Abstract
Xenon (Xe) is a noble gas that has been developed for use in people as an inhalational anesthestic and a diagnostic imaging agent. Xe inhibits glutamatergic N-methyl-D-aspartate (NMDA) receptors involved in learning and memory and can affect synaptic plasticity in the amygdala and hippocampus, two brain areas known to play a role in fear conditioning models of post-traumatic stress disorder (PTSD). Because glutamate receptors also have been shown to play a role in fear memory reconsolidation – a state in which recalled memories become susceptible to modification – we examined whether Xe administered after fear memory reactivation could affect subsequent expression of fear-like behavior (freezing) in rats. Male Sprague-Dawley rats were trained for contextual and cued fear conditioning and the effects of inhaled Xe (25%, 1 hr) on fear memory reconsolidation were tested using conditioned freezing measured days or weeks after reactivation/Xe administration. Xe administration immediately after fear memory reactivation significantly reduced conditioned freezing when tested 48 h, 96 h or 18 d after reactivation/Xe administration. Xe did not affect freezing when treatment was delayed until 2 h after reactivation or when administered in the absence of fear memory reactivation. These data suggest that Xe substantially and persistently inhibits memory reconsolidation in a reactivation and time-dependent manner, that it could be used as a new research tool to characterize reconsolidation and other memory processes, and that it could be developed to treat people with PTSD and other disorders related to emotional memory.
Introduction
Mitigation of persistent, intrusive, traumatic memories experienced by people with post-traumatic stress disorder (PTSD) remains a key therapeutic challenge [1]. Behavioral treatments such as extinction training – administered alone or in combination with cognitive-enhancing drugs (e.g. d-cycloserine) – attempt to inhibit underlying traumatic memories by facilitating a new set of learning contingencies, but often achieve limited success [2]. Another learning and memory phenomenon known as reconsolidation, a process by which reactivated (retrieved) memories temporarily enter a labile state (the reconsolidation window), has been studied to determine whether drug or behavioral interventions can prevent a traumatic memory trace from being re-incorporated back into the neural engram, inhibiting the memory [3]–[6]. Several chemical agents have been found to inhibit fear memory reconsolidation in animals [7] but unfortunately do not translate well to humans, limiting their clinical use. They either are toxic (e.g. protein synthesis inhibitors), induce unwanted side effects, are slow acting such that brain drug concentrations peak outside of the reconsolidation window, or are slowly eliminated such that they interfere with later onset memory processes including extinction [8]. A recent human study documented that a single electroconvulsive therapy (ECT) treatment administered to unipolar depressed subjects immediately after emotional memory reactivation disrupted reconsolidation, confirming that reconsolidation occurs in humans and that it can be inhibited by a brief treatment [9]. While ECT is indicated for therapeutic use in people with treatment-resistant major depression, it may not be a viable treatment for other clinical populations. Thus, there is a significant unmet need for a minimally invasive, safe and well-tolerated treatment that can be used clinically to inhibit fear memory reconsolidation in people with PTSD.
The noble gas xenon (Xe) inhibits glutamatergic N-methyl-D-aspartate (NMDA) receptors [10] known to play a role in memory reconsolidation [11]. Xe reduces NMDA-mediated synaptic currents and neuronal plasticity in the basolateral amygdala and CA1 region of the hippocampus [12], [13]; these brain areas are involved in Pavlovian fear conditioning, an animal model of PTSD used to elucidate learning and memory processes, including reconsolidation [14]–[16]. Xe already is used in humans at high concentration (>50%) as an anesthetic and at subsedative concentration (28%) as a diagnostic imaging agent; in both applications, Xe has excellent safety/side effect profiles and is well tolerated [17]–[19]. Further, NMDA receptor glycine antagonists like Xe [10] do not appear to have significant abuse liability and do not induce psychosis [20], [21], consistent with clinical experience [18], [19]. Thus, Xe has a number of favorable properties that might be beneficial for treating fear memory disorders. As fear memory reconsolidation is an “evolutionarily conserved memory-update mechanism” [5], we evaluated in rats whether administering a subsedative concentration of Xe (maximum concentration 25%, 1 h) via inhalation following conditioned fear memory reactivation could reduce subsequent expression of fear-like behavior. Here, we report that Xe impaired reconsolidation of fear memory demonstrated as a reduction in conditioned freezing, a behavioral readout used to measure fear in animals.
Methods and Materials
Experimental subjects
Male Sprague-Dawley rats (Charles River; Raleigh, NC) weighing 350–375 g were pair-housed in plastic Nalgene rat cages and acclimated to the main animal vivarium for two weeks before being randomly assigned to different treatment groups (below). Rats were maintained on 12/12 h light dark cycles and food and water were provided ad libitum. Experiments were performed from 10 a.m. to 3 p.m. All animal handling was limited to the time required to transport and place animals in the fear-conditioning chambers and air/xenon exposure chambers (i.e., no pre-study handling acclimation was used). The sample size was determined in concordance with our previous work examining reconsolidation mechanisms using the conditioned- freezing behavioral assay [22]. All animal procedures were approved by McLean Hospital's Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare Assurance number A3685–01) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (8th Edition).
Fear-conditioning apparatus
Conditioning and testing were conducted in four identical 19×9×14 cm Plexiglas behavioral chambers contained in a sound-attenuating cubicle (Med-Associates, Georgia VT). On the training day, rats were placed in chambers and after 2 min received two pairings of a 30 s, 5 kHz, 75 dB tone (conditioned stimulus; CS) co-terminating with a 0.6 mA, 0.5 s footshock (unconditioned stimulus; US) delivered through the floorbars of the chamber. Shock reactivity (cage movement in response to shock delivery) was measured after each training trial by an accelerometer at the base of the cage. Accelerometer analog output was amplified and digitized on a scale of 0–20 units by an analog-to-digital card interfaced with a PC computer (Med-Associates). Animals with shock reactivity levels <3 units (averaged across two training trials) were excluded as this can be used as an indicator of the strength of conditioning (i.e. weak shock reactivity) [23]; a total of 5 out of 99 animals were excluded based on this criteria. The intertrial interval of CS-US pairings was 30 s. After an additional 30 s in the chamber, animals were returned to their home cages. Memory was reactivated (Reactivation) 24 h after training by returning animals to testing chambers and after 2 min animals were exposed to the tone CS (5 kHz, 75 dB) for 60 s. Post-reactivation long-term memory (PR-LTM) was subsequently probed at 48 h (PR-LTM1), 96 h (PR-LTM2) or 18 d (PR-LTM3) using Reactivation day procedures. Freezing behavior was video-recorded on each day and scored by an experimenter blind to treatment conditions. Percent freezing was calculated as the % total time that animals remained immobile (frozen), other than breathing, during the first 2 min of re-exposure to the chamber (Context) and during 60 s CS presentation (Tone).
Xenon exposure apparatus
A custom-built system (Air Products and Chemicals, Inc.; Bethlehem, PA – APCI) was used to expose animals to 25% xenon (Xe) gas (Praxair, Inc.; Danbury, CT). The apparatus consisted of a 30×24×16 in. sealable Plexiglas chamber capable of housing two modified Nalgene rat cages (perforated along all sides to facilitate gas exchange) for exposure of up to four rats at a time (2 rats/cage). The delivery (rate and concentration) both of Xe and supplemental oxygen (as needed to maintain 20.9% concentration; Figure 1 ) was regulated by PC-interfaced mass-flow controllers using custom-designed software (APCI). Xenon, oxygen, carbon dioxide, pressure, temperature and humidity were all monitored by sensors in the system and compensated as needed by the internal control system and supporting equipment to maintain set levels. An identical system was used for air exposures except that only normal room air was supplied. Both the Xe and air-exposure apparatuses were located in a dedicated animal quarantine bay apart from the main vivarium but maintained under the same environmental conditions.
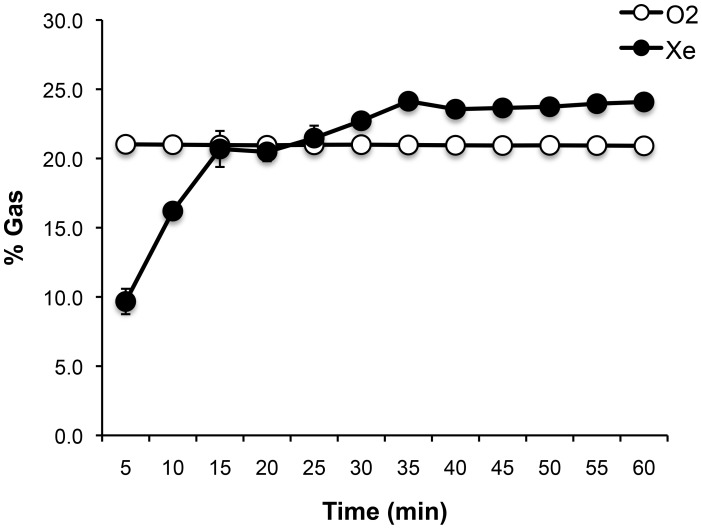
Figure 1. Xenon- (Xe) and oxygen (O2) gas concentration time course averaged across all exposures in this study.
Percent Xe reflects exposure chamber atmospheric Xe concentration along with oxygen (maintained at 20.9%) and balanced with nitrogen. The rate of Xe delivery was approximately 2.5 liters per minute (supplied as 100% Xe from an external tank) and continuously mixed with chamber atmosphere by circulating fans to reach a maximum preset concentration of 25%. Data included in the figure are from 14 total Xe exposures (Fig. 2B &C, n = 3 runs; Fig. 2D & E, n = 3 runs; Fig. 2F & G, n = 2 runs; Fig. 3 B & C, n = 3 runs after Reactivation test and 3 runs after PR-LTM1). Data are shown as mean ± s.e.m.
Experimental procedures
After two-weeks acclimation in the main vivarium, pairs of rats either were left in the rat housing room (Normal exposure group) or re-located to the Xe (Xenon group) or Air (Air group) chambers for further acclimation (1 week) to experimental-housing settings. Rats then were trained for contextual and cued fear conditioning using procedures adapted from Phillips and LeDoux [24]. Accordingly, this allowed us to evaluate the expression of conditioned freezing in the presence of a conditioned stimulus (CS, a tone) and the context (the conditioning/test chamber) present during the training (CS pairing with shock, the unconditioned stimulus; US), and to examine effects of Xe administered after memory reactivation on both components (freezing to context and tone).
The timeline of procedures used for fear conditioning, testing and Xe exposure is illustrated in Figures 2A & 3A . On Day 1, rats were fear conditioned as described above. Twenty-four hours later, immediately following reactivation testing, animals either were placed in Xe or Air-exposure chambers, lids were sealed, and animals were exposed to Xe (25%) or room air for 1 h. After 1 h, Xe was completely scavenged by the Xe-exposure system and chamber lids were opened to normal room air exposure for the duration of the study.
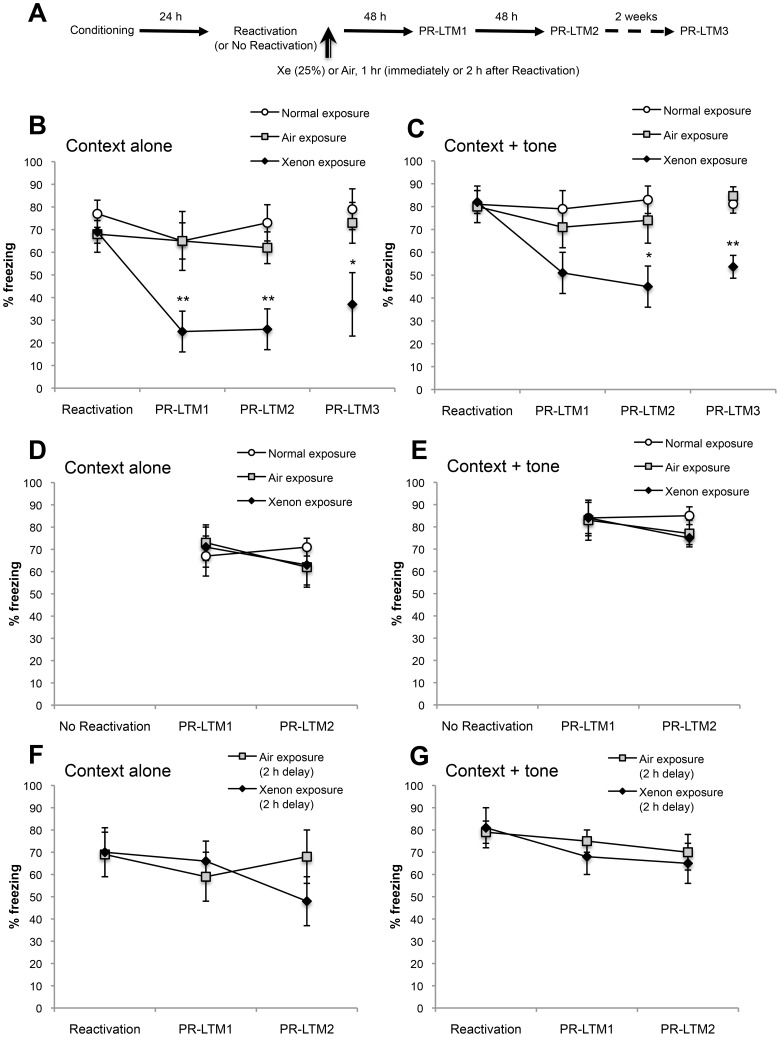
Figure 2. Xenon exposure impairs reconsolidation and reduces conditioned freezing in a reactivation- and time-dependent manner.
(A) Schematic of the experimental design. Twenty-four h after fear conditioning, fear memories either were or were not reactivated and rats were exposed either to Xe (25%) or Air for 1 h beginning either immediately or after a 2 h delay. An additional control group housed in the regular main rat vivarium (Normal exposure) also was included in some studies to control for any potential effects of housing in the exposure chambers. Post-reactivation long-term memory (PR-LTM) was subsequently probed 48 h (PR-LTM1) and 96 h (PR-LTM2) later. A subset of rats (n = 6–7/group) were further tested for spontaneous recovery of freezing by testing 18 d after Reactivation (PR-LTM3). (B & C) Percent freezing to context alone and context + tone (respectively) in animals exposed to Xe (25%) or Air immediately after Reactivation. **P<0.005; *P<0.05 compared to Air exposure. Normal exposure, n = 8; Air exposure, n = 11; Xe exposure, n = 11. (D & E) Percent freezing to context alone and context + tone in rats not receiving a Reactivation test. Normal exposure, n = 8; Air exposure, n = 11; Xe exposure, n = 11. (F & G) Percent freezing to context alone and context + tone in rats exposed either to Xe (25%) or Air beginning 2 h after Reactivation. Air exposure, n = 7; Xe exposure, n = 8. Data are shown as mean ± s.e.m.
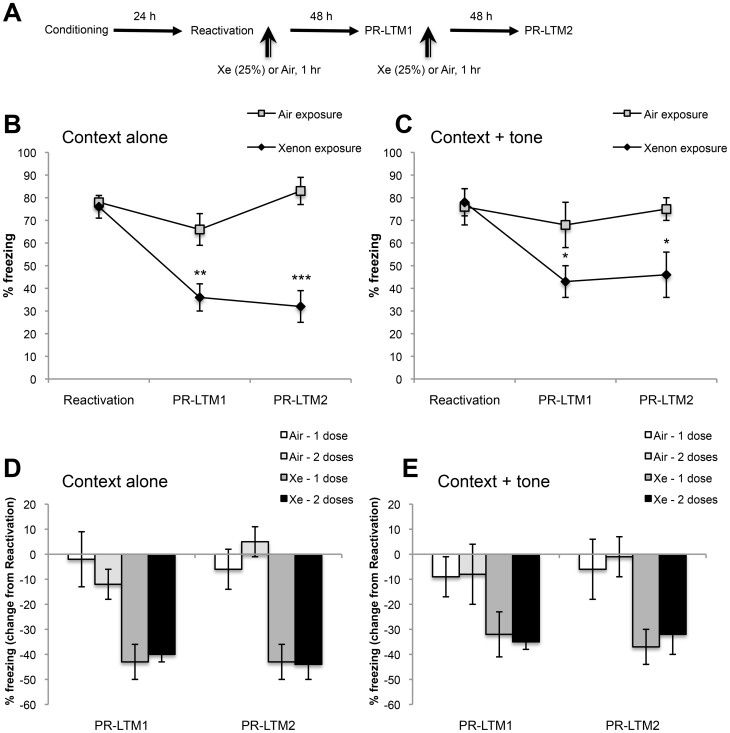
Figure 3. Multiple Xe exposures after fear memory reactivations do not enhance amnestic effects on conditioned freezing.
(A) Schematic of the experimental design for multiple Xe-exposure treatment. In addition to administering Xe (25%) or Air for 1 h after Reactivation, animals were exposed a second time to Xe (25%) or Air for 1 h immediately after PR-LTM1 and freezing was again probed 48 h later, (PR-LTM2). (B & C) Percent freezing to context alone and context + tone (respectively) in animals exposed to Xe (25%) or Air for 1 hr immediately after Reactivation and PR-LTM1. (D & E) Normalized freezing data to context and tone. Data are expressed as % differences from the first Reactivation test day in order to compare the effects of multiple Xe exposures. A second Xe exposure did not alter freezing either to context alone or context + tone at PR-LTM2 compared to PR-LTM1. ***P<0.0005; **P<0.005; *P<0.05; Air–1 exposure, n = 11; Air–2 exposures, n = 9; Xe–1 exposure, n = 11; Xe–2 exposures, n = 10. Data are shown as mean ± s.e.m.
A second set of animals was trained as described above but did not receive a reactivation test 24 h later. Instead, at this time-point, animals were exposed either to 25% Xe or Air for 1 h to determine whether Xe must be paired with memory reactivation for it to affect memory reconsolidation.
A third set of animals was trained as described above, underwent reactivation 24 hours later, and were exposed either to 25% Xe or Air (1 h, both types of exposure) beginning 2 h after the reactivation test, to determine whether delayed Xe exposure affected freezing at PR-LTM1 and PR-LTM2.
A fourth set of animals was trained as described above and exposed either to 25% Xe or Air for 1 h twice; immediately after reactivation and again after reactivation during PR-LTM1 testing, to determine whether multiple Xe exposures enhance reconsolidation blockade.
Statistics
Two-way ANOVAs for treatment group (between-subjects) × test day (within-subjects) comparisons were performed. Comparisons between treatment groups for the PR-LTM3 test day (long term test for spontaneous recovery), in a subset of animals, were performed using one-way ANOVA. For measurements yielding significant main effect, subsequent multiple pairwise comparisons were made using Dunn's test. All reported t tests are two-sided measures.
Results
Rats exposed to Xe (25%, 1 h) immediately after fear memory reactivation exhibited a significant reduction of freezing when tested 48 and 96 h after reactivation (PR-LTM1 and PR-LTM2, respectively) compared to air-exposed controls ( Fig. 2B & 2C ). Main effects: context alone (treatment group: F2,27 = 6.31, P = 0.006; test day: F2,54 = 10.41, P = 0.0001; interaction: F4,54 = 4.99, P = 0.002); context + tone (treatment group: F2,27 = 3.27, P = 0.05; test day: F2,54 = 4.41, P = 0.02; interaction: F4,54 = 2.63, P = 0.04). Xe-exposed rats exhibited a trend for reduced freezing in the context + tone condition 48 h after reactivation (P = 0.06 compared to Air-exposure; P = 0.02 compared to main vivarium (Normal)- exposure); the reduction attained statistical significance versus Air-exposed controls when reassessed 96 h after reactivation (i.e. at PR-LTM2; Fig. 2C ). In a separate cohort treated identically to the first cohort up through the PR-LTM1 test, the Xe effect on freezing to the context alone finding was replicated while Xe significantly reduced freezing to the context + tone at the first post-reactivation test (PR-LTM1; Fig. 3B & 3C ). When all observations of Xe effects on freezing at PR-LTM1 were pooled from these two independent experiments (Air-exposure, n = 20; Xe-exposure, n = 21) there was a highly significant reduction in freezing both to context alone (t 39 = 4.63, P<0.0001) and context + tone (t 39 = 2.16, P<0.01) compared to air-exposed controls. Freezing at PR-LTM1 and PR-LTM2 did not significantly differ.
In order to examine whether the amnestic effects of xenon were long-lasting, a subset of animals from each treatment group was further tested 18 days after the Reactivation test. On this test day (PR-LTM3), freezing both to the context alone and to context + tone was significantly reduced in Xe-exposed rats compared to control groups, indicating a lack of spontaneous recovery of the fear memory over time. Main effects: context alone (treatment group: F2,16 = 3.76, P = 0.04); context + tone (treatment group: F2,16 = 10.93, P = 0.001).
Figure 2D & E show that post-reactivation freezing to context alone and context + tone (respectively) in rats that were exposed to Xe but that did not receive a Reactivation test was not significantly different from controls (no significant main effects). Figure 2F & G show that post-reactivation freezing to context alone and context + tone (respectively) in rats exposed to Xe 2 h after Reactivation was not significantly different from controls (no significant treatment effects). Together, these data indicate that Xe was only effective at reducing long-term expression of freezing when administered in conjunction with memory reactivation and within the putative reconsolidation window.
Figure 3B & C show that multiple Xe exposures after fear memory reactivations do not further enhance the amnestic effects of Xe on conditioned freezing. Main effects: Percent freezing to context alone (treatment group: F1,17 = 14.9, P = 0.001; test day: F2,34 = 25.1, P<0.0001; interaction: F2,34 = 20.1, P<0.0001); percent freezing to context + tone (treatment group: F1,17 = 3.6, P = 0.07; test day: F2,34 = 6.9, P = 0.003; interaction: F2,34 = 4.2, P = 0.02). Figure 3D & E show normalized freezing data to context alone and context + tone. Data are expressed as % differences from the first Reactivation test day in order to compare the effects of multiple Xe exposures. As shown, a second Xe exposure did not alter freezing either to context alone or context + tone at PR-LTM2 compared to PR-LTM1 (no significant differences between PR-LTM1 and PR-LTM2).
Discussion
Here, we report for the first time, that inhaled administration of a subsedative concentration of Xe gas substantially and persistently inhibits a long-term fear memory, but only after memory reactivation and when administered within the putative reconsolidation window [25]. NMDA receptor dynamics appear to play key roles in both the destabilization and reconsolidation of memory [11], [26], [27] and Xe's rapid inhibition of these receptors post-reactivation could mediate the effects we observed. Xe directly reduces NMDA-mediated synaptic currents and affects neuronal plasticity in the basolateral amygdala and CA1 region of the hippocampus [12], [13], brain areas known to play a role in fear conditioning and which have been implicated in the pathophysiology of PTSD [28], [29]. Xe also may indirectly reduce NMDA receptor function by inhibiting the enzyme tissue plasminogen activator (tPA) [30]. tPA increases NMDA receptor activity by proteolytically cleaving the NR1 subunit amino terminal domain [31]. Although tPA is best known as a clot-busting drug used in acute stroke patients, tPA is released from dendrites during synaptic activity [32], especially during high frequency stimulation [33], tPA acts as a gliotransmitter [34], and tPA participates in synaptic plasticity and learning and memory processes including fear conditioning [35], [36]. Xe also had been reported to affect AMPA receptors [12] shown to play a role in memory reconsolidation [37], [38]. Collectively, Xe's direct and indirect inhibition of NMDA and AMPA receptor function may underlie its ability to impair fear memory reconsolidation.
Other targets of Xe also could mediate the effects we observed. For example, Xe has also been shown to have differential effects on excitatory and inhibitory ligand-gated ion channels; Xe reduces current through alpha4 beta2 (α4β2) nicotinic acetylcholine receptor-gated channels and increases current through glycine and GABAA receptor-gated channels [39]. Xe also targets other proteins known to play a role in contextual fear memory including alpha7 (α7) nicotinic acetylcholine receptors [40], [41] and ATP-dependent potassium (Kir6.2) channels [42], [43], and targets TREK-1 channels [44]. At this time, we cannot conclude which targets of Xe mediate its inhibition of fear memory reconsolidation, which is a limiting factor of this study. Future studies are planned, however, using selective agonists and antagonists of these and other receptors and proteins, to characterize the pharmacology and mechanism of action of Xe's effects on reconsolidation.
In our analysis of the pooled sample from all Xe-treated rats (n = 21) we found a within-subjects difference in Xe's effects at PR-LTM1 on freezing to context alone and context + tone whereby freezing in the presence of the tone was less sensitive to Xe (t 20 = 3.72, P<0.005). These data suggest that Xe's amnestic effects may be stronger for context- versus cue-induced freezing. This may reflect a stronger effect of Xe on the hippocampus than the amygdala, which play different roles in context and cued-fear conditioning [15], [24]. This differential effect could be related to Xe's apparently greater inhibition of hippocampal versus amygdala excitatory postsynaptic currents [12], [13]. Interestingly, other inhaled anesthetic agents, such as halothane, isoflurane, and nitrous oxide, which can also affect learning and memory and have amnestic effects, can alter hippocampal theta rhythms [45] which have been shown to contribute to reconsolidation of contextual fear memory by virtue of its synchronization with the amygdala [46]. Hence, a preferential action of Xe on hippocampal ensemble activities could account for the strong amnestic effect upon re-exposure to the conditioning context (hippocampal dependent), but which was reduced when the animal was then presented with a discrete cue (amygdala dependent) within the conditioning context. Clearly, a limitation of the current study is that animals were not tested for cue-induced freezing in a different context than that used for fear-conditioning, and that Xe was tested in only one fear-conditioning paradigm. Our intention in these initial studies was to elucidate the basic phenomenon using a paradigm similar to that used in the seminal studies of Phillips and LeDoux [24], which established a differential role for the hippocampus and amygdala in context versus cued fear-conditioning. Future studies investigating Xe's effects on reconsolidation for context- and cue-induced freezing, including freezing elicited in a different context are planned, as well as studies involving different fear conditioning paradigms.
As described in Tronson and Taylor [3], a number of control protocols can be employed to demonstrate that a specific treatment affects reconsolidation. The data presented in this report include several of these important comparison groups. First, we demonstrate that rats exposed to 25% Xe for 1 hour in the absence of fear memory reactivation exhibited no differences in freezing to context or tone versus air-exposed controls ( Fig. 2D & E ). These data suggest that the effects of Xe on reconsolidation and impairment of long-term fear memory are not due to non-specific effects of Xe gas inhalation, but that Xe's effects likely are having a direct effect on brain mechanisms engaged only after the fear memory is recalled. Second, when Xe administration was delayed until 2 hours after fear memory reactivation, a time point expected to be outside of the reconsolidation window for NMDA antagonists [25], Xe was ineffective at reducing freezing ( Fig. 2F & G ). Together, these results suggest that 25% Xe inhibits fear memory reconsolidation only after fear memory reactivation and only when administered within the reconsolidation window. These findings along with our data showing a lack of spontaneous recovery – a traditional test used to examine the enduring amnestic effect of a treatment [47] – document that Xe satisfies several requirements of a demonstrable reconsolidation-blocking agent.
Lastly, we examined whether a second 25% Xe exposure for 1 hour immediately after the PR-LTM1 test (which is, itself, another memory reactivation), could further impair reconsolidation. The second Xe exposure did not further affect freezing to context alone or context + tone ( Fig. 3B & 3C ) when compared with Xe administered only after first reactivation ( Fig. 3D & 3E ). Possible interpretations of this finding include that the Xe-sensitive component of reconsolidation may have a threshold for its amnestic capacity after blockade and/or that residual fear maintenance may occur by Xe-insensitive mechanisms. A recent study reported that a transition state may develop after multiple unreinforced CS exposures reflecting a dynamic shift from reconsolidation to extinction processes, during which NMDA receptor antagonists lose their ability to affect reconsolidation or extinction [48]. As both processes are NMDA-receptor dependent [11], it is possible that a first treatment with Xe and the ensuing amnestic effect may shift the reconsolidation-extinction boundaries to limit the effectiveness of a second treatment. Xe's rapid on-off kinetics [49] may facilitate its use both in animals and humans as a temporally precise tool to help characterize such transition states and other dynamic memory processes. Given that the timing of interventions aimed at blocking reconsolidation or enhancing extinction may significantly affect treatment outcomes [11], [48], Xe’s rapid kinetics also may enable temporally optimized treatment regimens.
In summary, we report in an animal model of PTSD that 25% Xe administered within the reconsolidation window after fear memory reactivation substantially reduced subsequent fear memory expression. This anxiolytic-like effect in rats has translational application to current clinical research aimed at modulating memory processes as a therapy for fear and anxiety disorders [50]–[53]. People with PTSD experience intrusive, persistent traumatic memories [54], impaired fear memory extinction [55], and may be locked in reconsolidation mode [56]. Given that fear memory reconsolidation is “evolutionarily conserved” [5] and that subsedative Xe inhalation is associated with well-established excellent safety and side-effect profiles in humans [18], [19] Xe appears to have potential for rapid development as a pharmacotherapy to inhibit traumatic memory reconsolidation in PTSD patients, and possibly treat other conditions involving reconsolidation, including addiction disorders [56], [57].
Acknowledgments
Xe and air exposure chambers were provided by Air Products and Chemicals, Inc. We thank Dr. William A. Carlezon Jr. and Erin Blanchfield for their technical and logistical support. We thank Drs. Scott Rauch and Kerry Ressler for reviewing a preliminary draft of the manuscript. MJK wishes to dedicate this work to the memory of Donald Julian Pollock.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by the following: National Institutes of Health grant R03NS080073 (MJK); http://www.nih.gov/; O'Keefe Family Junior Investigator Award (EGM); and McLean Hospital (MJK, EGM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Morrison FG, Ressler KJ (2014) From the neurobiology of extinction to improved clinical treatments. Depress Anxiety 31: 279–90 10.1002/da.22214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgerald PJ, Seemann JR, Maren S (2014) Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res. Bull. S0361–9230(13)00196–2 doi:10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed]
- 3. Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8: 262–275. [DOI] [PubMed] [Google Scholar]
- 4. Nader K, Hardt O (2009) A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10: 224–234. [DOI] [PubMed] [Google Scholar]
- 5. Agren T, Engman J, Frick A, Björkstrand J, Larsson EM, et al. (2012) Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science 337: 1550–1552. [DOI] [PubMed] [Google Scholar]
- 6. Auber A, Tedesco V, Jones CE, Monfils M-H, Chiamulera C (2013) Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharm 226: 631–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reichelt AC, Lee JLC (2013) Memory reconsolidation in aversive and appetitive settings. Front Behav Neurosci 7: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiller D, Phelps EA (2011) Does reconsolidation occur in humans? Front Behav Neurosci 5: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kroes MC, Tendolkar I, van Wingen GA, van Waarde JA, Strange BA, et al. (2014) An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat Neurosci 17: 204–206. [DOI] [PubMed] [Google Scholar]
- 10. Dickinson R, Peterson BK, Bank P, Simillis C, MartinJC, et al (2007) Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane. Anesthesiol 107: 756–767. [DOI] [PubMed] [Google Scholar]
- 11. Lee JL, Milton AL, Everitt BJ (2006) Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J Neurosci 26: 10051–10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haseneder R, Kratzer S, Kochs E, Eckle VS, Zieglgänsberger W, et al. (2008) Xenon reduces N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiol 109: 998–1006. [DOI] [PubMed] [Google Scholar]
- 13. Kratzer S, Mattusch C, Kochs E, Eder M, Haseneder R, et al. (2012) Xenon attenuates hippocampal long-term potentiation by diminishing synaptic currents and extrasynaptic N-methyl-D-aspartate receptor currents. Anesthesiol 116: 673–682. [DOI] [PubMed] [Google Scholar]
- 14. Johansen JP, Cain CK, Ostroff LE, LeDoux JE (2011) Molecular mechanisms of fear learning and memory. Cell 147: 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14: 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM (2013) From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobio Learn Mem S1074–7427(13)00242–6 doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed]
- 17. Dickinson R, Franks NP (2010) Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit Care 14: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlson AP, Brown AM, Zager E, Uchino K, Marks MP, et al. (2011) Xenon-enhanced cerebral blood flow at 28% xenon provides uniquely safe access to quantitative, clinically useful cerebral blood flow information: A multicenter study. Am J Neuroradiol 32: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bedi A, McCarroll C, Murray JM, Stevenson MA, Fee JP (2002) The effects of subanaesthetic concentrations of xenon in volunteers. Anaesthesia 57: 233–241. [DOI] [PubMed] [Google Scholar]
- 20. Beardsley PM, Ratti E, Balster RL, Willetts J, Trist D (2002) The selective glycine antagonist gavestinel lacks phencyclidine-like behavioral effects. Behav Pharmacol 13: 583–592. [DOI] [PubMed] [Google Scholar]
- 21. Nicholson KL, Balster RL (2009) The discriminative stimulus effects of N-methyl-D-aspartate glycine-site ligands in NMDA antagonist-trained rats. Psychopharm 203: 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Meloni EG, Carlezon WA, Milad MR, Pitman RK, et al. (2013) Learning and reconsolidation implicate different synaptic mechanisms. PNAS 110: 4798–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curzon P, Rustay NR, Browman KE (2009) Cued and contextual fear conditioning for rodents. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd ed. Boca Raton (FL): CRC Press, chap. 2. [PubMed]
- 24. Philips RG, LeDoux JE (1997) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Beh Neurosci 106: 274–285. [DOI] [PubMed] [Google Scholar]
- 25. Przybyslawski J, Sara SJ (1997) Reconsolidation of memory after its reactivation. Beh Brain Res 84: 241–246. [DOI] [PubMed] [Google Scholar]
- 26. Mamou CB, Gamache K, Nader K (2006) NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9: 1237–1239. [DOI] [PubMed] [Google Scholar]
- 27. Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, et al. (2013) Double dissociation of the requirement for GluN2B- and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. J Neurosci 33: 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elzinga BM, Bremner JD (2002) Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD). J Affect Disorders 70: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rauch SL, Shin LM, Phelps EA (2006) Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 60: 376–382. [DOI] [PubMed] [Google Scholar]
- 30. David HN, Haelewyn B, Risso J-J, Colloc'h N, Abraini JH (2010) Xenon is an inhibitor of tissue-plasminogen activator: adverse and beneficial effects in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab 30: 718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernández-Monreal M, Lopex-Atalya JP, Benchenane K, Cacquevel M, Dulin F, et al. (2004) Arginine 260 of the amino-terminal domain of NR1 subunits is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J Biol Chem 279: 50850–6. [DOI] [PubMed] [Google Scholar]
- 32. Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, et al. (2006) Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol 66: 564–77. [DOI] [PubMed] [Google Scholar]
- 33. Nagappan G, Zaitsev E, Senatorov VV Jr, Yang J, Hempstead BL, et al. (2009) Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A 106: 1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassé F, Bardou I, Danglot L, Briens A, Montagne A, et al. (2012) Glutamate controls tPA recycling by astrocytes, which in turn influences glutamatergic signals. J Neurosci 32: 5186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obiang P, Maubert E, Bardou I, Nicole O, Launay S, et al. (2011) Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol Learn Mem. 96: 121–9. [DOI] [PubMed] [Google Scholar]
- 36. Barnes P, Thomas KL (2008) Proteolysis of proBDNF is a key regulator in the formation of memory. PLoS ONE 3: e3248 10.1371/journal.pone.0003248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clem RL, Huganir RL (2010) Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330: 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong I, Kim J, Kim J, Lee S, Ko HG, et al. (2013) AMPA receptor exchange underlies transient memory destabilization on retrieval. Proc Natl Acad Sci U S A 110: 8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamakura T, Harris RA (2000) Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Anesthesiol 93: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki T, Ueta K, Sugimoto M, Uchida I, Mashio T (2003) Nitrous oxide and xenon inhibit the human (alpha7)5 nicotinic acetylcholine receptor expressed in xenopus oocyte. Anesth Analg 96: 443–448. [DOI] [PubMed] [Google Scholar]
- 41. Kenney JW, Raybuck JD, Gould TJ (2012) Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus 22: 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bantel C, Maze M, Trapp S (2010) Noble gas xenon is a novel adenosine triphosphate-sensitive potassium channel opener. Anesthesiol 112: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Betourne A, Bertholet AM, Labroue E, Halley H, Sun HS, et al. (2009) Involvement of hippocampal CA3 KATP channels in contextual memory. Neuropharmacology 56: 615–625. [DOI] [PubMed] [Google Scholar]
- 44. Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, et al. (2004) Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol 65: 443–452. [DOI] [PubMed] [Google Scholar]
- 45. Perouansky M, Rau V, Ford T, Oh SI, Perkins M, et al. (2010) Slowing of the hippocampal theta rhythm correlates with anesthetic-induced amnesia. Anesthesiol 113: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Narayanan RT, Seidenbecher T, Sangha S, Stork O, Pape H-C (2007) Theta resynchronization during reconsolidation of remote contextual fear memory. Neuroreport 18: 1107–1111. [DOI] [PubMed] [Google Scholar]
- 47. Duvarci S, Nader K (2004) Characterization of fear memory reconsolidation. J Neurosci 24: 9269–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Merlo E, Milton AL, Goozée JY, Theobald DE, Everitt BJ (2014) Reconsolidation and extinction are dissociable and mutually exclusive processes: Behavioral and molecular evidence. J Neurosci. 34: 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adolph O, Köster S, Georgieff M, Bäder S, Föhr KJ, et al. (2010) Xenon-induced changes in CNS sensitization to pain. Neuroimage 49: 720–730. [DOI] [PubMed] [Google Scholar]
- 50. Parsons RG, Ressler KJ (2013) Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 16: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cain CK, Maynard GD, Kehne JH (2012) Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs 21: 1323–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dunlop BW, Mansson E, Gerardi M (2012) Pharmacological innovations for posttraumatic stress disorder and medication-enhanced psychotherapy. Curr Pharm Des 18: 5645–5658. [DOI] [PubMed] [Google Scholar]
- 53. de Kleine RA, Rothbaum BO, van Minnen A (2013) Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. Eur J Psychotraumatol 4: 10.3402/ejpt.v4i0.21626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ehlers A, Hackman A, Michael T (2004) Intrusive re-experiencing in post-traumatic stress disorder: phenomenology, theory, and therapy. Memory 12: 403–415. [DOI] [PubMed] [Google Scholar]
- 55. Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, et al. (2009) Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nader K, Hardt O, Lanius R (2013) Memory as a new therapeutic target. Dialogues Clin Neurosci 15: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tronson NC, Taylor JR (2013) Addiction: a drug-induced disorder of memory reconsolidation. Curr Opin Neurobiol 23: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.