Abstract
The strong interfacial properties of selected plant polyphenols were recently exploited in forming functionally versatile nanocoatings via dip-coating. Here, we screened a library of ~20 natural and synthetic phenols and polyphenols, identifying eight catechol-, gallol- and resorcinol-rich precursors capable of forming coatings. Several newly identified compounds expand the molecular diversity of tannin-inspired coatings.
The use of bio-inspired approaches for forming multifunctional coatings has become commonplace in the last several years.1 Mussel adhesive proteins (MAPs) are polyphenolic biopolymers that have provided much inspiration for this work.2 MAPs with the highest known concentrations of 3,4-dihydroxyphenylalanine (DOPA) are found near the interface of the mussel byssus and substrate,3,4 suggesting an important interfacial role for DOPA. Due to the chemical versatility of its catechol (1,2-dihydroxyphenyl) side chain, DOPA is possibly unrivaled among amino acids in its ability to contribute adhesive properties to proteins.5 Because of this, DOPA has recently been employed as a molecular building block for anchoring biomimetic coatings onto substrates.6-8
Dopamine, a structural relative of DOPA, has also emerged as a convenient tool for depositing nanocoatings on an incredibly diverse array of substrates.9 Substrates immersed in aqueous solutions of dopamine spontaneously form conformal coatings, often referred to as polydopamine (pDA). pDA coating formation generally proceeds best at alkaline pH10 and is therefore thought to involve autoxidation to form a reactive o-quinone, in analogy to melanogenesis.11,12 Despite rapidly growing interest in the practical applications of pDA-like coatings,1 most reported studies rely on dopamine and a small number of other structurally related catecholamine coating precursors.13-18
Among other classes of phenolic biomolecules, plant phenols and polyphenols (hereafter, collectively referred to as ‘(poly)phenols’) stand out as possessing a remarkable abundance and density of catechol and gallol (1,2,3-trihydroxyphenyl) functional groups. Due to structural resemblance to DOPA and dopamine, plant (poly)phenols are expected to exhibit strong solid-liquid interfacial properties. Indeed, the common name for the class of plant (poly)phenols capable of binding, cross-linking, or precipitating proteins is tannins, and their strong interfacial activity is the basis of the astringency effect and their historical use in leather tanning.19 The use of plant-derived (poly)phenols as precursors for spontaneous, multifunctional, and substrate-independent nanocoatings was recently reported.20-23 These studies have revealed the potential of green tea catechins [epicatechin gallate (ECG) and epigallocatechin gallate (EGCG)], tannic acid (TA), and pyrogallol (PG) to form thin adherent films. Whereas some approaches to plant (poly)phenol-derived coatings involve the use of polymers, enzymes, trivalent metal ions, or multistep deposition processes,21-23 we favour an approach to coating formation that is analogous to pDA coatings, i.e., requiring only aqueous buffer and (poly)phenol precursor.20
In contrast to pDA coatings, where the molecular diversity of coating precursors is quite limited (primarily dopamine, norepinephrine, and DOPA), a vast number of (poly)phenols from plant tissues exist and can be considered potential precursors. Integration of more structurally and chemically diverse plant poly(phenols) into tannin-inspired nanocoatings should translate into novel and potentially useful coating properties, beyond those that have already been reported. In this study we investigated a number of plant-derived or plant-inspired (poly)phenol compounds, identifying several new molecules capable of forming coatings. Furthermore, the pH dependence of coating deposition was demonstrated, with the optimal coating pH generally being below the first phenolic pKa for each compound. In addition to significantly expanding the library of plant-inspired (poly)phenols useful as coating precursors, our results suggest that subtle changes in deposition conditions can significantly affect coating formation, even for structurally similar coating precursors.
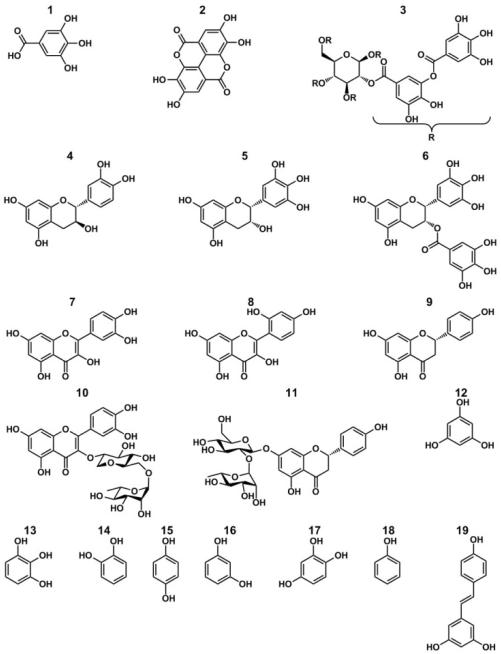
We investigated the coating potential of 19 natural and synthetic precursors containing gallol, catechol, or phenol structural units (Figure 1). The compounds studied were inspired by several different types of tannins: phlorotannins based on phloroglucinol, hydrolyzable tannins containing gallates, and condensed tannins composed of flavan-3-ols. The use of plant-based molecules was especially attractive due to the potential for fabricating multifunctional coatings from non-petroleum natural resources (Table 1), or even from current industrial waste and byproducts.24-27 In fact, we recently demonstrated that expended green tea leaves can be used to form coatings.20 As a basis for screening their coating-forming ability, each compound was dissolved in buffered 0.6 M NaCl, and TiO2 substrates were immersed with gentle agitation for 24-48 h. Anticipating that coating formation via autoxidation may be connected to phenolic deprotonation28 and recognizing that the molecular diversity of the compounds investigated would be reflected in a range of phenolic pKa values, pH values between 3-10 were investigated (not all compounds were soluble over the entire pH range). Coating thickness was monitored indirectly via XPS, using the reduction of signal from the underlying TiO2 substrate as an indicator of coating formation. For screening purposes only, we interpreted a reduction in Ti2p signal of at least 50 % as confirmation of a successful coating, though we recognize that thinner coatings may perform well for some intended applications.29
Fig. 1.
Chemical structures of tannin-inspired compounds studied as coating precursors. The names and plant sources of all compounds can be found in Table 1.
Table 1.
Summary of coating precursors studied, their plant sources, and their ability to form spontaneous coatings
| Precursor | Numbera | Common Plant Sourceb | Coating Former? |
|---|---|---|---|
| Gallic acid | 1 | Oak | NO |
| Ellagic acid | 2 | Oak | NO |
| TA | 3 | Oak | YES |
| Ctn | 4 | Cocoa, Green tea | YES |
| EGC | 5 | Green tea | YES |
| EGCG | 6 | Green tea | YES |
| Quercetin | 7 | Caper | NO |
| Morin | 8 | Guava | YES |
| Naringenin | 9 | Citrus fruit | NO |
| Rutin | 10 | Citrus fruit | NO |
| Naringin | 11 | Citrus fruit | NO |
| Phloroglucinol | 12 | Brown algae | NO |
| PG | 13 | Eurasian watermilfoil | YES |
| Ctl | 14 | Argan | YES |
| Hydroquinone | 15 | Broccoli | NO |
| Resorcinol | 16 | Argan | NO |
| HHQ | 17 | None | YES |
| Phenol | 18 | None | NO |
| Resveratrol | 19 | Grapes | NO |
See figure 1 for structures.
Not intended to be exhaustive list of all known natural sources.
Eight molecules were discovered to form coatings (Supplementary Table S1), including three identified earlier20 (TA, PG and EGCG) and five that were previously unknown [catechol (Ctl), catechin (Ctn), hydroxyhydroquinone (HHQ), epigallocatechin (EGC), and morin]. The carbon-to-oxygen (C/O) ratios of the coatings were determined by XPS, yielding C/O values very similar to those of the molecules from which the coatings were derived (Supplementary Table S2). An exception was morin, where the experimental C/O ratio of the coating formed at pH 8 (1.11) was substantially dissimilar to the C/O value of morin (2.14). This may reflect a relatively thin coating, as the adlayer thickness on TiO2 and polycarbonate (PC) were determined by ellipsometry to be less than 5 nm (Supplementary Table S3). For certain precursors, even small changes in pH strongly influenced the ability to form coatings, an observation exemplified by TA, PG and EGC. In our previous study, coatings based on TA and PG formed at pH 7.8.20 However, in this work, a minor increase to pH 8 largely abolished the coating ability of PG and TA (Supplemental Table S1 and S2). EGC showed little coating ability at pH 7.8,20 but was much better at pH 9.
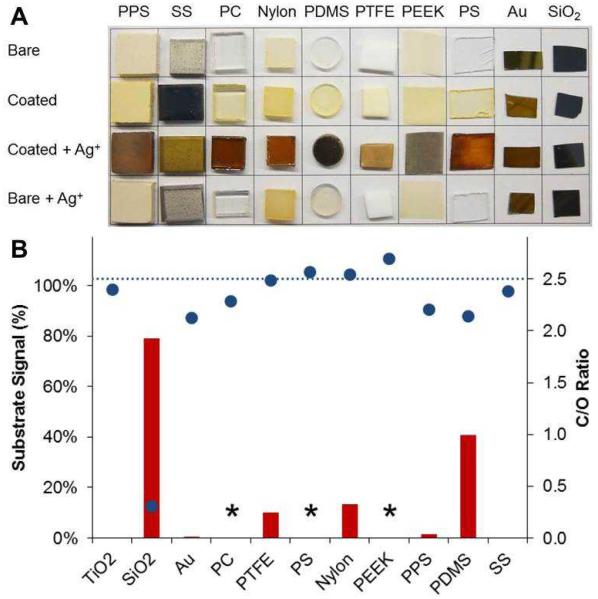
On the basis of initial screening results, TA, PG, Ctl, Ctn, HHQ, and EGCG were further examined for their ability to form coatings on a wide variety of metal, ceramic, and polymer substrates. Representative findings for Ctn are shown in Figure 2 and in Supplementary Figures S1-S5 for TA, PG, Ctl, HHQ, and EGCG. With the exception of SiO2, which is known to be solubilized by catechols near neutral pH,30 coatings formed on all substrates investigated, as indicated by silver staining.20 After exposure to silver nitrate (AgNO3), modified samples darkened because of the formation of silver nanoparticles via a redox couple between Ag+ and the polyphenolic coating. Chemical analysis of the modified surfaces by XPS revealed good agreement between the C/O ratios of coatings and the C/O ratios of the corresponding phenolic precursors. As reported previously,20 (poly)phenol coatings produce less substrate discoloration than pDa for coatings of comparable thickness (Supplementary Figure S6). TA-, PG-, Ctl-, Ctn-, HHQ-, and EGCG-derived coatings could also be deposited onto porous poly(ε-caprolactone) (PCL) foams (Figure 3) and PEEK mesh (Supplementary Figures S7 – S13) in a straightforward manner.
Fig. 2.
Spontaneous deposition of catechin-derived coatings on a variety of substrates. (A) Digital images of uncoated, coated, and AgNO3-treated substrates demonstrating versatility of catechin as a coating former on metal, ceramic, and polymer substrates. Darkening of substrates upon treatment with AgNO3 results from silver nanoparticle formation, confirming the presence and redox-active nature of the polyphenolic coating. (B) Surface chemical analysis of coated substrates by XPS. Inherent substrate signals are represented by red bars (100 % = no coating; * indicates the inability to differentiate coating from substrate by XPS), and carbon/oxygen (C/O) ratios are represented by blue symbols. Attenuation of substrate signal in conjunction with a C/O ratio similar to that of catechin (C/O = 2.5, blue dashed line) is indicative of successful coating formation.
Fig. 3.

Spontaneous deposition of coatings on porous PCL substrates. PCL foams were immersed in solutions containing TA, PG, Ctl, Ctn, HHQ, and EGCG under buffered conditions. As-deposited coatings (− Ag+) were further visualized by immersing in AgNO3 (+ Ag+).
The stability of tannin-inspired nanocoatings toward sonication was substrate dependent (Supplementary Table S3). Coatings deposited on TiO2 were partly removed upon sonication in water, while coatings applied on PC remained essentially unchanged after sonication. EGCG, which decreased from 65 nm to 4 nm after sonication, was the exception. Immersion of coated PC substrates in aqueous buffer and assessment of coating thickness by ellipsometry at various times led to the finding that coating stability depended on pH and the presence of esters in the precursor (Supplementary Figures S14-S17). Coatings derived from ester-free precursors (PG, Ctl, Ctn, HHQ) were stable or increased slightly in thickness at acidic pH over 7 days. However, TA- and EGCG-derived coatings decreased in thickness at acidic pH, presumably due to hydrolysis. Hydrolysis of coatings based on TA and EGCG could be employed for release of gallic acid (GA), which has antibacterial, anti-inflammatory, and antioxidant properties.31-33 A recent report suggested that PG and TA coatings were unstable toward sonication.34 However, those coatings were formed under different pH conditions than those shown to be optimal in this report. Our data show that pH during coating formation has a significant influence on coating thickness and properties (Tables S1-S3).
Noting our previous success with PG, TA and EGCG,20 we anticipated the aromatic vicinal diol (catechol) to emerge as a common structural feature of coating-forming precursors. Indeed, seven of the eight coating precursors identified in this study contain at least one aromatic vicinal diol ring. However, having this structural component is clearly not sufficient, as GA, ellagic acid, quercetin, and rutin did not produce coatings under our conditions. Quercetin was recently reported to reduce the contact angle of a hydrophobic substrate, but the thickness of the coating was not reported.34 Additionally, the reported method required the use of an organic co-solvent which was not necessary in our method.
The failure of GA to form coatings is especially interesting, as it is very similar to PG and essentially the monomeric unit of TA, which both coat very efficiently. When comparing GA to PG, the obvious difference is the presence of a carboxylic acid in GA, which contributes charge, alters the electronic structure of the aromatic ring, affects the pKa, and eliminates one potential reaction site on the phenyl ring.
The presence of vicinal diols was expected to be a requisite for forming coatings due to the need to form reactive o-quinones. Aryl-aryl couplings, which proceed through o-quinones, are considered vital to oxidative reactions among plant phenols.19 The ability of morin to form coatings is notable among the catecholamines and plant polyphenols as the first precursor that does not contain a vicinal diol. Among the six precursors containing a 1,3-dihydroxybenzene motif but lacking an aromatic vicinal diol (morin, naringenin, phloroglucinol, resorcinol, and resveratrol), only morin formed coatings. Associating the presence of the α,β-unsaturated ketone in morin with successful coating formation is tempting, though such a generalization is not supported by the inability of quercetin to form coatings.
For the (poly)phenol coating precursors identified in this study, we reasonably expected a relationship between coating pH, phenol deprotonation, and autoxidation.28,35-38 Oxygen-free titrations of Ctn, TA, EGCG, Ctl, PG, and HHQ were performed to identify pKa values of the precursors (Supplementary Table S4). No clear trend emerged when these values were compared to the optimal coating pH (pHcoating), though all successful coatings were noted to occur at pH values less than the first phenolic pKa of the precursor. In most cases, the optimal coating pH was within approximately 2 pH units of the pKa value of the precursor, suggesting a role for deprotonation and autoxidation in coating deposition.
Acknowledging that the titration and coating experiments were performed at different salt concentrations, which may influence the protonation of phenols, additional studies are needed to fully understand the relationship between optimal coating pH, phenol deprotonation, and autoxidation. Nevertheless, our observations already provide some guidance to those seeking to identify ideal conditions to produce coatings from a (poly)phenol precursor.
Lastly, a brief comment on precursor solubility is warranted. The coating method requires at least some solubility of the molecular precursor in the coating medium, and all of the coating precursors identified here – TA, PG, EGCG, Ctl, Ctn, HHQ, EGC, and morin – met this requirement within at least part of the pH range studied. However, limited solubility over a wide pH range essentially precluded the use of certain compounds. This is well illustrated by ellagic acid and quercetin, which were insoluble below pH 10 and 9, respectively. In the future, it may be possible to overcome such solubility limitations with mixed aqueous/organic solvent systems,34 though we did not investigate this.
The identification of new polyphenol coating precursors is important because the immense structural diversity of this family of natural compounds may lead to coatings with novel chemical and biological properties. With this in mind, we investigated a library of (poly)phenols as precursors. One important outcome of this study is the expansion of known molecular precursors to include tannic acid, epigallocatechin gallate, pyrogallol, catechin, epigallocatechin, morin, catechol, and hydroxyhydroquinone. Although the establishment of strict rules associating coating ability with precursor molecular features was elusive, most precursors were found to contain at least one aromatic vicinal diol. Morin was a notable exception, emerging as the first known example of a (poly)phenol coating precursor that does not contain a catechol or gallol group. Finally, compound-specific ideal conditions for substrate modification were established, revealing a loose relationship between coating pH and phenolic pKa and providing general guidance for preparing tannin-inspired nanocoatings from these and other (poly)phenol precursors. In contrast to pDA coatings, where precursor choice is quite limited, the vast molecular diversity in plant-inspired (poly)phenols offers many opportunities for tailoring chemical, physical, and biological properties of (poly)phenol coatings in the future.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health grant R37 DE014193.
Footnotes
Electronic Supplementary Information (ESI) available. See DOI: 10.1039/b000000x/
References
- 1.Ye Q, Zhou F, Liu W. Chem Soc Rev. 2011;40:4244–4258. doi: 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- 2.Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Annu Rev Mater Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papov VV, Diamond TV, Biemann K, Waite JH. J Biol Chem. 1995;270:20183–20192. doi: 10.1074/jbc.270.34.20183. [DOI] [PubMed] [Google Scholar]
- 4.Waite JH, Qin X. Biochemistry. 2001;40:2887–2893. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Scherer NF, Messersmith PB. Proc Natl Acad Sci USA. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalsin JL, Hu BH, Lee BP, Messersmith PB. J Am Chem Soc. 2003;125:4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 7.Paunesku T, Rajh T, Wiederrecht G, Maser J, Vogt S, Stojicevic N, Protic M, Lai B, Oryhon J, Thurnauer M, Woloschak G. Nat Mater. 2003;2:343–346. doi: 10.1038/nmat875. [DOI] [PubMed] [Google Scholar]
- 8.Zürcher S, Wäckerlin D, Bethuel Y, Malisova B, Textor M, Tosatti S, Gademann K. J Am Chem Soc. 2006;128:1064–1065. doi: 10.1021/ja056256s. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball V, Frari DD, Toniazzo V, Ruch D. J Colloid Interface Sci. 2012;386:366–372. doi: 10.1016/j.jcis.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Ball V. Bionanoscience. 2012;2:16–34. [Google Scholar]
- 12.d'Ischia M, Wakamatsu K, Napolitano A, Briganti S, Garcia-Borron JC, Kovacs D, Meredith P, Pezzella A, Picardo M, Sarna T, Simon JD, Ito S. Pigm Cell Melanoma R. 2013;26:616–633. doi: 10.1111/pcmr.12121. [DOI] [PubMed] [Google Scholar]
- 13.Kang SM, Rho J, Choi IS, Messersmith PB, Lee H. J Am Chem Soc. 2009;131:13224–13225. doi: 10.1021/ja905183k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L-P, Yu J-Z, Xu Y-Y, Xi Z-Y, Zhu B-K. Colloids Surf B. 2009;69:152–155. doi: 10.1016/j.colsurfb.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Sun P, Lu H, Yao X, Tu X, Zheng Z, Wang X. J Mater Chem. 2012;22:10035–10041. [Google Scholar]
- 16.Ham HO, Liu Z, Lau KHA, Lee H, Messersmith PB. Angew Chem Int Ed. 2011;50:732–736. doi: 10.1002/anie.201005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai A, Perry CC. J Mater Chem. 2012;22:4790–4796. [Google Scholar]
- 18.Kuang J, Messersmith PB. Langmuir. 2012;28:7258–7266. doi: 10.1021/la300738e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslam E. Plant polyphenols: vegetable tannins revisited. Cambridge University Press; 1989. [Google Scholar]
- 20.Sileika TS, Barrett DG, Zhang R, Lau KHA, Messersmith PB. Angew Chem Int Ed. 2013;52:10766–10770. doi: 10.1002/anie.201304922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ejima H, Richardson JJ, Liang K, Best JP, van Koeverden MP, Such GK, Cui J, Caruso F. Science. 2013;341:154–157. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 22.Jeon J-R, Kim J-H, Chang Y-S. J Mater Chem B. 2013;1:6501–6509. doi: 10.1039/c3tb21161d. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Kim DS, Kang SM. Chem Asian J. 2013;9:63–66. doi: 10.1002/asia.201301291. [DOI] [PubMed] [Google Scholar]
- 24.Schieber A, Stintzing FC, Carle R. Trends Food Sci Technol. 2001;12:401–413. [Google Scholar]
- 25.Arvanitoyannis IS, Ladas D, Mavromatis A. Int J Food Sci Technol. 2006;41:475–487. [Google Scholar]
- 26.Balasundram N, Sundram K, Samman S. Food Chem. 2006;99:191–203. [Google Scholar]
- 27.Yamamoto A, Rohumaa A, Kontturi E, Hughes M, Saranpää P, Andberg M, Vuorinen T. ACS Sustainable Chemistry & Engineering. 2013;1:1075–1082. [Google Scholar]
- 28.Odiatou EM, Skaltsounis AL, Constantinou AI. Cancer Lett. 2013;330:113–121. doi: 10.1016/j.canlet.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Han G, Zhang S, Li X, Widjojo N, Chung T-S. Chem Eng Sci. 2012;80:219–231. [Google Scholar]
- 30.Belton DJ, Deschaume O, Patwardhan SV, Perry CC. The Journal of Physical Chemistry B. 2010;114:9947–9955. doi: 10.1021/jp101347q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chanwitheesuk A, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Food Chem. 2007;100:1044–1048. [Google Scholar]
- 32.Kroes BH, van den Berg AJJ, Quarles van Ufford HC, van Dijk H, Labadie RP. Plant Med. 1992;58:499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz Y, Toledo RT. J Agric Food Chem. 2003;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 34.Hong S, Yeom J, Song IT, Kang SM, Lee H, Lee H. Adv Mater Interfaces. DOI: 10.1002/admi.201400113. [Google Scholar]
- 35.Siegel SM, Siegel BZ. Nature. 1958;181:1153–1154. doi: 10.1038/1811153a0. [DOI] [PubMed] [Google Scholar]
- 36.Kalyanaraman B, Sealy RC, Sivarajah K. J Biol Chem. 1984;259:14018–14022. [PubMed] [Google Scholar]
- 37.Gao R, Yuan Z, Zhao Z, Gao X. Bioelectrochem Bioenerg. 1998;45:41–45. [Google Scholar]
- 38.Mochizuki M, Yamazaki S.-i., Kano K, Ikeda T. Biochim Biophys Acta. 2002;1569:35–44. doi: 10.1016/s0304-4165(01)00230-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.