Abstract
We tested whether the thecosome pteropod Limacina helicina from Puget Sound, an urbanized estuary in the northwest continental US, experiences shell dissolution and altered mortality rates when exposed to the high CO2, low aragonite saturation state (Ωa) conditions that occur in Puget Sound and the northeast Pacific Ocean. Five, week-long experiments were conducted in which we incubated pteropods collected from Puget Sound in four carbon chemistry conditions: current summer surface (∼460–500 µatm CO2, Ωa≈1.59), current deep water or surface conditions during upwelling (∼760 and ∼1600–1700 µatm CO2, Ωa≈1.17 and 0.56), and future deep water or surface conditions during upwelling (∼2800–3400 µatm CO2, Ωa≈0.28). We measured shell condition using a scoring regime of five shell characteristics that capture different aspects of shell dissolution. We characterized carbon chemistry conditions in statistical analyses with Ωa, and conducted analyses considering Ωa both as a continuous dataset and as discrete treatments. Shell dissolution increased linearly as aragonite saturation state decreased. Discrete treatment comparisons indicate that shell dissolution was greater in undersaturated treatments compared to oversaturated treatments. Survival increased linearly with aragonite saturation state, though discrete treatment comparisons indicated that survival was similar in all but the lowest saturation state treatment. These results indicate that, under starvation conditions, pteropod survival may not be greatly affected by current and expected near-future aragonite saturation state in the NE Pacific, but shell dissolution may. Given that subsurface waters in Puget Sound’s main basin are undersaturated with respect to aragonite in the winter and can be undersaturated in the summer, the condition and persistence of the species in this estuary warrants further study.
Introduction
Global oceans have absorbed about a third of the anthropogenically released carbon dioxide [1], [2], [3]. The accumulation of carbon dioxide in seawater has lowered average global ocean pH from 8.2 to 8.1 and decreased the concentration of carbonate ions [1]. Projections of carbon dioxide emissions indicate that by year 2100, surface ocean pH will be 0.3–0.4 units lower than today, and carbonate ion concentration will decrease by about 50% [4], [5], [6], [7]. Changes in the carbonate ion concentration of seawater can affect how readily calcium carbonate structures accrete or dissolve, a property reflected in seawater saturation state, Ω. Once the carbonate ion concentration is low enough (Ω<1), dissolution of calcium carbonate structures is favored.
Ocean acidification can affect a variety of physiological processes in marine organisms, from photosynthesis to neuronal signaling [8], [9]. Although there is much variation in response to ocean acidification among marine species, in general, laboratory studies indicate that ocean acidification has negative effects on survival, calcification, growth, and reproduction; these effects are larger for calcifying species than for non-calcifiers [10]. Differential response of marine species to ocean acidification has the potential to change the structure of marine communities, as has been observed in natural experiments, modeling exercises, and Earth’s history [11], [12], [13], [14].
Pteropods are small, holoplanktonic marine snails with aragonitic calcium carbonate shells, and are important prey species in many marine food webs [15], [16], [17], [18]. All available research indicates that growth rate and calcification of shelled pteropods decline with ocean acidification [19], [20], [21], [22], [23]. Pteropods can build shell when seawater is undersaturated with respect to aragonite, but their shells also experience dissolution [20], [21], [22], [23], [24], [25], [26]. Pteropod mortality may increase as pCO2 increases, but studies done to date, only in high-latitude regions, have yielded uncertain and contradictory results [22], [23], [24]. Over the past few decades, the abundance of subarctic pteropods (Limacina spp.) in the North Pacific off of Canada’s Vancouver Island has declined in 3 of 4 locations, while abundance of subtropical planktivorous pteropods (Clio spp.) and predatory pteropods (Clione spp.) has increased offshore of the southern part of Vancouver Island and remained unchanged elsewhere in the region [27].
L. helicina is distributed throughout polar and some subpolars seas, and is the most abundant pteropod species in marine waters of the US Pacific Northwest. L. helicina uses a mucus web to gather food, and, in Arctic waters, the species is an omnivore in autumn and winter and an herbivore in spring and summer [28]. In North Pacific waters, pteropods are prey for fishes such as anchovy (Engraulis mordax), herring (Clupea pallasii), jack mackerel (Trachurus symmetricus), sablefish (Anoplopoma fimbria), and pink, coho, chum, and sockeye salmon (Oncorhynchus gorbuscha, O. kisutch, O. keta, O. nerka) [15], [16], [17], [18]. Pteropods are also eaten by other zooplankton, squid, whales, and birds. Many pteropod populations undergo diel vertical migration, feeding in surface waters at night and retreating to deeper waters during the day to avoid predation. The diel migration for L. helicina is shallower in the Pacific Northwest region (100 m) [27] than other parts of the species’ range (150–250 m) [29]. L. helicina forms a delicate aragonitic shell with a periostracum covering [30], and the species is globally a major contributor of inorganic and organic carbon fluxes to the deep ocean [31], [32]. In Arctic waters, it has a 1-yr life-cycle [28]. Natural history information on North Pacific L. helicina is scant.
Due to natural oceanographic processes, aragonite saturation horizons in North Pacific waters are some of the shallowest in the world [5], [33], [34], [35]. Changes in ocean circulation in the California Current have compounded recent declines in aragonite saturation state [36]. Large-scale, coastal upwelling occurs each spring and summer in the California Current System. Upwelled waters are naturally high in both nutrients and CO2, and ocean acidification adds to the CO2 load of these waters [34]. Global climate change may increase upwelling favorable winds in this region [37]. Upwelling has intensified in the southern California Current System in recent decades [38]. Recent observations of the California Current found that ocean acidification has changed ocean chemistry enough that waters undersaturated with respect to aragonite now reach the surface in some locations when upwelling occurs [34]. Without ocean acidification, undersaturated waters would be 50 m deeper than they are currently [34]. Models project that by 2050, large sections of nearshore (0–10 km from coast) water from 0–60 m will be undersaturated with respect to aragonite during the entire summer upwelling season and more than half of the nearshore water mass will be undersaturated throughout the year [39]. Thus, upper, nearshore waters of the California Current System are likely to develop undersaturated conditions at a similar timeframe to the Southern Ocean and potentially faster than surface Arctic waters [39].
Low pH water from the California Current feeds into Puget Sound, a fjordal estuary in the northwestern continental US. Once in Puget Sound, ocean carbon chemistry is influenced by low-pH, low-alkalinity freshwater inputs; nutrient run-off caused by agriculture and urban development and the influence of these nutrients on biological activity; and local oceanographic processes that lead to stratification and restrictions in flow [40]. Because of these inputs and processes, Puget Sound waters have low pH and aragonite saturation states. Since the Industrial Revolution, ocean acidification in Puget Sound has caused a decrease in pH of 0.05–0.15 units and surface aragonite saturation state of 0.09–0.33 [40]. In Puget Sound’s main basin in the summer, surface waters (depth<8 m) are supersaturated with respect to aragonite (Ωa = 1.01–2.79), but waters below 50 m range between under- and supersaturated (Ωa = 0.86–1.35) [40]. In the winter, the entire water column in the main basin is undersaturated with respect to aragonite (Ωa = 0.79–0.95) [40].
We test whether the vertically migrating, thecosome pteropod Limacina helicina collected in Puget Sound is sensitive to levels of pCO2 found currently in the estuary and expected more frequently in the future [41]. Such conditions are also representative of current and future upwelling events in the North Pacific. This is the first study to test the sensitivity of temperate North Pacific L. helicina to different current pCO2 levels and those expected with ocean acidification. By evaluating their sensitivity to carbon chemistry conditions, it is also a first step towards understanding how North Pacific pteropods persist in the corrosive conditions that typify the northern California Current. Adaptation to different environmental conditions has been documented previously between populations of pteropods [42], and differential sensitivity to ocean acidification has also been found within other invertebrate species and among pteropod species [43], [44], [45]. We test the hypothesis that shell condition is linked directly to aragonite saturation state, and expect dissolution only in the treatments where Ωa<1. Due to uncertainties in the relationship between survival and carbon chemistry conditions from prior research [22], [23], [24], we expect that L. helicina survival will not be affected by carbon chemistry treatment in laboratory conditions.
Materials and Methods
Pteropods used in this study were collected from Puget Sound using collection permits from the Washington Department of Fish and Wildlife. No specific requirements for planktonic molluscs are included in national animal care guidelines.
We conducted five experiments on L. helicina in the summer of 2012, two in May and three in July. We collected adult pteropods (mean shell diameter = 1.94 mm, standard deviation = 0.25 mm, N = 68 individuals) from Port Susan in Puget Sound, Washington (48.1° N, 122.4° W), using 1 m-diameter ring nets with mesh sizes of 335, 580, or 2000 µm towed obliquely at 2–3 knots between 5–40 m depth. Collecting trips occurred from twilight to early night. Pteropods were transported to the NOAA Northwest Fisheries Science Center in a cooler filled with aerated seawater. For the May experiments, pteropods were kept in the cooler overnight. For the July experiments, pteropods were placed into 4.5-L PET plastic jars immediately upon arrival in the lab, and those jars were connected to a recirculating seawater system with ∼460–500 µatm CO2. The morning following collection, we haphazardly distributed swimming or otherwise active pteropods into 4.5-L PET plastic jars at densities of 33–40 pteropods per jar, depending on the experiment (Table 1). This density is higher than reported L. helicina density in the wild [27], [46], [47], but lower than the maximum density reported for the congener L. retroversa [48]. Compared to other studies that explore the impact of ocean acidification on pteropod survival, density was similar to that used in one study [24] and 2.5 to 20 times less than the two others [22], [23]. Each jar acted as a replicate, and replicates were spread equally across all treatments in a given experiment (Table 1). We gradually increased dissolved CO2 from ∼460–500 µatm to treatment conditions in 2 h, which resembles the rapid drop in pH and aragonite saturation state that coastal organisms experience when they vertically migrate downward or upwelled waters invade the upper ocean [35]. We did not feed the pteropods due to the technical difficulties of developing proper feeding conditions for pteropods in laboratory settings, especially in jars with water flow.
Table 1. Treatment conditions during experiments.
| Exp. | Dates | Nreplicates | Njar | Nshells | Salinity | Temp. | System pH | Jar pH | pCO2 * | Ωa * | TA | DIC | |
| (psu) | (°C) | Durafet | Spec | Spec | (µatm) | (µmol/kg) | (µmol/kg) | ||||||
| 1 | 5/10–5/16 | 3 | 40 | 35 | 31.5 | 12.12±0.15 | 8.01±0.02 | 7.97 | – | 491 | 1.7 | 2239.6 | 2092.9 |
| 42 | 31.5 | 12.13±0.18 | 7.49±0.01 | 7.50 | – | 1576 | 0.62 | 2235.3 | 2236.5 | ||||
| 2 | 5/17–5/23 | 4 | 40 | 23 | 31.8 | 12.04±0.07 | 7.98±0.05 | 7.95±0.03 | 7.92 | 502±34 | 1.64±0.10 | 2237.8 | 2076.3 |
| 33 | 31.8 | 12.04±0.06 | 7.76±0.00 | 7.79±0.02 | 7.81 | 766±40 | 1.17±0.06 | 2239.2 | 2140.2 | ||||
| 23 | 31.8 | 12.05±0.07 | 7.48±0.01 | 7.46±0.03 | – | 1725±122 | 0.58±0.04 | 2230.5 | 2246.7 | ||||
| 3 | 6/29–7/5 | 4 | 33 | 46 | 31.9 | 12.08±0.08 | 8.02±0.00 | 7.97 | 7.99 | 462 | 1.50 | 2092.1 | 1940.5 |
| 26 | 31.9 | 12.07±0.08 | 7.47±0.01 | 7.46 | 7.46 | 1614 | 0.50 | 2085.6 | 2080.5 | ||||
| 4 | 31.9 | 12.09±0.09 | 7.19±0.01 | 7.22 | 7.21 | 2815 | 0.30 | 2084.5 | 2147.0 | ||||
| 4 | 7/3–7/9 | 2 | 39 | 13 | 29.6 | 12.09±0.09 | 8.02±0.00 | 7.98±0.00 | 7.97 | 460±3 | 1.51±0.01 | 2101.5 | 1943.6 |
| 14 | 29.6 | 12.08±0.07 | 7.47±0.00 | 7.47±0.02 | 7.47 | 1579±50 | 0.52±0.02 | 2097.4 | 2093.4 | ||||
| 0** | 29.6 | 12.11±0.12 | 7.19±0.01 | 7.19±0.05 | 7.12 | 3057±342 | 0.28±0.03 | 2092.2 | 2165.3 | ||||
| 5 | 7/6–7/12 | 3 | 40 | 24 | 29.7 | 12.12±0.13 | 8.02±0.00 | 7.97±0.00 | 7.96 | 460±4 | 1.53±0.01 | 2114.3 | 1956.6 |
| 21 | 29.7 | 12.09±0.09 | 7.47±0.00 | 7.47±0.02 | 7.46 | 1585±59 | 0.53±0.01 | 2108.1 | 2100.7 | ||||
| 7 | 29.7 | 12.12±0.12 | 7.18±0.01 | 7.14±0.02 | 7.15 | 3421±174 | 0.25±0.01 | 2101.7 | 2211.1 | ||||
Exp. stands for experiment, temp. for temperature, spec. for spectrophotometer, TA for total alkalinity, and DIC for dissolved inorganic carbon. Nreplicates is the number of replicate jars in each treatment, Njar is the number of pteropods per jar, and Nshells is the number of shells in a treatment scored for shell condition.
* Ωa and pCO2 values calculated from DIC and pH measured via spectrophotometry.
** No pteropods survived this treatment in experiment 4.
We chose the experimental treatments based on pCO2 conditions observed in Puget Sound [40] and projected for the future. The ∼460–500 µatm pCO2 treatment represents summer surface conditions. Because of the variability in pCO2 in Puget Sound, we use two treatments, ∼760 and ∼1600–1700 µatm pCO2, to represent either current deep water conditions or surface conditions during upwelling. The ∼2800–3400 µatm pCO2 treatment represents future deep water conditions or future surface conditions during upwelling. Future pCO2 conditions in Puget Sound were approximated by assuming an addition of dissolved inorganic carbon (DIC) at a concentration of 90 µmol/kg seawater above current levels (P. McElhany and J. Reum, unpublished data).
After seven days of incubation, we terminally sampled each experiment. Live and dead pteropods in each jar were counted and preserved separately in 70% ethanol. Most live individuals were clearly identifiable by movement of the wings. All pteropods without wing movement were visualized under a dissecting microscope to detect movement of the internal organs. We rated the shell condition of pteropods that lived through the seven-day experiment, using the methodology presented in Lischka et al. [22] with one modification: we immersed samples in 6% sodium hypochlorite for 60 hr to sufficiently clean tissue from the shells. The scoring regime presented in Lischka et al. [22] assesses five shell characteristics, with either four or five point scales (scores 0–3 or 0–4): shell transparency/opaqueness, shell transparency/brownness, scarred structures, corrosion, and number of perforations [22]. Higher scores indicate more shell corrosion. We consider overall shell condition to be the sum of the scores for these five characteristics. Selection of shells for scoring was done by two observers who were blind to treatment condition and selected shells haphazardly. All shells selected for scoring were scored by a single observer, who was blind to treatment conditions, using a Nikon SMZ 745T stereoscope. We preserved pteropods from 4 of the 5 collection trips in 70% ethanol on day 0 of the experiments to examine shell state of the free-living population, and rated 17 haphazardly chosen shells from this collection for shell condition.
Experimental system and carbon chemistry measurements
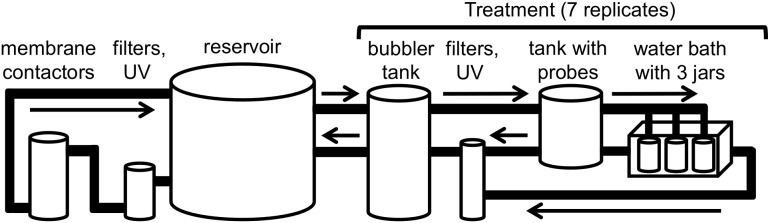
We conducted these experiments at the NOAA Northwest Fisheries Science Center ocean acidification laboratory (Figure 1). Seawater used in the ∼20,000 L experimental system was collected from Elliot Bay, Seattle, Washington, and the majority of it housed in a large reservoir. Water in the reservoir was filtered to 1 µm, exposed to UV, and degassed using Liqui-Cel membrane contactors (Membrana, Charlotte, North Carolina). A Honeywell conductivity probe monitored seawater salinity in the system. Treatment conditions were controlled by a program built in LabView Software (National Instruments, Austin, Texas) and maintained by bubbling one of four gases: air, CO2-free air, CO2, and O2. CO2-free air was generated with CO2 adsorbers (Twin Towers Engineering, Broomfield, Colorado). Temperature, pH, and dissolved oxygen in each treatment were continuously monitored with temperature, Durafet pH, and dissolved oxygen transmitter probes, respectively (Honeywell Process Solutions). The flow-through, pteropod-rearing jars (4.5 L, CO2-impermeable PET plastic) were connected to the treatment systems, and flow through each jar was approximately 6 L/hr. Jars were immersed in water baths with sufficient flow to maintain the target temperature (12°C) and kept in darkness behind black-out curtains. In each experiment, all jars for a given pCO2 treatment were in the same water bath; technical difficulties prevented the interspersing of pCO2 treatments among water baths. When we conducted these experiments, the experimental system contained some components that were later found toxic to herring (Clupea pallasii), copepod (Calanus pacificus), krill (Euphausia pacifica) larvae, but not to oyster (Crassostrea gigas) and geoduck (Panopea generosa) larvae or early life stages of Dungeness (Metacarcinus magister) and pygmy rock crab (Cancer oregonensis).
Figure 1. Simplified schematic of ocean acidification experimental system at NOAA’s Northwest Fisheries Science Center.
We measured three carbon-chemistry parameters (pH, DIC, and total alkalinity) for each study. Durafet pH probes in each experimental treatment continuously recorded pH, and every probe was calibrated at 12°C with a pH-certified Tris buffer (Dickson Laboratory, Scripps Institution of Oceanography). pH conditions in all treatments and rearing jars were verified by taking discrete samples for pH measurement using a spectrophotometer (Ocean Optics USB 2000+ Fiber Optic Spectrometer) and m-cresol purple dye (Sigma Aldrich). Discrete water samples for measurement of total alkalinity and DIC were taken once during each experiment, and these samples were analyzed at NOAA Pacific Marine Environmental Laboratory or the University of Washington’s Friday Harbor Laboratory. Standard operating procedures were followed for all carbon chemistry analyses [49]. We used DIC and spectrophometric pH measurements to estimate pCO2 and aragonite saturation state (Ωa) with CO2sys version 2.1 [50], using the K1 and K2 constants from Lueker et al. [51], KHSO4 constant from Dickson [52], [B]T from Uppstrom [53], and the total pH scale.
Statistical analysis
We assessed whether survival and shell condition over the 7-day study varied with treatment conditions using mixed-effects logistic regression with the lme4 package (method glmer) in the R statistical program [54]. Ωa was used as the metric to characterize treatments (fixed effect), and, in separate sets of models for each dataset, we considered Ωa as either a continuous variable or as a discrete treatment. Considering Ωa continuously allows evaluation of linear trends in the data, and considering Ωa as discrete treatments enables analyses that can distinguish significant differences among the four carbon chemistry treatments. We included two random effects in the model sets: experiment and jar within experiment. The best-fit models for the discrete and continuous Ωa model sets were selected based on AICc values of alternative models [55]. A logit scale was used for survival data, and a linear scale for the shell condition data. Hosmer-Lemeshow tests were used to assess if survival data (a binomial data set) met the assumptions of the statistical models. A Fligner-Killeen test was used to assess homogeneity of variance of the residuals from the discrete model on the shell condition data set. The sum score of the five shell condition characteristics was used for shell condition statistical analysis.
Results
The 4 treatments included in these experiments were distinct in pH, DIC, pCO2, and Ωa (Table 1). pH values for each treatment were consistent among the 5 experiments and varied little in each treatment over the course of each experiment (standard deviation ≤0.05). Mean pH values from the Durafet sensors were consistent with discrete spectrophotometric measurements of pH from each system and, when available, inside each jar, indicating that the Durafet pH probe measurements were accurate and that carbon chemistry conditions in the rearing jars were consistent with treatment system pH. Alkalinity in all treatments within each experiment was similar, but varied by ∼140 µmol/kg between the experiments in May and July due to use of different batches of seawater. Differences in alkalinity among experiments led to variation in DIC, pCO2, and Ωa for each treatment among experiments. Two of the treatments were oversaturated with respect to aragonite and two of the treatments were undersaturated with respect to aragonite. Mean temperature in the treatments ranged from 12.04–12.13°C, with small variation in each treatment over each experiment (standard deviation <0.2°C).
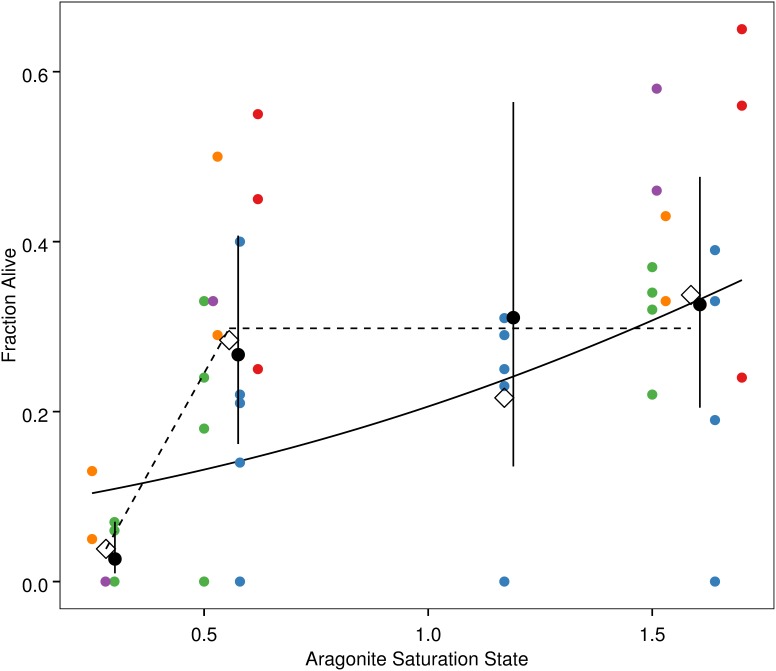
The best-fit model for the continuous Ωa analysis of survival data included Ωa as a fixed effect and jar within experiment as a random effect (Table 2). The slope of this relationship was positive (proportion live in a jar = Ωa * 1.07–2.52) and significantly different from zero (Z = −4.056, p<0.001; Figure 2). For the discrete Ωa analyses of survival data, the best-fit model included Ωa as a fixed effect and experiment and jar within experiment as random effects. Based on comparison of 95% confidence intervals, survival rates in the Ωa≈1.59, Ωa≈1.17, and Ωa≈0.56 treatments were similar and higher than in the Ωa≈0.28 treatment. A hypothetical relationship between Ωa and survival based on the discrete analysis is illustrated in Figure 2 as a “broken stick”, which characterizes survival in treatments with Ωa≥0.56 using the mean for these treatments and highlights the potentially rapid decrease in survival below this value by drawing a line between mean survival in treatments with Ωa≥0.56 to mean survival of the Ωa≈0.28 treatment. Results from Hosmer-Lemeshow tests indicate that the survival data are consistent with the assumptions of the models (χ2 = 0.67 for the continuous Ωa model, χ2 = 0.16 for the discrete Ωa model; number of groups = 10).
Table 2. AICc scores for generalized linear mixed effects models of pteropod survival and shell dissolution with aragonite saturation state (Ωa) treated as either a continuous or discrete variable.
| AICc score | ||||
| Survival | Shell dissolution | |||
| Model Treatment condition data organization: | Continuous | Discrete | Continuous | Discrete |
| Response = Ωa+(exp) +intercept | 1803 | 1727 | 1127 | 1100 |
| Response = Ωa+(jar) +intercept | 1687 | 1676 | 1094 | 1082 |
| Response = Ωa+(exp) +(jar)+intercept | 1688 | 1674 | 1097 | 1084 |
| Response = Ωa+(exp)+(jar) +(exp)×Ωa+intercept | 1690 | 1676 | 1099 | 1086 |
| Response = Ωa+(exp)+(jar) +(jar)×Ωa+intercept | 1690 | 1676 | 1099 | 1086 |
| Response = Ωa+(exp)+(jar) +(jar)×Ωa+(exp)×Ωa+intercept | 1692 | 1678 | 1101 | 1088 |
The lowest AICc score for each data set (column) is in bold. In the model equations, parentheses indicate random effects. Exp stands for experiment.
Figure 2. Pteropod survival as a function of Ωa.
Small colored circles: fraction surviving within an individual jar, with May experiments in warm colors and July experiments in cool colors; open diamonds: overall fraction of pteropods surviving at the mean Ωa for a given treatment; small black circles with vertical bars: expected value and 95% confidence intervals calculated from the best-fit discrete mixed effects model, with small x-axis offset from the treatment mean Ωa; solid line: best-fit linear relationship from continuous mixed effects model; dashed line: hypothetical “broken-stick” relationship.
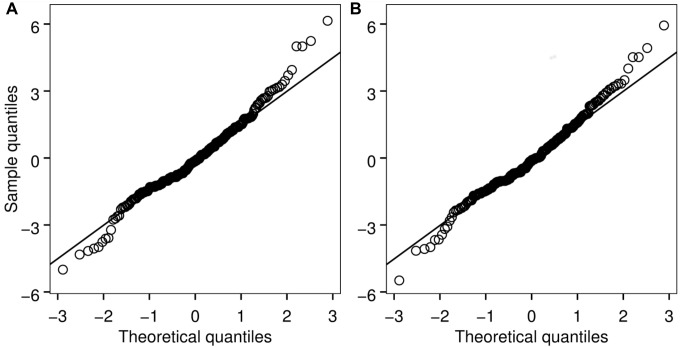
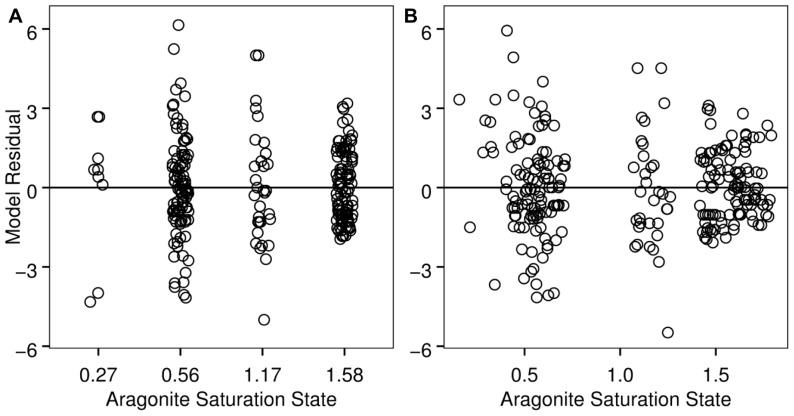
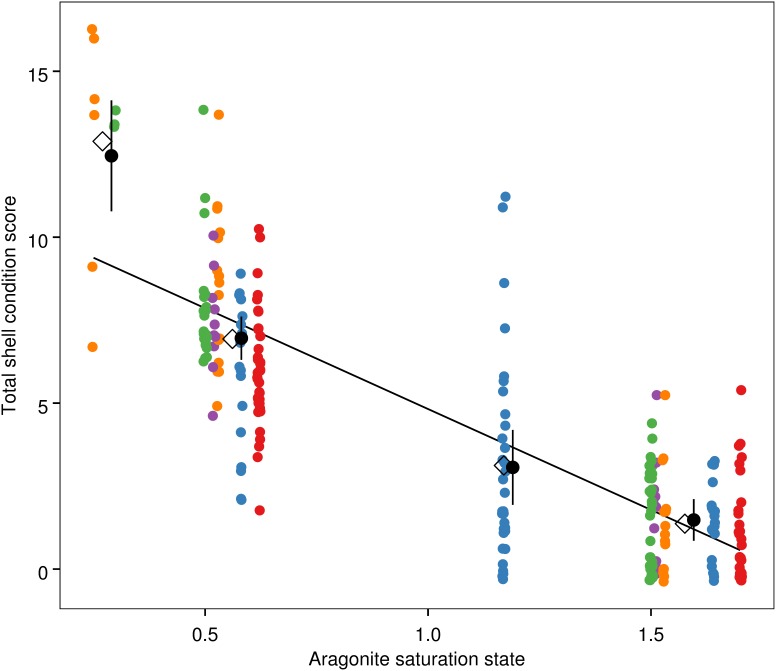
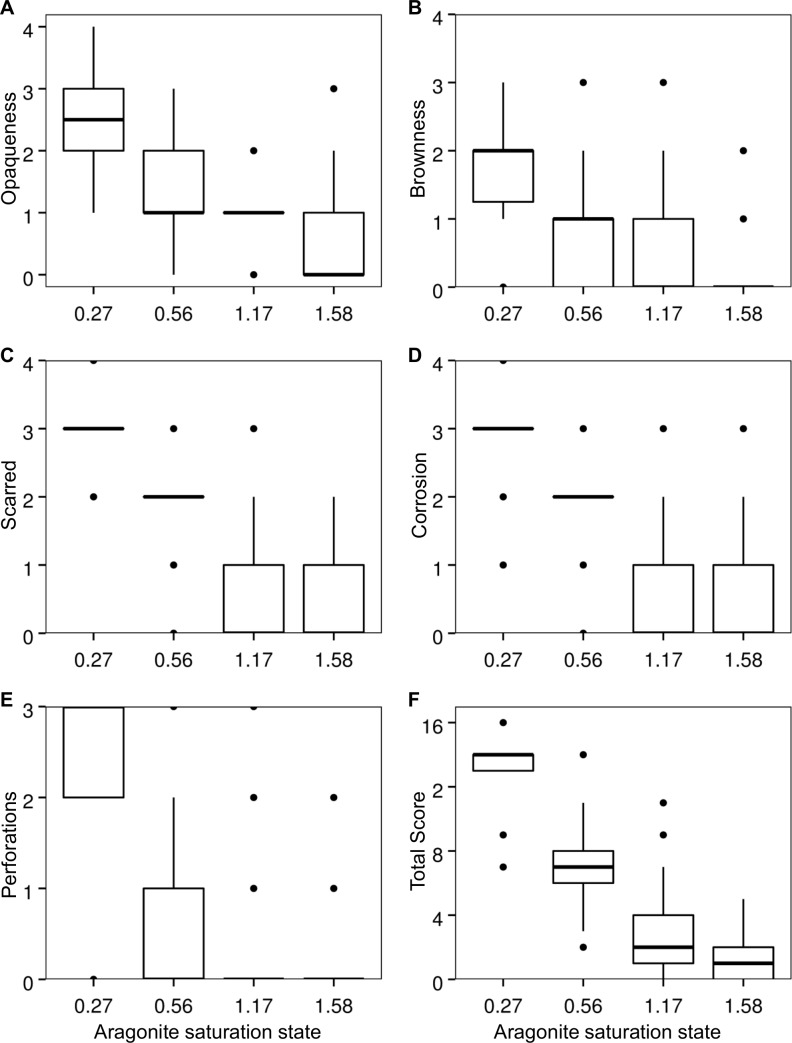
The best-fit models for shell condition and both continuous and discrete Ωa data included Ωa as a fixed effect and jar within experiment as a random effect (Table 2). Although residuals from the models deviate from a normal distribution (Figure 3), they show a reasonable approximation of normality such that inference is possible given the robustness of the regression approach to normality assumptions [56]. Consistent with model assumptions, a Fligner-Killeen test indicated similar variance of residuals among treatments in the discrete model (p = 0.10; Figure 4). The slope of the equation for the continuous Ωa data set was negative (shell condition score = −6.08 * Ωa+10.09) and significantly different from zero (t = −12.56, p<0.001; Figure 5). Based on the discrete model confidence intervals, dissolution scores for the Ωa≈1.59 and Ωa≈1.17 treatments were not significantly different from each other, but scores from the Ωa≈0.56 and Ωa≈0.27 treatments differed from one another and were significantly higher than those from the oversaturated treatments. Shell condition score decreased with Ωa treatment for all five shell condition characteristics (Figure 6). Pteropods preserved prior to the experiment showed little to no shell dissolution (mean shell condition score ± standard deviation = 0.5±0.6). One shell was slightly opaque (score = 1), 5 had scarred structures (score = 1 for 4 shells and 2 for 1 shell), and one had mild corrosion (score = 1).
Figure 3. Normal quantile-quantile plot of shell condition model residuals (discrete model, a; continuous model, b).
Solid line shows theoretical conditions in which model residuals are normally distributed. Open circles show actual sample values. Shapiro-Wilks test results indicate that the model residuals deviate from exact normality (p = 0.001 for models).
Figure 4. Shell condition model residuals as a function of Ωa (discrete model, a; continuous model, b).
Data are shown with a small x-axis offset from the treatment mean Ωa for better visualization of overlapping points. The plots indicate that the data are reasonably consistent with model assumptions of homoscedasticity.
Figure 5. Pteropod sum shell condition score as a function of Ωa.
Small, colored circles show the sum score for individual pteropods, with May experiments in warm colors and July experiments in cool colors. These points were plotted with small random offsets to prevent overlap. Open diamonds: treatment mean at mean Ωa for a given treatment; small, black circles with vertical bars: expected value and 95% confidence intervals calculated from the best-fit discrete mixed effects model, with a small x-axis offset from the treatment mean Ωa; solid line: linear relationship from continuous mixed effects model.
Figure 6. Observed pteropod shell condition scores as a function of Ωa treatment.
Each box plot shows the median with the upper and lower first and third quartiles. The whiskers extend to the highest and lowest values within 1.5 * the distance between the first and third quartiles. Outliers are plotted as points.
Discussion
We observed significant shell dissolution in adult L. helicina after one week of exposure to seawater with aragonite saturation states that occur today in Puget Sound and along the US West Coast (Ωa≈0.56) and are expected to occur more frequently in the future (Ωa≈0.28; Figure 5–7) [34], [40]. The data suggest that L. helicina from Puget Sound, like L. helicina from other locales, is sensitive to aragonite saturation state conditions below 1, which supports our hypothesis that shell corrosion is linked to undersaturated conditions. Recent work has documented shell corrosion in pteropods collected along the US West Coast, likely from exposure to corrosive conditions [57]. Previous studies suggest that shells of living pteropods dissolve in slightly oversaturated conditions [24], [25], [26]; we found little to no evidence of this phenomenon in these experiments. Corrosion in the Ωa≈0.56 treatment was most notable on the ribs of the shell, indicating that these shell areas might be most prone to dissolution (Figure 7). We observed little to no shell dissolution in the treatment with the highest Ωa (Ωa≈1.59), nor in shells of pteropods observed prior to the experiments.
Figure 7. Representative shells from pteropods incubated in the (a) Ωa≈1.59, (b) Ωa≈0.56, and (c) Ωa≈0.28 treatments.

Note corrosion on the ribs of the shell in image (b) and the shell perforations in image (c). Tissue that was not dissolved during the sodium hypochlorite incubation is visible as yellow-white material inside of the shells.
Starvation conditions may have exacerbated shell dissolution during this and other studies on the response of pteropods to ocean acidification by reducing the energy available for calcification (e.g., shell repair). Feeding during the incubations could have mitigated some or all of the influence of aragonite saturation state on shell dissolution. The influence of food availability on species response to carbon chemistry conditions has been documented multiple times in molluscs [58], [59], [60], including L. helicina [61], and other species, such as corals ([62], but see [63]). Exposure to laboratory starvation conditions for more than several days is known to suppress the metabolism of L. helicina and other pteropods [61], [64], [65], which could influence processes such as calcification. Taking a longer view, food availability throughout the season could also have influenced our results, for feeding history throughout a season influences the metabolic response of Antarctic L. helicina to ocean acidification conditions [61]. Unfortunately, we do not have data on primary productivity where the pteropods for this study were captured, so do not know if the study subjects were energy limited or if food conditions varied substantially among the different batches of pteropods used in this study.
While the discrete analysis found reduced survival with aragonite saturation state <0.3, the treatment condition representative of the future, it was unable to detect any effects that current carbon chemistry conditions may have on survival (broken stick model in Figure 2) [22], [23], [24]. While the latter result supports our expectation and is consistent with uncertainty in the relationship between ocean acidification and pteropod survival from prior work [22], [23], [24], the significant negative effect of the lowest saturation state treatment was counter to our expectation. No prior studies have assessed pteropod survival in response to such a low aragonite saturation state treatment.
Our ability to detect subtle impacts of carbonate chemistry on survival in current carbon chemistry conditions is limited due to the high variance in survival for a given treatment within and among experiments. Some of the variance among experiments is likely due to differing environmental conditions experienced by the pteropods prior to capture. Effects on survival at the intermediate Ωa levels used in this study may be masked by the inherently sub-optimal environment of the laboratory, which may have promoted high mortality rates regardless of treatment. Specifically, in our experimental setup, pteropods suffered from a lack of food and, potentially, chemicals released by plastics in the experimental system (high mortality has been observed for newly hatched fish and crustacean larvae for some species reared in the system). Furthermore, in captivity, pteropods typically do not deploy mucus nets, which negatively influences their ability to hold position in the water column. Thus, while large effects of strongly acidified treatments on survival may be detectable in the laboratory, mesocosm studies are likely needed to more accurately test how small changes in carbon chemistry conditions affect survival in free-living pteropods. That said, shell dissolution was negatively related to Ωa and, presumably, survival in the wild is related to shell condition.
The mechanism behind low survival in the highest CO2/lowest aragonite saturation state treatment is unclear. Mortality in this treatment could be related to mechanical and/or energetic complications caused by severe shell corrosion or due to other physiological effects of high CO2 conditions, such as those caused by regulation of acid-base balance. High CO2 conditions can impair acid-base balance in molluscs, in some cases leading to internal shell dissolution while compensating for extracellular acidosis [66]. This phenomenon could have caused the mean shell dissolution score for the highest CO2 treatment to be higher than what was predicted by the best-fit linear model (Figure 3, compare open diamond to solid line). Furthermore, in some pilot work using the same experimental setup described here (July 2012), we found that the shell-less, gymnosome pteropod Clione limacina also experienced low survival in the highest CO2 treatment compared to the lowest CO2 treatment, suggesting that pteropod mortality in this treatment may be due to processes unrelated to the shell (highest CO2 treatment: 0 of 12 alive at day 3 of incubation; lowest CO2 treatment: 11 of 12 alive at day 3 of incubation, 11 alive at day 9, 2 alive at day 15).
Given the sensitivity of north Pacific L. helicina shell condition to current carbon chemistry conditions demonstrated by this study and observations from the field [57], the microhabitat in which L. helicina lives and its natural history may be important for the persistence of the species in this region. Shell dissolution in L. helicina from the US West Coast and Southern Ocean is related to the prevalence of undersaturated conditions, indicating that some L. helicina populations are already affected by ocean carbon chemistry conditions, though potentially in a patchy manner [26], [57]. Little is known about the basic natural history of the species in Puget Sound, including its seasonal and spatial distribution and whether populations persist in the estuary year-round. Puget Sound populations of some zooplankton species (e.g., Calanus mashallae) appear to be largely re-initiated every season via transport from the Pacific Ocean (Frost and Pierson, unpublished data). Pteropods may follow a similar mechanism, flushing into Puget Sound every spring following the increased estuary circulation produced by spring freshwater run off [67]. A highly relevant question is whether the pervasive extent of waters undersaturated with respect to aragonite in the winter months makes Puget Sound a population sink that requires reseeding from the coast for persistence. Research on overwintering, juvenile L. helicina from other regions finds shell dissolution at carbon chemistry conditions currently found in Puget Sound in the winter [23], [24]. Laboratory research on whether calcification can balance dissolution in the variable conditions found in Puget Sound could inform research on this hypothesis [22].
Northeast Pacific L. helicina are typically found above 100 m [27], which is shallower than in other ocean regions [29]. Investigations to explore whether this is a behavioral modification to avoid low pH, corrosive waters could be a fruitful line of research for understanding how current variation in and future changes of carbon chemistry condition may influence free-living populations. Behavioral research could also explore whether pteropods in Puget Sound and elsewhere seek out energy-rich phytoplankton blooms, a strategy that would simultaneously lead them to low CO2 waters. Unlike Bednaršek et al. (2014), we observed little to no corrosion in pteropod shells preserved soon after collection. This finding suggests that the carbon chemistry conditions experienced by these pteropods were non-corrosive, pteropods with shell corrosion repaired their shells, or pteropods with corroded shells do not persist long in free-living populations.
We found that, under starvation conditions in the laboratory, pteropods collected from Puget Sound are sensitive to current and future local carbon chemistry conditions. We note that these laboratory data are insufficient to even conjecture about the trajectory of regional pteropod populations because of the potential influence of starvation conditions on response to our treatments and other influences on pteropod population dynamics. For example, changes in ocean chemistry projected to occur due to climate change could impact pteropod survival in Puget Sound. Low salinity events, which will be more frequent in the estuary under the projections of higher winter rainfall [68], may exacerbate the impacts of ocean acidification on wintertime mortality and shell dissolution, as they do in a closely related species, L. retroversa [69]. Furthermore, laboratory experiments on other species that are highly sensitive to acidification do show variation in the response of individuals to low pH conditions, indicating that the potential for evolution in response to ocean acidification should not be ignored [43], [44], [70].
Acknowledgments
We thank Julie Keister, Rebecca Lauzon, Anna McLaskey, Natasia Poinsatte, and Amanda Winans for collecting pteropods for this study; Rebecca Lauzon, Sarah Norberg, and Audrey Djunaedi for help setting up and collect data from these experiments; Jason Miller for pteropod shells measurements; Cynthia Peacock for carbon chemistry analysis; and the University of Washington’s Friday Harbor Laboratory for access to their carbon chemistry analysis equipment. Nina Bednaršek and Chris Harvey provided comments that improved this manuscript.
Funding Statement
This project was funded by NOAA’s Ocean Acidification Program, Northwest Fisheries Science Center, and Office of Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sabine CL, Feely RA, Gruber N, Key RM, Lee K, et al. (2004) The oceanic sink for anthropogenic CO2 . Science 305: 367–371. [DOI] [PubMed] [Google Scholar]
- 2. Le Quéré C, Takahashi T, Buitenhuis ET, Rödenbeck C, Sutherland SC (2010) Impact of climate change and variability on the global oceanic sink of CO2 . Global Biogeochemical Cycles 24: GB4007. [Google Scholar]
- 3. Canadell JG, Le Quéré C, Raupach MR, Field CB, Buitenhuis ET, et al. (2007) Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proceedings of the National Academy of Sciences 104: 18866–18870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orr JC, Fabry VJ, Aumont L, Bopp L, Doney SC, et al. (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681–686. [DOI] [PubMed] [Google Scholar]
- 5. Feely RA, Doney SC, Cooley SR (2009) Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22: 36–47. [Google Scholar]
- 6. Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425: 365–365. [DOI] [PubMed] [Google Scholar]
- 7.Solomon S, Qin D, Manning M, Chen Z, Marquis M, et al., editors (2007) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge and New York.
- 8. Beardall J, Stojkovic S, Larsen S (2009) Living in a high CO2 world: impacts of global climate change on marine phytoplankton. Plant Ecology & Diversity 2: 191–205. [Google Scholar]
- 9. Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sorensen C, et al. (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Climate Change 2: 201–204. [Google Scholar]
- 10. Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters 13: 1419–1434. [DOI] [PubMed] [Google Scholar]
- 11. Ainsworth CH, Samhouri JF, Busch DS, Chueng WWL, Dunne J, et al. (2011) Potential impacts of climate change on Northeast Pacific marine fisheries and food webs. ICES Journal of Marine Science 68: 1217–1229. [Google Scholar]
- 12. Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, et al. (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454: 96–99. [DOI] [PubMed] [Google Scholar]
- 13. Hönisch B, Ridgwell A, Schmidt DN, Thomas E, Gibbs SJ, et al. (2012) The geological record of ocean acidification. Science 335: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 14. Busch DS, Harvey CJ, McElhany P (2013) Potential impacts of ocean acidifcation on the Puget Sound food web. ICES Journal of Marine Science 70: 823–833. [Google Scholar]
- 15.Brodeur RD, Lorz RD, Pearcy WG (1987) Food habits and dietary variability of pelagic nekton off Oregon and Washington, 1979–1984. U.S. Dept. Commerce, NOAA Tech. Memo. NMFS-57. 32 p. [Google Scholar]
- 16. Aydin KY, McFarlane GA, King JR, Megrey BA, Myers KW (2005) Linking oceanic food webs to coastal production and growth rates of Pacific salmon (Oncorhynchus spp.), using models on three scales. Deep Sea Research Part II: Topical Studies in Oceanography 52: 757–780. [Google Scholar]
- 17. Armstrong JL, Boldt JL, Cross AD, Moss JH, Davis ND, et al. (2005) Distribution, size, and interannual, seasonal and diel food habits of northern Gulf of Alaska juvenile pink salmon, Oncorhynchus gorbuscha . Deep Sea Research Part II: Topical Studies in Oceanography 52: 247–265. [Google Scholar]
- 18. Brodeur RD, Daly EA, Sturdevant MV, Miller TW, Moss JH, et al. (2007) Regional comparisons of juvenile salmon (Oncorhynchus spp.) feeding in coastal marine waters off the West Coast of North America. American Fisheries Society Symposium 57: 183–203. [Google Scholar]
- 19. Comeau S, Gorsky G, Alliouane S, Gattuso JP (2010) Larvae of the pteropod Cavolina inflexa exposed to aragonite undersaturation are viable but shell-less. Marine Biology Letters 157: 2341–2345. [Google Scholar]
- 20. Comeau S, Gorsky G, Jeffree R, Teyssié J-L, Gattuso JP (2009) Impact of ocean acidification on a key Arctic pelagic mollusc (Limacina helicina). Biogeosciences 6: 1877–1882. [Google Scholar]
- 21. Comeau S, Jeffree R, Teyssié J-L, Gattuso J-P (2010) Response of the arctic pteropod Limacina helicina to projected future environmental conditions. PLoS ONE 5: e11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lischka S, Budenbender J, Boxhammer T, Riebesell U (2011) Impact of ocean acidification and elevated temperatures on early juveniles of the polar shelled pteropod Limacina helicina: mortality, shell degradation, and shell growth. Biogeosciences 8: 919–932. [Google Scholar]
- 23. Comeau S, Alliouane S, Gattuso JP (2012) Effects of ocean acidification on overwintering juvenile Arctic pteropods Limacina helicina . Marine Ecology Progress Series 456: 279–284. [Google Scholar]
- 24. Lischka S, Riebesell U (2012) Synergistic effects of ocean acidification and warming on overwintering pteropods in the Arctic. Global Change Biology 18: 3417–3528. [Google Scholar]
- 25. Bednaršek N, Tarling GA, Bakker DCE, Fielding S, Cohen A, et al. (2012) Description and quantification of pteropod shell dissolution: a sensitive bioindicator of ocean acidification. Global Change Biology 18: 2378–2388. [Google Scholar]
- 26. Bednaršek N, Tarling GA, Bakker DCE, Fielding S, Jones EM, et al. (2012) Extensive dissolution of live pteropods in the Southern Ocean. Nature Geosciences 5: 881–885. [Google Scholar]
- 27. Mackas DL, Galbraith MD (2012) Pteropod time-series from the NE Pacific. ICES Journal of Marine Science 69: 448–459. [Google Scholar]
- 28. Gannefors C, Böer M, Kattner G, Graeve M, Eiane K, et al. (2005) The Arctic sea butterfly Limacina helicina: lipids and life strategy. Marine Biology 147: 169–177. [Google Scholar]
- 29. Comeau S, Gattuso JP, Nisumaa AM, Orr J (2012) Impact of aragonite saturation state changes on migratory pteropods. Proceedings of the Royal Society B-Biological Sciences 279: 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato-Okoshi W, Okoshi K, Sasaki H, Akiha F (2010) Shell structure characteristics of pelagic and benthic molluscs from Antarctic waters. Polar Science 4: 257–261. [Google Scholar]
- 31. Collier R, Dymond J, Honjo S, Manganini S, Francois R, et al. (2000) The vertical flux of biogenic and lithogenic material in the Ross Sea: moored sediment trap observations 1996–1998. Deep Sea Research Part II: Topical Studies in Oceanography 47: 3491–3520. [Google Scholar]
- 32. Honjo S (2004) Particle export and the biological pump in the Southern Ocean. Antarctic Science 16: 501–516. [Google Scholar]
- 33. Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, et al. (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305: 362–366. [DOI] [PubMed] [Google Scholar]
- 34. Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B (2008) Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320: 1490–1492. [DOI] [PubMed] [Google Scholar]
- 35. Hauri C, Gruber N, Plattner G-K, Alin S, Feely RA, et al. (2009) Ocean acidification in the California current system. Oceanography 22: 61–71. [Google Scholar]
- 36. Feely RA, Sabine CL, Byrne RH, Millero FJ, Dickson AG, et al. (2012) Decadal changes in the aragonite and calcite saturation state of the Pacific Ocean. Global Biogeochem Cycles 26: GB3001. [Google Scholar]
- 37. Bakun A (1990) Global climate change and intensification of coastal ocean upwelling. Science 247: 198–201. [DOI] [PubMed] [Google Scholar]
- 38. Rykaczewski RR, Checkley DM (2008) Influence of ocean winds on the pelagic ecosystem in upwelling regions. Proceedings of the National Academy of Sciences 105: 1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gruber N, Hauri C, Lachkar Z, Loher D, Frölicher TL, et al. (2012) Rapid progression of ocean acidification in the California Current System. Science 337: 220–223. [DOI] [PubMed] [Google Scholar]
- 40. Feely RA, Alin SR, Newton J, Sabine CL, Warner M, et al. (2010) The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuarine, Coastal and Shelf Science 88: 442–449. [Google Scholar]
- 41. Reum JCP, Alin SR, Feely RA, Newton J, Warner M, et al. (2014) Seasonal carbonate chemistry covariation with temperature, oxygen, and salinity in a fjord estuary: implications for the design of ocean acidification experiments. PLoS ONE 9: e89619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosenthal JJC, Seibel BA, Dymowska A, Bezanilla F (2009) Trade-off between aerobic capacity and locomotor capability in an Antarctic pteropod. Proceedings of the National Academy of Sciences 106: 6192–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, et al. (2012) Adult exposure influences offspring response to ocean acidification in oysters. Global Change Biology 18: 82–92. [Google Scholar]
- 44. Sunday JM, Crim RN, Harley CDG, Hart MW (2012) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6: e22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maas AE, Wishner KF, Seibel BA (2012) The metabolic response of pteropods to acidification reflects natural CO2-exposure in oxygen minimum zones. Biogeosciences 9: 747–757. [Google Scholar]
- 46. Ohman MD, Lavaniegos BE, Townsend AW (2009) Multi-decadal variations in calcareous holozooplankton in the California Current System: Thecosome pteropods, heteropods, and foraminifera. Geophysical Research Letters 36: L18608. [Google Scholar]
- 47. Hunt BPV, Pakhomov EA, Hosie GW, Siegel V, Ward P, et al. (2008) Pteropods in Southern Ocean ecosystems. Progress in Oceanography 78: 193–221. [Google Scholar]
- 48. Bathmann UV, Noji TT, von Bodungen B (1991) Sedimentation of pteropods in the Norwegian Sea in autumn. Deep Sea Research Part A Oceanographic Research Papers 38: 1341–1360. [Google Scholar]
- 49.Dickson AG, Sabine CL, Christian JR, editors (2007) Guide to best practices for ocean CO2 measurements: PICES Special Publication 3. 191 p. [Google Scholar]
- 50.Lewis E, Wallace DWR (2012) Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee.
- 51. Lueker TJ, Dickson AG, Keeling CD (2000) Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Marine Chemistry 70: 105–119. [Google Scholar]
- 52. Dickson AG (1990) Standard potential of the reaction: AgCl(s)+1/2 H2(g) = Ag(s)+HCl(aq), and the standard acidity constant of the ion HSO4 − in synthetic seawater from 273.15 to 318.15 K. Journal of Chemical Thermodynamics. 22: 113–124. [Google Scholar]
- 53. Uppstrom LR (1974) The boron/chloronity ratio of deep-sea water from the Pacific Ocean. Deep-Sea Research 21: 161–162. [Google Scholar]
- 54.R Development Core Team (2013) R: a language and environment for statistical computing, version 2.15.2.
- 55.Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theorhetic approach, Second edition. New York: Springer-Verlag. 488 p. [Google Scholar]
- 56.van Belle G (2002) Statistical rules of thumb. New York: John Wiley and Sons. 304 p. [Google Scholar]
- 57. Bednaršek N, Feely RA, Reum JCP, Peterson B, Menkel J, et al. (2014) Limacina helicina shell dissolution as an indicator of declining habitat suitability due to ocean acidification in the California Current Ecosystem. Proceedings of the Royal Society B: Biological Sciences 281: 20140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Melzner F, Stange P, Trübenbach K, Thomsen J, Casties I, et al. (2011) Food supply and seawater pCO2 impact calcification and internal shell dissolution in the Blue Mussel Mytilus edulis . PLoS ONE 6: e24223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F (2013) Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Global Change Biology 19: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 60. Hettinger A, Sanford E, Hill TM, Hosfelt JD, Russell AD, et al. (2013) The influence of food supply on the response of Olympia oyster larvae to ocean acidification. Biogeosciences 10: 6629–6638. [Google Scholar]
- 61. Seibel BA, Maas AE, Dierssen HM (2012) Energetic plasticity underlies a variable response to ocean acidification in the pteropod, Limacina helicina antarctica . PLoS ONE 7: e30464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Holcomb M, McCorkle DC, Cohen AL (2010) Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). Journal of Experimental Marine Biology and Ecology 386: 27–33. [Google Scholar]
- 63. Holcomb M, Cohen A, McCorkle D (2012) An investigation of the calcification response of the scleractinian coral Astrangia poculata to elevated pCO2 and the effects of nutrients, zooxanthellae and gender. Biogeosciences 9: 29–39. [Google Scholar]
- 64. Maas A, Elder L, Dierssen H, Seibel B (2011) Metabolic response of Antarctic pteropods (Mollusca: Gastropoda) to food deprivation and regional productivity. Marine Ecology Progress Series 441: 129–139. [Google Scholar]
- 65. Seibel BA, Dierssen HM (2003) Cascading trophic impacts of reduced biomass in the Ross Sea, Antarctica: just the tip of the iceberg? The Biological Bulletin 205: 93–97. [DOI] [PubMed] [Google Scholar]
- 66. Parker L, Ross P, Connor W, Pörtner H, Scanes E, et al. (2013) Predicting the response of molluscs to the impact of ocean acidification. Biology 2: 651–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cannon CA (1983) An overview of circulation in the Puget Sound estuarine system. U.S. Dept. Commerce, NOAA Tech. Memo. ERL PMEL-48. 30 p. [Google Scholar]
- 68.Snover AK, Mote PW, Whitely Binder L, Hamlet AF, Mantua NJ (2005) Uncertain future: climate change and its effects on Puget Sound. A report for the Puget Sound Action Team by the Climate Impacts Group (Center for Science in the Earth System, Joint Institute for the Study of the Atmosphere and Oceans, University of Washington, Seattle). 37 p. [Google Scholar]
- 69. Manno C, Morata N, Primicerio R (2012) Limacina retroversa’s response to combined effects of ocean acidification and sea water freshening. Estuarine, Coastal and Shelf Science 113: 163–171. [Google Scholar]
- 70. Pespeni MH, Sanford E, Gaylord B, Hill TM, Hosfelt JD, et al. (2013) Evolutionary change during experimental ocean acidification. Proceedings of the National Academy of Sciences 110: 6937–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]