Abstract
The vasculature of an organism has the daunting task of connecting all the organ systems to nourish tissue and sustain life. This complex network of vessels and associated cells must maintain blood flow but constantly adapt to acute and chronic changes within tissues. While the vasculature has been studied for over a century, we are just beginning to understand the processes that regulate its formation and how genetic hierarchies are influenced by mechanical and metabolic cues to refine vessel structure and optimize efficiency. As we gain insights into the developmental mechanisms, it is clear that the processes that regulate blood vessel development can also enable the adult to adapt to changes in tissues that can be elicited by exercise, aging, injury, or pathology. Thus, research in vessel development has provided tremendous insights into therapies for vascular diseases and disorders, cancer interventions, wound repair and tissue engineering, and in turn, these models have clearly impacted our understanding of development. Here we provide an overview of the development of the vascular system, highlighting several areas of active investigation and key questions that remain to be answered.
INTRODUCTION
The cardiovascular system (CV) is the first functional organ system formed during vertebrate development. The major function of the CV system is to enable gas exchange, supply nutrients and remove waste from tissues in order to properly nourish cells within the body and sustain organism growth and viability. In addition to these obvious physiological roles, it is now appreciated that endothelial cells and vessels can also provide key regulatory and guidance cues to support the development of other systems, for instance in the pancreas or the nervous system1–5. As the embryo develops, the cardiovascular system also plays an important role in lymph regulation, systemic functions of the endocrine system, as well as immunological surveillance and inflammation.
In rodents and humans, the cardiovascular system is composed of a four-chambered heart connected to the rest of the vasculature through the pulmonary arteries and veins (to circulate blood to and from the lungs) and the dorsal aorta and sinus venosus (to circulate blood throughout the rest of the body). The vasculature can be subdivided into three main vessel systems. The arterial system carries blood away from the heart, with larger arteries such as the pulmonary artery or the dorsal aorta feeding into progressively smaller diameter arteries, arterioles, and capillaries. In contrast, the venous system transports blood back to the heart by collecting it from capillaries and transporting it through progressively larger venules and veins. Finally, the lymphatic system of vessels transports interstitial fluid from tissues and organs and returns it to the circulation ultimately through drainage into the subclavian veins. Together, these three vascular beds form a closed system of vessels that comprise the circulatory system.
The vessels themselves are composed of several different cell types. The inner lining of vessels (the endothelium) is made up of endothelial cells arranged in a simple squamous epithelial layer that surrounds the internal lumen of the blood vessel. In small vessels and capillaries, the endothelium is also often supported by vascular smooth muscle cells and pericytes, collectively known as mural cells, that associate with the abluminal side of the vessel6, although mural cell associations are sparse in many of the smallest vessels. Larger arteries and veins take on an even more complex structure, with the endothelium (also referred to as the tunica intima) surrounded by both a thick stabilizing layer of smooth muscle cells (tunica media) and an outermost layer of connective tissue, collagen, and elastic fibers (tunica adventitia)7. This structure confers stability to these vessels, while still allowing them to dynamically respond to changing metabolic demands by altering blood flow using both acute and chronic adaptations. Acute changes in vessel diameter and blood flow are regulated by contractile mural cells, which contract or relax depending on signals from the tissue. In contrast, chronic changes in the vasculature require the assembly, disassembly, or remodeling of vascular beds.
Development of the circulatory system involves the orchestration of several overlapping events to build then remodel the vasculature into mature vessels. Endothelial cells must be specified and assembled or added into growing vessels either through vasculogenesis, the de novo formation of vessels from aggregated endothelial precursors, or angiogenesis, the formation of neovessels from existing vessels. Vessels are then remodeled to refine the structure based on metabolic need and structural constraints of the tissue (Figure 1). Remodeling can also facilitate vessel regression to eliminate transient or unnecessary vessels during morphogenesis. Interestingly, although these events are required to establish the functional circulatory system in the embryo, many of these processes continue to be active throughout the life of the organism. The CV system must remain constantly adaptive to changes in the body triggered by tissue growth (including adipose expansion or loss), muscle addition/atrophy, cyclic remodeling of the reproductive system, and wound repair, as well as during pathological conditions, such as diabetes or cancer8–13. The same principle processes that regulate development thus continue throughout life to maintain one of the most dynamic organ systems within the body.
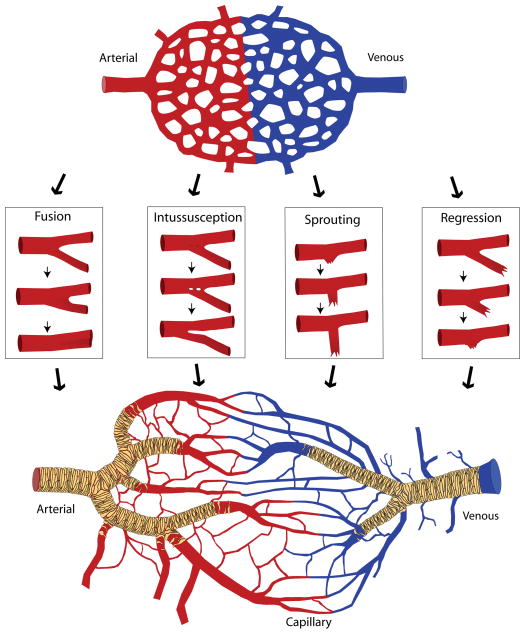
Figure 1.
The vascular plexus, mechanisms of angiogenesis and the remodeled vasculature. The vascular/capillary plexus, composed of an interwoven capillary network with intervening avascular spaces, and in which the presumptive arterial (red) and venous (blue) sides have been specified, is primarily generated by vasculogenesis. Various mechanisms of angiogenesis – fusion, intussusception, sprouting, and regression – contribute to changes in vessel diameter and vessel density. Remodeling of the plexus results in the formation of a hierarchical vasculature tree with large diameter arteries and veins that are connected to progressively smaller diameter/distal capillaries. Simultaneously, vessels become stabilized by the recruitment of mural cells (pericytes and smooth muscle cells) (tan).
Though many informative reviews have been written about vasculogenesis and angiogenesis in vertebrates7, 14–17, utilization of new markers and experimental strategies have revealed novel insights into the mechanisms of blood vessel formation. Thus, the purpose here is to highlight the latest advances in research on vasculogenesis and angiogenesis. Examples from several vertebrate model systems, including avians, teleosts and mammals are discussed, with an emphasis on data from mouse studies.
ENDOTHELIAL CELLS: THE BUILDING BLOCKS OF VESSELS
The first step in building blood vessels is to specify endothelial cells. Though it is currently unclear precisely how endothelial cells are specified, early endothelial cells (also referred to as angioblasts) can be identified in avian embryos using MB1 and QH1 immunochemical markers18, 19. Classical approaches in avians, including surgical ablation and chick-quail transplants, have revealed that extraembryonic angioblasts arise from extraembryonic mesoderm that exits the posterior primitive streak14, 15, 20–23. In contrast, intraembryonic angioblasts form from both somatic and paraxial mesoderm14, 24. In mice, angioblasts have been identified as non-aggregated TAL1+(t-cell acute lymphocytic leukemia protein 1)/KDR+(kinase insert domain protein receptor, also known as vascular endothelial growth factor receptor 2 or fetal liver kinase 1) mesodermal cells of the E6.5 extraembryonic yolk sac, and after E7.5, endothelial cell differentiation is marked by the upregulation of Pecam1 (platelet endothelial cell adhesion molecule 1), Tek (endothelial-specific receptor tyrosine kinase, also known as tyrosine kinase with immunoglobin-like and egf-like domains 2 [Tie2]) and Laminin, followed by the downregulation of Tal114, 25.
In both mice and avians, extraembryonic angioblasts are found in the yolk sac clustered with hematopoietic cells in blood islands, whereas in zebrafish, blood islands are found intraembryonically in the lateral mesoderm14, 26–29. Due to this close association between these early endothelial and hematopoietic cells, it has long been postulated that the two cell types arise from a common bipotent progenitor termed a hemangioblast28, 29. However, the exact nature of this lineage relationship is controversial, particularly because different experimental models for exploring their lineage have often yielded multiple outcomes. For example, recent fate mapping and live imaging experiments have shown that at least a subset of all hematopoietic cells are derived from an endothelial cell intermediate, known as the hemogenic endothelium30–36. Further complicating the picture is the fact that in some cases, endothelial and hematopoietic precursors arise independently14, 37–39,40, most notably during intraembryonic vasculogenesis, where angioblasts arise alone14, 37, 38, 40. Future work will hopefully reconcile some of the opposing results observed using these different systems and further illuminate the subtleties of this relationship between vasculogenesis and hematopoiesis.
At present, the regulatory mechanisms that drive angioblast differentiation are not completely clear. Many factors that are required for the normal specification of endothelial cells are also required for hematopoietic development16, 41 so it is not clear how these two different cell types emerge in mammals. Although specific transcription factors expressed exclusively in hematopoietic cells have been identified, there is no known single transcription factor expressed in endothelial cells that could act to control endothelial specific transcription41. That said, it is clear that the regulatory regions of endothelial-specific genes share similarities in binding site motifs, and it has been proposed that a specific combination of factors may be required for endothelial specification41. Moreover, since there is a diversity of endothelial cell sub-types (arterial, venous and hemogenic endothelial cells), some of which arise in very early development, it is possible that there may be distinct pathways that regulate these different sub-types. More studies need to be performed to determine if all endothelial cells are specified via the same pathway, or if endothelial cell sub-types arise from lineage-restricted populations under the control of distinct factors.
While initial endothelial cell specification is established during gastrulation in the embryo, it is less clear how endothelial cell differentiation is regulated in the adult. Studies over the past 15 years have suggested that circulating endothelial progenitor cells (EPCs) that derive from hematopoietic and mesenchymal stem cells in the bone marrow42, 43 can contribute to new blood vessel formation or blood vessel repair in adult tissues44–52. However, conflicting studies make it difficult to ascertain whether EPCs are directly integrated into vessels, or if they contribute indirectly by releasing localized paracrine factors that promote proliferation of endogenous endothelial cells53–58.
VASCULOGENESIS: ASSEMBLY OF VESSELS FROM INDIVIDUAL CELLS
New vessels can be generated via two different, well-recognized processes. Vasculogenesis describes the process by which new vessels form from the coalescence and assembly of endothelial cells into functional vessels. Later, we will also discuss angiogenesis, which is the sprouting of new vessels from existing vessels. In some cases vasculogenesis and angiogenesis can occur together, but in the very early embryo, the first vessels including the presumptive dorsal aortae and aortic arches, the internal carotid arteries, and the anterior and posterior cardinal veins59, are assembled de novo from individual endothelial cells. For some other vessel networks, vasculogenesis results in the formation of an intermediate network of similar sized vessels with polygonally-shaped avascular spaces – known as a vascular or capillary plexus (Figure 1). These networks are often remodeled into branched tree-like hierarchies as the surrounding tissues mature, demanding more perfusion. Examples include the perineural plexus that surrounds the developing neural tube, the capillary plexus in the allantois that is remodeled into the umbilical vein and artery, and one of the first vessel structures in the embryo, the primitive capillary plexus of the visceral yolk sac in mammals and avians.
Surprisingly, little is known about the precise mechanisms that drive vasculogenesis in vivo. In the extraembryonic yolk sac of the quail, blood island clusters are composed of an outer layer of angioblasts that differentiate into flattened endothelial cells and surround an inner group of rounded primitive blood cells. Here, differentiating endothelial cells in the blood islands form sprouts and anastomose (interconnect) to other blood islands, ultimately forming the primitive yolk sac vascular plexus19, 23, 60–62. In this way, the yolk sac plexus serves the important function of connecting the first site of blood development, the blood islands, to the embryo proper. Similarly, in mice, primitive blood cells form one large band around the proximal yolk sac that is apparent by E7.5-E7.75, but here they only loosely associate with angioblasts, until eventually the differentiating endothelial cells ensheathe the blood cells through an unknown mechanism63. In contrast, intraembryonic vessels initially form independently of such an association with primitive blood cells by the de novo formation and coalescence of angioblasts into the major intraembryonic vessels and plexuses. It should be noted that though the coalescence of angioblasts is critical to the formation of the founding extraembryonic and intraembryonic vessel plexuses, it is unknown what extent angiogenesis plays in the formation of these early plexuses. Further studies need to be performed at the appropriate resolution to more clearly distinguish when these different mechanisms are enacted. It is clear, however, that as development proceeds angiogenesis becomes an increasingly important means of vessel formation.
Due to the complexity of studying vasculogenesis in a live animal, in vitro models using two-dimensional (2D) cultures or three-dimensional (3D) extracellular matrices have been used to enhance our understanding of the mechanisms of vasculogenesis64, 65. Such cultures have been implemented using human or murine embryonic stem cells that, under the appropriate culturing conditions, differentiate into a vasculature64. While many in vitro systems have been used to study endothelial cell biology, assays in 2D and 3D cultures have been able to recapitulate many fundamental aspects of early vasculogenic processes such as the dynamic migration of endothelial cells and pericytes, cord formation, as well as the formation of lumenized vessels. Using a combination of time-lapse microscopy, molecular genetic approaches and biochemical analysis, these studies have revealed that key interactions of isolated endothelial cells with various extracellular matrix (ECM) components, such as collagen and fibrin, can induce changes in endothelial morphogenesis that promote vascular assembly65.
ANGIOGENESIS: THE GROWTH OF NEW BLOOD VESSELS FROM A PREEXISTING VASCULATURE
Sprouting angiogenesis
In addition to vasculogenesis, new vessels can form by sprouting from the existing vasculature. This process underlies the formation of many vessel structures during development, including but not limited to formation of the intersegmental vessels, and vessels of the developing retina, limbs, yolk sac, heart and CNS15, 66–72. Similar processes are also utilized in response to hypoxia, tumor growth and wound healing in the adult organism11, 12, 73.
Vessel sprouting was first recognized in vivo in the late 1800s using light microscopy. Vessel sprouts were observed in tadpole tails and in the newt by Platner and Meyer, as characterized by the presence of thin fibers which expanded in diameter and later became hollow74–76. Such observations were also described during wound healing and in tumor angiogenesis in early studies77.
Despite these very early observations, the cellular and molecular mechanisms that regulate sprouting angiogenesis have only recently begun to be elucidated. Moreover, advances in genetic models and tools to analyze cell-signaling have revealed five key events associated with sprouting angiogenesis. First, cell-cell signaling initiates new vessel formation. Second, a subset of endothelial cells takes on the role of endothelial tip cells, which make up the distal-most cells of a temporarily close-ended vessel and extend lamellipodia and filopodia into the avascular space. Third, juxtaposed endothelial cells form endothelial stalk cells whose main function is to proliferate and provide more cells to extend the growing vessel73, 78, 79. Fourth, vessel outgrowth propels the sprout into the tissue perpendicular to the axis of the parent vessel. Last, individual sprouts anastomose with other vessels. These events (1–5) are illustrated in Figure 2, and are further described below. Though these events are demonstrated in a linear manner, changes in the vasculature and the extracellular environment may elicit a reprogramming of the angiogenic response; thus, there is some fluidity in endothelial tip versus stalk cell identity during vessel outgrowth and fusion.
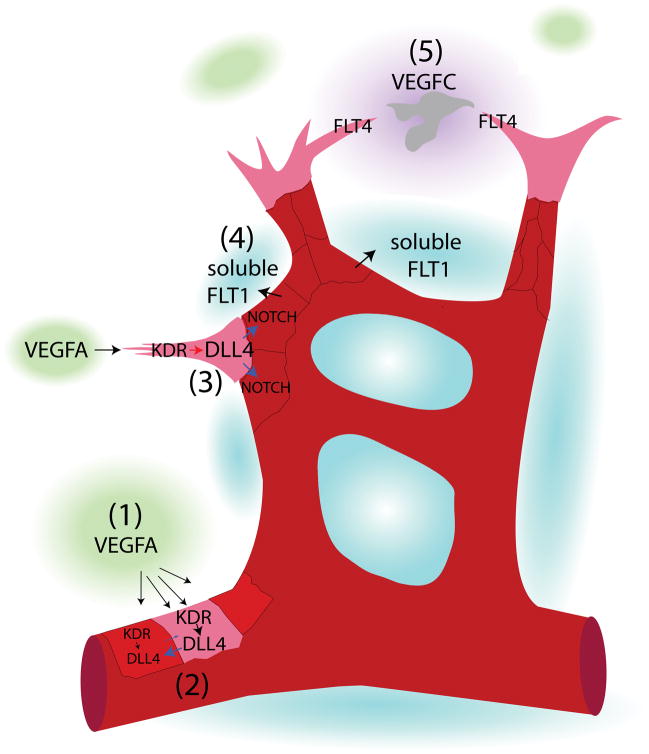
Figure 2.
The stages of sprouting angiogenesis. Sprouting angiogenesis occurs in stages. (1) Signaling to induce new vessel formation: VEGFA is secreted from tissues, resulting in KDR activation in nearby vessels. (2) Induction of a tip cell: an endothelial cell exposed to the highest concentration of VEGFA becomes a tip cell and exhibits the highest expression of DLL4. (3) Formation of stalk cells: in turn, the tip cell induces NOTCH activation in the juxtaposed neighboring cells to induce stalk cell fate. (4) Vessel outgrowth: stalk cells release soluble FLT1, which functions to bind available VEGFA in the vicinity. This increases the steepness of the VEGFA gradient, and stalk cell proliferation and tip cell pulling drives vessel outgrowth perpendicular to the length of the vessel. (5) Vessel fusion to other vessels: as the vessel grows toward VEGFA, release of VEGFC from macrophages (gray cell) activates FLT4 on endothelial tip cells to guide opposing tip cells together, to promote the conversion of tip cells to stalk cells, and to complete vessel anastamosis.
1) Signaling to induce new vessel formation
The induction of new vessels can be initiated by several known pro-angiogenic factors that are used in development, repair, homeostasis, and tumor angiogenesis80. Perhaps the most well-studied factors are those of the vascular endothelial growth factor (Vegf) family, which together with NOTCH signaling, are known to regulate new vessel outgrowth in a variety of different sites throughout embryogenesis80. There are several members of the Vegf family (Vegfa, Vegfb, Vegfc, Figf and Pgf), and multiple isoforms of VEGFA protein with different molecular weights that can each have different activities6, 81. VEGF ligands signal primarily through VEGF receptors (transmembrane receptor tyrosine kinases) and have diverse roles in endothelial cell differentiation, survival, vessel diameter regulation, vessel barrier integrity and endothelial cell chemotaxis82–88. In addition to these roles, VEGF also functions in mediating sprout induction and vessel outgrowth. Differences in the response of endothelial cells to VEGFA are likely to relate to signaling through different receptors and the activation of different intracellular pathways, including importantly the NOTCH pathway (discussed further below)16.
It is thought that the establishment of a VEGF gradient, initiated by hypoxia or cell-cell signaling, directs growing vessels89 (Figure 2). Though VEGF is a widely known mediator of sprouting angiogenesis, other factors have also been shown to direct vessel outgrowth. For instance, FGF2 (basic fibroblast growth factor) is a potent angiogenic factor in addition to playing diverse roles in many different tissues90, 91. Recently, it has been shown that FGF2 induces Kdr expression, adding to our understanding of how these factors may interact during angiogenesis92.
2) Induction of a tip cell
Tip cells are induced by high concentrations of VEGFA signaling through KDR to specify a single endothelial cell to lead the vessel sprout 93 (Figure 2). Endothelial tip cells are so-named because they are located at the distal end of a close-ended outgrowing vessel. Morphologically, they are characterized by the presence of lamellipodia and filopodia extensions, and they rarely proliferate. Molecularly, they express high levels of Dll4 (delta-like 4) mRNA93 and secrete proteinases which break down the basement membrane94.
3) Formation of stalk cells
Tip cells are flanked by endothelial stalk cells. In contrast to tip cells, stalk cells maintain adherens junctions and do not extend processes. These cells proliferate in response to VEGFA, and unlike tip cells, do have lumens93. Since only one endothelial tip cell is formed at the leading edge of a sprout, a mechanism must exist to prevent neighboring endothelial cells from also forming tip cells. Competition for tip cell versus stalk cell specification occurs via a NOTCH-mediated lateral inhibition mechanism, whereby the cell exposed to the highest amount of VEGF signaling upregulates Dll4 expression to become the NOTCH signal-sending cell and the tip cell (Figure 2). Then, neighboring NOTCH signal-receiving cells downregulate Kdr expression, reducing their ability to respond to VEGFA; these cells form endothelial stalk cells66, 79, 95.
4) Vessel outgrowth
After establishment of tip and stalk cells, vessel outgrowth begins. Recent studies in mouse embryoid bodies and mouse retinal vessels have shown that perpendicular vessel outgrowth occurs due to the production of soluble FLT1 (a soluble isoform of FMS-like tyrosine kinase 1, a VEGF receptor) in endothelial cells that neighbor the tip cell (Figure 2). Upregulation of soluble FLT1 in neighboring stalk cells provides a sink for VEGF ligand to increase the sharpness of the VEGF gradient and direct outgrowth perpendicular to the existing vessel96.
5) Vessel fusion to other vessels
Finally, once vessel outgrowth occurs, the sprouting vessels eventually undergo anastomosis to fuse to neighboring vessels. Though our understanding of the mechanisms that regulate anastomosis is incomplete, studies in the mouse hindbrain and retina, as well as in intersomitic vessels of zebrafish, show that macrophages can act as cell chaperones for vessel anastomosis (Figure 2)97–99. For example, the release of VEGFC by macrophages can activate FLT4 in endothelial tip cells to promote the rapid conversion of tip cells to stalks cells at vessel fusion points98. Though macrophages have also been implicated in aiding angiogenesis during repair100, 101 and facilitating interactions between tumor cells and vessels,100, 101 it is unknown whether macrophages also play a physical role in chaperoning vessel anastamosis.
Intussusceptive (splitting) angiogenesis
Another type of angiogenesis is called intussusception. Prior to the discovery of intussusception, it was thought that all vessel angiogenesis occurs via a sprouting angiogenesis mechanism. Though the process of intussusception was initially seen with longitudinal observations of the rabbit ear vasculature102, intussusception has now been characterized in different species (humans, rats, and birds), and in many different tissues and organs (lung, chorioallantoic membrane, kidney, heart, endometrium, ovary, retina, brain), as well as in disease (tumor induced angiogenesis)37, 103–114. In its simplest form, intussusception (also known as splitting angiogenesis) occurs in a vessel by the formation of an endothelial-lined pillar that extends into the middle of a vessel, followed by the expansion of pillar diameter, resulting in the splitting of the vessel (Figure 1). Studies within the past 25 years have provided the spatial resolution required to discern the cellular mechanisms of intussusception104, 105, 109–111, 115–120 (see below), but little is known about the mechanisms of transcapillary pillar induction. Analysis of computational models reveals that pillars form in areas of either low shear stress or oscillatory blood flow106, 121.
There are three major forms of intussusceptive angiogenesis – intussusceptive microvascular growth, intussusceptive arborization and intussusceptive branching remodeling. Intussusceptive microvascular growth is a mechanism for capillary expansion that was first identified in EM studies of pulmonary capillaries, but has been found in many tissue types116, 117. Observations in chick chorioallantoic membrane (CAM) vessels have directly documented pillar formation and confirmed the impact of the mechanism on microvascular growth104, 105, 109, 110. Other studies have shown that intussusceptive microvascular growth can allow for rapid expansion (on the order of hours) in the number of capillaries in a vessel bed with little contribution from cell proliferation or apoptosis, thus minimizing the amount of energy required for vessel expansion115.
Intussusception is utilized not only for the expansion of capillaries, but also for shaping vessel morphology. In intussusceptive arborization, vessel hierarchies are established by the formation and fusion of transcapillary pillars from unremodeled capillaries105. Thus, initially large capillaries are parsed into proximal feeding vessels that extend into progressively smaller distal capillaries. In intussusceptive branching remodeling, transcapillary pillars are formed at vessel bifurcations which eventually fuse to the connective tissue to decrease branch angles106.
LUMEN FORMATION IN DEVELOPING BLOOD VESSELS
Regardless of whether new vessels form by vasculogenesis or angiogenesis, they must eventually support blood flow, which requires that a lumen forms de novo or by extension from the parent vessel. Models of lumen formation in blood vessels date back to the early 1900s. Studies by Clark and Clark in the 1930s showed that newly sprouted vessels contained discontinuous vacuolation in rabbit ear vessels122–124, raising the idea that vacuole formation was a key mechanism to generating vessel lumens. Time-lapse microscopy studies in 3D in vitro cultures have beautifully shown that indeed, isolated endothelial cells undergo simultaneous sprouting and intracellular vacuolation. Interestingly, anastomosing endothelial cells undergo exocytosis of intracellular vacuoles to produce an internal lumen65. Here intracellular vacuoles ultimately coalesce, span the length of the cell and subsequently fuse to the plasma membrane and connect with the newly formed lumens in neighboring cells125. Work in 3D cultures has clearly shown that this process mechanistically depends on INTEGRIN/ECM interactions which signal through RAC1, RHOA and CDC42 GTPases65.
Though in vivo studies using time-lapse microscopy initially suggested that lumen formation in zebrafish occurs via the coalescence of intracellular vacuoles within endothelial cells126, follow up studies showed that these intersegmental vessels actually form by intercellular lumenogenesis127. Close inspection of intersegmental vessels reveals that they are comprised of more than one cell, and lumen formation occurs at cell-cell contacts. Still, intracellular vacuoles may undergo exocytosis with this extracellular space to at least partially contribute to lumen formation 127. Another model for intercellular lumen formation has recently been demonstrated in the developing mouse dorsal aortae. Here the dorsal aortae lumenize by the aggregation of angioblasts due to the establishment of specific extracellular interactions, followed by the reorganization of junctions at lateral regions between the cells, repulsion of the apical surface of adjacent cells, and the subsequent change in EC shape established by the apical actomyosin complexes128. Taken together, it appears as if an intercellular lumenogenesis mechanism is adopted in vivo, but whether intracellular lumenogenesis occurs in other vascular beds remains to be determined.
ESTABLISHING ARTERIAL, VENOUS AND LYMPHATIC IDENTITIES
Three distinct types of vessels are formed during development: the arterial network that carries blood away from the heart, the venous network that carries blood to the heart, and the lymphatic network that regulates lymph drainage by carrying small molecules and solutes from the tissues. As soon as the first vessels are established within the embryo, and in some cases even before blood flow begins, heterogeneity among endothelial cells is initiated by the specification of arterial-venous fates, although these fates are not irreversibly determined. In many experiments, arterial-venous fates have been assessed by the analysis of marker gene expression. In the avian yolk sac plexus, NRP1 (neuropilin 1, a KDR coreceptor expressed in arteries) and NRP2 (neuropilin 2, a KDR coreceptor expressed in veins) are found to be segregated in some blood islands and later in the presumptive arterial and venous regions before blood flow begins129. Separate arterial and venous expression in the yolk sac plexus is similarly observed in the mouse yolk sac with Efnb2 (ephrinB2) and EphB4 (eph receptor B4), respectively130. However in intraembryonic vessels of the mouse, expression of arterial markers (for example, Efnb2, Gja4/5, Hey1/2, Nrp1, Notch1/4, Dll4) precedes that of venous markers, and is found both in endothelial cells of the arteries (which form first) as well as endothelial cells that give rise to veins131. At a slightly later stage, the endothelial cells that give rise to veins start to coalesce to form the future cardinal veins, and these endothelial cells begin to express venous markers (for example, Nrp2, Aplnr [apelin receptor], and Flt4)131. Thus, within the mouse embryo, a specific spatiotemporal sequence is beginning to emerge for how arteries and veins are specified.
Recent studies have further illuminated the underlying molecular mechanisms of arterial/venous (A/V) specification. Studies in zebrafish have revealed that Shha (sonic hedgehog a), Vegfaa and Notch signaling may specify arterial and venous fates of the presumptive dorsal aorta and posterior cardinal vein132, 133. First, Shha released from the notochord stimulates the expression and release of Vegfaa from the paraxial mesoderm133. Second, exposure of the adjacent presumptive dorsal aortae to high levels of Vegfaa induces arterial differentiation and efnb2a expression through Notch activation. In contrast, the more distal presumptive cardinal vein is exposed to a lower amount of Vegfaa, Notch is not activated, and a program of venous differentiation begins132, 133. Supporting the zebrafish studies, mouse embryonic stem cells exposed to high or low concentrations of VEGFA begin expressing arterial (Efnb2, Nrp1) or venous (Nr2f2, also known as CoupTFII, and Nrp2) specific markers, respectively134. Another recent study has shown that the presumptive dorsal aorta and cardinal vein first form as a single cord, where the dorsal domain of the vessel is specified by NOTCH signaling to become arterial, and the ventral domain becomes venous 135. Then, via Efnb2/Ephb4 bidirectional signaling, venous-fated angioblasts sprout ventrally and segregate from the developing dorsal aorta to form the posterior cardinal vein135. Other studies in mice and zebrafish have further characterized NOTCH as a mediator of arterial specification, as downstream effectors of NOTCH (Hey1, Hey2 and gridlock) are required for proper arterial specification, while NOTCH activation and effector expression are absent in veins136, 137. Despite this genetic control in A/V specification, however, Moyon et al. have demonstrated plasticity in cell fates, as A/V grafts from the quail can incorporate into both arteries and veins of the chick138. Also, Le Noble et al. have revealed that the initiation of artery specification is flow independent, but that flow is important in maintaining A/V cell fates, as ligated (flow reduced) arteries differentiate into and connect with veins139. Similarly, in the mouse dorsal aortae, flow is required for expression of a select group of arterial markers131, 140. Thus, to some extent, hemodynamics and interactions with neighboring endothelial cells can influence the plasticity of endothelial cell fates.
A third endothelial cell subtype that arises after A/V specification is that of the lymphatic endothelial cell (LEC). Lymphatic vessels make up a tree-like vascular system that functions to return extravasated fluid from the interstitial environment back to the blood circulation, to aid in immune surveillance by transporting white blood cells, and to absorb lipids in the digestive tract141, 142 (Figure 3). LEC fate is specified in mice around E9.75 (embryonic day) in a subset of venous endothelial cells that make up the walls of the anterior cardinal vein (Figure 3). This process is controlled by Prox1 (prospero related homeobox 1), which has been shown to be both necessary and sufficient for inducing lymphatic differentiation of endothelial cells143–145. Transcriptional control of Prox1 expression is regulated by SOX18 (sry-related hmg-box transcription factor) and NR2F2. Though transient expression of Sox18 is found in the dorsal aorta, blood islands, cranial surface vessels, and microvasculature of the caudal trunk and hind limb146, 147, the convergent expression of Sox18 in the dorsal lateral region of the anterior cardinal vein together with venous expression of Nr2f2 is necessary for Prox1 expression in this region148–150. Following induction of Prox1 expression in venous endothelial cells of the cardinal vein, they begin to downregulate genes specific to blood endothelial cells and upregulate genes specific to LECs, including Flt4, Pdpn (podoplanin), and Foxc2 (forkhead box c2) transcription factor151–155. Importantly, Flt4 expression in these newly specified LECs makes them competent to respond to VEGFC signaling from the nearby mesenchyme, and at E10.5 they begin to bud off from the cardinal vein in response. During this process, clot formation by platelets is essential for closing off the connection between the cardinal vein and the nascent lymphatic vasculature151, 156. Once this process is complete, the LECs coalesce to form the lymphatic sacs, which will eventually give rise to the rest of the lymphatic vasculature via sprouting angiogenesis (see above)157–159. Studies in zebrafish and Xenopus have shown that many of these pathways regulating lymphatic development are highly conserved160–163.
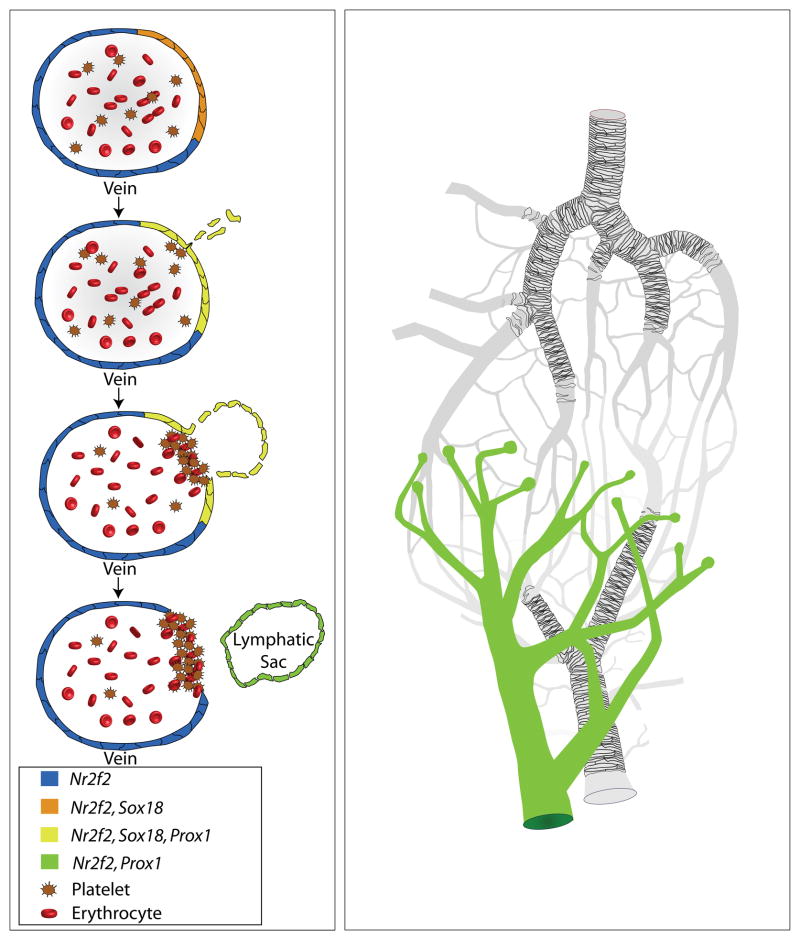
Figure 3.
Lymphangiogenesis. During development, lymphatic vessels form from veins. Veins entirely expressing Nr2f2 (blue and orange cells) exhibit a subpopulation of cells that also express Sox18 (orange cells). The coexpression of Nr2f2 and Sox18 confers expression of Prox1 (yellow cells). Prox1 expressing cells migrate away from the vein and downregulate Sox18 (green cells). These cells then connect to one another to form the lymphatic sac, which ultimately forms the lymphatic vasculature.
VESSEL REMODELING AND MATURATION: CREATING STABILIZED VESSEL HIERARCHIES
Vascular remodeling
Simultaneous to embryonic vasculogenesis in the mouse, the heart tube also forms, connects to the dorsal aortae by 0-3 somites, and becomes functional by 3 somites, where functionality is first determined by the onset of plasma flow164. Then, as blood cells enter the circulation at 5–6 somites165, 166, hemodynamic action marks the onset of an aspect of vascular development that is important for the maturation and stabilization of vessels—vascular remodeling. In the adult, vessel remodeling is usually a term used to describe a change in the size of a vessel (inward remodeling, which is a reduction in vessel diameter, and outward remodeling, which is an enlargement of vessel diameter) to enable vessels to cope with changes in blood flow167, 168. However, during early embryonic development, vascular remodeling mainly serves in the maturation of blood vessels from a primitive vasculature to a hierarchical network of large-diameter proximal arteries and veins that feeds into the more extensive network of small-diameter distal capillaries (Figure 1).
For over one hundred years it has been posited that physical forces imparted by blood flow directly influence changes in the endothelium that are responsible for vascular remodeling169–171. Recently, it has been shown that viscous forces imparted by blood flow are necessary and sufficient for normal vascular remodeling of the mouse yolk sac vascular plexus, independently of changes in oxygenation or a circulating signal164, 172. Other studies also implicate mechanical forces imparted by blood flow in regulating the remodeling of the heart, aortic arches, and other vessels in a variety of animal models169, 172–177. In all of these cases, it is still unclear how mechanical forces are interpreted by cells at the molecular level in vivo. A large body of work shows that exposure of endothelial cells in cell culture to fluid shear stress can activate a large number of mechanotransduction pathways and induce changes in several EC behaviors that could influence remodeling, including endothelial cell migration, alignment, proliferation and apoptosis178–183. However, it remains to be determined which mechanisms are utilized in vivo during development. This has proven to be difficult for several reasons. First, it is highly likely that signaling pathways that regulate mechanotransduction in vivo are also used to regulate other events such as growth factor signaling, interactions with the ECM, survival, and proliferation. Thus, the role in mechanotransduction is not likely to be revealed by simple genetic analysis or through the use of inhibitors unless sophisticated assays are used. Second, we have only begun to understand exactly which cellular behaviors are altered by mechanical signals in vivo. Understanding these questions would be a tremendous benefit to the design of more specific experiments to test this hypothesis.
Vessel Maturation
Once vessel formation and remodeling are complete, vessel stability and tissue homeostasis becomes crucial for a new vessel. This is partially achieved through the recruitment of smooth muscle cells and pericytes, collectively known as mural cells, to the maturing vessel wall6. During maturation, endothelial cells recruit and promote the proliferation of mural cell precursors by secreting PDGFB (platelet-derived growth factor subunit b), which binds to and activates PDGFRB (PDGF receptor beta) expressed by mural cell precursors (Figure 4). Once mural cell precursors are recruited to the endothelium, they undergo reciprocal signaling by secreting ANGPT1 (angiopoietin1), which then binds to the TEK receptor expressed on the surface of endothelial cells184–186. Ultimately, this promotes the formation of cell-cell adhesions that allow mural cells to associate with the vessel wall187. In parallel, mural cell precursors undergo endothelial-contact dependent mural cell differentiation. This requires gap junction communication via GJA1 (gap junction protein alpha1)188 and GJC1 (gap junction protein gamma1),189 and TGFB1 (transforming growth factor-beta1) signal activation190. Currently, it is thought that the latent form of TGFB1 ligand is secreted by endothelial cells and differentiating mural cells. Upon endothelial-mural cell interaction, the latent form is cleaved and activated to induce TGFB1 receptor activation, in both cell types191–193, through the TGFB1 receptors, ACVRL1 (activin A receptor, type2 like) and TGFBR1 (transforming growth factor, beta receptor 1). TGFB1 signaling has been shown to induce mural cell marker expression such as Acta2 (smooth muscle alpha actin) via upregulation of Srf (serum response factor)194, to inhibit proliferation of endothelial and mural cells193, and to induce secretion of elastin from smooth muscle cells as well as fibroblasts195–197. It is worth noting that TGFB1 receptor activation appears to depend on gap junction formation (via connexins), but it is unclear how this occurs. Ultimately, the association of differentiated mural cells with endothelial cells allows the vessel wall to be stabilized and creates quiescent vessel beds where remodeling has ceased187. This vessel stabilization is reversible to allow for further remodeling when needed. Secretion of ANGPT2, an antagonist of ANGPT1/TEK signaling that is expressed in regions of pronounced vascular remodeling, is thought to be one mechanism by which this disruption of vessel homeostasis is achieved187, 198.
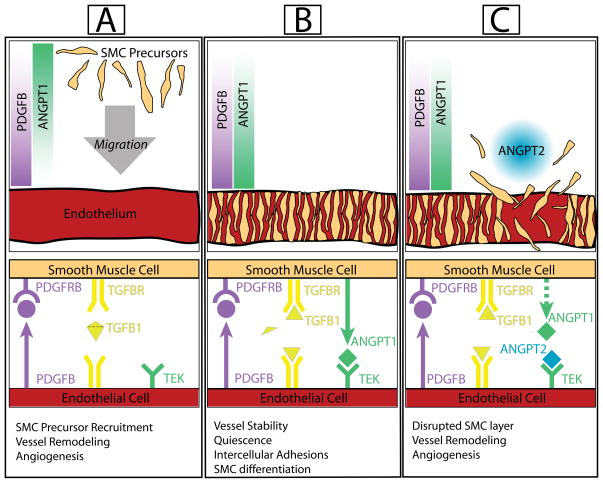
Figure 4.
Vessel maturation. New vessels (created during remodeling and angiogenesis) begin maturation by the recruitment of differentiating mural cells (pericytes and smooth muscle cells). For example, differentiating smooth muscle cells (SMC precursors) migrate toward a gradient of PDGFB (released from endothelial cells) resulting in activation of PDGFB receptors on SMC precursors (a). Upon the close proximity of SMC precursors to endothelial cells, SMC precursor-released ANGPT1 induces TEK activation on endothelial cells, which enhances SMC-endothelial adhesion. Simultaneously, the close proximity of these cells allows for latent TGFB1 (released by both cell types) to become activated. This induces proliferation arrest (quiescence) in SMC precursors and endothelial cells, and induces differentiation of SMCs, yielding vessel stability (b). When a new remodeling or angiogenic mechanism is enacted, competition of ANGPT2 with ANGPT1 for the TEK receptor can disrupt the endothelial-SMC interaction (c).
As development proceeds, mural cells become crucial for the maintenance of vessel integrity. Contractile pericytes and smooth muscle cells become critical for enabling the vessels to withstand the increasing pressures 199–201. Fittingly, mural cell recruitment is differentially regulated according to endothelial subtype, as well as hemodynamics, where arterial endothelial cells exposed to higher shear stress recruit more support cells in order to strengthen the arterial wall and distribute the force of the flow202, 203. Furthermore, by coupling smooth muscle cells to endothelial cells with gap junctions, smooth muscle cell contractions induced by vasodilators and vasoconstrictors can be coordinated with the endothelium204–207. Later, this becomes an important method for regulating blood flow.
One aspect of mural cell biology that is still poorly understood, however, is the developmental origins of these cells. Intriguingly, fate mapping studies suggest that during development, vascular smooth muscle cells are derived from a wide variety of different cell types208, including mesothelium209, 210, mesoangioblasts211, proepicardium212–214, secondary heart field215, 216, neural crest217, 218, and somites219, 220. The mechanisms by which the members of this diverse set of cell types retain the competency to differentiate into mural cells are still largely a mystery, as are the implications that these lineage relationships have for our understanding of how mural cell types first arose during evolution. Additionally, the role that this heterogeneity plays in smooth muscle function in the adult is still under investigation. Studies examining TGFB1 signaling in vitro have shown that smooth muscle cells from different origins have different functional responses to TGFB1, and a few published studies offer clues that this is true for in vivo systems as well221, 222, but a full understanding of this functional heterogeneity is still lacking.
PATTERNING BLOOD VESSELS DURING ORGANOGENESIS
Vasculogenesis, angiogenesis, remodeling, and mural cell recruitment are all mechanisms by which new vessels form and mature. However, the ultimate pattern that vessels adopt in various tissues can be quite different. Figure 5 shows examples of three different microvasculatures in the cerebral cortex, the heart, and the kidney, showing that even similarly-sized vessels can be arranged quite differently to adapt to tissue structure and function. Recently it has been shown that vessel pattern, like neuron outgrowth, can be guided by specific signals. For example, various transmembrane axonal guidance molecules (eph family receptor tyrosine kinases, roundabouts, slits, semaphorins, netrins, UNC5B, plexins, and neuropilins) can direct vessel outgrowth away from or towards non-vascular cells by juxtacrine or paracrine signaling79, 80. A paracrine approach is often utilized via the release of VEGFA from non-vascular cells that are already patterned in order to create a similar vascular pattern by guiding vessel sprouts89. Incidentally, Vegfa expression can be stimulated by a flow-induced genetic pathway, implicating a role for hemodynamic force in regulating vessel patterning as well176. Other molecules, such as the FGF2 that is released from podocytes in the kidney, can induce intussusceptive microvascular growth120. In addition to promoting angiogenesis, signals from non-vascular cells can also specify endothelial heterogeneity. For instance, both VEGFA (which is expressed highly by the glomerular podocytes) and TGFB1 can regulate vessel permeability by inducing the formation of endothelial fenestrae in the glomerular capillaries223–225. Taken together, vessel patterning during angiogenesis seems to occur by distinct molecular interactions with neighboring cells and their environment.
Figure 5.
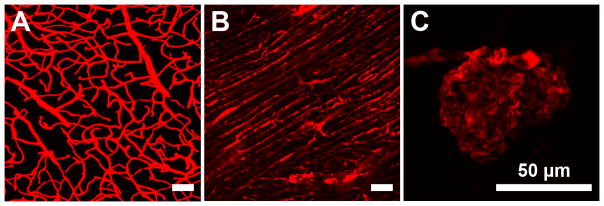
Variability in vessel morphology and function. Differences in vessel bed morphology, induced by organ-specific patterning, are easily discernible. Vessels of the adult mouse cerebral cortex show a tree-like pattern that maximizes blood flow to all of the cells in the brain (a). Vessels of the adult mouse heart are organized in a manner that aligns with the cardiomyocytes (b). Finally, vessels in the adult mouse kidney show a convoluted vascular structure (c).
SUMMARY
The last century of research has defined many of the events required for normal vessel development. These insights have led to a greater understanding of not only how vessels form and undergo repair, but also how the same molecular pathways influence adult vessel physiology and cardiovascular disease. However, despite the advances in our knowledge, there are still many open questions. As discussed above, the upstream mechanisms controlling initial endothelial cell specification are not well understood. Persistent mysteries surround the origins of pericytes, and how endothelial cells sense and respond to mechanical force still remains unclear. These and many other open questions continue to fuel research in this area with profound implications for clinical translation. New tools and methods to assess cell lineage relationships, to image angiogenesis or to measure how forces are interpreted by cells are leading the charge into the next decade of vessel research, and will undoubtedly add to our understanding of vessel development, likely adding additional unanticipated layers of complexity that drive future investigation.
References
- 1.Yebra M, Diaferia GR, Montgomery AM, Kaido T, Brunken WJ, Koch M, Hardiman G, Crisa L, Cirulli V. Endothelium-derived Netrin-4 supports pancreatic epithelial cell adhesion and differentiation through integrins alpha2beta1 and alpha3beta1. PLoS One. 2011;6:e22750. doi: 10.1371/journal.pone.0022750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, et al. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011;138:4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serini G, Bussolino F. Common cues in vascular and axon guidance. Physiology (Bethesda) 2004;19:348–354. doi: 10.1152/physiol.00021.2004. [DOI] [PubMed] [Google Scholar]
- 4.Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Rossant J, Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- 7.Eichmann A, Yuan L, Moyon D, Lenoble F, Pardanaud L, Breant C. Vascular development: from precursor cells to branched arterial and venous networks. Int J Dev Biol. 2005;49:259–267. doi: 10.1387/ijdb.041941ae. [DOI] [PubMed] [Google Scholar]
- 8.Augustin HG, Braun K, Telemenakis I, Modlich U, Kuhn W. Ovarian angiogenesis. Phenotypic characterization of endothelial cells in a physiological model of blood vessel growth and regression. Am J Pathol. 1995;147:339–351. [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser HM, Duncan WC. Vascular morphogenesis in the primate ovary. Angiogenesis. 2005;8:101–116. doi: 10.1007/s10456-005-9004-y. [DOI] [PubMed] [Google Scholar]
- 10.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 12.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 13.Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1059–1067. doi: 10.1152/ajpregu.00347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 15.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 16.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 17.Beck L, Jr, D’Amore PA. Vascular development: cellular and molecular regulation. Faseb J. 1997;11:365–373. [PubMed] [Google Scholar]
- 18.Labastie MC, Poole TJ, Peault BM, Le Douarin NM. MB1, a quail leukocyte-endothelium antigen: partial characterization of the cell surface and secreted forms in cultured endothelial cells. Proc Natl Acad Sci U S A. 1986;83:9016–9020. doi: 10.1073/pnas.83.23.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardanaud L, Altmann C, Kitos P, Dieterlen-Lievre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- 20.Miller AM, McWhorter JE. Experiments on the development of blood vessels in the Area Pellucida and embryonic body of the chick. Anatomical Record. 1914;8:203–227. [Google Scholar]
- 21.Pardanaud L, Luton D, Prigent M, Bourcheix LM, Catala M, Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- 22.Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool. 1989;251:224–231. doi: 10.1002/jez.1402510210. [DOI] [PubMed] [Google Scholar]
- 23.Sabin FR. Contr Embryol. 1917;6:61–124. [Google Scholar]
- 24.Coffin JD, Poole TJ. Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordia in quail embryos. Development. 1988;102:735–748. doi: 10.1242/dev.102.4.735. [DOI] [PubMed] [Google Scholar]
- 25.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9. 5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 26.Chen AT, Zon LI. Zebrafish blood stem cells. J Cell Biochem. 2009;108:35–42. doi: 10.1002/jcb.22251. [DOI] [PubMed] [Google Scholar]
- 27.Flamme I, Breier G. The role of vascular endothelial growth factors and their receptors during embryonic vascular development. Boston: Birhauser; 2002. [Google Scholar]
- 28.His W. Lechitoblast und angioblast der wirbeltiere. Abhandl Math-Phys Ges Wiss. 1900;26:171–328. [Google Scholar]
- 29.Sabin FR. Studies on the origin of blood vessels and of red blood corpuscles as seen in the living blastoderm of chick during the second day of incubation. Contr Embryology. 1920;9:215–262. [Google Scholar]
- 30.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 32.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 33.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 35.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zovein AC, Turlo KA, Ponec RM, Lynch MR, Chen KC, Hofmann JJ, Cox TC, Gasson JC, Iruela-Arispe ML. Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood. 2010;116:3435–3444. doi: 10.1182/blood-2010-04-279497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardanaud L, Yassine F, Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- 38.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 39.Xiong JW. Molecular and developmental biology of the hemangioblast. Dev Dyn. 2008;237:1218–1231. doi: 10.1002/dvdy.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 41.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patenaude A, Parker J, Karsan A. Involvement of endothelial progenitor cells in tumor vascularization. Microvasc Res. 2010;79:217–223. doi: 10.1016/j.mvr.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 44.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 45.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 46.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 47.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 49.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 50.Sata M. Role of circulating vascular progenitors in angiogenesis, vascular healing, and pulmonary hypertension: lessons from animal models. Arterioscler Thromb Vasc Biol. 2006;26:1008–1014. doi: 10.1161/01.ATV.0000206123.94140.f3. [DOI] [PubMed] [Google Scholar]
- 51.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 53.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 54.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 55.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 56.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 57.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 58.Caplice NM, Doyle B. Vascular progenitor cells: origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev. 2005;14:122–139. doi: 10.1089/scd.2005.14.122. [DOI] [PubMed] [Google Scholar]
- 59.Cleaver O, Krieg PA. Molecular Mechanisms of Vascular Development. In: Harvey P, Rosenthal N, editors. Heart Development. Boston: Academic Press; 1999. pp. 221–252. [Google Scholar]
- 60.Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- 61.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 62.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. J Hematother Stem Cell Res. 2002;11:5–7. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- 63.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Jakobsson L, Kreuger J, Claesson-Welsh L. Building blood vessels--stem cell models in vascular biology. J Cell Biol. 2007;177:751–755. doi: 10.1083/jcb.200701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268:252–275. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- 66.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 67.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 69.Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 70.Breier G, Risau W. The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol. 1996;6:454–456. doi: 10.1016/0962-8924(96)84935-x. [DOI] [PubMed] [Google Scholar]
- 71.Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 72.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudlicka O, Tyler KR. Angiogenesis: the growth of the vascular system. London; Orlando, FL: Academic Press; 1986. [Google Scholar]
- 75.Meyer J. Ann Charite. 1853;4:41–140. [Google Scholar]
- 76.Platner EA. Müller’s archiv für anat, phys und wissensch medicin. 1844. Einige beobachtungen über die entwicklung der kapillargefäße (Some observations on the development of capillaries) [Google Scholar]
- 77.Billroth T. Untersuchungen über die entwicklung der blutgefäße (studies on the development of blood vessels) 1856. [Google Scholar]
- 78.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 79.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 80.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mac Gabhann F, Popel AS. Systems biology of vascular endothelial growth factors. Microcirculation. 2008;15:715–738. doi: 10.1080/10739680802095964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. Exs. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 83.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 85.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 86.Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- 87.Kohn S, Nagy JA, Dvorak HF, Dvorak AM. Pathways of macromolecular tracer transport across venules and small veins. Structural basis for the hyperpermeability of tumor blood vessels. Lab Invest. 1992;67:596–607. [PubMed] [Google Scholar]
- 88.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 89.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M. FGF-dependent regulation of VEGF receptor 2 expression in mice. J Clin Invest. 2011;121:2668–2678. doi: 10.1172/JCI44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 94.Glaser BM, Kalebic T, Garbisa S, Connor TB, Jr, Liotta LA. Degradation of basement membrane components by vascular endothelial cells: role in neovascularization. Ciba Found Symp. 1983;100:150–162. doi: 10.1002/9780470720813.ch10. [DOI] [PubMed] [Google Scholar]
- 95.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 100.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 101.Rahat MA, Bitterman H, Lahat N. Molecular mechanisms regulating macrophage response to hypoxia. Front Immunol. 2011;2:45. doi: 10.3389/fimmu.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark ER, Clark EL. Microscopic observations on the extra-endothelial cells of living mammalian blood vessels. Am J Anat. 1940;66:1–49. [Google Scholar]
- 103.Djonov V, Andres AC, Ziemiecki A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001;52:182–189. doi: 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 104.Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res. 2000;86:286–292. doi: 10.1161/01.res.86.3.286. [DOI] [PubMed] [Google Scholar]
- 105.Djonov VG, Galli AB, Burri PH. Intussusceptive arborization contributes to vascular tree formation in the chick chorio-allantoic membrane. Anat Embryol (Berl) 2000;202:347–357. doi: 10.1007/s004290000126. [DOI] [PubMed] [Google Scholar]
- 106.Djonov VG, Kurz H, Burri PH. Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev Dyn. 2002;224:391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- 107.Gambino LS, Wreford NG, Bertram JF, Dockery P, Lederman F, Rogers PA. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum Reprod. 2002;17:1199–1206. doi: 10.1093/humrep/17.5.1199. [DOI] [PubMed] [Google Scholar]
- 108.Gargett CE, Lederman F, Heryanto B, Gambino LS, Rogers PA. Focal vascular endothelial growth factor correlates with angiogenesis in human endometrium. Role of intravascular neutrophils. Hum Reprod. 2001;16:1065–1075. doi: 10.1093/humrep/16.6.1065. [DOI] [PubMed] [Google Scholar]
- 109.Patan S, Alvarez MJ, Schittny JC, Burri PH. Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch Histol Cytol. 1992;55 (Suppl):65–75. doi: 10.1679/aohc.55.suppl_65. [DOI] [PubMed] [Google Scholar]
- 110.Patan S, Haenni B, Burri PH. Evidence for intussusceptive capillary growth in the chicken chorio-allantoic membrane (CAM) Anat Embryol (Berl) 1993;187:121–130. doi: 10.1007/BF00171743. [DOI] [PubMed] [Google Scholar]
- 111.Patan S, Haenni B, Burri PH. Implementation of intussusceptive microvascular growth in the chicken chorioallantoic membrane (CAM): 1. pillar formation by folding of the capillary wall. Microvasc Res. 1996;51:80–98. doi: 10.1006/mvre.1996.0009. [DOI] [PubMed] [Google Scholar]
- 112.Patan S, Munn LL, Tanda S, Roberge S, Jain RK, Jones RC. Vascular morphogenesis and remodeling in a model of tissue repair: blood vessel formation and growth in the ovarian pedicle after ovariectomy. Circ Res. 2001;89:723–731. doi: 10.1161/hh2001.097870. [DOI] [PubMed] [Google Scholar]
- 113.van Groningen JP, Wenink AC, Testers LH. Myocardial capillaries: increase in number by splitting of existing vessels. Anat Embryol (Berl) 1991;184:65–70. doi: 10.1007/BF01744262. [DOI] [PubMed] [Google Scholar]
- 114.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 115.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 116.Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec. 1990;228:35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- 117.Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec. 1986;216:154–164. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- 118.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–117. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 119.Makanya AN, Hlushchuk R, Baum O, Velinov N, Ochs M, Djonov V. Microvascular endowment in the developing chicken embryo lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1136–1146. doi: 10.1152/ajplung.00371.2006. [DOI] [PubMed] [Google Scholar]
- 120.Makanya AN, Stauffer D, Ribatti D, Burri PH, Djonov V. Microvascular growth, development, and remodeling in the embryonic avian kidney: the interplay between sprouting and intussusceptive angiogenic mechanisms. Microsc Res Tech. 2005;66:275–288. doi: 10.1002/jemt.20169. [DOI] [PubMed] [Google Scholar]
- 121.Styp-Rekowska B, Hlushchuk R, Pries AR, Djonov V. Intussusceptive angiogenesis: pillars against the blood flow. Acta Physiol (Oxf) 2011;202:213–223. doi: 10.1111/j.1748-1716.2011.02321.x. [DOI] [PubMed] [Google Scholar]
- 122.Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1939;64:251–301. [Google Scholar]
- 123.Clark ER, Clark EL. Observations of living preformed blood vessels as seen in a transparent chamber inserted into the rabbit ear. Am J Anat. 1932;49:441–447. [Google Scholar]
- 124.Clark ER, Clark EL. Observations on changes in blood vascular endothelium in the living animal. Am J Anat. 1935;57:385–438. [Google Scholar]
- 125.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 127.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 128.Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 129.Herzog Y, Guttmann-Raviv N, Neufeld G. Segregation of arterial and venous markers in subpopulations of blood islands before vessel formation. Dev Dyn. 2005;232:1047–1055. doi: 10.1002/dvdy.20257. [DOI] [PubMed] [Google Scholar]
- 130.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 131.Chong DC, Koo Y, Xu K, Fu S, Cleaver O. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn. 2011;240:2153–2165. doi: 10.1002/dvdy.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 133.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 134.Lanner F, Sohl M, Farnebo F. Functional arterial and venous fate is determined by graded VEGF signaling and notch status during embryonic stem cell differentiation. Arterioscler Thromb Vasc Biol. 2007;27:487–493. doi: 10.1161/01.ATV.0000255990.91805.6d. [DOI] [PubMed] [Google Scholar]
- 135.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]
- 138.Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- 139.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 140.Nguyen HL, Lee YJ, Shin J, Lee E, Park SO, McCarty JH, Oh SP. TGF-beta signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab Invest. 2011;91:1554–1563. doi: 10.1038/labinvest.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oliver G, Srinivasan RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development. 2010;137:363–372. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 144.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Downes M, Koopman P. SOX18 and the transcriptional regulation of blood vessel development. Trends Cardiovasc Med. 2001;11:318–324. doi: 10.1016/s1050-1738(01)00131-1. [DOI] [PubMed] [Google Scholar]
- 147.Olsson JE, Kamachi Y, Penning S, Muscat GE, Kondoh H, Koopman P. Sox18 expression in blood vessels and feather buds during chicken embryogenesis. Gene. 2001;271:151–158. doi: 10.1016/s0378-1119(01)00505-4. [DOI] [PubMed] [Google Scholar]
- 148.Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 149.Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, Oliver G. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24:696–707. doi: 10.1101/gad.1859310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells. 2009;14:425–434. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 151.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 153.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 155.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. Embo J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carramolino L, Fuentes J, Garcia-Andres C, Azcoitia V, Riethmacher D, Torres M. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res. 2010;106:1197–1201. doi: 10.1161/CIRCRESAHA.110.218073. [DOI] [PubMed] [Google Scholar]
- 157.Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- 158.Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1:367–389. [Google Scholar]
- 159.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 160.Hogan BM, Herpers R, Witte M, Helotera H, Alitalo K, Duckers HJ, Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- 161.Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires VEGFC signaling. Curr Biol. 2006;16:1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 162.Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- 163.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 164.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jones EA, Baron MH, Fraser SE, Dickinson ME. Dynamic in vivo imaging of mammalian hematovascular development using whole embryo culture. Methods Mol Med. 2005;105:381–394. doi: 10.1385/1-59259-826-9:381. [DOI] [PubMed] [Google Scholar]
- 166.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol. 2004;287:H1561–1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 167.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 168.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 169.Chapman W. The effect of the heart-beat upon development of the vascular system in chick. Am J Anat. 1918;23:175–203. [Google Scholar]
- 170.Murray CD. The Physiological Principle Of Minimum Work Applied To The Angle Of Branching Of Arteries. J Gen Physiol. 1926;9:835–841. doi: 10.1085/jgp.9.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thoma R. Untersuchungen uber die Histogenese and Histomechanik des Gefassystems. 1893. [Google Scholar]
- 172.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17:164–178. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res. 1999;41:87–99. doi: 10.1016/s0008-6363(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 174.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 175.Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu PH, Evans S, Chen J. Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development. 2003;130:6111–6119. doi: 10.1242/dev.00831. [DOI] [PubMed] [Google Scholar]
- 176.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]
- 178.Califano JP, Reinhart-King CA. Exogenous and endogenous force regulation of endothelial cell behavior. J Biomech. 2010;43:79–86. doi: 10.1016/j.jbiomech.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 179.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 180.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 181.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 182.Simmers MB, Pryor AW, Blackman BR. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. Am J Physiol Heart Circ Physiol. 2007;293:H1937–1946. doi: 10.1152/ajpheart.00534.2007. [DOI] [PubMed] [Google Scholar]
- 183.Ziegler T, Nerem RM. Effect of flow on the process of endothelial cell division. Arterioscler Thromb. 1994;14:636–643. doi: 10.1161/01.atv.14.4.636. [DOI] [PubMed] [Google Scholar]
- 184.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 185.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 186.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 187.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 188.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 189.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 190.Carvalho RL, Jonker L, Goumans MJ, Larsson J, Bouwman P, Karlsson S, Dijke PT, Arthur HM, Mummery CL. Defective paracrine signalling by TGFbeta in yolk sac vasculature of endoglin mutant mice: a paradigm for hereditary haemorrhagic telangiectasia. Development. 2004;131:6237–6247. doi: 10.1242/dev.01529. [DOI] [PubMed] [Google Scholar]
- 191.Hirota S, Liu Q, Lee HS, Hossain MG, Lacy-Hulbert A, McCarty JH. The astrocyte-expressed integrin alphavbeta8 governs blood vessel sprouting in the developing retina. Development. 2011;138:5157–5166. doi: 10.1242/dev.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hirschi KK, Lai L, Belaguli NS, Dean DA, Schwartz RJ, Zimmer WE. Transforming growth factor-beta induction of smooth muscle cell phenotpye requires transcriptional and post-transcriptional control of serum response factor. J Biol Chem. 2002;277:6287–6295. doi: 10.1074/jbc.M106649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. Embo J. 2007;26:3283–3295. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marigo V, Volpin D, Vitale G, Bressan GM. Identification of a TGF-beta responsive element in the human elastin promoter. Biochem Biophys Res Commun. 1994;199:1049–1056. doi: 10.1006/bbrc.1994.1335. [DOI] [PubMed] [Google Scholar]
- 197.Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, Jacob MP. Localization of elastin mRNA and TGF-beta1 in rat aorta and caudal artery as a function of age. Cell Tissue Res. 1998;291:305–314. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- 198.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 199.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oishi K, Kamiyashiki T, Ito Y. Isometric contraction of microvascular pericytes from mouse brain parenchyma. Microvasc Res. 2007;73:20–28. doi: 10.1016/j.mvr.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 201.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hsieh HJ, Li NQ, Frangos JA. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991;260:H642–646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- 203.Mitsumata M, Fishel RS, Nerem RM, Alexander RW, Berk BC. Fluid shear stress stimulates platelet-derived growth factor expression in endothelial cells. Am J Physiol. 1993;265:H3–8. doi: 10.1152/ajpheart.1993.265.1.H3. [DOI] [PubMed] [Google Scholar]
- 204.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 205.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- 206.Sokoya EM, Burns AR, Setiawan CT, Coleman HA, Parkington HC, Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2006;291:H385–393. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- 207.Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 209.Perez-Pomares JM, Macias-Lopez D, Garcia-Garrido L, Munoz-Chapuli R. Immunohistochemical evidence for a mesothelial contribution to the ventral wall of the avian aorta. Histochem J. 1999;31:771–779. doi: 10.1023/a:1003997919311. [DOI] [PubMed] [Google Scholar]
- 210.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 211.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 212.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 214.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 215.Maeda J, Yamagishi H, McAnally J, Yamagishi C, Srivastava D. Tbx1 is regulated by forkhead proteins in the secondary heart field. Dev Dyn. 2006;235:701–710. doi: 10.1002/dvdy.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 217.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 218.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 219.Christ B, Huang R, Scaal M. Formation and differentiation of the avian sclerotome. Anat Embryol (Berl) 2004;208:333–350. doi: 10.1007/s00429-004-0408-z. [DOI] [PubMed] [Google Scholar]
- 220.Esner M, Meilhac SM, Relaix F, Nicolas JF, Cossu G, Buckingham ME. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133:737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- 221.Rosenquist TH, Kirby ML, van Mierop LH. Solitary aortic arch artery. A result of surgical ablation of cardiac neural crest and nodose placode in the avian embryo. Circulation. 1989;80:1469–1475. doi: 10.1161/01.cir.80.5.1469. [DOI] [PubMed] [Google Scholar]
- 222.Topouzis S, Majesky MW. Smooth Muscle Lineage Diversity in the Chick Embryo. Dev Biol. 1996;178:430–445. [PubMed] [Google Scholar]
- 223.Ballermann BJ, Stan RV. Resolved: capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol. 2007;18:2432–2438. doi: 10.1681/ASN.2007060687. [DOI] [PubMed] [Google Scholar]
- 224.Datta K, Li J, Karumanchi SA, Wang E, Rondeau E, Mukhopadhyay D. Regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF-A) expression in podocytes. Kidney Int. 2004;66:1471–1478. doi: 10.1111/j.1523-1755.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- 225.Matsunaga Y, Yamazaki Y, Suzuki H, Morita T. VEGF-A and VEGF-F evoke distinct changes in vascular ultrastructure. Biochem Biophys Res Commun. 2009;379:872–875. doi: 10.1016/j.bbrc.2008.12.129. [DOI] [PubMed] [Google Scholar]