.5) | 28.7 (−8.5, 65.9) |
| Barthel Index | 15.9 (3.7) | 15.6 (4.0) | −0.3 (2.4) | 15.8 (4.1) | 15.0 (4.0) | −0.8 (3.1) | 0.6 (−0.7, 1.8) | 0.6 (−0.7, 1.8) |
| EQ-5D | 0.53 (0.30) | 0.51 (0.34) | −0.02 (0.30) | 0.52 (0.25) | 0.46 (0.26) | −0.06 (0.23) | 0.04 (−0.04, 0.06) | 0.04 (−0.09, 0.18) |
| GDS | 3.8 (2.7) | 3.4 (3.3) | 0.4 (2.0) | 5.0 (3.2) | 4.8 (3.0) | 0.2 (3.2) | 0.2 (−1.1, 1.5) | 0.2 (−1.1, 1.5) |
Key: SD, standard deviation; CI, confidence interval; EQ-5D, EuroQol Group 5-Dimension Self-Report Questionnaire; GDS, geriatric depression scale
There were no differences in ADL, measured using the Barthel Index, quality of life, measured using the EQ-5D or depression, measured using the geriatric depression scale.
Sensitivity analyses
Two participants in the intervention group and five participants in the control group who had been mobile at baseline were unable to complete the TUGT at follow-up (risk ratio for new immobility, RR, 0.30, 95% CI 0.06, 1.44). An exploratory sensitivity analysis was performed whereby the data were re-analysed with results from these participants excluded. The overall trend was maintained, but considerably diminished (mean adjusted between group difference 3.2s, 95% CI −9.8, 16.2s).
To analyse patterns of missing data we did an exploratory sensitivity analysis using multiple imputation procedures for all randomised participants (29). Results were similar for the primary outcome (mean adjusted between group difference in TUGT 25.9s, 95% CI −13.9, 65.7) and secondary outcomes, indicating that the results from the primary intention to treat analysis were likely robust.
Adverse outcomes
Seven participants in the intervention arm and eight in the control arm fell at least once (risk ratio, RR, 0.66, 95% CI 0.27, 1.61). Two participants in the intervention arm and four in the control arm were admitted to hospital on at least one occasion (RR 0.38, 95% CI 0.07, 1.91). One participant in the control group was admitted to a care home.
Rates of researcher unblinding
Researchers were unblinded by participants during 62% of follow-up visits. Unblinding was more common when participants were in the intervention group (73%) compared to the control group (48%).
Discussion
The pilot HOPE trial has provided important process, resource, management and scientific data, including a preliminary estimate of effectiveness to guide the design of a future definitive RCT.
Process data
Approximately 1/5 of those approached were successfully recruited. Recruitment rates were lower than the majority of RCTs identified in an earlier systematic review (11), but this may reflect different inclusion/exclusion criteria and methods of approach. Given that the trial recruited a population of older people with frailty at high risk of adverse outcomes, retention rates were relatively high, with only seven out of 84 participants (8%) withdrawing from the trial. Only three participants (4%) were lost to follow-up. The high rates of completion of data items provide reassurance that the selected outcome measures were appropriate and broadly acceptable to participants.
Although mean recorded partial intervention adherence was relatively high at 67%, total recorded adherence was lower, with a mean of 46%. This implies that a home-based exercise intervention intensity of three times per day on five days of the week may not be realistic for some older people with frailty. However, one limitation of self-completed adherence diaries is that low rates of diary completion do not conclusively confirm low rates of exercise participation. Use of wearable functional activity monitors, which continuously monitor participant activity, should be considered for a future definitive trial.
Resource data
On the basis of the recorded data, an estimated total direct staff time of around 220 minutes is required for delivery of the 12 week HOPE programme. A formal cost-effectiveness evaluation was not possible in this pilot trial.
Management data
Maintenance of assessor blinding was challenging. Assessors were frequently unblinded, both directly and indirectly, at the follow-up assessment. The high rates of assessor unblinding highlight the need to explore including a sham intervention or alternative methods of outcome assessment when designing RCTs of complex interventions for older people with frailty.
Scientific data
Both an adjusted between group TUGT difference of 28.6s and 3.2s (derived from the sensitivity analysis) are greater than the TUGT MCID of 1.4s. These preliminary estimates are therefore likely to be clinically important for future change in health status and provide the necessary data to design a future definitive trial.
Strengths of the trial
The HOPE trial was methodologically rigorous and followed international guidelines for the development and evaluation of complex interventions (12) and trials involving older people with frailty (15). A number of recruitment methods were used to identify older people with frailty and, on the basis of current knowledge (11), the HOPE trial is the first RCT of a home-based exercise intervention for older people with frailty that has reported baseline frailty using a validated measure.
The mean baseline EFS score of 8.1 indicates that most participants could be considered frail, but a proportion may have had milder frailty. However, more recent evidence has identified that, compared to a reference standard phenotype model, a TUGT cut-point of ≥15s has a very high specificity for identifying frailty, indicating very few false positive results (18). Therefore, the mean baseline TUGT of 51.0s provides additional support that, on the whole, our participants can reasonably be considered frail. Taken together, these results provide reassurance that our recruitment methods were appropriate.
Limitations of the trial
Previous research has demonstrated that the EQ-5D may be less sensitive to change than alternative quality of life measures (30) and there is uncertainty regarding the sensitivity to change of the Barthel Index (31). The relatively short duration of the trial, absence of long-term follow up and lack of statistical power meant that it was not possible to investigate whether these measures are likely to be sufficiently responsive for use in a future definitive trial.
The trial was characterised by high rates of assessor unblinding, which increases the risk of detection bias. However, it is important to identify this limitation so that appropriate measures to reduce risk of bias can be incorporated into the design of future trials.
Conclusion
The pilot HOPE trial has provided valuable process, resource, management and scientific data, including preliminary evidence that the deterioration in mobility experienced by older people with frailty may potentially be diminished through a 12 week home-based exercise intervention. This preliminary evidence requires confirmation in a future definitive, adequately powered RCT that incorporates long-term follow-up of important outcomes including disability, quality of life and admission to hospital and long-term care.
Acknowledgement
This work was supported by a Dunhill Medical Trust and Royal College of Physicians Joint Research Fellowship awarded to the lead author (AC), which is gratefully acknowledged. We would also like to express our gratitude to all the HOPE trial participants and to the physiotherapy team at Bradford Teaching Hospitals NHS Foundation Trust who delivered the HOPE programme.
Web appendix 1
A schematic representation of an evidence synthesis using information from four key domains to develop the HOPE programme.
Web table 1.
Recruitment rates from different sources
| Method of identification | Number approached | Number recruited | Recruitment rate |
|---|---|---|---|
| Outpatient clinic | 3 | 1 | 0.33 |
| Case manager | 174 | 54 | 0.31 |
| Day centre | 54 | 9 | 0.17 |
| Respite care | 16 | 3 | 0.16 |
| Intermediate care | 37 | 4 | 0.11 |
| GP practice | 182 | 13 | 0.07 |
| Assisted living housing | 8 | 0 | 0 |
Footnotes
Conflict of interest
AC, SB, JY, SI and AF all declare that they have no conflict of interest.
Ethical and organisational review
The Bradford Research Ethics Committee (application number 09/H1302/55) granted ethical approval for the HOPE trial. NHS Bradford and Airedale and Bradford Teaching Hospitals NHS Foundation Trust granted NHS Research & Development (R&D) approval.
Trial registration and date of first participant randomisation
Current Controlled Trials – International Standard Randomised Controlled Trial Number ISRCTN57066881. Date of trial registration 19/05/2010. Date of first participant randomisation 15/07/2010.
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–7. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 4.van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32(5):283–90. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber SE, Clegg AP, Young JB. Is there a role for physical activity in preventing cognitive decline in people with mild cognitive impairment? Age Ageing. 2012;41(1):5–8. doi: 10.1093/ageing/afr138. [DOI] [PubMed] [Google Scholar]
- 6.Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- 7.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh MA, Ding W, Manfredi TJ, Solares GS, O’Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol. 1999;277(1 Pt 1):E135–43. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Thomas S, Mackintosh S, Halbert J. Does the ‘Otago exercise programme’ reduce mortality and falls in older adults?: a systematic review and meta-analysis. Age Ageing. 2010;39(6):681–7. doi: 10.1093/ageing/afq102. [DOI] [PubMed] [Google Scholar]
- 11.Clegg A, Barber S, Young J, Forster A, Iliffe S. Do home-based exercise interventions improve outcomes for frail older people? Findings from a systematic review. Reviews in clinical gerontology. 2012;22(1):68–78. doi: 10.1017/S0959259811000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medical Research Council . Developing and evaluating complex interventions: new guidance. MRC; London: 2008. [Google Scholar]
- 13.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg A, Barber S, Young J, Forster A, Iliffe S. The Home-Based Older People’s Exercise (HOPE) trial: study protocol for a randomised controlled trial. Trials. 2011;12:143. doi: 10.1186/1745-6215-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr., Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs B. Clinical and laboratory studies of falls in old people. Prospects for prevention. Clin Geriatr Med. 1985;1(3):513–24. [PubMed] [Google Scholar]
- 17.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 18.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2012;68(4):441–6. doi: 10.1093/gerona/gls190. [DOI] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Iersel MB, Munneke M, Esselink RA, Benraad CE, Olde Rikkert MG. Gait velocity and the Timed-Up-and-Go test were sensitive to changes in mobility in frail elderly patients. J Clin Epidemiol. 2008;61(2):186–91. doi: 10.1016/j.jclinepi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, Hsieh CL. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840–50. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 23.Wright AA, Hegedus EJ, David Baxter G, Abbott JH. Measurement of function in hip osteoarthritis: Developing a standardized approach for physical performance measures. Physiother Theory Pract. 2011;27(4):253–62. doi: 10.3109/09593985.2010.491150. [DOI] [PubMed] [Google Scholar]
- 24.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–3. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 25.The EuroQol Group EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh JI, Yesavage JA, Brooks JO, 3rd, Friedman L, Gratzinger P, Hill RD, Zadeik A, Crook T. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3(1):23–8. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- 27.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123–4. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allison P. Missing data. Sage Publications Ltd; London: 2002. [Google Scholar]
- 29.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eurich DT, Johnson JA, Reid KJ, Spertus JA. Assessing responsiveness of generic and specific health related quality of life measures in heart failure. Health Qual Life Outcomes. 2006;4:89. doi: 10.1186/1477-7525-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hocking C, Williams M, Broad J, Baskett J. Sensitivity of Shah, Vanclay and Cooper’s modified Barthel Index. Clin Rehabil. 1999;13(2):141–7. doi: 10.1191/026921599668105029. [DOI] [PubMed] [Google Scholar]