Abstract
Terrestrial protected areas (PAs) are cornerstones of global biodiversity conservation. Their efficacy in terms of maintaining biodiversity is, however, much debated. Studies to date have been unable to provide a general answer as to PA conservation efficacy because of their typically restricted geographic and/or taxonomic focus, or qualitative approaches focusing on proxies for biodiversity, such as deforestation. Given the rarity of historical data to enable comparisons of biodiversity before/after PA establishment, many smaller scale studies over the past 30 years have directly compared biodiversity inside PAs to that of surrounding areas, which provides one measure of PA ecological performance. Here we use a meta-analysis of such studies (N = 86) to test if PAs contain higher biodiversity values than surrounding areas, and so assess their contribution to determining PA efficacy. We find that PAs generally have higher abundances of individual species, higher assemblage abundances, and higher species richness values compared with alternative land uses. Local scale studies in combination thus show that PAs retain more biodiversity than alternative land use areas. Nonetheless, much variation is present in the effect sizes, which underscores the context-specificity of PA efficacy.
Introduction
Nearly 12% of the world’s terrestrial surface is now classified as some form of protected area (PA) [1]. Indeed, the designation and maintenance of PAs are considered key global strategies to address the growing extinction crisis [1]. The unprotected world has been so transformed by human activity that it can now be characterized more readily by a set of human biomes than by the classic biogeographic regions [2]. Therefore, affording an area protection, a long-standing and current centrepiece of conservation strategy [3], appears to be an effective means of conserving its biodiversity features. Conservation scientists have rightly been concerned, however, that simple assumptions of positive ecological performance may be misleading [4]–[9]. Studies have recognized that effective PA management is key to biodiversity protection [7], and demonstrated that PA designation achieves good conservation return on investment at a relatively low cost [10]. Evidence exists, however, that in many cases PA systems are inefficiently planned to maximize benefits to biodiversity often owing to their spatial location [4], [6], and worrying declines in biodiversity even within PAs in particular regions have been identified [5], [8].
Much interest has focused on determining PA effectiveness in terms of preventing landscape cover changes (e.g. [11]–[13]), but these assessments serve only as a proxy for PA performance, as the measures used cannot necessarily capture the implications of land use change for biodiversity features. Where the latter are investigated, outcomes are typically available for specific areas, such as the tropics [8], or particular taxa, such as birds [14] or mammals [5]. Given that negative pressures on biodiversity and evidence for population declines are global in extent [15], [16], the overall significance of terrestrial PAs for maintaining biodiversity values thus remains unclear. Protected area policy demonstrating their efficacy should ideally be evidence-based, that is, informed by rigorously established objective scientific evidence, as should be the case for conservation policy generally [17]. However, such evidence is not as well developed as it should be [18], despite urgent calls for so doing both in the scientific [18], [19] and policy [3] arenas.
Protected area ecological performance would best be assessed by determining for every established PA what the overall biodiversity status is compared with what would have happened in the absence of protection. Plainly such a comparison cannot readily be achieved. One experimentally tractable alternative is the assessment of biodiversity before and after land cover change, but such studies are extremely rare (although see [20] for a notable exception). The scarcity of data to enable comparisons before/after PA establishment almost invariably necessitates comparisons of each PA with some other area that is unprotected, but similar in all but this designation. As a consequence, assessments of PA performance are typically restricted to small spatial scales and particular taxa (e.g. [14], [21]), but there is no clear indication of the generality of their often-contrasting outcomes [18], [21], [22]. The biodiversity response when comparing a PA with some other area that is unprotected can vary widely, with different studies finding both higher and/or lower biodiversity values across areas [8], [20]–[22]. Results from such studies suggest that PA ecological performance is context-specific and can be influenced by several local factors [8], [18], [22]. As a consequence, the generality of PA efficacy in maintaining biodiversity across regions remains unclear.
Here we use local scale studies comparing biodiversity between PAs and surrounding alternative land use areas to test if PAs contain higher biodiversity values than surrounding areas, using a meta-analysis. Specifically, our aim is to assess the ecological performance of terrestrial PAs, compared with areas in close proximity that are not protected, thus outside PAs, using three key biodiversity attributes: the abundances of individual species (hereafter ‘species abundances’), assemblage abundances (summed across species) and assemblage species richness. These are key measures of biodiversity [23]. We also use an information theoretic approach with candidate explanatory variables to explore reasons for the variation in effect size. We then consider sources of bias in interpreting results, and highlight the benefits and shortcomings of our approach in determining PA efficacy.
Methods
Literature search
Our search and data extraction protocol follows best practice guidelines in conducting meta-analysis (see Appendix S1). We used keyword searches in Web of Science, Scopus, and Google Scholar for relevant papers published from 1975–2011, and their references, and included those reporting pairwise comparisons of biodiversity measurements either inside and outside protected areas (PAs) or between areas within PAs (details follow). The initial search string was: (bird* OR mammal* OR reptile* OR amphibia* OR arthropod* OR insect* OR fish* OR plant* OR vegetation*) AND (“protected area” OR “protected areas” OR “national park” OR “national parks” OR “reserve” OR “reserves” OR “game reserve” OR “game reserves”) AND (effective* OR inside* OR performance* OR assessment* OR evaluation* OR estimate* OR comparison* OR contrast*) AND (outside* OR adjacent* OR neighbour* OR adjoining* OR bordering* OR near*). We also used unstructured and opportunistic literature searches with sections of the initial string (particularly to identify studies on different taxonomic groups), expert knowledge of available data sets, and results of a recent multi-database systematic review on protected area efficacy [9]. Grey literature (informally published written material [such as reports, theses and books]) was targeted but exceedingly rare, as found by others [9].
Data capture
From suitable papers we retained studies that measured biodiversity responses inside PAs, and outside PAs, in a replicated study design. These data represent three response variables: (i) species abundances (the abundances of individual species), (ii) abundances per assemblage, (iii) species richness per assemblage, following [24]. Species abundance represents indices of abundance; for example counts, density, capture frequency, occupancy estimates and biomass, for a single species both inside and outside PAs, but only in cases where taxonomy was resolved to the species level (species included are listed in the Dataset S1; N = 243). Assemblage abundance represents indices of abundance; for example counts, density, capture frequency, occupancy estimates and biomass, from cases where abundance was reported across species assemblages or could be calculated across sampling sites. We included these data as estimated by the original authors, but ensured that authors followed a replicated study design, and identical calculations of such indices both inside and outside PAs, and so we consider this response variable as an additional indicator of abundance across species groups (following [24]). Species richness included, for example, observed/estimated/rarefied richness, species density and genera/family richness, from cases where richness was reported or could be calculated across sampling sites. The use of these three variables as estimates of biodiversity was constrained by the approaches adopted by the studies we examined. Although biodiversity can be measured in a variety of ways, these three measures are commonly used as effective measures of biodiversity [23].
Most data we used included mean, standard deviation and sample sizes both inside and outside PAs. Where standard deviation was not reported (20%; 300/1484), we calculated it from either standard error or confidence intervals using imputation methods [25]. Studies with no suitable variance measures were omitted. Where the species could not be correctly identified to species level, data were omitted from the species abundance analysis, but included in the assemblage abundance analyses in cases where these values could be summed across groups of unidentified species. Invasive and domesticated species were omitted as they are not considered here to be of conservation interest. Observations on presence or absence of species were omitted as effect size cannot be calculated. Where data were reported across mixed habitats and/or vegetation types, we only took those from matched pairs, i.e. in areas where the same habitat type was reported. To avoid inadequate treatment of fragmentation effects, data from habitat fragments, typically forest fragments, were excluded following [24]. We did not find studies comparing biodiversity features before and after PA establishment. Few studies considered census-area effects [26] directly, but as individual studies had similar (often identical) sampling designs inside and outside the PAs and in consequence comparable sampling areas, we consider the potential confounding effects of sampling area negligible. Data reporting diversity indices were omitted to avoid pseudoreplication [24], as they are secondary (or derived) measures of species richness and/or abundance, and data on demographics or community structure were omitted because the direction of the expected response was not straightforward to interpret [24], [27]. WebPlotDigitizer v2.4 [28] was used to capture data from figures, which is considered a robust technique [29].
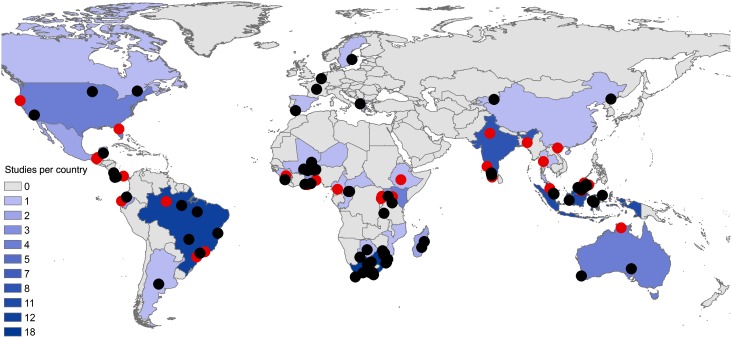
We found 861 pairwise observations inside and outside PAs from 86 studies distributed amongst 32 countries and 57 PAs that met our criteria (Figure 1; all studies used in the meta-analysis are in Dataset S1; Figure S1 in File S1). During the search, we also discovered comparisons within PAs only, that typically included a pristine baseline site (as judged by the authors) in the PA and an anthropogenically disturbed area also inside the PA (disturbances such as logging, clearing or hunting pressure). To determine if PA designation may offset negative anthropogenic influences, such as land transformation, we compared this portion of the dataset to the comparisons made inside and outside PAs. If the effect sizes of comparisons between areas within PAs are lower than for comparisons made inside and outside PAs, we can infer that PAs offset negative anthropogenic influences, such as land transformation, to a greater degree than no PA designation. We identified an additional 623 such pairwise comparisons from 41 studies between sites within PAs only.
Figure 1. Map of the study sites by the centroid coordinates of protected areas for inside-outside pairwise comparisons (black dots; n = 71) and inside only comparisons (red dots; n = 32).
Both categories include data where studies reported across clusters of protected areas.
Data analysis
To estimate effect size, we calculated the Hedges g metric for pairwise comparisons. This is the weighted average of the mean standardized difference (based on pooled variance measures). The metric is the most commonly used, and preferred, to compare pairs of means where variance is available, and unlike others is insensitive to unequal sampling variances in paired groups [27], [30]. It is defined as
where
 |
Since Hedges g is a biased estimator of population effect size [27], [30], we used the commonly applied conversion factor J to compute the bias-corrected Hedge’s g* metric [27], or g* = gJ, where
We then calculated effect sizes using a random-effects model that weights individual comparisons by the inverse of within-study variance plus between study variance, following [24], [27], [30], with a maximum likelihood variance estimator. Random effects models encompass the variance both between-studies and within-studies, and as such, they are the most appropriate for the majority of ecological meta-analyses because they account for variation in study-specific effects [27]. All analyses were conducted in the “metafor” package [31] in R [32].
We set the direction of the sign of the effect size as positive when the biodiversity value for PAs was greater than outside PAs, implying that PAs contain higher biodiversity values [33]. We selected a single effect size measure that could incorporate variance, which was standardized across response variables. We calculated effect sizes for pairwise comparisons across data inside and outside PAs (N = 861), and also for comparisons inside PAs only (N = 623). We then calculated effect sizes for the three response variables. For inside and outside comparisons only we further calculated effect sizes for (i) five major taxonomic groups (mammals, birds, herptiles [reptiles and amphibians combined], arthropods, plants), (ii) continents (excluding Antarctica with no data), and (iii) International Union for Conservation of Nature’s Protected Area Management Categories (a globally recognised PA categorisation system [34]). The categories are primarily based on their management objectives, in which categories 1–4 reflect stricter goals for biodiversity conservation [1 being the strictest], and 5–6 generally allow extractive use via exploitation of biodiversity features [34], see full definitions in Appendix S2). Finally, (v) we calculated effect sizes for the status of species on the IUCN Red List (a global inventory of the threat status of species according to predetermined criteria [35]).
We calculated two commonly used metrics to characterize heterogeneity between pair-wise comparisons as employed in the “metafor” package [31] in R [32]: the Q-statistic and the I 2 value. Total heterogeneity in effect size can be tested with a Q-statistic where a significant value indicates that the estimated effect size is more heterogeneous than expected by chance [27]. The total heterogeneity of the study, QTOTAL or QT can be calculated as
The I 2 value is presented as the total percentage of heterogeneity that can be attributed to between-study variance [27]. The metric quantifies the heterogeneity by comparing the calculated QT to its expected value under the assumption of homogeneity [27], as
We conducted three commonly used tests for detecting bias in meta-analysis in the “metafor” package [31] in R [32]: funnel plot, cumulative meta-analysis and Orwin’s fail safe N [27], [30], [31]. A funnel plot graphs effect size against standard error, and assumes that studies with the largest sample sizes will have lower standard error, and so will be near the average effect size, while studies with smaller sample sizes will be spread on both sides of the average effect size. Variation from this assumption can indicate bias, although the source of such bias may be unclear from a funnel plot [27]. However, positive asymmetry is typically taken to mean publication bias, in that those studies with positive effects are submitted and/or accepted for publication with a greater frequency then those with negative effects [27]. A cumulative meta-analysis sorts all pair-wise comparisons by precision, thus starting with the studies with the largest standard error, after which the comparison with the next largest standard error is added and the effect size is recalculated, and so continues iteratively [27]. The resulting graph enables inspection of the development of the observed effect size with the addition of more precise data. Orwin’s fails safe N is a metric of the number of studies averaging null results that would have to be added to the observed outcomes to reduce the average effect size to half the observed effect size. All tests for publication bias in this study showed that it was negligible [27] (Table S1; Figure S2 & S3 in File S1).
To address the potential spatial pseudoreplication in the dataset that could arise from multiple responses reported within studies [22], PAs, countries or species, we recalculated effect sizes after sampling one pairwise comparison only per study, PA, country or species, respectively. This resampling was repeated 10 000 times for each of these four parameters and the estimated mean and 95% confidence intervals compared with the overall effect size, which was conducted in R [32].
We used an information theoretic approach [36] to assess the influence of a candidate set of models and variables to explain the variation in effect size, where data were available for all variables. Models tested the influence of (i) pre-planned subgroups in the meta-analysis (variables: response variable, taxonomic group, PA IUCN Category), (ii) design, location and structural attributes of the PAs (variables: continent, latitude, longitude, PA area in km2, and PA establishment date, using [34]) and (iii) influence of socio-economic conditions of the countries in which PAs are located (variables: World Governance Index [37], Gross Domestic Product (GDP), Country Population size and the Gini coefficient of income inequality [38]). We followed an exhaustive search approach, which entails fitting all possible model formulations, with a Generalized Linear Model (GLM; [39]). We assumed a Gaussian distribution with a log link function, which was identified as the appropriate family and link function by visual inspection of quantile-quantile plots, using the “glmulti” package [40], in R [32]. We selected the highest ranked model based on the lowest Akaike Information Criterion [39], [40] value. Furthermore, to address possible pseudoreplication, one pairwise comparison per study was selected at random and the respective GLM model refitted as above. We selected the highest ranked model based on the lowest Akaike Information Criterion value [39], [40], and repeated this procedure 1000 times, to calculate the proportionally highest ranked model for each candidate dataset.
At least some of the variation in effect size may also be accounted for by the scale over which studies were conducted. To test the influence of distance between PA boundaries, and the maximum distance between pair-wise comparisons, a best GLM model by exhaustive fit was performed as above [40]. These independent variables were (i) the maximum distance to protected area boundary within studies, and (ii) the maximum distance between pair wise comparisons within studies, meaning, within each study, the maximum distance between sampling points assigned to all points in that study. Since the data on the distance between comparisons were only available for a reduced subset of pair-wise comparisons (N = 569), we performed a separate GLM as above with only the distance variables. We also estimated Pearson’s product moment correlation coefficient between Hedges g* metric values for pair-wise comparisons and the two distance variables in R [32].
Results
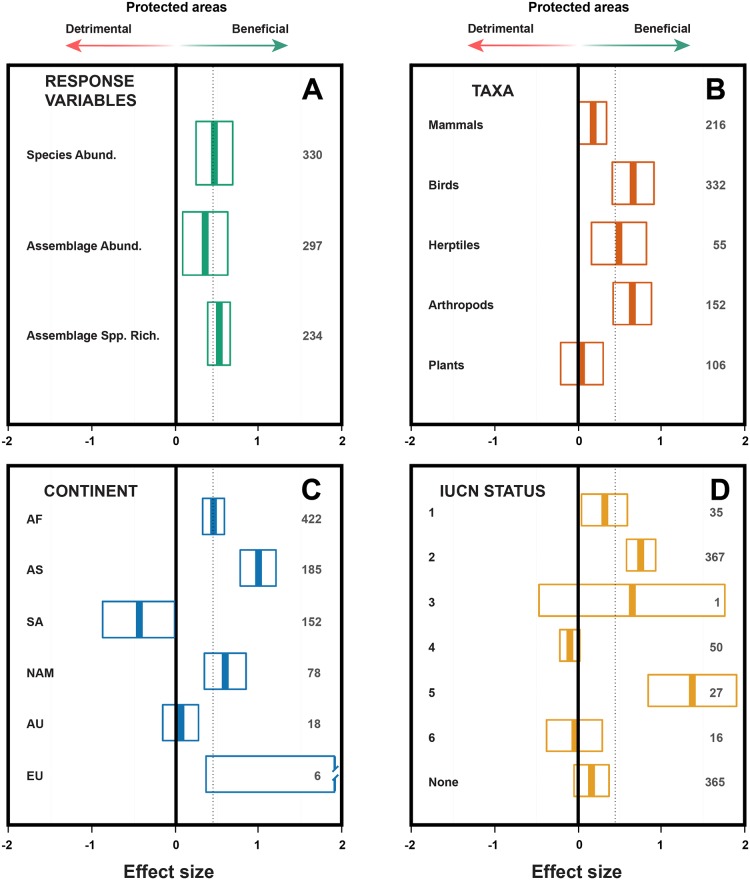
The mean effect size using the random effects model, which provides an indication of the general trend across all 861 comparisons, was 0.444 (95% confidence intervals 0.324–0.564; Table 1). Substantial variation was present in the direction and size of effects in response variables for different pairwise comparisons (I2>87.9%; Q-statistic significant at <0.01; Table 1). However, when fitting the random effects model, PAs had higher species abundances (Figure 2A; N = 330), assemblage abundances (Figure 2A; N = 297) and assemblage species richness (Figure 2A; N = 234) than land use areas outside PAs (see Table 1).
Table 1. Effect sizes (ES), lower bound (lbCI) and upper bound (ubCI) confidence intervals and sample sizes (N), Tau square (I2), Q statistic (Q) with its p-value (Qp) for at different subgroup designations.
| Description | Subgroup | ES | lbCI | ubCI | N | I2 | Q | Qp |
| Overall | Inside Outside PAs | 0.444 | 0.324 | 0.564 | 861 | 94.9 | 8620.1 | <0.01 |
| Overall | Comparisons insidePAs only | 0.172 | 0.083 | 0.261 | 623 | 46.8 | 1439.9 | <0.01 |
| Overall | PAs with IUCNdesignation | 0.621 | 0.488 | 0.755 | 496 | 92.9 | 3622.3 | <0.01 |
| Overall | PAs with no IUCNdesignation | 0.161 | –0.050 | 0.372 | 365 | 95.9 | 4910.5 | <0.01 |
| Overall | Clusters of PAs | 0.560 | 0.400 | 0.719 | 189 | 86.1 | 980.9 | <0.01 |
| Overall | Unique PAs identified | 0.413 | 0.264 | 0.562 | 672 | 95.8 | 7638.9 | <0.01 |
| Variable | Species abundance | 0.517 | 0.382 | 0.652 | 330 | 87.9 | 1869.6 | <0.01 |
| Variable | Assemblage abundance | 0.349 | 0.083 | 0.615 | 297 | 97.6 | 4997.7 | <0.01 |
| Variable | Assemblage speciesrichness | 0.457 | 0.238 | 0.676 | 234 | 93.2 | 1666.9 | <0.01 |
| Variable | Species abundance(inside) | −0.086 | −0.235 | 0.063 | 295 | 50.2 | 721.4 | <0.01 |
| Variable | Assemblage abundance(inside) | 0.165 | 0.044 | 0.286 | 152 | 9.9 | 270.8 | <0.01 |
| Variable | Assemblage speciesrichness (inside) | 0.529 | 0.364 | 0.694 | 176 | 51.7 | 418.5 | <0.01 |
| Taxon | Mammals | 0.179 | 0.010 | 0.344 | 216 | 88.2 | 1129.6 | <0.01 |
| Taxon | Birds | 0.657 | 0.410 | 0.910 | 332 | 97.2 | 5415.6 | <0.01 |
| Taxon | Herptiles | 0.487 | 0.159 | 0.816 | 55 | 89.6 | 425.4 | <0.01 |
| Taxon | Arthropods | 0.654 | 0.421 | 0.882 | 152 | 64.1 | 406.1 | <0.01 |
| Taxon | Plants | 0.043 | −0.214 | 0.301 | 106 | 95.4 | 1167.8 | <0.01 |
| Continent | Africa | 0.450 | 0.319 | 0.581 | 422 | 92.9 | 3320.0 | <0.01 |
| Continent | Asia | 0.986 | 0.769 | 1.203 | 185 | 71.2 | 571.4 | <0.01 |
| Continent | South America | −0.441 | −0.883 | 0.002 | 152 | 98.2 | 3950.6 | <0.01 |
| Continent | North America | 0.587 | 0.340 | 0.835 | 78 | 84.0 | 387.2 | <0.01 |
| Continent | Australasia | 0.056 | −0.160 | 0.273 | 18 | 4.2 | 16.9 | <0.01 |
| Continent | Europe | 2.544 | 0.362 | 4.726 | 6 | 97.1 | 133.3 | <0.01 |
| IUCN Cat. | IUCN 1 | 0.316 | 0.038 | 0.594 | 35 | 0.0 | 32.1 | 0.560 |
| IUCN Cat. | IUCN 2 | 0.754 | 0.581 | 0.926 | 367 | 94.8 | 3204.3 | <0.01 |
| IUCN Cat. | IUCN 3 | 0.646 | −0.473 | 1.764 | 1 | NA | 0.0 | 0.999 |
| IUCN Cat. | IUCN 4 | −0.099 | −0.222 | 0.025 | 50 | 42.1 | 104.5 | <0.01 |
| IUCN Cat. | IUCN 5 | 1.371 | 0.838 | 1.904 | 27 | 83.8 | 112.6 | <0.01 |
| IUCN Cat. | IUCN 6 | −0.044 | −0.382 | 0.295 | 16 | 0.0 | 6.1 | 0.978 |
| IUCN Cat. | No IUCNDesignation | 0.161 | −0.050 | 0.372 | 365 | 95.9 | 4910.5 | <0.01 |
| Red List | Not Evaluated | 0.596 | 0.234 | 0.957 | 62 | 91.0 | 402.8 | <0.01 |
| Red List | Data deficient | −0.479 | −1.170 | 0.212 | 6 | 85.2 | 32.4 | <0.01 |
| Red List | Least Concern | 0.460 | 0.291 | 0.628 | 168 | 83.4 | 744.8 | <0.01 |
| Red List | Near Threatened | 1.027 | 0.262 | 1.791 | 27 | 95.2 | 323.8 | <0.01 |
| Red List | Vulnerable | 0.814 | 0.333 | 1.294 | 28 | 89.2 | 195.4 | <0.01 |
| Red List | Endangered | 0.557 | 0.266 | 0.848 | 22 | 60.9 | 50.1 | <0.01 |
| Red List | CriticallyEndangered | −0.119 | −0.548 | 0.311 | 6 | 66.3 | 14.5 | 0.013 |
| Small mammals | 0.042 | −0.236 | 0.320 | 25 | 3.4 | 21.0 | 0.637 | |
| Large mammals | 0.372 | 0.131 | 0.613 | 114 | 93.1 | 796.6 | <0.01 |
PA = Protected area. Cat = Category.
Figure 2. Effect sizes and 95% confidence intervals of response variables (A), taxa (B), continent (C) and the Protected Areas IUCN category (D).
Positive boxplot values indicate a net positive impact of protected areas (PAs) on biodiversity. Sample sizes are in grey, the vertical black lines show a zero effect size, while the dashed lines show the overall effect size of 0.444. The effect size for the truncated bar (Europe; Panel C) with large variance due to low sample sizes is 2.54 CI: 0.36–4.73. Abund = Abundance; Spp. Rich. = Species richness; AF = Africa; AS = Asia; SA = South America; NAM = North America; AU = Australasia; EU = Europe. IUCN categories are detailed in Appendix S2.
Effect sizes for PAs with no IUCN category designation were lower than those with a designation, but remained positive and overlapped with the overall effect size and so we included them here (Table 1). Studies that reported across clusters of PAs rather than individual PAs remained positive and were thus included in the overall assessment of effect size (Table 1). When resampling effect sizes to account for pseudoreplication, they remained positive and overlapped with the overall effect size for both inside-outside PAs and inside PA only comparisons, but were less positive for species responses (Table S2). The variance of these resampled effect sizes also increased, but we note that effect size precision increases with additional data (Figure S3 in File S1). Thus, the present results overall can be considered robust to pseudoreplication.
Although variable, the mean effect sizes confirm that on average PAs contain significantly higher numbers of species and more individuals for mammals, birds, herptiles and arthropods, but the effect is non-significant for plants (Figure 2B; Table 1). Small mammals showed a smaller effect size for species abundance (<1 kg; N = 25; 0.042; CI: −0.236–0.320) than did large mammals (>1 kg; N = 114; 0.372; CI: 0.131–0.613). These results suggest that while most species benefit from PA establishment, a suite of them, typically plants, fare better outside PAs in typically anthropogenically transformed habitat.
Protected area efficacy by continent generally showed positive effect sizes, apart from South America (strongly negative and only just non-significant) and Australia (also non-significant; Figure 2C). We note that sample sizes for Europe and Australia are low.
Improved biodiversity outcomes with an increase in IUCN management category would seem an obvious a priori outcome, but such a simple relationship was not clear (Figure 2D). IUCN Category 2 PAs, and to a lesser extent Category 1 PAs, had a high positive effect size. However, although the sample sizes are low, so did IUCN Category 5 PAs that allow much extractive use within their borders. PAs with no IUCN designation, and those of categories 4 and 6 had no significant effect and few data are available on Category 3 PAs.
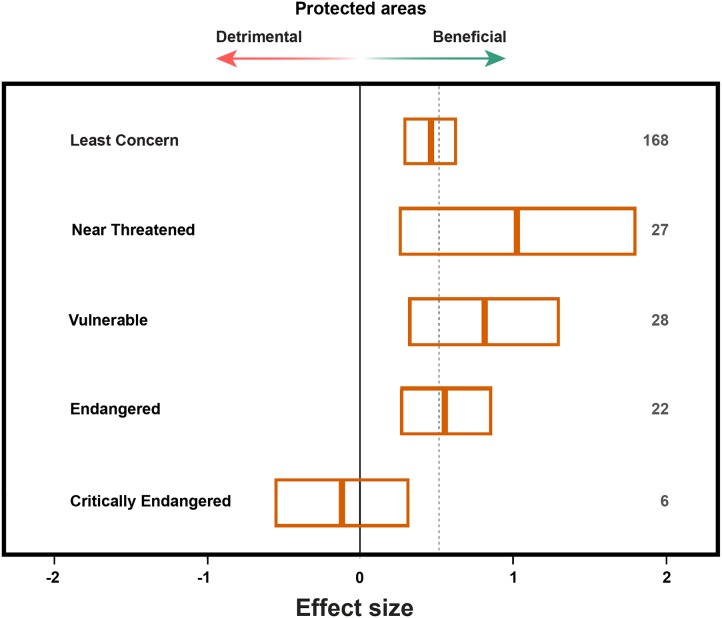
Species listed as Least Concern, Near Threatened, Vulnerable or Endangered by the IUCN generally had greater abundances inside than outside PAs (Figure 3; Table 1). A small sample size of only six observations from one species (Gorilla gorilla) that is Critically Endangered was non-significant. However, at least for species abundance, few data on those species of greatest conservation concern, as measured by their Red List status, are available (Figure 3). Indeed, published studies comparing the abundances of highly threatened species both inside and outside PAs are rare, and many such species do not occur in PAs [18], [41].
Figure 3. Effect sizes and 95% confidence intervals for species abundance responses by their IUCN Red List status.
Positive boxplot values indicate a net positive impact of protected areas on species abundance. Note that multiple responses may be reported for one species (unique species = 168, total cases = 251) and taxonomic uncertainties, Not Evaluated and Data Deficient species are excluded (n = 79). The vertical black line shows a zero effect size while the dashed line indicates the overall effect size for species abundance responses (0.517). Taxa included mammals, birds, herptiles and plants.
The overall effect size from pairwise comparisons inside PAs only (0.172; 95% confidence intervals: 0.083–0.261) was lower than that of the inside and outside comparisons only (their 95% confidence intervals did not overlap). When fitting the random effects model, pristine areas in PAs only, did not have significantly higher species abundances (−0.086; CI: −0.235–0.063; n = 295), but did have significantly higher assemblage abundances (0.165; CI: 0.044–0.286; n = 152) and significantly higher assemblage species richness (0.529; CI: 0.364–0.694; n = 176) than anthropogenically disturbed areas.
The variation explained by the fitted explanatory models was low, with the meta-analytical and socio-economic models each accounting for about 5% and 7%, respectively, of the variation in effect size (Table S3). By contrast, the PA-model accounted for 25% of the variation (Table S3). For all candidate explanatory variables there were multiple competing best-fit models (Table S4). Distance among comparison sites explained only c. 1% of the variation in effect size for studies included in our meta-analysis (Table S5). Indeed, despite being significant the relationship between effect size and the greatest distance between comparison sites was weak (Pearson’s r = 0.146; p<0.001) as was the relationship with distance to PA boundary (r = 0.085; p<0.05).
Discussion
An initial assessment of global PA efficacy should be to determine if differences exist in biodiversity between PAs and unprotected land in a direction demonstrating higher biodiversity values in the former. Most of the studies included here did indeed find higher species abundances, assemblage abundance, and species richness inside PAs compared to areas outside them. This pattern holds across taxonomic groups (although non-significant for plants) and continents (although non-significant for South America and Australia).
What mechanisms underlie the non-significant effects for plants and for South America and Australia are not entirely obvious. Clearly, habitat change has major effects on plants, with many studies documenting replacement of particular species and changes in habitat structure [2], [24], [42], [43]. Likewise there has been a growing focus on the scarcity of large old trees [44]. The plant studies analysed here included 53 pairwise comparisons of species richness, 41 of assemblage abundance and 12 of species abundances. All of the 12 species abundance studies were concerned with trees. This balance of investigations to date may account for the lack of an effect, and may rather reflect the globally emerging fingerprint for biodiversity loss under global change, where community turnover, but not necessarily species richness change is observed [43]. In the case of Australia, the small sample size may be driving the absence of an effect size. Alternatively, most data from Australia (84%; 16/19) came from only one study that documented the recovery of small mammal populations after their isolation from invasive predators [45], and so it is not clear to what extent this outcome reflects general patterns on the continent. For South America the situation is also difficult to explain. The taxon with the most data for South America was the mammals (53%; 81/152), many of them large (>1 kg; 54%; N = 44), and their increase outside PAs contributes to the non-significant outcome. One explanation may be hunting pressure. Carrilo et al. [46] found that increasing hunting pressure inside a PA diminished large mammal populations in Costa Rica. In contrast to our general finding, Negroes et al. [47] found that private forest reserves in Brazil were responsible for conserving medium to large-sized vertebrates, more so than PAs. While primary forest is globally irreplaceable for conserving biodiversity [24], land use areas under low extraction, or regenerating forests, seem to contribute to a degree to an integrated landscape level conservation strategy, which may be particularly true in South America [48]. Furthermore, in a comparable meta-analysis, Gibson et al. [24] found that primary forests in South America retain more biodiversity than transformed forests, but since they did not focus on PAs exclusively, they had a larger sample size (N = 909). This finding emphasises that greater sample sizes could increase a positive effect size signal between transformed and more natural areas, a finding that our data corroborate (Figure S3 in File S1).
Despite effect sizes generally being positive, PA efficacy clearly varies considerably amongst PAs, species and local contexts, as demonstrated here and in region-specific studies [5], [8], [17], [21]. Determining the mechanisms driving the pattern of higher species abundances, assemblage abundance, and species richness inside compared to outside PAs remains challenging. Using GLMs we sought to explore a range of proximate factors that might explain this variation. We included explanatory variables such as spatial structure, socio-economic conditions, and structural attributes of PAs, which have been shown elsewhere to have effects on biodiversity values [4], [6], [49]–[55]. These assessments provided some insight, but did not account for much of the variation in effect sizes found here. Since comparisons inside and outside PAs in our database were mainly made at the local scale, the geographic context explained little of the observed effect size variation. In consequence, the extent of site matching, which may play a role in increasing estimates of PA efficacy outcomes in some cases [56], may be less important in our database comparing biodiversity features themselves, rather than proxies such as deforestation (but see [56]). Socio-economic factors influence conservation outcomes in some regions [50]–[52], but here also fared relatively poorly at explaining variation. Likewise structural variables such as PA size and location explained little of the variation. Some of these variables are important drivers of biodiversity variation generally, such as area and latitudinal position (mostly via energy availability) [57], [58], [59]. However, they have much less of an influence per se on differences in diversity inside and outside PAs. Such an outcome suggests that PA efficacy itself is invariate, at least with regard to these variables. In other words the largest effect is of the PAs themselves, as we found here. Our meta-analysis of studies which compare pristine and transformed areas within PAs themselves bears out this suggestion. The pristine areas typically have higher biodiversity values than the transformed areas. Moreover, the effect size here is weaker than the effect size found when comparisons are between sites inside and outside PAs. Together with evidence that pristine forests retain biodiversity features to a greater degree than transformed areas [24], these outcomes suggest that PAs must offset negative anthropogenic influences to a greater degree than no PA designation.
Nonetheless, additional variation may be attributable to other factors such as characteristics of the organisms themselves. For mammals at least, we were able to show that larger species in particular are reduced outside PAs, in keeping with other evidence that smaller mammals are typically better able to tolerate conditions outside PAs [8], [21], [53], [60]. Others have also highlighted the contrasting responses in biodiversity documented by assessments among PAs, but comparing different taxonomic groups [8], [21]. Interspecific variability in population responses to landscape change is well-known (e.g. [61]), and some taxa obviously fare better in transformed landscapes, even within PAs. Whether this is the case for species that have a high threat status is more difficult to discern given that information for such species is so scarce [41]. Unfortunately, the scope of our data did not enable us to pursue in more detail possible factors underlying effect size variation among continents and other taxa, largely because of the risk of misleading subgroup effects [25], [27]. A range of factors could explain the variation we found given substantial life history differences among these taxa (e.g. dispersal distances, life span, migration propensity, tolerance of disturbance [62]–[64]), and considerable differences in the evolutionary history of species on continents [59], [65].
Similarly, the relationship between the designated management status of a PA and effective conservation of its biodiversity features also seems to be unclear. This outcome provides further evidence for calls that the IUCN PA management categories should be reassessed to reflect biodiversity outcomes rather than management objectives (see [66]). Thus, challenges for conservation science include determining which mechanisms drive positive PA efficacy, under which conditions PAs fail species with differential responses to threats, and concomitantly, IUCN threat designations, and how management categories and biodiversity outcomes can best be aligned.
In addition to these proximate mechanisms that might influence effect size, several ultimate mechanisms may also be important. These include (i) the observation that PAs are non-randomly placed, typically biased towards areas of inaccessibility, which in itself would reduce threatening anthropogenic processes [54]; (ii) the persistence of existing differences in abundance and richness between the areas at the time of PA designations, as a result of the choice of location [18]; (iii) that lower levels of threatening processes, such as habitat alteration or exploitation, have prevailed inside PAs than elsewhere; (iv) that active management of PAs has maintained or increased abundance and richness relative to outside PAs [18]; and/or (v) leakage effects, where threatening processes are displaced from PAs to surrounding areas [55].
Given that data on biodiversity condition before and after establishment of PAs is so rare, studies have understandably had to focus on some comparison of PAs to other areas that may embody likely outcomes if the area had not received PA designation. However, the effect size as calculated here can inherently not explicitly consider the counterfactual (the biodiversity outcome that would have occurred if there had been no PA designation, see [18], [56], [67]). Site matching approaches attempt to address this bias arising when observable biophysical and socioeconomic factors may affect biodiversity in addition to PA designation [56], [67]. While these studies may detect a weaker signal for the influence of PA designation itself, they still typically focus on proxies of biodiversity to determine performance outcomes, such as deforestation offsets. Although the irreplaceability of primary forests for biodiversity has been established [24], measures of deforestation cannot characterize changes in species richness and abundance itself. As a consequence, the vast majority of published studies on biodiversity have taken a broader view of the counterfactual, and made what for the investigators seemed a priori sensible comparisons between PAs and other areas that they held to embody likely outcomes if the former had not received designation. However, due to the way the original studies were designed, the dataset developed here cannot be analysed with site matching approaches. Thus some concern might remain that the observed effects have been influenced by any one or many of the mechanisms above. Nonetheless, the ultimate mechanisms that drive patterns of higher biodiversity retention within PAs are clearly far from universal, and their geographic distribution and intensity is poorly known [18], [67]. Therefore, a comprehensive assessment of PA effectiveness can benefit from assessments that consider the net outcomes of both observed effects (as is the case here) in addition to those approaches that can better quantify assessments of effects of bias (i.e. site matching approaches [56] or experimental designs [20]).
In conclusion, despite the ultimate mechanisms underlying our findings not being firmly established, and much variation in effect sizes across regions and among taxa remaining unexplained, a signal is clear: PAs have positive biodiversity values compared with alternative land uses. In consequence, our results, together with emerging qualitative evidence [9], suggest that in general PA establishment itself may confer a net benefit to biodiversity. Thus, our approach provides a quantitative demonstration of the value of PAs as an effective strategy for conserving biodiversity. In other words, studies undertaken at a local scale to date clearly indicate that an ecological foundation exists from which the economic, political and social benefits of PAs are being realized [68]. This outcome provides evidence in support of the value of the Aichi Biodiversity Target 11 of the Convention on Biological Diversity’s (CBD) new Strategic Plan for Biodiversity [69], the nationally agreed goals to fulfil signatory countries commitments under the CBD [69]. Target 11 calls for the expansion and effective management of PAs. The outcomes of our analyses show why this Target is worth achieving.
Supporting Information
Contains the following files: Table S1. Orwin’s fail safe N is 1238 to reach an overall effect size of 0.222. Table S2. Effect sizes determined by resampling one pairwise comparison per unit of study, per species, per country and per protected area (PA), to assess the potential spatial pseudoreplication in our dataset arising from multiple responses. Table S3. Best GLM models by exhaustive fit for the Meta Analysis model, Protected Areas (PA) model and Socio-Economic model. Table S4. Proportion of five highest ranked models for Meta Analysis model, PA model and the Socio-Economic model. Table S5. Best GLM model by exhaustive fit for two variables, the maximum distance to protected area boundary within studies, and the maximum distance between pair wise comparisons within studies. Figure S1. PRISMA flow diagram, depicting the flow of information through different phases of the search process conducted. Figure S2. Funnel plot of effect size standard error plotted against effect size for all inside-outside pairwise comparisons. Figure S3. Cumulative meta-analysis of the dataset sorted by precision, with effect sizes and 95% confidence intervals (n = 861). Appendix S1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist. Appendix S2. Detailed descriptions of IUCN protected area management categories.
(DOCX)
Complete dataset and references included in the meta-analysis.
(XLSX)
Acknowledgments
Comments by Melodie A. McGeoch, Richard A. Fuller and anonymous reviewers improved the manuscript. This paper is dedicated to Cor J.S. Coetzee.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the National Research Foundation of South Africa, Stellenbosch University, and by a Harry Crossley grant to BWTC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jenkins CN, Joppa L (2009) Expansion of the global terrestrial protected area system. Biol Conserv 142: 2166–2174. [Google Scholar]
- 2. Ellis EC, Ramankutty N (2008) Putting people in the map: anthropogenic biomes of the world. Front Ecol Environ 6: 439–447. [Google Scholar]
- 3.Bertzky B, Corrigan C, Kemsey J, Kenney S, Ravilious C, et al.. (2012) Protected Planet Report 2012: Tracking progress towards global targets for protected areas. UNEP-WCMC, Cambridge. Available: http://www.unep-wcmc.org/ppr2012_903.html. Accessed February 2014.
- 4. Rodrigues ASL, Andelman SJ, Bakarr MI, Boitani L, Brooks TM, et al. (2004) Effectiveness of the global protected area network in representing species diversity. Nature 428: 640–643. [DOI] [PubMed] [Google Scholar]
- 5. Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, et al. (2010) Large mammal population declines in Africa’s protected areas. Biol Conserv 143: 2221–2228. [Google Scholar]
- 6. Fuller RA, McDonald-Madden E, Wilson KA, Carwardine J, Grantham HS, et al. (2010) Replacing underperforming protected areas achieves better conservation outcomes. Nature 466: 365–367. [DOI] [PubMed] [Google Scholar]
- 7.Leverington F, Hockings M, Paveses H, Costa K, Courrau J (2010) Management effectiveness evaluation in protected areas - a global study. The University of Queensland, Australia. Available: http://www.wdpa.org/me/PDF/global_study_2nd_edition.pdf. Accessed February 2014.
- 8. Laurance WF, Useche DC, Rendeiro J, Kalka M, Bradshaw CJA, et al. (2012) Averting biodiversity collapse in tropical forest protected areas. Nature 489: 290–294. [DOI] [PubMed] [Google Scholar]
- 9. Geldmann J, Barnes M, Coad L, Craigie ID, Hockings M, et al. (2013) Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol Conserv 61: 230–238. [Google Scholar]
- 10. Balmford A, Bruner A, Cooper P, Costanza R, Farber S, et al. (2002) Economic reasons for conserving wild nature. Science 297: 950–953. [DOI] [PubMed] [Google Scholar]
- 11. Defries R, Hansen A, Newton AC, Hansen MC (2005) Increasing isolation of protected areas in tropical forests over the past twenty years. Ecol Appl 15: 19–26. [Google Scholar]
- 12. Joppa LN, Loarie SR, Pimm SL (2008) On the protection of “protected areas.”. Proc Natl Acad Sci USA 105: 6673–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagendra H (2008) Do parks work? Impact of protected areas on land cover clearing. Ambio 37: 330–337. [DOI] [PubMed] [Google Scholar]
- 14. Greve M, Chown SL, van Rensburg BJ, Dallimer M, Gaston KJ (2011) The ecological effectiveness of protected areas: a case study for South African birds. Anim Cons 14: 295–305. [Google Scholar]
- 15. Butchart SHM, Walpole M, Collen B, van Strien A, Scharlemann JPW, et al. (2010) Global biodiversity: indicators of recent declines. Science 328: 1164–1168. [DOI] [PubMed] [Google Scholar]
- 16. Chown SL, Lee JE, Hughes KA, Barnes J, Barrett PJ, et al. (2012) Challenges to the future conservation of the Antarctic. Science 337: 158–159. [DOI] [PubMed] [Google Scholar]
- 17. Sutherland WJ, Pullin AS, Dolman PM, Knight TM (2004) The need for evidence-based conservation. Trends Ecol Evol 19: 4–7. [DOI] [PubMed] [Google Scholar]
- 18. Gaston KJ, Jackson SF, Cantú-Salazar G, Cruz-Piñón G (2008) The ecological performance of protected areas. Annu Rev Ecol Syst 39: 93–113. [Google Scholar]
- 19. Ferraro PJ, Pattanayak SK (2006) Money for nothing? A call for empirical evaluation of biodiversity conservation investments. PLoS Biol 4: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laurance WF, Camargo JLC, Luizão RCC, Laurance SG, Pimm SL, et al. (2011) The fate of Amazonian forest fragments: a 32-year investigation. Biol Cons 144: 56–67. [Google Scholar]
- 21. Gardner TA, Caro T, Fitzherbert EB, Banda T, Lalbhai P (2007) Conservation value of multiple-use areas in East Africa. Cons Biol 21: 1516–2155. [DOI] [PubMed] [Google Scholar]
- 22. Caro TM, Gardner TA, Stoner C, Fitzherbert E, Davenport TRB (2009) Assessing the effectiveness of protected areas: paradoxes call for pluralism in evaluating conservation performance. Divers Distrib 15: 178–182. [Google Scholar]
- 23.Magurran AE, McGill BJ (2011) Biological diversity: Frontiers in measurement and assessment. Oxford University Press, Oxford. 345 p.
- 24. Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478: 378–381. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions. Version 5.0.2 [updated September 2009]. Available: http://www.cochrane.org/sites/default/files/uploads/Handbook4.2.6Sep2006.pdf. Accessed February 2014.
- 26. Gaston KJ, Blackburn T, Gregory RD (1999) Does variation in census area confound density comparisons? J Appl Ecol 36: 191–204. [Google Scholar]
- 27.Koricheva J, Gurevitch J, Mengersen K (2013) Handbook of meta-analysis in ecology and evolution. Princeton University Press, New Jersey, 498 p. [Google Scholar]
- 28.Rohatgi A (2014) WebPlotDigitizer v2.4. Available: http://arohatgi.info/WebPlotDigitizer. Accessed February 2014.
- 29.Schmid CH, Stewart GB, Rothstein HR, Lajeunesse MJ, Gurevitch J (2013) Software for statistical meta-analyis. In: Eds Koricheva J, Gurevitch J, Mengersen K (2013) Handbook of meta-analysis in ecology and evolution. Princeton University Press, New Jersey, 498 p. [Google Scholar]
- 30.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to meta-analysis. Wiley-Blackwell, UK. 450 p. [Google Scholar]
- 31. Viechtbauer W (2012) Conducting meta-analyses in R with the metafor package. J Stat Soft 3: 1–48. [Google Scholar]
- 32.R Foundation (2014) R: A language and environment for statistical computing. Available: http://www.R-project.org. Accessed February 2014.
- 33. Gaston KJ, Charman K, Jackson SF, Armsworth PR, Bonn A, et al. (2006) The ecological effectiveness of protected areas: the United Kingdom. Biol Conserv 132: 76–87. [Google Scholar]
- 34.IUCN UNEP (2014) The world database on protected areas. Available: http://www.protectedplanet.net. Accessed February 2014.
- 35.IUCN (2014) The IUCN Red List of threatened species. Available: www.iucnredlist.org. Accessed February 2014.
- 36.Burnham K, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer-Verlag, New York. 520 p. [Google Scholar]
- 37.World Governance Index (2014) Available: www.govindicators.org. Accessed February 2014.
- 38.CIA (2012). The World Factbook Available: https://www.cia.gov/library/publications/the-world-factbook/index.html. Accessed February 2014.
- 39.Bolker BM (2008) Ecological models and data in R. Princeton University Press, New Jersey. 396 p. [Google Scholar]
- 40. Calcagno V, Mazancourt C (2010) glmulti: An R package for easy automated model selection with (Generalized) Linear Models. J Stat Soft 34: 1–29. [Google Scholar]
- 41. Ricketts TH, Dinerstein E, Boucher T, Brooks TM, Butchart SHM, et al. (2005) Pinpointing and preventing imminent extinctions. Proc Natl Acad Sci USA 102: 18497–18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foley JA, Defries R, Asner GP, Barford C, Bonan G, et al. (2005) Global consequences of land use. Science 309: 570–574. [DOI] [PubMed] [Google Scholar]
- 43. Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, et al. (2014) Assemblage time series reveal biodiversity change but not systematic loss. Science 344: 296–299. [DOI] [PubMed] [Google Scholar]
- 44. Lindenmayer D, Laurance W, Franklin J (2012) Global decline in large old trees. Science 338: 1305–1306. [DOI] [PubMed] [Google Scholar]
- 45. Moseby KE, Hill BM, Read JL (2009) Arid recovery - a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia. Austral Ecol 34: 156–169. [Google Scholar]
- 46. Carrillo E, Wong G, Cuarón AD (2000) Monitoring mammal populations in Costa Rican protected areas under different hunting restrictions. Conserv Biol 14: 1580–1591. [DOI] [PubMed] [Google Scholar]
- 47. Negrões N, Revilla E, Fonseca C, Soares AMVM, Jácomo ATA, et al. (2011) Private forest reserves can aid in preserving the community of medium and large-sized vertebrates in the Amazon arc of deforestation. Biodivers Conserv 20: 505–518. [Google Scholar]
- 48. Barlow J, Gardner TA, Araujo IS, Ávila-Pires TC, Bonaldo AB, et al. (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci USA 104: 18555–18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomson JR, Weiblan G, Thomson BA, Alfaro S, Legendre P (1996) Untangling multiple factors in spatial distributions: lilies, gophers and rocks. Ecology 77: 1698–1715. [Google Scholar]
- 50. Reyers B, van Jaarsveld AS, McGeoch MA, James AN (1998) National biodiversity risk assessment: a composite multivariate and index approach. Biodivers Conserv 7: 945–965. [Google Scholar]
- 51. Veech JA (2003) Incorporating socioeconomic factors into the analysis of biodiversity hotspots. Appl Geog 23: 73–88. [Google Scholar]
- 52. Balmford A, Moore JL, Brooks TM, Burgess N, Hansen LA, et al. (2001) Conservation conflicts across Africa. Science 291: 2616–2619. [DOI] [PubMed] [Google Scholar]
- 53. Woodroffe R, Ginsberg JA (1998) Edge effects and the extinction of populations inside protected areas. Science 280: 2126–2128. [DOI] [PubMed] [Google Scholar]
- 54. Joppa LN, Pfaff A (2009) High and far: biases in the location of protected areas. PLoS ONE 4: e8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ewers RM, Rodrigues ASL (2008) Estimates of reserve effectiveness are confounded by leakage. Trends Ecol Evol 23: 113–116. [DOI] [PubMed] [Google Scholar]
- 56. Andam KS, Ferraro PJ, Pfaff A, Sanchez-Azofeifa GA, Robalino JA (2008) Measuring the effectiveness of protected area networks in reducing deforestation. Proc Natl Acad Sci USA 105: 16089–16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press, Cambridge.
- 58. Gaston KJ (2000) Global patterns in biodiversity. Nature 405: 220–227. [DOI] [PubMed] [Google Scholar]
- 59. Jetz W, Fine PVA (2012) Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol 10: e1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peres CA (2004) Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conserv Biol 14: 240–253. [Google Scholar]
- 61. Henle K, Davies KF, Kleyer M, Margules CR, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13: 207–251. [Google Scholar]
- 62. Nicolakakis N, Sol D, Lefebvre L (2003) Behavioural flexibility in birds predicts species richness, but not extinction risk. Anim Behav 65: 445–452. [Google Scholar]
- 63. Hays GC, Scott R (2013) Global patterns for upper ceilings on migration distance in sea turtles and comparisons with fish, birds and mammals. Func Ecol 27: 748–756. [Google Scholar]
- 64. Di Marco M, Buchanan GM, Szantoi Z, Holmgren M, Grottolo Marasini G, et al. (2014) Drivers of extinction risk in African mammals: the interplay of distribution state, human pressure, conservation response and species biology. Proc R Soc B 369: 20130198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hawkins BA, McCain CM, Davies TJ, Buckley LB, Anacker BL, et al. (2012) Different evolutionary histories underlie congruent species richness gradients of birds and mammals. J Biogeogr 39: 825–841. [Google Scholar]
- 66. Boitani L, Cowling RM, Dublin HT, Mace GM, Parrish J (2008) Change the IUCN protected area categories to reflect biodiversity outcomes. PLoS Biol 6: 436–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joppa LN, Pfaff A (2010) Global protected area impacts. Proc R Soc B 278: 1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stolton S, Dudley N (2010) Arguments for protected areas: multiple benefits for conservation and use. Routledge, London. 296 p. [Google Scholar]
- 69.CBD (2012) Convention on Biological Diversity: Aichi Biodiversity Targets. Available: http://www.cbd.int/sp/targets/. Accessed February 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains the following files: Table S1. Orwin’s fail safe N is 1238 to reach an overall effect size of 0.222. Table S2. Effect sizes determined by resampling one pairwise comparison per unit of study, per species, per country and per protected area (PA), to assess the potential spatial pseudoreplication in our dataset arising from multiple responses. Table S3. Best GLM models by exhaustive fit for the Meta Analysis model, Protected Areas (PA) model and Socio-Economic model. Table S4. Proportion of five highest ranked models for Meta Analysis model, PA model and the Socio-Economic model. Table S5. Best GLM model by exhaustive fit for two variables, the maximum distance to protected area boundary within studies, and the maximum distance between pair wise comparisons within studies. Figure S1. PRISMA flow diagram, depicting the flow of information through different phases of the search process conducted. Figure S2. Funnel plot of effect size standard error plotted against effect size for all inside-outside pairwise comparisons. Figure S3. Cumulative meta-analysis of the dataset sorted by precision, with effect sizes and 95% confidence intervals (n = 861). Appendix S1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist. Appendix S2. Detailed descriptions of IUCN protected area management categories.
(DOCX)
Complete dataset and references included in the meta-analysis.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.