Abstract
Heterothermic insects like honeybees, foraging in a variable environment, face the challenge of keeping their body temperature high to enable immediate flight and to promote fast exploitation of resources. Because of their small size they have to cope with an enormous heat loss and, therefore, high costs of thermoregulation. This calls for energetic optimisation which may be achieved by different strategies. An ‘economizing’ strategy would be to reduce energetic investment whenever possible, for example by using external heat from the sun for thermoregulation. An ‘investment-guided’ strategy, by contrast, would be to invest additional heat production or external heat gain to optimize physiological parameters like body temperature which promise increased energetic returns. Here we show how honeybees balance these strategies in response to changes of their local microclimate. In a novel approach of simultaneous measurement of respiration and body temperature foragers displayed a flexible strategy of thermoregulatory and energetic management. While foraging in shade on an artificial flower they did not save energy with increasing ambient temperature as expected but acted according to an ‘investment-guided’ strategy, keeping the energy turnover at a high level (∼56–69 mW). This increased thorax temperature and speeded up foraging as ambient temperature increased. Solar heat was invested to increase thorax temperature at low ambient temperature (‘investment-guided’ strategy) but to save energy at high temperature (‘economizing’ strategy), leading to energy savings per stay of ∼18–76% in sunshine. This flexible economic strategy minimized costs of foraging, and optimized energetic efficiency in response to broad variation of environmental conditions.
Introduction
Honeybees are heterothermic insects which change from the ectothermic to the endothermic state for foraging. They have to keep their body temperature high throughout the entire foraging cycle to stay ready for immediate flight, and to promote fast exploitation of resources [1]–[6]. Endothermy in insects of this small size, however, means enormous efforts to compensate for the high heat loss because of the large surface to volume ratio [7]. This calls for energetic optimisation.
During a foraging trip the challenge is especially high because not only the ambient temperature but also solar radiation may vary in a broad range within a day and during a foraging season [4], [8], [9]. In order to assess the energetic demand of foraging bees under variable ambient temperatures there have been measurements of metabolism in the shade, both at artificial flowers [10]–[14], and during flight [15]–[17]. On many flowers, however, or at water sources honeybees are often not airborne for long periods of time [4], [5], [18], [19]. Since they need not to stay airborne their ability of thermoregulation via regulation of heat production with the thoracic flight muscles is much more pronounced than in flight. Thorax temperature is regulated at different levels depending on several parameters like food quality and demand in the colony [3], [20]–[22]. If weather conditions are fine honeybees prefer foraging in sunshine to get additional heat from solar radiation [5]. Thoracic temperature of foragers in sunshine is usually about 1–3°C higher than in shade [1], [5], [19]. The balancing of body temperature regulation during foraging with the own energetic effort and heat gain from the environment, however, is not well known [6]. The main question is what energetic optimisation strategy honeybee foragers follow. Do they follow general economic principles? Is their energetic and thermoregulatory strategy constant or variable throughout the natural range of ambient temperature variation? An ‘economizing’ strategy of energetic optimisation would be to use external heat gain or high ambient temperatures to minimize foraging costs directly by investing it to save energy via a reduction of the own metabolism. An alternative, more forward directed; ‘investment-guided’ strategy would be to invest heat production and external heat gain to optimize physiological parameters like body temperature which might speed up foraging. Though this would mean an instantaneous increase of costs it might nevertheless optimize foraging indirectly in the longer term by speeding up feeding, pollen gathering and flight, which in turn would decrease costs of a foraging trip. To answer these questions we here present a novel approach of simultaneous measurement of body temperature and energy turnover (from CO2 production) of bees foraging sucrose solution from an artificial flower under outdoor conditions, in a broad range of environmental temperature and radiation variation.
Materials and Methods
Energetics, thermoregulation and environmental conditions
The experiments were conducted on 11 days in August and September 2004, on 2 days in early October 2005 and on one day in September 2006, between 10∶00 and 16∶00 hours. In order to allow a simultaneous comparison of foraging energetics and thermoregulation in sunshine and shade, 20 individually marked honeybees originating from 15 colonies in an apiary about 10–20 m away were trained to forage 1.5 M sucrose solution ad libitum from inside a brass measurement chamber of ∼7.9 ml inner volume, immersed in a water bath (Julabo F33 HT) outside the laboratory. The chamber lid could be opened and closed quickly to give the bees fast access to an artificial flower inside (for details see [6]). 15 of the 20 bees could be tested both in shade and in sunshine, up to 12 times per radiation condition.
The CO2 production was measured with a differential infrared gas analyser (DIRGA; URAS 14, ABB) with a flow-through measurement setup in serial mode according to Stabentheiner et al. [6], operated at a flow rate of 240 ml/min. The loss of measurement gas during chamber opening after the insects’ visits was compensated for by calibrations as described in [6]. Briefly, this procedure compares the washout volumes from the chamber containing certain concentrations of CO2 with and without chamber opening.
The ambient air temperature near the foragers was measured by a thermocouple inside the chamber at the air outlet below the bees. The effect of radiation on thermocouple readings was corrected according to [6] if necessary. Solar radiation reaching the bees through the plastic film window of the measurement chamber lid was measured by a photoelectric miniature global radiation sensor in a second chamber beside that containing the artificial flower (FLA613GS/Mini spezial; Ahlborn; see [6]). Convection around the bees was measured with an omnidirectional flow sensor (FV A605 TA, Ahlborn). Environmental data were recorded by ALMEMO data loggers (2690–8 or 2890–9; Ahlborn).
Observation of behaviour and measurement of body surface temperature were done with infrared thermography at a rate of 3–5 Hz (FLIR ThermaCam SC2000 NTS) without behavioural impairment. The infrared camera was calibrated against a peltier-driven reference radiator placed close to the insects (Figure 1; [6]). The attenuation of the infrared radiation by the plastic film was compensated for by covering part of the reference source head with a stripe of the same film. This also minimised errors resulting from ambient reflections via the film surface. In addition, several layers of corrugated cardboard were placed above the infrared (IR) camera. So even in sunshine the lowest cardboard surface temperature resembled the ambient air temperature, which is usually used for correction of reflected ambient radiation. Body surface temperature was calibrated using the cuticular emissivity of the honeybee (0.97; [23]).
Figure 1. Thermogram of a honeybee foraging sucrose from an artificial flower inside a respiratory measurement chamber.
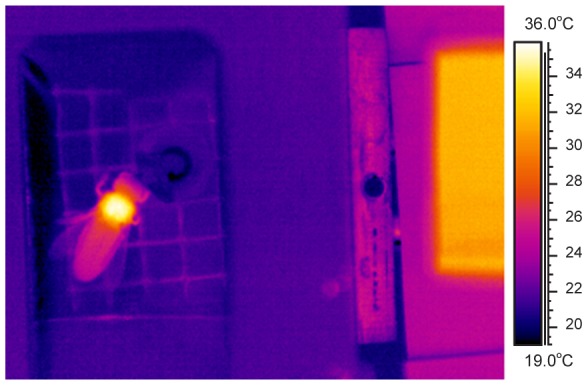
Air inlet is at the bottom of the image, outlet is in the chamber floor right to the bee. The thorax is heated by activation of the flight muscles, part of the heat has reached the head and the abdomen. Ta = 21°C. Right-hand rectangle: proprietary infrared reference radiator.
Energy gain
The energy gain from sucrose foraging was determined by training bees to forage from a balance (Mettler Toledo) where their landing and takeoff weight was measured to the nearest 0.1 mg at ambient temperatures of 15–35°C (in shade and in sunshine). The difference was calculated as crop load (in mg). Energy gain from sugar was determined by correcting for density variation due to temperature and using a calorific value of 16.8 kJ/g sucrose [24].
Data evaluation and statistics
Respiratory data were evaluated in Microsoft Excel and Origin (OriginLab) software. Surface temperatures of head, thorax and abdomen, and of the sucrose solution the bees imbibed, were evaluated at intervals of 3(−5) seconds with ThermaCam Researcher software (FLIR) controlled by a proprietary Excel VBA macro which extracted the stored environmental data automatically from the logger files at the time of thermographic measurement. Statistics and curve fitting was done with Statgraphics (Statpoint Technologies) and Origin software.
All work was conducted according to relevant national and international guidelines.
Results
Interrelation of thermoregulation and energetics
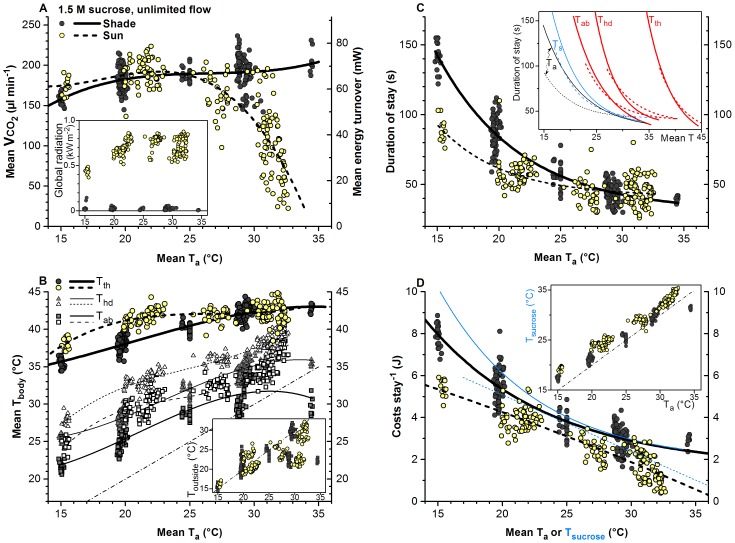
Once trained properly, the honeybees entered the measurement chamber immediately after arrival and started to drink the sucrose solution from the artificial flower. From a total of 400 visits to the artificial flower 217 measurements were made in shade and 183 in sunshine. The foragers remained endothermic during the whole stay (Figure 1). To our surprise, however, the bees foraging in shade did not decrease energy turnover with increasing ambient temperature (Ta) but kept it at a high level of ∼56–69 mW on average throughout the investigated range of Ta (Figure 2A). This way they were able to increase the thorax surface temperature (Tth) from ∼35–36°C at Ta = 15°C to ∼42–43°C at Ta = 30–35°C (Figure 2B).
Figure 2. Energetics and thermoregulation of honeybees foraging sucrose in shade (grey/filled symbols) and in sunshine (yellow/open symbols).
One symbol represents one mean per stay (N = 217 stays in shade and 183 in sunshine; 20 bees). (A) CO2 production rate (VCO2), (B) body surface temperature of head (Thd), thorax (Tth) and abdomen (Tab), (C) duration of stay, (D) costs per stay, and environmental parameters were measured simultaneously in all individuals. Dashed-dotted line in (B): isoline. Ta = ambient air temperature near the bees in the measurement chamber, Toutside = temperature in shade outside the measurement chamber, Ts = sucrose temperature. Regression lines (all P<<0.0001, ANOVA): (A, B) cubic (y = A+B*x+C*x2+ D*x3); (C, D) exponential decay (y = A1*e(−x/t1)+y0)), for constants and statistics see Table 1. Inserts: if not given, axes labelings as in main graphs; insert in (C): Ta, Ts, Tab, Thd, Tth refer to x-axis temperature to be used, respectively.
In sunshine (701 W m−2 on average; see insert in Figure 2A) the foragers changed the energetic and thermoregulatory strategy in dependence on Ta. Below about 25°C they did not reduce energy turnover but kept it at a similar level as in shade (∼62–65 mW; Figure 2A). This way Tth increased by ∼1–3°C in comparison to the shade (Figure 2B). Above 25°C, by contrast, the bees reduced their energy turnover with increasing Ta (∼66–∼6 mW; Figure 2A) in a way that their Tth remained at a similarly high level of ∼42–43°C as in shade (Figure 2B). The temperature excess over Ta increased in all body parts with decreasing Ta (Figure 2B). In the bees exposed to the sun the temperatures of head and abdomen were by about 1–5°C higher than in the bees foraging under shaded conditions. External convection around the bees at our flow rate setting of 240 ml/min amounted to 4.3 cm/s.
Duration of stay
Both in sunshine and in shade the duration of stay decreased approximately exponential with increasing Ta (Figure 2C; Table 1). In shade it changed from ∼140 s at Ta = 15°C to ∼40 s at Ta = 30–35°C. In sunshine it was considerably lower than in the shade in the lower range of Ta (<25°C) but similar at high Ta (>25°C), decreasing from ∼90 s at Ta = 15°C (∼36% of shade value) to ∼40 s at Ta = 30–35°C.
Table 1. Constants and statistics for regression functions in Figure 2.
| y = A+B*x+C*x2+ D*x3 | constants | R2 | |||||
| (cubic) | A | B | C | D | |||
| Figure 2A) | shade | −162.1361 | 39.14352 | −1.463 | 0.01839 | 0.2762 | |
| sun | 455.04686 | −51.09818 | 2.97696 | −0.05445 | 0.7089 | ||
| Figure 2B) | Thd | shade | 41.35954 | −2.925 | 0.15998 | −0.00231 | 0.94005 |
| sun | −28.58677 | 6.83706 | −0.25986 | 0.00351 | 0.93368 | ||
| Tth | shade | 34.94919 | −0.51212 | 0.04957 | –0.0008097 | 0.88119 | |
| sun | −6.95259 | 5.44539 | −0.20204 | 0.0025 | 0.5122 | ||
| Tab | shade | 33.13349 | −2.61309 | 0.16308 | −0.00259 | 0.81108 | |
| sun | −65.26133 | 11.57206 | −0.47612 | 0.00669 | 0.87559 | ||
| y = A1*e(−x/t1)+y0) | constants | R2 | |||||
| (exponential decay) | y0 | A1 | t1 | ||||
| Figure 2C)* | duration vs. Ta | shade | 30.62809 | 1026.245 | 6.76459 | 0.91215 | |
| sun | 41.59258 | 866.42933 | 5.30143 | 0.58617 | |||
| duration vs. Tsucrose | shade | 33.69157 | 2791.07346 | 5.34167 | 0.90384 | ||
| (insert) | sun | 41.72591 | 3531.23814 | 4.45765 | 0.57871 | ||
| duration vs. Thd | shade | 34.64229 | 65179.5756 | 3.95205 | 0.87412 | ||
| (insert) | sun | 36.90517 | 9201.64316 | 5.35922 | 0.56006 | ||
| duration vs. Tth | shade | 15.50437 | 199780.804 | 4.77369 | 0.83793 | ||
| (insert) | sun | 23.41157 | 206487.229 | 4.66765 | 0.47277 | ||
| duration vs. Tab | shade | 24.04419 | 4112.32539 | 5.97249 | 0.82327 | ||
| (insert) | sun | 40.11864 | 7351.28361 | 4.79935 | 0.47877 | ||
| Figure 2D)* | costs vs. Ta | shade | 1.58628 | 30.94792 | 9.49157 | 0.85673 | |
| sun | 22.8322 | −14.58653 | −82.78026 | 0.83342 | |||
| costs vs. Tsucrose | shade | 1.80662 | 61.13779 | 7.54753 | 0.86228 | ||
| sun | 105668.232 | 105657.722 | −389503.053 | 0.85918 | |||
Ta = ambient air temperature; Thd, Tth, Tab = surface temperatures of head, thorax, abdomen. N = 217 in shade and 183 in sun; all regressions significant at P<<0.0001, ANOVA, df = N-4 for cubic and N-3 for exponential decay functions. *: ANOVA linear regression analysis revealed significant differences in slope and intercepts between shade and sunshine at P<0.05, except intercepts for duration vs. Tth (n.s.).
The duration of stay decreased also approximately exponential with increasing temperature of the sucrose solution (Ts) but the correlations were somewhat less pronounced than the correlations with Ta (see R2 values in Table 1). The regressions for shade and sunshine still differed at low Ta (<25°C; see insert in Figure 2C). This difference between nonlinear sunshine and shade regressions became much smaller when we correlated the duration of stay with body temperature (insert in Figure 2C). The correlations were best with the temperature of the head (Thd; R2 = 0.87412 and 0.56006 in shade and in sunshine, respectively), and less pronounced with the temperature of the abdomen (Tab; R2 = 0.82327 and 0.47877) and of the thorax (Tth; R2 = 0.83793 and 0.47277) (Table 1). ANOVA regression analysis revealed considerable differences between shade and sunshine in most cases (Table 1).
Costs, gain and efficiency per stay
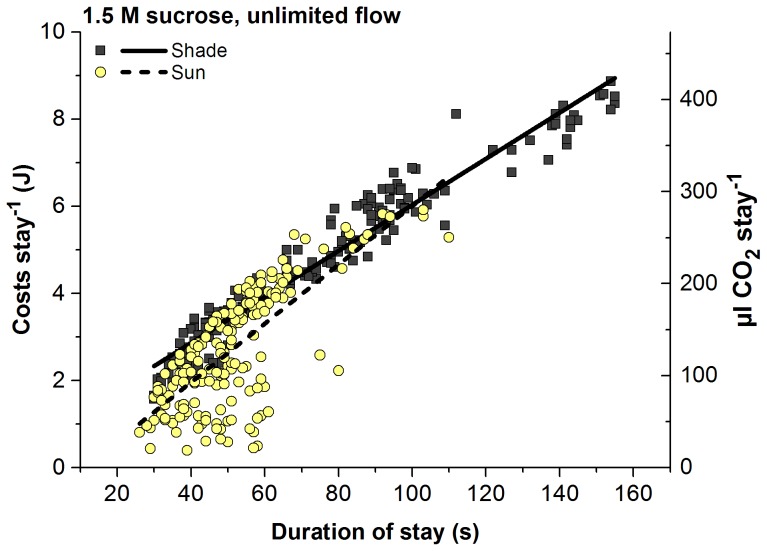
Energetic costs per stay decreased with increasing Ta (Figure 2D; Table 1). Values in shade (as derived from the fitted curves in Figure 2D) amounted to ∼8.0 J at Ta = 15°C and ∼2.36 J at Ta = 35°C, and in sunshine from ∼5.35 J to ∼0.57 J, respectively. The lower costs in sunshine equal energy savings from external heat gain of 2.65 J, 0.71 J and 1.78 J at a Ta of 15, 25 and 35°C if one compares the curves in Figure 2D. This equals savings of ∼33.1%, ∼18.6% and ∼75.7%, respectively. In a similar way as with Ta energetic costs per stay decreased with increasing temperature of the sucrose solution (Ts; thin lines in Figure 2D; Table 1). Figure 3 shows that the energetic costs per stay were a rather straight function of the duration of stay, with similar regressions in sunshine and in shade. In sunshine, however, the range of durations was smaller.
Figure 3. Dependence of costs per stay on duration of stay in shade and in sunshine.
Regression line (y = A+B*x) constants A/B: 0.74577/0.05285 (R2 = 0.94911) in shade, and −0.74292/0.06753 (R2 = 0.55439) in sun (both P<<0.0001, ANOVA; N = 217 in shade and 183 in sun). Regression lines significantly different between shade and sunshine in slope and intercept (P<0.0001, ANOVA).
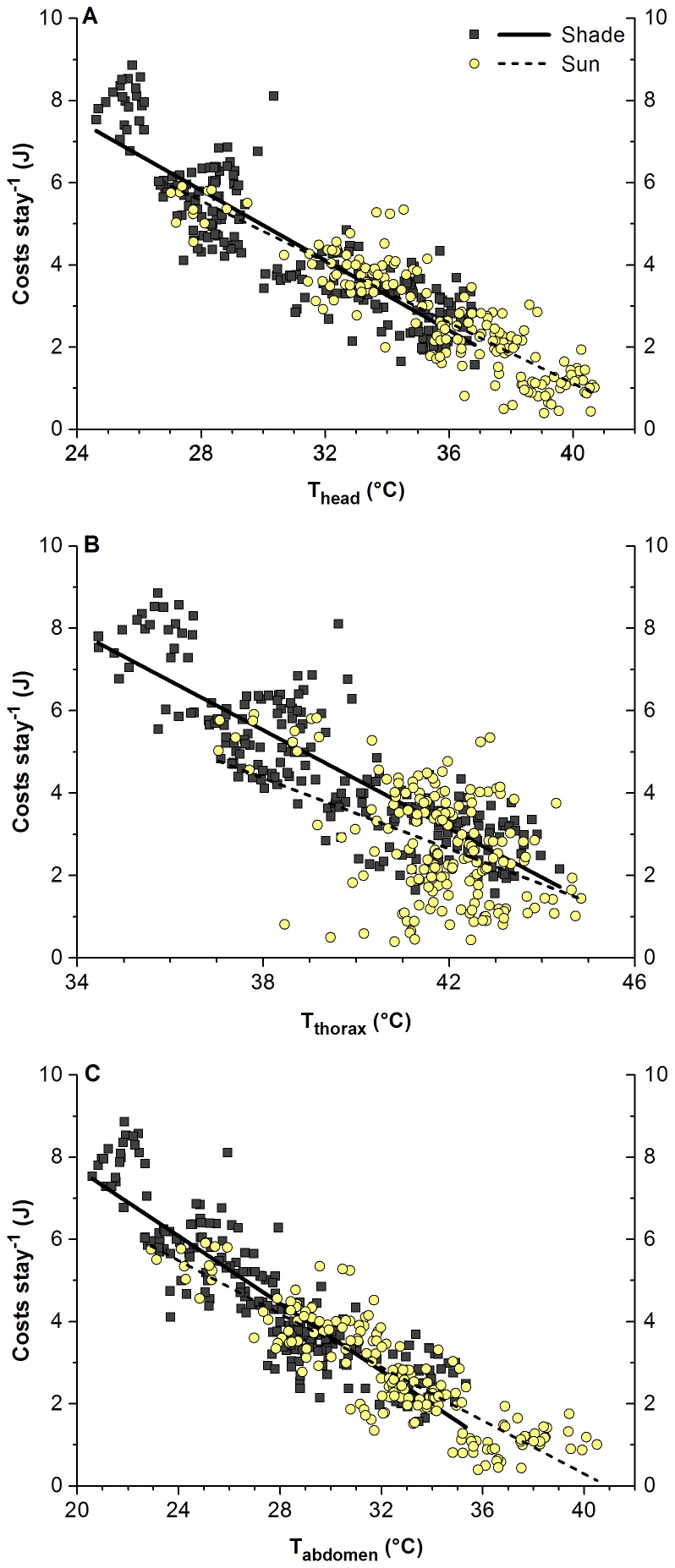
Since body temperature influences duration of stay we analysed the costs per stay in dependence on body part temperature. Costs correlated linearly with the temperatures of all body parts (Figure 4). The best correlations were found with Thead (R2 = 0.76945 and 0.79089 in shade and in sunshine, respectively) and with Tabdomen (R2 = 0.79066 and 0.76387). Correlations were less pronounced with Tthorax (R2 = 0.71848 and 0.20125). The regression lines for shade and sunshine were, though similar, significantly different in slope (P<0.05) but did not differ in intercept for Thead (P = 0.4652) and Tabdomen (P = 0.6066).
Figure 4. Costs per stay in dependence on temperature of body parts, in shade and in sunshine.
Regressions (y = A+B*x; all P<<0.0001, ANOVA; N = 217 in shade and 183 in sun), constants A/B: (A) 17.75233/−0.42623 (R2 = 0.76945) in shade, 15.92754/−0.37025 (R2 = 0.79089) in sun; (B) 28.17624/−0.59599 (R2 = 0.71846) in shade, 20.68964/−0.42959 (R2 = 0.20125) in sun; (C) 15.9186/−0.40997 (R2 = 0.79066) in shade, 13.26462/−0.32425 (R2 = 0.76387) in sun. Regression lines for shade and sunshine significantly different in slope (P<0.05, ANOVA) but not different in intercept for Thead (P = 0.4652) and Tabdomen (P = 0.6066).
Bees trained to forage 1.5 M sucrose (unlimited flow) from an artificial flower on a balance at ambient temperatures of 15–30°C imbibed a mean amount of sucrose solution of 64.9 mg in shade (SD = 8.38, N = 64) and 64.2 mg in sunshine (SD = 8.69, N = 28) (difference n.s., t test). The imbibed amount was independent of Ta in shade (amount (mg) = 65.7741–0.03975*Ta; R2 = −0.01504, P = 0.797) and increased with Ta in sunshine (amount (mg) = 45.70473+0.85964*Ta; R2 = 0.37022, P<0.001). Using the mean values we got a mean energetic gain per stay of ∼464 J in shade, and of ∼470 J in sunshine. The measured costs of 8 J down to 0.57 J (Figure 2D) make up only 1.7%, 0.8% and 0.5% of the mean harvested gain in shade, and 1.1%, 0.7% and 0.1% of the mean gain in the sun, at Ta = 15, 25 and 35°C, respectively.
Energetic efficiency (gain-costs/costs) (J/J) per stay at our artificial flower was calculated by using the above regressions for imbibed sucrose solution (converted to energy gain) in dependence on Ta, and the regressions of Figure 2D. Efficiency increased with ambient temperature, in shade from ∼58 to ∼122 and ∼197 (J/J) at Ta = 15, 25 and 35°C, and in sunshine from ∼78 to ∼156 and ∼961 (J/J), respectively. Foraging in the sun increased efficiency in the entire range of Ta investigated, by ∼35%, ∼28% and ∼387% in comparison to shade, respectively.
Discussion
Foraging motivation and energetics
Motivation is an important modulating parameter in foraging honeybees’ thermoregulation and energetics (e.g. [3], [12], [20], [23], [25]). Under our experimental conditions with unlimited flow of 1.5 M sucrose solution, a very high-quality resource, the foraging bees displayed a high energy turnover under most environmental conditions, in shade amounting to about 55–70 mW on average (Figure 2A), which is higher than the 57–60 mW reported by Stabentheiner et al. [6]. Despite foraging 1.5 M sucrose, in shade our bees displayed only a moderately high thorax surface temperature (Tth) at low to medium Ta, which was considerably lower than in bees which had foraged a lower concentration (1 M) at a similar distance from the hive [23], and similar to or even somewhat lower than measured in bees foraging only 0.5 M sucrose [3]. We suggest that such differences originate from differences in the bees’ motivational status, which modulates thermoregulation [3], [9], [20], [23], [25] and energy turnover [10]–[13]. In bees foraging from a patch of artificial flowers at limited flow rates, which surely decreases foraging motivation (e.g. [26], [27]), metabolic rate was considerably lower than in the present investigation [12], [13].
Energy turnover, thermoregulation and environmental variation
It is clear that bees foraging in the shade at low ambient temperature have to be ‘economizing’ in some way because of the high heat loss due to their unfavourable surface to volume ratio [2], [7]. To our surprise the bees foraging under shaded conditions did not follow an ‘economizing’ strategy to reduce the energy turnover with increasing ambient temperature (Ta) [14], [28] but decided for a graded change to an ‘investment-guided’ strategy: they kept energy turnover rather constant or even increased it (Figure 2A). This resembles independent measurements of oxygen consumption under similar environmental temperature and radiation conditions [6]. Such rather flat energetic curves were also found in flying honeybees [17], in flying carpenter bees (Xylocopa; [29]), and in many other flying insects (e.g. [30]; for more literature see [2]). Conversely, studies have shown that honeybees may decrease metabolic rate and wingbeat frequency during flight at high air temperatures [15], [16]. In flying endothermic moths and honeybees these flat curves are thought to be the result of the limited ability to regulate heat production with the flight muscles because the insects have to stay airborne [2], [17]. In our bees drinking sucrose solution, however, this rather flat energetic curve is surprising, because they do have the ability to decrease energy turnover at high Ta if necessary as is shown by the values measured in sunshine (Figure 2A). Obviously, the bees changed their energetic strategy more and more to an ‘investment-guided’ one the higher the ambient temperature.
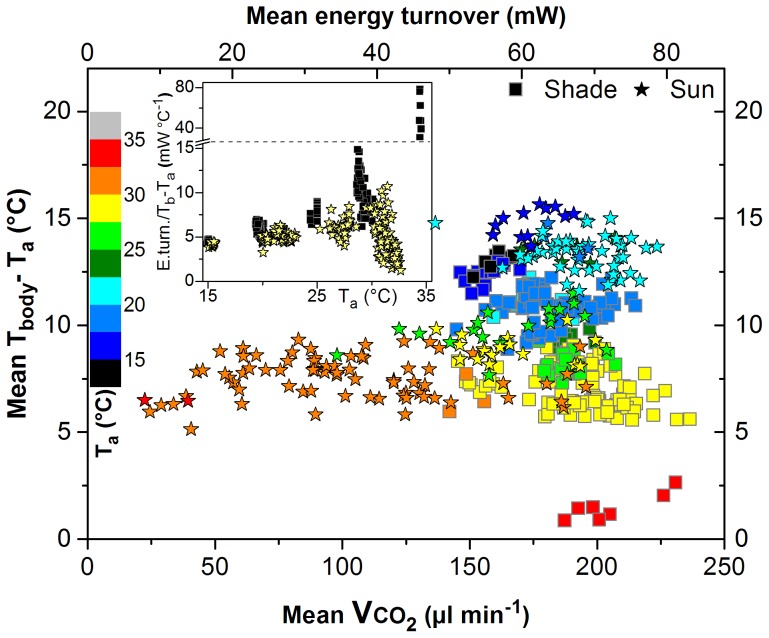
Though in shade the CO2 production was nearly independent of Ta (Figure 2A) and Tth decreased with decreasing Ta (Figure 2B), the bees managed to thermoregulate to some extent: the thoracic temperature excess over Ta (Tth–Ta) increased with decreasing Ta (Figure 2B). One might assume that body temperature and energy turnover are in a simple interrelationship. In honeybees heating their thorax up in flight preparation Goller and Esch [31] reported a straight increase of Tth–Ta with the metabolic turnover. Our simultaneous measurements of body temperature and CO2 production uncovered a considerable variability with no simple relationship (Figure 5; compare [32]). The dependence of Tth-Ta on the energy turnover necessary to reach a certain excess temperature changed with Ta both in shade and in sunshine (Figure 5). Therefore, the bees foraging in the shade must have regulated body temperature in reaction to changes of Ta primarily not by regulation of heat production but by regulation of heat loss. The decrease of a ‘conductance’ estimate in shade with decreasing Ta (energy turnover per degree body temperature difference to Ta; insert in Figure 5, dark symbols) supports this interpretation. We suggest that the bees did not have much regulatory ability left at the lowest Ta but utilized this ability at higher Ta. The low and constant external convection of 4.3 cm/s around the bees was probably not a major source of heat loss. Regulation of heat loss via cooling of the head by regurgitated fluid droplets at high Ta [33]–[35] was probably also not of much importance because the bees imbibing the sucrose solution had wet mouthparts anyway, suggesting a rather constant cooling effect. The two remaining pathways of heat loss regulation are the heat transport to the abdomen, which is not so much pronounced in honeybees [2], and respiration. Regulation of respiratory heat loss might be accomplished by modulation of ventilation frequency for example.
Figure 5. Mean body surface temperature excess over ambient temperature (Tbody–Ta) per stay, in dependence on mean CO2 production rate (VCO2) or energy turnover, and Ta (colour scale), in shade (squares) and in sunshine (asterisks).
Bees foraged 1.5 M sucrose solution from an unlimited flow feeder. Insert: Quotient of energy turnover (E.turn.) and body temperature excess (Tb–Ta) in dependence on Ta. N = 217 in shade and 183 in sun. Tb = Tbody (mean of head, thorax and abdomen).
Our investigation shows for the first time that honeybees follow a flexible strategy concerning the use of solar radiation. Instead of reducing energy turnover at low Ta they invested solar heat to increase the temperature of all body parts considerably (Figure 2, ‘investment-guided’ strategy). At high Ta, by contrast, they decreased their energy turnover in the sun (‘economizing’ strategy), probably to prevent overheating. Though honeybees are rather heat tolerant insects [36], [37] a further increase of body temperature might nevertheless have been unfavourable in the long term [37].
Body temperature and suction speed
The energy turnover measured in the present study was considerably higher than the turnover measured in most studies of agitated or hovering flight, where means amounted to about 38–63 mW [15]–[17], [38], [39]. This is surprising in so far as in our experiments the bees had not to lift their weight in flight. What is the purpose of this extreme investment? Figure 2 shows that the foragers used both the high energy turnover and solar heat to increase body temperature in a flexible manner, which led to the concurrent exponential decrease of the duration of stay (increase of suction speed) with increasing Ta (Figure 2C; [40]). The function of the musculature involved in ingestion of fluids (‘suction pump’, cibarium with associated structures) is suggested to be strongly dependent on body temperature [19].
The extraordinarily high values of Tth at high Ta (Tth = 40–44.5°C; Figure 2B) were not primarily a means to achieve maximum lift for immediate readiness for takeoff. Coelho [41] has shown that bees reach their maximum achievable lift already with a Tth of ∼39°C. A higher Tth has an inhibitory effect on flight muscle performance. In bees foraging from flowers where they often must remain prepared for immediate commencement of flight, Tth usually remains below 40°C [1], [4], [5]. On water sources, on the other hand, where the bees can speed up foraging with a higher body temperature like at our artificial flower (unlimited flow of resources in both cases), a Tth in sunshine higher than 40°C was nearly as common as in our experiments [19].
Costs, gain and efficiency
The balance between energy investment and energy gain is crucial in foraging insects. With their flexible strategy the bees’ costs per stay decreased considerably with increasing Ta both in shade and in sunshine (Figure 2D). The main parameter determining the costs per stay under our experimental conditions of unlimited sucrose flow was time: costs increased linearly with the duration of stay (Figure 3). The linear decrease of the costs with body temperature (Figure 4) supports the hypothesis that the body temperature, especially that of head and abdomen, was the main factor determining efficiency of foraging [19]. The temperature of the thorax is of course also important because the heat produced there is transferred to the head and to the abdomen in part. It is noteworthy that the costs for sunny conditions are closely in the trend of those from the shade, with identical intercepts for head and abdomen temperature (Figure 4). This again emphasizes the interpretation that the bees use solar radiation in a flexible way to optimize body temperature for the purpose of optimisation of foraging efficiency.
Beside the body temperature, also the temperature of the imbibed food (of nectar or water) influences honeybee foraging [19], [42]–[44]. On flowers and cold water sources bees always prefer the warmer or sunny patches over colder or shaded ones [5], [19]. Beside the direct effect of the sun on body temperature [5], [19] warmer nectar or water will cool the mouthparts and suction pump less and this way probably contributes to an improved function. This way the bees can make more foraging trips per time interval, which increases the harvested amount of sugar (energy) per day.
In any case, foraging in the sun enabled the foragers to reduce the energetic costs per stay considerably, by about 19% to 76% (compare Figure 2D). At low Ta this was achieved by an increase of body temperature and the resulting increase of the suction speed. This points to an ‘investment-guided’ strategy under these conditions which promises additional gain in return. This maximising of returns would not be accessible with an energy-saving (‘economizing’) strategy. At high Ta, by contrast, it was the reduction of the energy turnover which made these savings possible. This equals an ‘economizing’ strategy.
It has to be kept in mind, however, that experiments with unlimited flow of highly concentrated sugar solution provide the bees with an enormous gain per unit of time [45]. The costs of 8 J down to 0.57 J (Figure 2D) make up a rather small fraction of the energy gain (1.7% to 0.5% in shade and only 1.3% to 0.1% in sunshine). The relation of gain to costs will be much less favourable under conditions of low (limited) nectar flow [12]. The high turnover observed not only in shade but even in sunshine is not a waste of energy but an investment which maximizes the profitability of foraging by optimizing energetic efficiency (gain-costs/costs) [45]–[50]. Foraging in the sun increased efficiency in the entire range of Ta investigated (by ∼28% to ∼387%). However, this was not accomplished by a constant but by a flexible physiological and behavioural strategy of own energetic investment and use of external (solar) heat.
We conclude that foraging honeybees follow a flexible economic strategy. They change between an ‘economizing’ or an ‘investment-guided’ strategy on demand. This optimizes body temperature in a graded manner in reaction to environmental variation, and this way maximizes intake rate of the colony.
Acknowledgments
We greatly appreciate the help with data evaluation by M. Ablasser and for partial help with bee training by R. Brodschneider.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research was funded by the Austrian Science Fund (FWF) (http://www.fwf.ac.at/): P16584-B06 to AST and HK, P20802-B16 to HK and AST, and P25042-B16 to HK and AST. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Heinrich B (1979a) Thermoregulation of African and European honeybees during foraging, attack, and hive exits and returns. J Exp Biol 80: 217–229. [Google Scholar]
- 2.Heinrich B (1993) The Hot-Blooded Insects. Springer-Verlag, Berlin, Heidelberg, Germany; London, U.K.; Paris, France.
- 3. Schmaranzer S, Stabentheiner A (1988) Variability of the thermal behaviour of honeybees on a feeding place. J Comp Physiol B 158: 135–141. [Google Scholar]
- 4. Kovac H, Schmaranzer S (1996) Thermoregulation of honeybees (Apis mellifera) foraging in spring and summer at different plants. J Insect Physiol 42: 1071–1076. [Google Scholar]
- 5. Kovac H, Stabentheiner A (2011) Thermoregulation of foraging honeybees on flowering plants. Ecol Entomol 36: 686–699 10.1111/j.13652311.2011.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stabentheiner A, Kovac H, Hetz SK, Käfer H, Stabentheiner G (2012) Assessing honeybee and wasp thermoregulation and energetics – New insights by combination of flow through respirometry with infrared thermography. Thermochimica Acta 534: 77–86 10.1016/j.tca.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bishop JA, Armbruster WS (1999) Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecology. Funct Ecol 13: 711–724. [Google Scholar]
- 8. Germ M, Stabentheiner A, Kastberger G (1997) Seasonal and daily variation of honeybee dancing temperature under constant feeding conditions. Apidologie 28: 385–398. [Google Scholar]
- 9. Stabentheiner A (2001) Thermoregulation of dancing bees: thoracic temperature of pollen and nectar foragers in relation to profitability of foraging and colony need. J Insect Physiol 47: 385–392. [DOI] [PubMed] [Google Scholar]
- 10. Balderrama NM, Almeida LO, Núnez JA (1992) Metabolic rate during foraging in the honey bee. J Comp Physiol B 162: 440–447. [DOI] [PubMed] [Google Scholar]
- 11. Moffatt L, Núnez JA (1997) Oxygen consumption in the foraging honeybee depends on the reward rate at the food source. J Comp Physiol B 167: 36–42. [DOI] [PubMed] [Google Scholar]
- 12. Moffatt L (2000) Changes in the metabolic rate of the foraging honeybee: effect of the carried weight or of the reward rate? J Comp Physiol A 186: 299–306 10.1007/s003590050430 [DOI] [PubMed] [Google Scholar]
- 13. Moffatt L (2001) Metabolic rate and thermal stability during honeybee foraging at different reward rates. J Exp Biol 204: 759–766. [DOI] [PubMed] [Google Scholar]
- 14. Blatt J, Roces F (2001) Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J Exp Biol 204: 2709–2716. [DOI] [PubMed] [Google Scholar]
- 15. Harrison JF, Fewell JH, Roberts SP, Hall HG (1996) Achievement of thermal stability by varying metabolic heat production in flying honeybees. Science 274: 88–90. [DOI] [PubMed] [Google Scholar]
- 16. Roberts SP, Harrison JF (1999) Mechanisms of thermal stability during flight in the honeybee, Apis mellifera, J Exp Biol. 202: 1523–1533. [DOI] [PubMed] [Google Scholar]
- 17. Woods Jr WA, Heinrich B, Stevenson RD (2005) Honeybee flight metabolic rate: does it depend upon air temperature? J Exp Biol 208: 1161–1173 10.1242/jeb.01510 [DOI] [PubMed] [Google Scholar]
- 18. Schmaranzer S (2000) Thermoregulation of water collecting honeybees (Apis mellifera) J Insect Physiol. 46: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 19. Kovac H, Stabentheiner A, Schmaranzer S (2010) Thermoregulation of water foraging honeybees – balancing of endothermic activity with radiative heat gain and functional requirements. J Insect Physiol 56: 1834–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyer CD, Seeley TD (1987) Interspecific comparison of endothermy in honey-bees (Apis): deviations from the expected size-related patterns. J Exp Biol 127: 1–26. [Google Scholar]
- 21. Waddington KD (1990) Foraging profits and thoracic temperatures of honey bees (Apis mellifera). J Comp Physiol B 160: 325–329. [Google Scholar]
- 22. Sadler N, Nieh JC (2011) Honey bee forager thoracic temperature inside the nest is tuned to broad-scale differences in recruitment motivation J Exp Biol. 214: 469–475 10.1242/jeb.049445 [DOI] [PubMed] [Google Scholar]
- 23. Stabentheiner A, Schmaranzer S (1987) Thermographic determination of body temperatures in honey bees and hornets: calibration and applications. Thermology 2: 563–572. [Google Scholar]
- 24.Hartfelder K, Bitondi MMG, Brent C, Guidugli-Lazzarini KR, Simões ZLP et al.. (2013) Standard methods for physiology and biochemistry research in Apis mellifera. J Apic Res 52: 26–47. (In “V. Dietemann, J. D. Ellis, P. Neumann (Eds) The COLOSS BEEBOOK, Volume I: standard methods for Apis mellifera research.”) doi:10.3896/IBRA.1.52.1.06.
- 25. Stabentheiner A, Hagmüller K (1991) Sweet food means ‘hot dancing’ in honey bees. Naturwissenschaften 78: 471–473. [Google Scholar]
- 26. Seeley TD, Tovey CA (1994) Why search time to find a food storer bee accurately indicates the relative rates of nectar collecting and nectar processing in honey bee colonies. Anim Behav 47: 311–316. [Google Scholar]
- 27. Schmickl T, Crailsheim K (2004) Costs of environmental fluctuations and benefits of dynamic decentralized foraging decisions in honey bees. Adaptive Behavior 12: 263–277 10.1177/105971230401200311 [DOI] [Google Scholar]
- 28. Stabentheiner A, Vollmann J, Kovac H, Crailsheim K (2003) Oxygen consumption and body temperature of active and resting honeybees. J Insect Physiol 49: 881–889 10.1016/S0022-1910(03)00148-3 [DOI] [PubMed] [Google Scholar]
- 29.Nicolson SW, Louw GN (1982) Simultaneous measurement of evaporative water loss, oxygen consumption, and thoracic temperature during flight in a carpenter bee. J Exp Zool 222, 287–296.
- 30. Casey TM (1981) Energetics and thermoregulation of Malacosoma americanum (Lepidoptera, Lasiocampidae) during hovering flight. Physiol Zool 54: 362–371. [Google Scholar]
- 31. Goller F, Esch HE (1991) Oxygen consumption and flight muscle activity during heating in workers and drones of Apis mellifera . J Comp Physiol B 161: 61–67. [Google Scholar]
- 32. Esch HE, Goller F, Burns JE (1994) Honeybee waggle dances: the energy hypothesis and thermoregulatory behavior of foragers. J Comp Physiol B 163: 621–625. [Google Scholar]
- 33. Heinrich B (1979b) Keeping a cool head: honeybee thermoregulation. Science 205: 1269–1271. [DOI] [PubMed] [Google Scholar]
- 34. Cooper PD, Schaffer WM, Buchmann SL (1985) Temperature regulation of honey bees (Apis mellifera) foraging in the Sonoran desert. J Exp Biol 114: 1–15. [Google Scholar]
- 35. Heinrich B (1980) Mechanisms of body-temperature regulation in honeybees, Apis mellifera. II. Regulation of thoracic temperature at high air temperature. J Exp Biol 85: 73–87. [Google Scholar]
- 36. Käfer H, Kovac H, Stabentheiner A (2012) Resting metabolism and critical thermal maxima of vespine wasps (Vespula sp.). J Insect Physiol 58: 679–689 10.1016/j.jinsphys.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovac H, Käfer H, Stabentheiner A, Costa C (2014) Metabolism and upper thermal limits of Apis mellifera carnica and A. m. ligustica. Apidologie, in press. doi:10.1007/s13592-014-0284-3. [DOI] [PMC free article] [PubMed]
- 38. Harrison JF, Hall HG (1993) African-European honeybee hybrids have low nonintermediate metabolic capacities. Nature 363: 258–260. [Google Scholar]
- 39. Feuerbacher E, Fewell JH, Roberts P, Smith EF, Harrison JF (2003) Effects of load type (pollen or nectar) and load mass on hovering metabolic rate and mechanical power output in the honey bee Apis mellifera . J Exp Biol 206: 1855–1865 10.1242/jeb.00347 [DOI] [PubMed] [Google Scholar]
- 40. Afik O, Shafir S (2007) Effect of ambient temperature on crop loading in the honey bee, Apis mellifera (Hymenoptera: Apidae). Entomol Gener 29: 135–148 doi:0171-8177/07/0029-0135 [Google Scholar]
- 41. Coelho JR (1991) The effect of thorax temperature on force production during tethered flight in the honeybee (Apis mellifera) drones, workers and queens. Physiol Zool 64: 823–835. [Google Scholar]
- 42. Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L (2006) Bees associate warmth with floral colour. Nature 442: 525 10.1038/442525a [DOI] [PubMed] [Google Scholar]
- 43. Nicolson SW, de Veer L, Köhler A, Pirk CWW (2013) Honeybees prefer warmer nectar and less viscous nectar, regardless of sugar concentration. Proc R Soc B 280: 20131597 10.1098/rspb.2013.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan K, Latty T, Hu Z, Wang Z, Yang S, et al. (2014) Preferences and tradeoffs in nectar temperature and nectar concentration in the Asian hive bee Apis cerana . Behav Ecol Sociobiol 68: 13–20 10.1007/s00265-013-1617-3 [DOI] [Google Scholar]
- 45. Seeley TD (1994) Honeybee foragers as sensory units of their colonies. Behav Ecol Sociobiol 34: 51–62. [Google Scholar]
- 46. Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Quart Rev Biol 52: 137–154. [Google Scholar]
- 47. Schmid-Hempel P, Kacelnik A, Houston AJ (1985) Honeybees maximize efficiency by not filling their crop. Behav Ecol Sociobiol 17: 61–66. [Google Scholar]
- 48. Schmid-Hempel P, Schmid-Hempel R (1987) Efficient nectar-collecting by honeybees II. response to factors determining nectar availability. J Anim Ecol 56: 219–227. [Google Scholar]
- 49. Seeley TD (1986) Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav Ecol Sociobiol 19: 343–354. [Google Scholar]
- 50. Waddington KD, Holden LD (1979) Optimal foraging: on flower selection by bees. Am Nat 114: 179–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.