Abstract
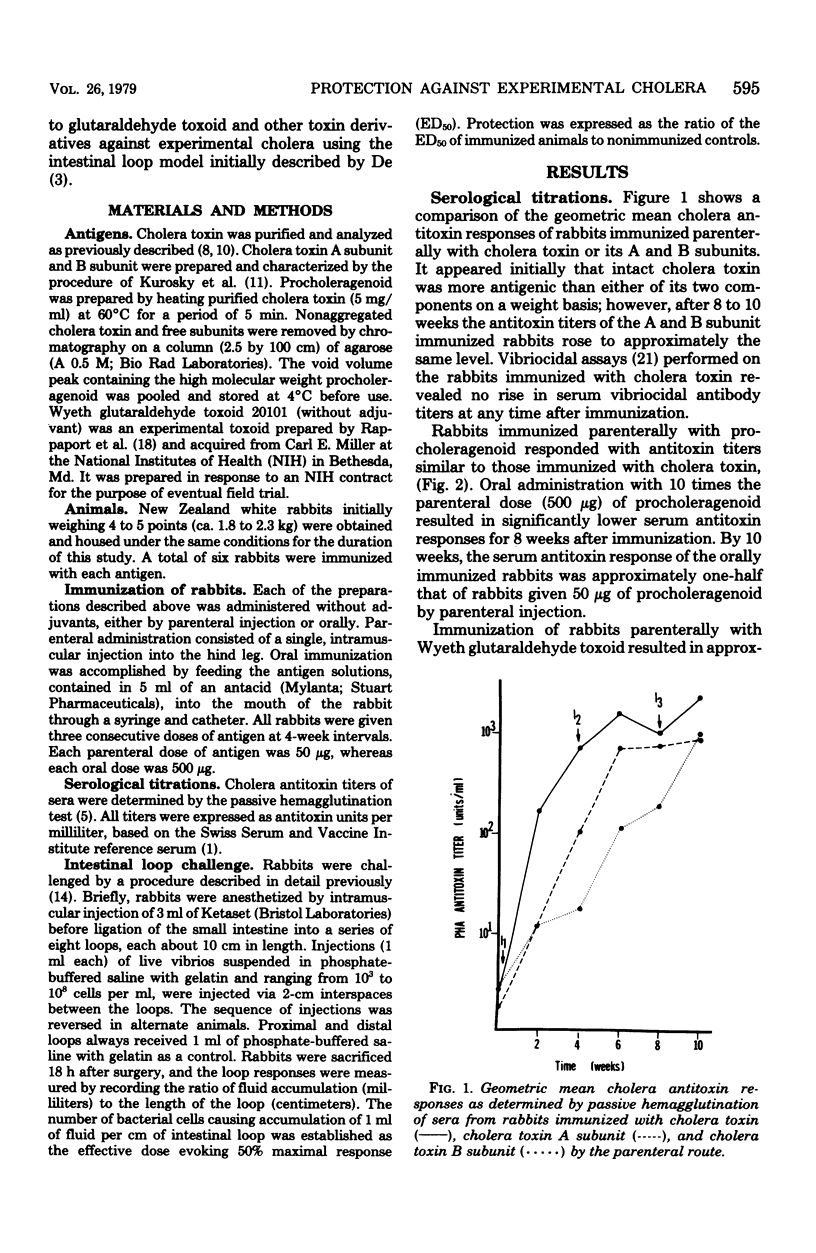
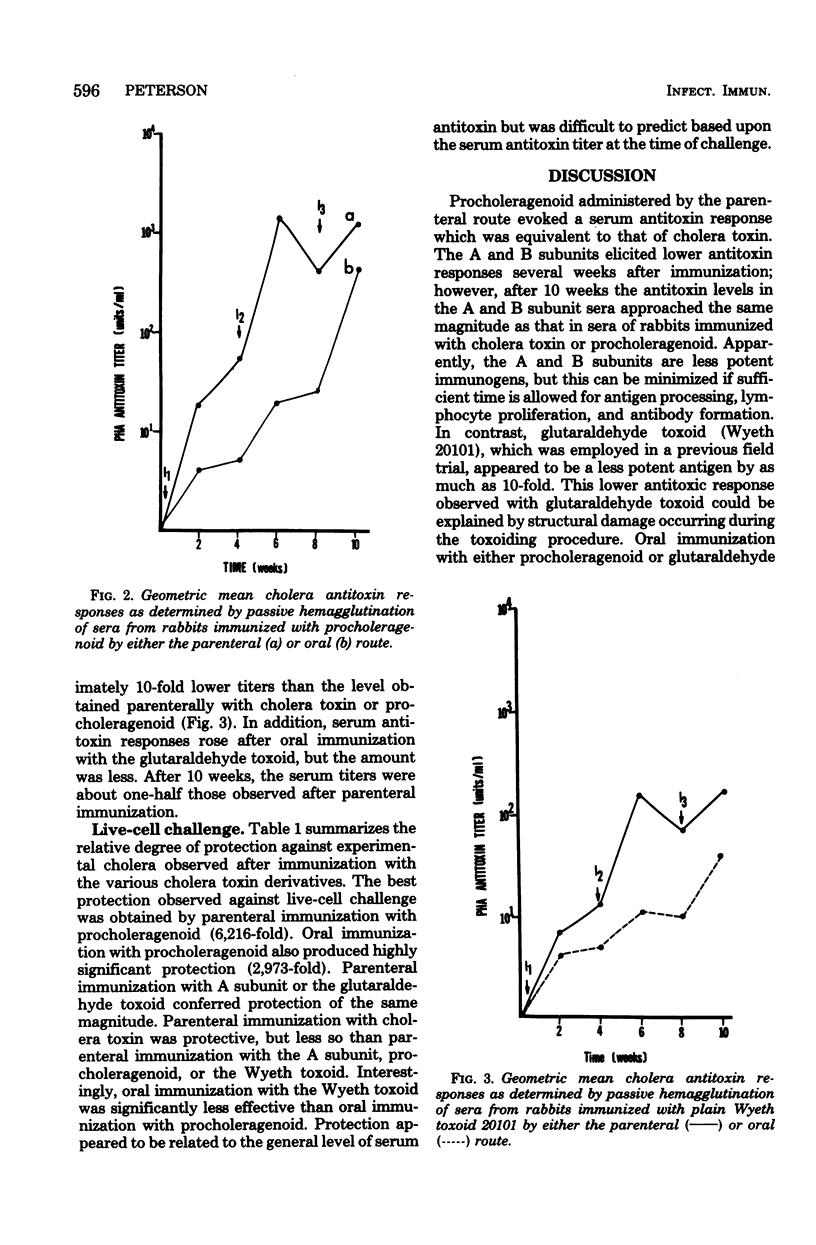
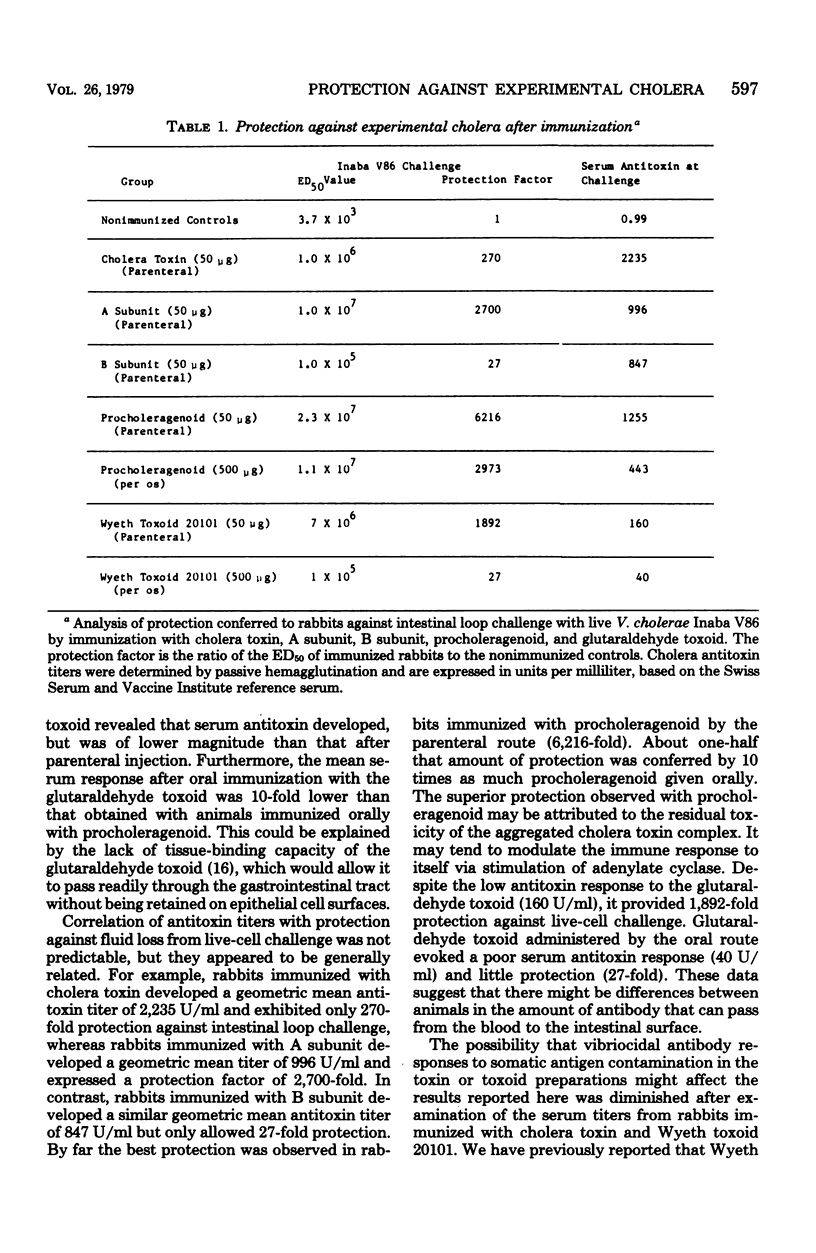
Comparisons were made between the antigenic potency and protective capacity of several cholera toxin derivatives. Rabbits were immunized parenterally with 50 microgram of cholera toxin, A subunit, B subunit, procholeragenoid, or Wyeth glutaraldehyde toxoid 20101. Examination of the antibody response curves revealed that cholera toxin elicited serum antitoxin responses that rose more quickly than in the subunit-immunized animals; however, antitoxin levels were of the same magnitude after 10 weeks. Parenteral immunization with procholeragenoid evoked antibody titers that were similar to the toxin, whereas Wyeth toxoid yielded only one-tenth the level of antitoxin. Oral immunization with procholeragenoid as well as Wyeth toxoid resulted in lower serum antitoxin titers than that achieved with parenteral immunization, despite the oral administration of 10 times the parenteral dose. Analysis of protection against live-cell challenge revealed that parenteral administration of procholeragenoid provided the best protection against fluid accumulation. Oral immunization with procholeragenoid also was very effective, whereas oral immunization with B subunit or Wyeth toxoid resulted in minimal protection. Also, the A subunit provided surprisingly more protection than did cholera toxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig J. B. Toward the development of a standard reference cholera antitoxin. Dev Biol Stand. 1978;41:415–422. [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W. In vitro detection of antibody to cholera enterotoxin in cholera patients and laboratory animals. Infect Immun. 1970 Jan;1(1):21–29. doi: 10.1128/iai.1.1.21-29.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E., Varallyay S., Inderbitzin T. M. Preparation of a purified antigenic cholera toxoid. Infect Immun. 1976 Jun;13(6):1692–1698. doi: 10.1128/iai.13.6.1692-1698.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik K. E., Peterson J. W., Markel D. E., Kurosky A. Radioimmunoassay for the antigenic determinants of cholera toxin and its components. Infect Immun. 1977 Sep;17(3):621–628. doi: 10.1128/iai.17.3.621-628.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W. Covalent structure of the beta chain of cholera enterotoxin. J Biol Chem. 1977 Oct 25;252(20):7257–7264. [PubMed] [Google Scholar]
- Markel D. E., Hejtmancik K. E., Peterson J. W., Martin F. B., Kurosky A. Characterization of the antigenic determinants of cholera toxin subunits. Infect Immun. 1979 Aug;25(2):615–626. doi: 10.1128/iai.25.2.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Hejtmancik K. E., Markel D. E., Craig J. P., Kurosky A. Antigenic specificity of neutralizing antibody to cholera toxin. Infect Immun. 1979 Jun;24(3):774–779. doi: 10.1128/iai.24.3.774-779.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Synergistic protection against experimental cholera by immunization with cholera toxoid and vaccine. Infect Immun. 1979 Nov;26(2):528–533. doi: 10.1128/iai.26.2.528-533.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Verwey W. F. Radiolabeled toxin for studying binding of cholera toxin and toxoids to intestinal mucosal receptor sites. Proc Soc Exp Biol Med. 1974 Apr;145(4):1187–1191. doi: 10.3181/00379727-145-37978. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Pierzchala W. A., Bonde G., McCann T., Rubin B. A. Development of a purified cholera toxoid. III. Refinements in purification of toxin and methods for the determination of residual somatic antigen. Infect Immun. 1976 Sep;14(3):687–693. doi: 10.1128/iai.14.3.687-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletti M., Ricci A. Experiments with cholera toxin detoxified with glutaraldehyde. Bull World Health Organ. 1974;51(6):633–639. [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



