Abstract
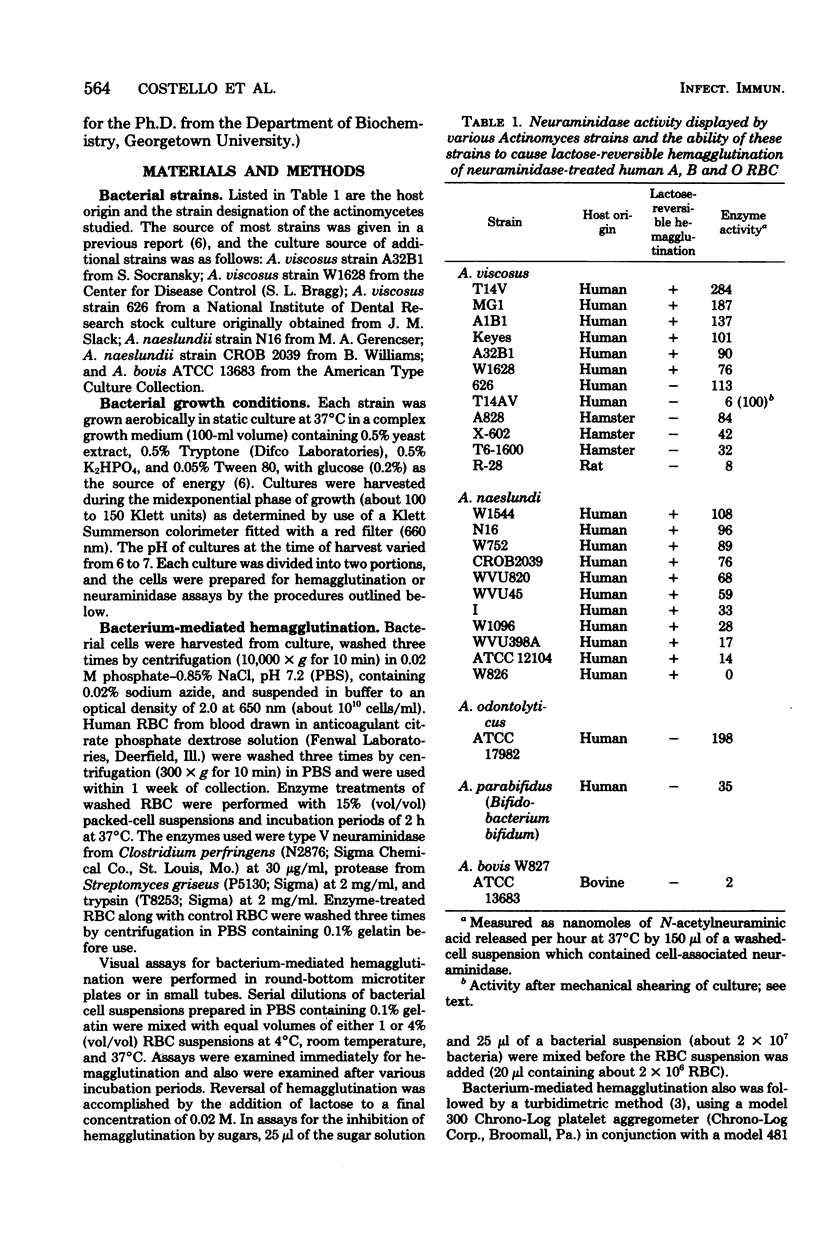
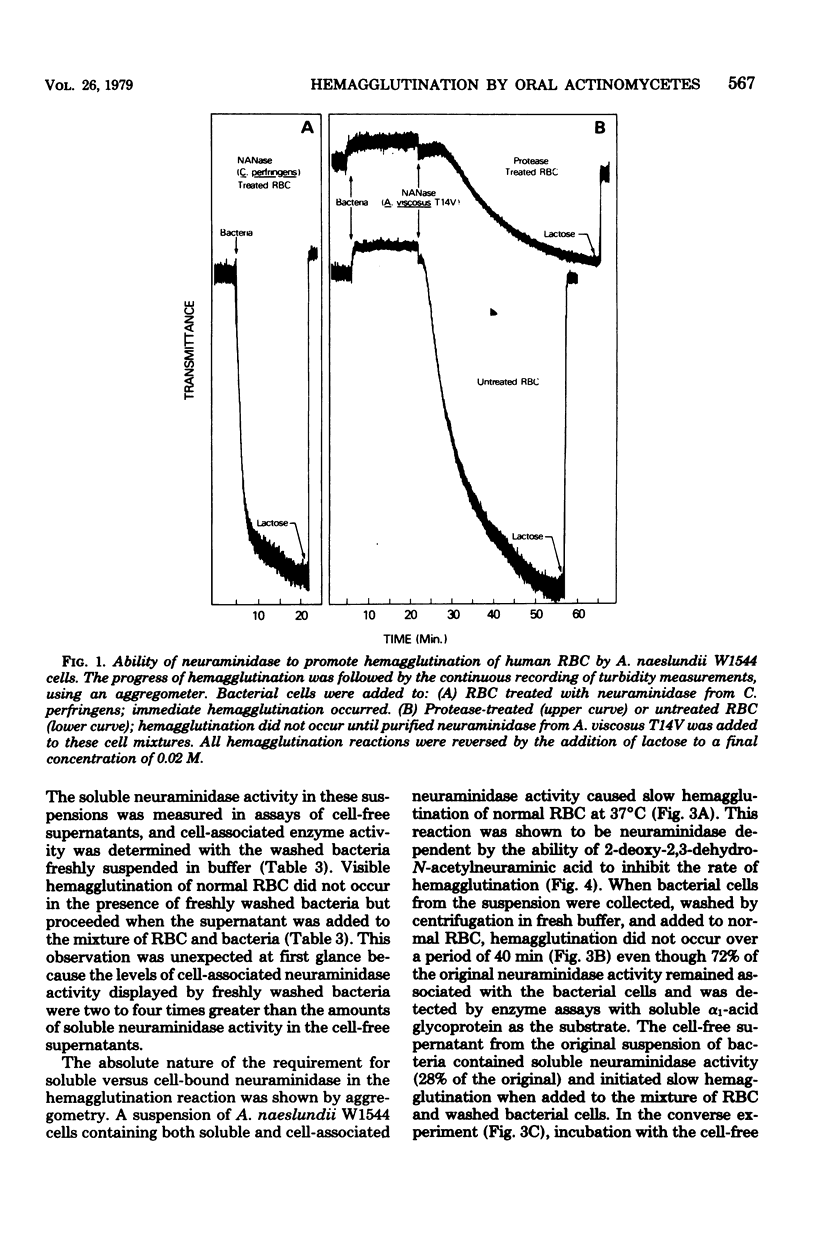
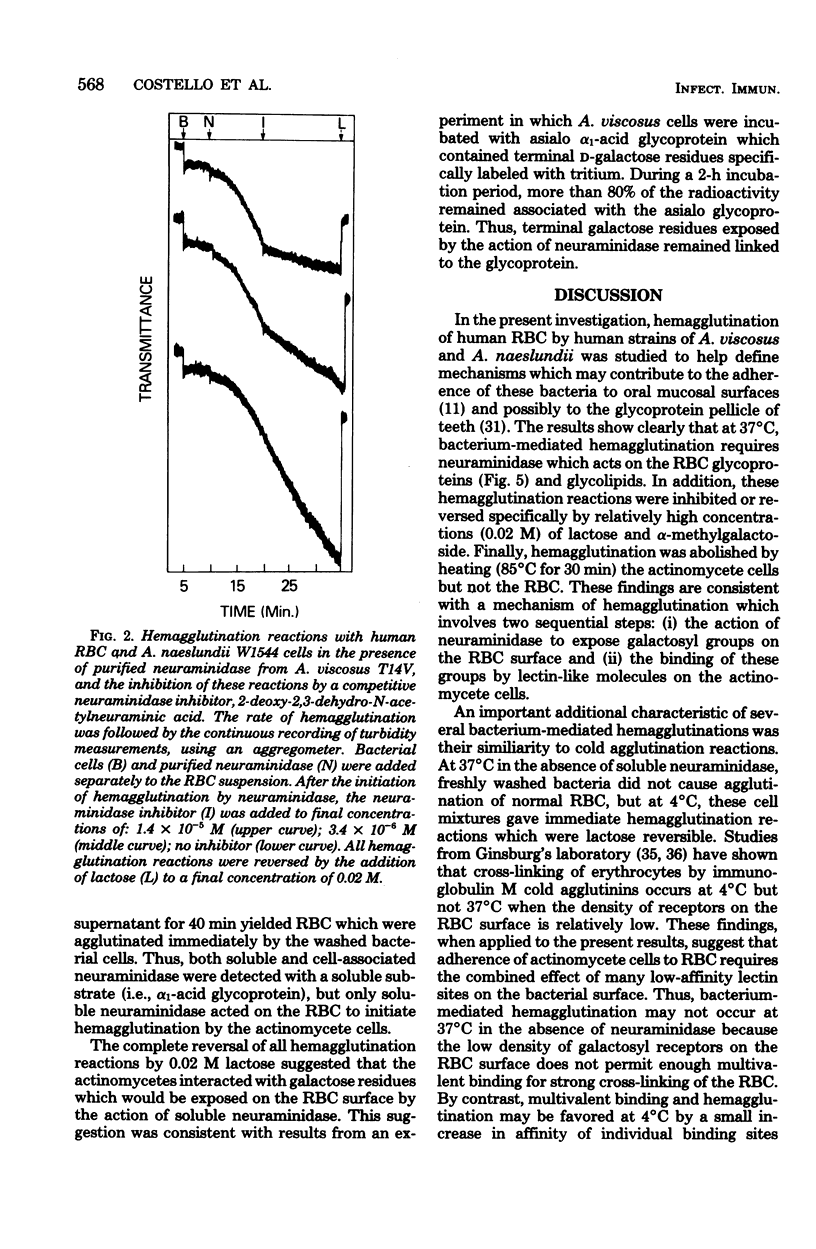
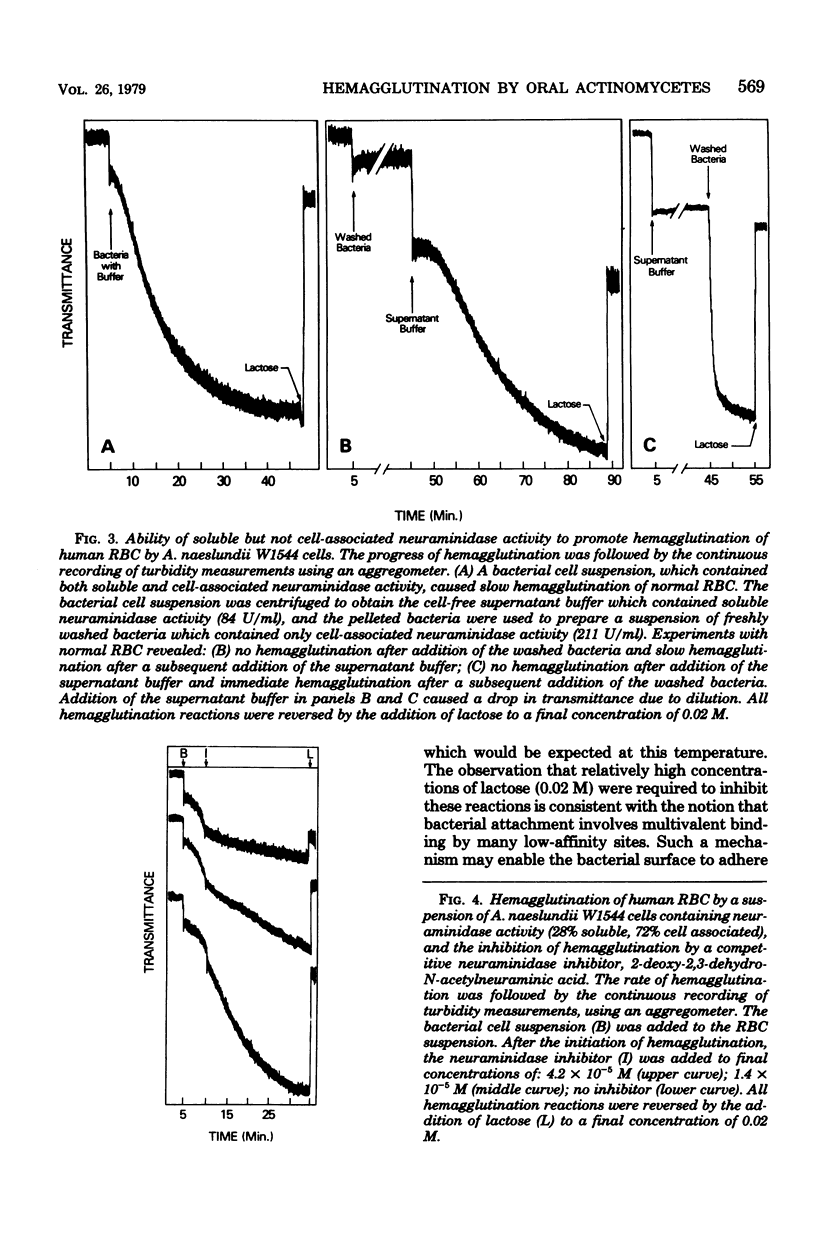
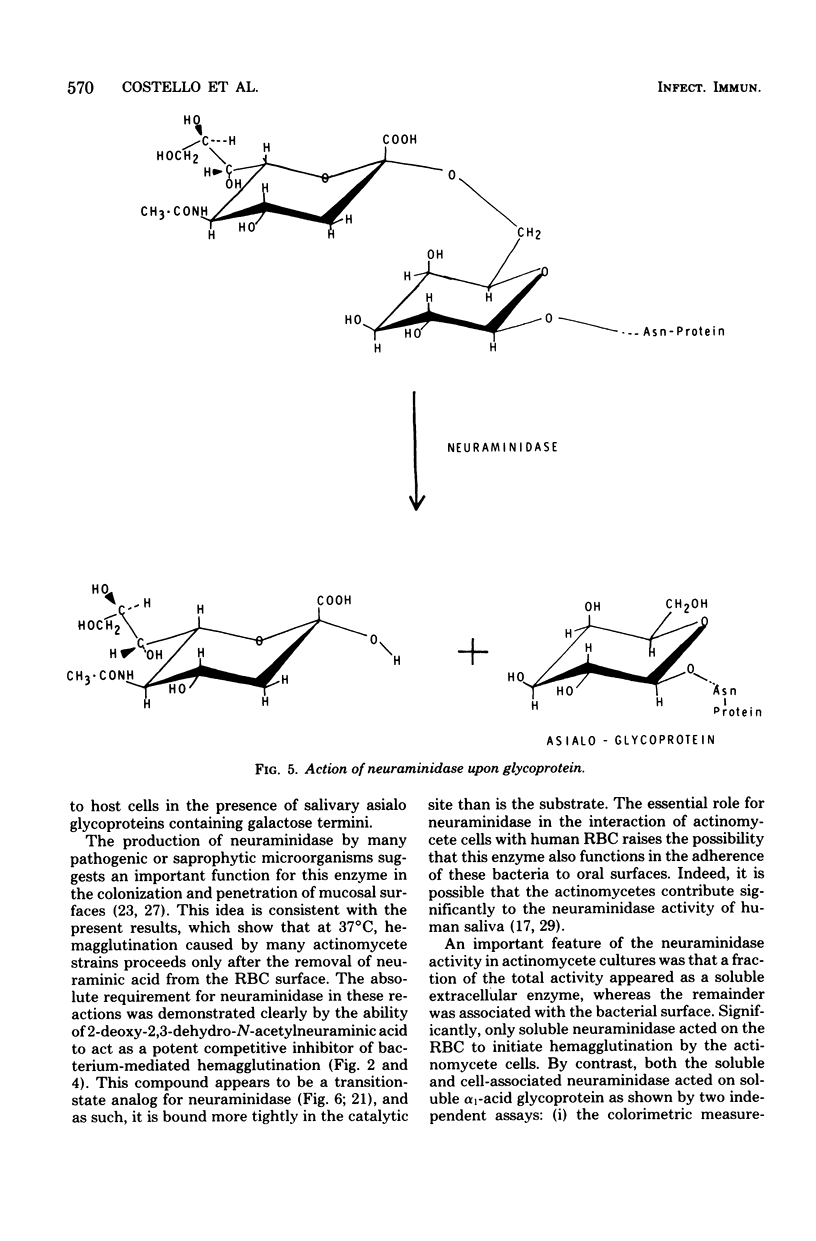
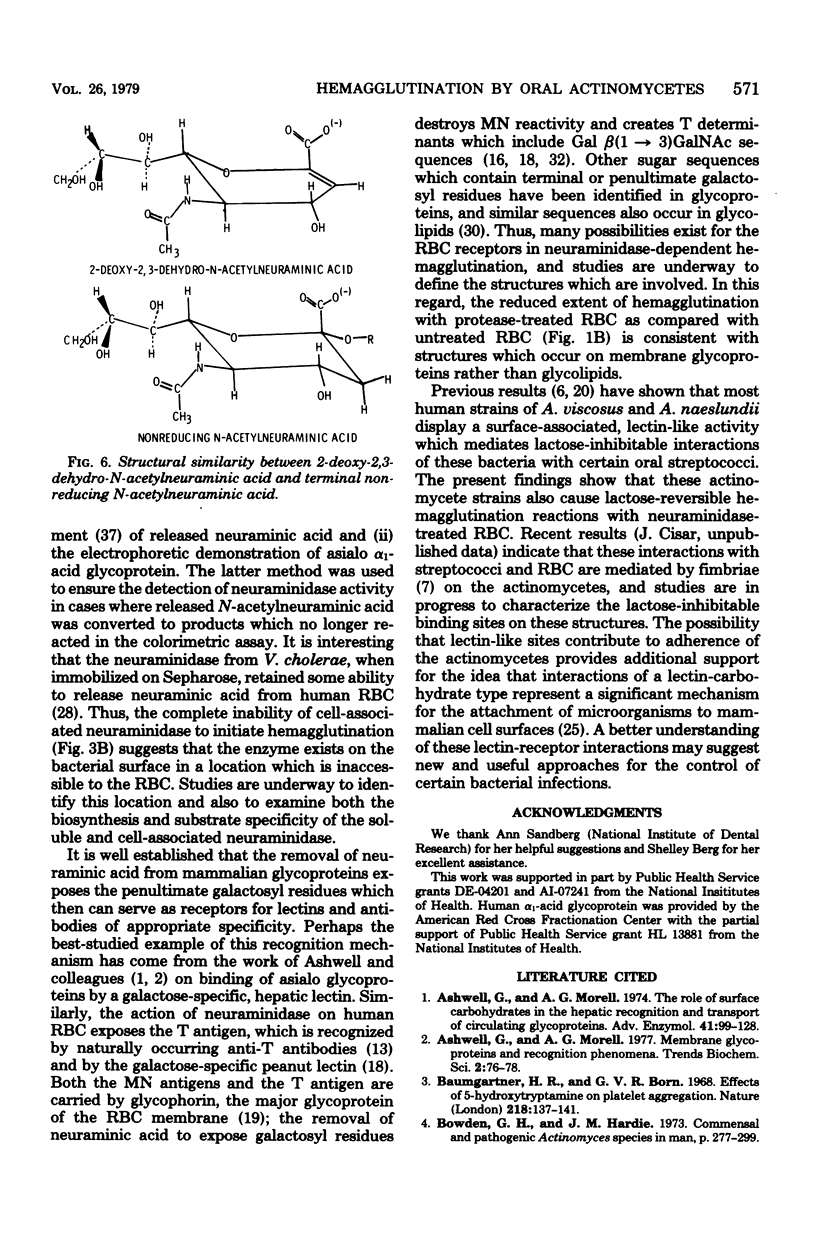
Human A, B, and O erythrocytes (RBC) were agglutinated by many human strains of Actinomyces viscosus and A. naeslundii. At 37 degrees C, these bacterium-mediated hemagglutination reactions required the action of bacterial neuraminidase upon the RBC; however, at 4 degrees C, the requirement for neuraminidase was not as striking. Bacterial cell suspensions which caused hemagglutination at 37 degrees C contained both soluble extracellular and cell-associated neuraminidase activities as shown by enzyme assays using a soluble substrate (i.e., alpha 1-acid glycoprotein). Bacterium-mediated hemagglutination occurred only in the presence of soluble neuraminidase activity, and the rate of hemagglutination could be inhibited by 2-deoxy-2,3-dehydro-N-acetylneuraminic acid, a competitive inhibitor of purified soluble neuraminidase from A. viscosus T14V. Suspensions of bacteria which contained only cell-associated neuraminidase activity were unable to initiate hemagglutination, but they caused immediate hemagglutination when mixed with neuraminidase-treated RBC. All hemagglutination reactions were reversible in the presence of 0.02 M lactose and were abolished by heating (85 degrees C for 30 min) the actinomycete cells but not the RBC. The proposed mechanism of hemagglutination involves two sequential steps: (i) the action of neuraminidase to unmask galactose-containing receptors on the RBC and (ii) the multivalent binding of these receptors by many low-affinity lection sites on the bacterial surface.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Born G. V. Effects of 5-hydroxytryptamine on platelet aggregation. Nature. 1968 Apr 13;218(5137):137–141. doi: 10.1038/218137a0. [DOI] [PubMed] [Google Scholar]
- Bowden G. H., Hardie J. M. Commensal and pathogenic Actinomyces species in man. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:277–299. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E. Surface fibrils (fimbriae) of Actinomyces viscosus T14V. Infect Immun. 1979 May;24(2):523–531. doi: 10.1128/iai.24.2.523-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLENK E., UHLENBRUCK G. [On the isolation of mucoids containing neuraminic acid from human erythrocyte stroma, a contribution to the chemistry of agglutinogens]. Hoppe Seylers Z Physiol Chem. 1960;319:151–160. doi: 10.1515/bchm2.1960.319.1.151. [DOI] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A., Wang P., Flashner M. Mechanism of Arthrobacter sialophilus neuraminidase: the binding of substrates and transition-state analogs. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1479–1487. doi: 10.1016/0006-291x(78)91388-8. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Van den Hamer C. J., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal D-galactose residues. J Biol Chem. 1966 Aug 25;241(16):3745–3749. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Parker T. L., Corfield A. P., Veh R. W., Schauer R. Immobilized Clostridium perfringens neuraminidase. Substrate cleavage and enzyme release during incubation. Hoppe Seylers Z Physiol Chem. 1977 Jul;358(7):789–795. doi: 10.1515/bchm2.1977.358.2.789. [DOI] [PubMed] [Google Scholar]
- Perlitsh M. J., Glickman I. Salivary neuraminidase. I. The presence of neuraminidase in human saliva. J Periodontol. 1966 Sep-Oct;37(5):368–373. doi: 10.1902/jop.1966.37.5.368. [DOI] [PubMed] [Google Scholar]
- Rauvala H., Finne J. Structural similarity of the terminal carbohydrate sequences of glycoproteins and glycolipids. FEBS Lett. 1979 Jan 1;97(1):1–8. doi: 10.1016/0014-5793(79)80039-3. [DOI] [PubMed] [Google Scholar]
- Rölla G., Kilian M. Haemagglutination activity of plaque-forming bacteria. Caries Res. 1977;11(2):85–89. doi: 10.1159/000260253. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Paulson J. C., Hill R. L. The role of sialic acid in the expression of human MN blood group antigens. J Biol Chem. 1979 Mar 25;254(6):2112–2119. [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Zopf D. A., Ginsburg V. The molecular basis for cold agglutination: effect of receptor density upon thermal amplitude of a cold agglutinin. Biochem Biophys Res Commun. 1978 Feb 28;80(4):905–910. doi: 10.1016/0006-291x(78)91330-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Zopf D. A., Yu R. K., Wistar R., Jr, Ginsburg V. A Waldenström macroglobulin that is both a cold agglutinin and a cryoglobulin because it binds N-acetylneuraminosyl residues. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4591–4594. doi: 10.1073/pnas.74.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]



