Abstract
The clinical syndrome comprised of heart failure symptoms but with a left ventricular ejection fraction that is not diminished, e.g. heart failure with a preserved ejection fraction (HFpEF), is increasingly the predominant form of HF in the developed world, and soon to reach epidemic proportions. It remains among the most challenging of clinical syndromes for the practicing clinician and scientist alike, with a multitude of proposed mechanisms involving the heart and other organs and complex interplay with common co-morbidities. Importantly, its morbidity and mortality is on par with heart failure and a reduced ejection fraction, and as the list of failed treatments continues to grow, HFpEF clearly represents a major unmet medical need. The field is greatly in need of a more unified approach to its definition and view of the syndrome that engages integrative and reserve pathophysiology beyond that related to the heart alone. We need to reflect on prior treatment failures and the message this is providing, and re-direct our approaches likely with a paradigm shift in how the disease is viewed. Success will require interactions between clinicians, translational researchers, and basic physiologists. Here, we review recent translational and clinical research into HFpEF, give perspectives on its evolving demographics and epidemiology, the role of multi-organ deficiencies, potential mechanisms that involve the heart and other organs, clinical trials, and future directions.
Keywords: heart failure, hypertension, hypertrophy, clinical ccardiology, therapy
Introduction
Heart failure(HF) is a clinical syndrome characterized by breathlessness (dyspnea) at normal or low-level exertion, fatigue, and fluid retention. As it’s name implies, HF centrally involves impaired heart function and the percent of blood volume ejected with each beat, or ejection fraction, has traditionally served as an indicator of pump dysfunction, being low in dilated hearts with depressed systolic performance. However, nearly half of all patients with HF symptoms have an EF that is preserved (exceeding 50%), or HFpEF).1 Importantly, the prevalence of HFpEF is rising, with morbidity, mortality, and healthcare costs on par with HF with a reduced ejection fraction (HFrEF).2-5 This syndrome has proven particularly challenging on virtually every front: A) consensus-based diagnostic criteria results in an very heterogeneous population that has proven very challenging for clinical studies and trials; B) multiple mechanisms have been proposed but many remain hypothetical due to limited access to live human heart tissue; C) good experimental models do not really exist, as many capture components of the human disease but do not reflect its integrative complexity; and D) patients suffer from multiple common comorbidities such as hypertension, diabetes, vasculopathy, renal disease, atrial fibrillation, metabolic syndrome, etc., that have an major impact on the syndrome and mortality. Given this, it is perhaps not surprising that we have yet to find an evidence-based HFpEF therapy beyond diuretics for fluid overload, and conventional treatments for co-morbidities.
In this article, we provide an overview of HFpEF for both the clinical and basic research scientist that includes a brief examination of its diagnostic criteria and evolving epidemiology, a summary of proposed mechanisms involving the heart and other organs, a discussion of our valiant but unsuccessful prior efforts to develop an effective therapy, and a review of newer potential approaches. The literature refers to HFpEF by several names including diastolic heart failure (DHF) and heart failure with normal ejection fraction (HFnlEF). HFpEF is currently the accepted form and we stick to that here. The companion review in this issue by Loffredo et al.6 focuses on the basic science underlying age-related cardiac disease, most notably diastolic dysfunction. Many of these changes are thought to be relevant to HFpEF, though direct evidence remains limited for most of them. In this presentation, we focus on the major human data findings.
HFpEF: What’s in a name?
Until fairly recently, patients with clinical HF yet with a normal-range EF and evidence of slow chamber relaxation were given a diagnosis of DHF.7-9 However, subsequent studies of such patients revealed minimal diastolic dysfunction in many10-12 or similar abnormalities in elderly patients with hypertensive heart disease but no HF,13,14 as well as key non-diastolic features such as limited systolic reserve, abnormal volume regulation, and maladaptive ventricular-arterial interaction.12, 15-17 In other words, a normal-range EF did not imply normal systolic function. As these and other non-cardiac features were recognized, the disease was re-named HFnlEF, though as of only 8 years ago, there was sufficient debate that DHF and HFnlEF were suggested to be used interchangeably.18 As more studies questioned whether systole is truly normal,19-21 the name changed to HFpEF22, 23 which is now the accepted standard.
Making the Diagnosis of HFpEF
To an extent, the diagnostic criteria for HFpEF have evolved along with its name. By the late 1990’s, this included signs and symptoms of HF with an objective measurement of exercise intolerance; “normal left ventricular (LV) function” defined as LVEF > 45%; and abnormal LV relaxation, filling, diastolic distensibility, or diastolic stiffness.24 Several embellishments were made involving morphological changes in the heart (e.g. hypertrophy, atrial enlargement, diastolic dysfunction),25 but these have gradually been removed as many patients often lacked a particular diastolic or structural defect, yet had all the hallmarks of a HF syndrome. Recent guidelines from the 2013 American College of Cardiology/American Heart Association consensus statement reconfirm that in practice, the diagnosis of HFpEF is based on typical symptoms and signs of HF in a patient with a normal LVEF and no significant valvular abnormalities by echocardiography.26 Diastolic abnormalties are mentioned but nothing specific. The European Society of Cardiology requires normal or mildly abnormal LV function and evidence of abnormal LV relaxation, filling, diastolic distensibility, and diastolic stiffness.27 We agree that while patients with clinical HF and preserved EF often have diastolic dysfunction, this should not be required for the diagnosis. In cases where dyspnea of unknown cause is present and EF is >50%, then objective evidence of cardiac dysfunction at rest or more likely with exertion would be important to demonstrate to assign a HF diagnosis. It is important for experimental biologists to appreciate that many humans have abnormal diastolic function with a normal EF – and this combination per se does not mean they have HF. Too often one sees animal models presented as HFpEF where diastolic pressures are elevated or relaxation delayed and EF is in the normal range. This may be a model of diastolic abnormalities, but it is not a priori HFpEF.
Epidemiology of HFpEF
Cross-sectional studies from westernized countries have established a view of HFpEF as elderly, predominantly female patients, and small hypertrophied hearts and a high prevalence of hypertension, diabetes, and atrial fibrillation.3, 4,28-30 Those reporting race have found a Caucasian predominance.29, 30 However, growing evidence suggests HFpEF patients are far more diverse (Table 1). Melenovsky et al. studied HFpEF in an urban population, finding a somewhat younger, predominantly African American (AA, 76%) population with very high rates of hypertension, marked ventricular hypertrophy, and obesity.13 Similar findings were reported by the New York Heart Failure Registry, with the addition of worse renal function in AA-HFpEF patients.31 These differences as recently reviewed by Shah32 likely impact therapy responses and net outcome. Increasingly, epidemiologic data report a much more balanced sex distribution,33 and this is seen in most clinical trials.34-36 The National Ambulatory Cohort of Veterans study examined nearly all men with HF; 30% had HFpEF.37 Compared to HFrEF, they were older, more likely Caucasian, had higher systolic blood pressure, and a higher prevalence of co-morbidities (diabetes, hypertension, anemia, chronic obstructive pulmonary disease, cancer, and psychiatric disorders). Internationally, HFpEF can be more common than HFrEF, as in Hong Kong where it accounts for 67% of HF admissions,38 occurring in men and women equally with high rates of hypertension. In Germany, HF is more common in elderly women, largely due to HFpEF.39 These data reveal HFpEF spans sex, race, and ethnicity, and is affecting increasingly younger patients. The traditional concept that hypertension and hypertrophy are dominant features conflicts with clinical studies finding this in a minority of recruited patients33-35, but may apply to some populations such as AA. This impacts our understanding of the disease and patient selection for clinical trials.
Table 1.
Comparison of clinical characteristics from population-based studies of HFpEF
| Characteristics | Olmsted Co., MN48 |
Olmsted Co, MN (2006)48 |
Ontario, CA4 |
Framingham 171 |
OPTIMIZE29 | ADHERE30 | Baltimore, MD13 |
NY HF Consortium31 |
Chicago, IL33 |
China38 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size, n | 244 | 2,167 | 880 | 220 | 10,072 | 26,322 | 37 | 619 | 419 | 132 |
| Age, yr | 76 | 74.4 ± 14.4 | 75.4 ±11.5 | 80 | 75.6 ± 13.1 | 73.9 ± 13.2 | 65 ± 10 | 71.7 ± 14.1 | 65 ± 13 | 72.3 |
| Women, % | 55 | 55.7 | 65.7 | 65 | 68 | 62 | 84 | 72.5 | 62 | 55.3 |
| African American, % | 15 | 17 | 76 | 30 | 39 | |||||
| LV ejection fraction, % | 62 ± 6 | 61 ± 7 | 62.4 | ≥ 45 | 61.8 ± 7.0 | ≥ 40 | 72 ± 11 | 60 | ≥ 50* | ≥ 45 |
| Outcomes | ||||||||||
| % 1yr survival | 71 | 78 | 80** | 65** | 86 (1.5yr) | |||||
| Comorbidities | ||||||||||
| Hypertension, % | 96 | 62.7 | 55.1 | 77 | 77 | 100 | 78.2 | 77 | 57 | |
| CAD % | 53 | 52.9 | 35.5 | 37 | 32 | 50 | 42 | 43.1 | 48 | 39 |
| Diabetes, % | 37 | 33.1 | 31.7 | 22 | 41 | 45 | 61 | 45.9 | 33 | 35 |
| Chronic kidney disease, % | 26 | 9.5 | 33 | 9 (ESRD) | ||||||
| Atrial fibrillation, % | 41.3 | 31.8 | 29 | 32 | 21 | 23.4 | 26 | |||
| SBP, mmHg | 132 ± 23 | 156 | 145 ± 24 | 150 ± 33 | 152.5 ± 33 | 143 ± 25 | 159.7 ± 35.5 | 125 ± 20 | ||
| DBP, mmHg | 67 ± 14 | 76 ± 13 | 75 ± 19 | 78.7 ± 21 | 69 ± 14 | 83.9 ± 20.4 | 70 ± 12 | |||
| BMI, kg/m2 | 32.2 ± 20.7 | 29.7 ± 7.8 | 27 ± 5 | 37 ± 8 | 30.6 ± 8.8 | 32.5 ± 9.3 | ||||
| Laboratory Values | ||||||||||
| Hemoglobin (g/dl) | 11.8 ± 2.1 | 12.4 ± 2.2 | 11.8 ± 2.2 | 11.9 ± 1.9 | ||||||
| Serum CR mg/dl | 1.6 ± 1.1 | 1.5 ± 0.9 | 1.2 | 1.7 ± 1.5 | 1.4 ± 0.7 | 1.6 ± 1.5 | ||||
| Medications | ||||||||||
| Diuretic, % | 57 | 64.8 | 87 | 63 | 74 | |||||
| ACE-I, % | 34 | 36.1 | 68 | 40 | 55 (ACE-I or ARB) | |||||
| ARB, % | 14 | 12.7 | 14 | 10 | ||||||
| Beta-blocker % | 50 | 45.5 | 81 | 35 | 67 | |||||
| Digoxin, % | 15 | 18.7 | 20 | |||||||
| Aldosterone antagonist, % | 4 | 5.4 | 5 | 5 | ||||||
| Statin, % | 37 | 23 |
OPTIMIZE: Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; ADHERE: Acute Decompensated Heart Failure National Registry; LV: left ventricular; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; ACE-I: angiotensin converting enzyme-inhibitor; ARB: angiotensin receptor blocker; CR – creatinine.
Mean/median values not given; enrollment criteria LVEF values reported.
Estimated survival based on Kaplan-Meier curves
The clinical outcomes of HFpEF are similar to those with HFrEF, including in-hospital morbidity and hospital readmission rates.4, 29,30 While in-hospital mortality may be slightly higher in HFrEF, 30-day to 1-year mortality post discharge is similar between groups.4, 29,30 Patients with either HF syndrome suffer from comparable functional limitations and poor quality of life.40, 41 Risk factors for mortality in HFpEF include advanced age, renal impairment, and hemodynamic instability (hypotension, tachycardia).30 There are differences in the etiology of morbidity and mortality between the groups, with morbidity in HFpEF being often driven more by non-HF cardiovascular conditions,37, 42, 43 and ~40% of deaths being linked to non-cardiac causes.44, 45
Mechanisms of Disease
Given the multi-faceted constellation of comorbidities that are almost invariably present in HFpEF patients, its underlying pathophysiology remains subject to debate. Among the leading contenders are diastolic dysfunction, impaired systolic reserve and perhaps even resting dysfunction, abnormal ventricular-arterial coupling, inflammation and endothelial dysfunction, depressed heart rate response (chronotropic incompetence), altered myocardial energetics and peripheral skeletal muscle metabolism and perfusion, pulmonary hypertension, and renal insufficiency. Several of these mechanisms are non-cardiac. A major challenge to the field is that truly representative experimental models of HFpEF do not exist, yet human data particularly direct myocardial analysis remains very limited. There are no data from beating muscle or cells from human hearts. Animal models usually focus on one or two features common to HFpEF such as pressure-overload (aortic banding or hypertension), obesity, diabetes, renal disease, aging, or ischemic heart disease without infarction. For practical reasons, however, multiple defects are rarely combined, and in this sense, existing animal models fall short of capturing the complexity of the human disease. Finally, there has long been a debate that HFrEF and HFpEF differ only in the letters r and p; that they are part of a continuum sharing key mechanisms. As attractive as this seems, we believe that mechanistic data and trial experience to date would suggest otherwise. In this section, we will address current cellular/tissue and integrative mechanisms, relying principally on data obtained in humans. These mechanisms are summarized in two cartoons, shown in Figures 1 and 2.
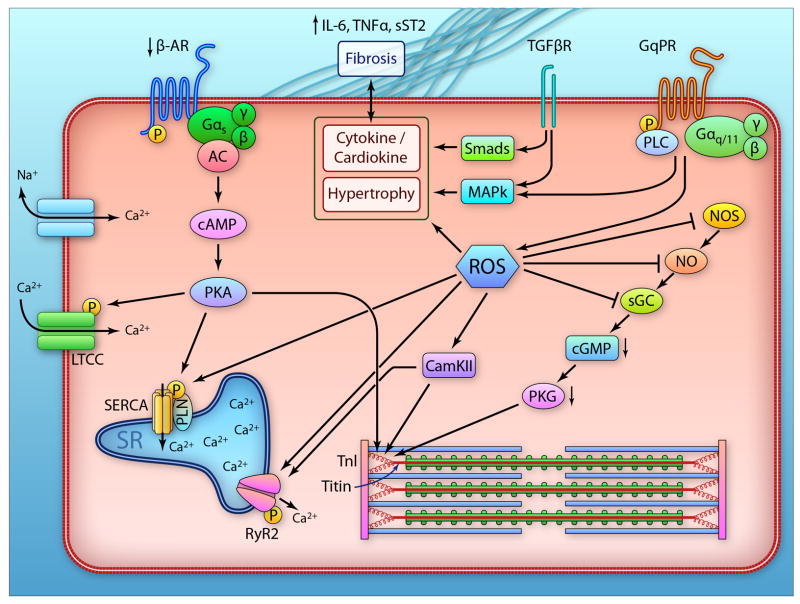
Figure 1.
Schematic of myocardial abnormalties revealed in human HFpEF. The left side shows components of the beta-adrenergic (b-AR) pathway from the receptor to adenylcyclase (AC), generation of cyclic AMP (cAMP) to activation of protein kinase A (PKA). The latter is involved with modification of L-type calcium channels, phospholamban (PLN), titin, and other regulatory thin filament proteins (e.g. troponin I, TnI) which influence myofilament stiffness and contractile activation. Evidence suggests a deficiency in this signaling pathway in HFpEF, with increased titin stiffness and depressed β-AR responsiveness. The middle section shows transforming growth factor b (TGFb) and Gq-protein coupled receptor (GqPR) signaling involving transcription factors (Smad), phospholipase C (PLC) and mitogen activated kinases (MAPk) which are involved with activation of pro-fibrotic and hypertrophic cascades. At the right is the nitric oxide synathase (NOS) pathway resulting in NO activation of soluble guanylatecyclase (sGC), generation of cyclic GMP (cGMP) and activation of protein kinase G (PKG). In the middle is reactive oxygen species (ROS) activated by TGFb, b-AR, and GqPR coupled signaling – which inhibits the NOS-cGMP generation and thereby PKG activity, stimulates CamKII which can render sarcoplasmic reticular (SR) calcium release by the ryanodine receptor (RyR2) more promiscuous. ROS and CamKII also impact titin to influence stiffening. Lastly, the upper right depicts the role of matrix modulation by cytokines/inflammation, and the by-directional interaction of these factors with the myocyte. (Illustration credit: Ben Smith)
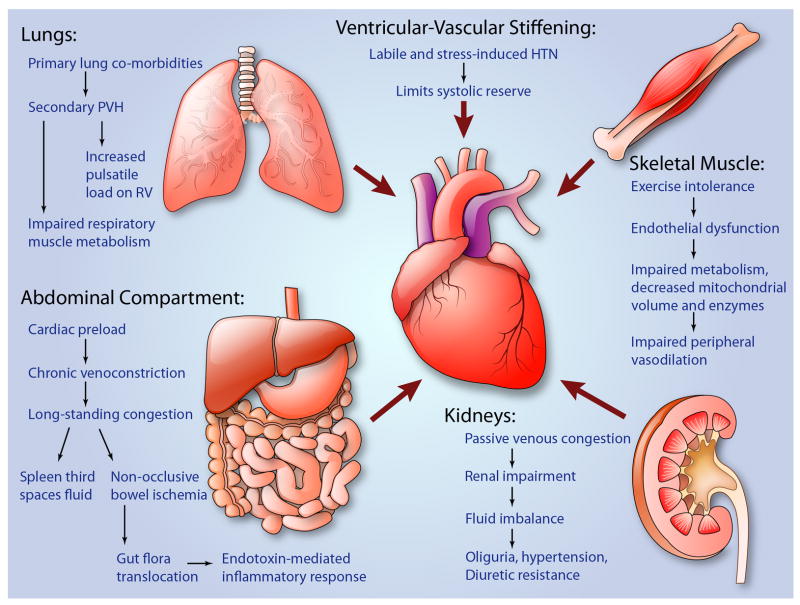
Figure 2.
Schematic of the integrative physiology of HFpEF showing various extracardiac mechanisms and how they are involved. From top left, counterclockwise: lung involvement including primary lung disease leading to PAH, secondary PVH, impaired lung muscle mechanics, and eventual increase pulsatile RV load; abdominal compartment mechanisms including splanchnic circulation (preload), bowel congestion leading to endotoxin translocation and systemic inflammation; skeletal muscle mechanisms including impaired metabolism and peripheral vasodilation; renal mechanisms including passive congestion leading to renal impairment, changes in neurohormonal axis activation, hypertension, abnormal fluid homeostasis, eventual oliguria/renal insufficiency; ventricular-vascular mechanisms including ventricular stiffening leading to systolic and diastolic impairment, diminished systolic reserve, increased cardiac energetic demands and fluid-pressure shift sensitivity. (Illustration credit: Ben Smith)
Myocardial Abnormalities
Diastolic Relaxation
HFpEF often presents with diastolic abnormalities including delayed early relaxation, myocardial and myocyte stiffening, and associated changes in filling dynamics. Slow relaxation has been documented in patients by means of invasive pressure recordings or echo-Doppler imaging parameters.11, 13, 15, 46-49 The magnitude of delay is such that its impact on resting diastolic pressures, particularly in mid to late diastole, is slight, but at faster heart rates,46 and/or conditions of increased vascular loading,15 this delay can become a more prominent contributor to elevated pressures. Most of the reported data compares relaxation rates to that of age-matched normotensive subjects or hypertensive patients without LV hypertrophy (LVH); however, the combination of LVH and hypertension without HF generates similar delay.13
The mechanisms for slowed chamber relaxation in HFrEF include reduction in the expression and regulation of proteins involved with calcium cycling into and out of the sarcoplasmic reticulum,50 depression of β-adrenergic signaling, oxidative stress targeting calcium handling proteins,51 and reduced recoil of elastic elements compressed during systole.52 Many of the same abnormalities are suspected in HFpEF, though direct proof remains limited given the lack of live tissue for human myocardial analysis. Clinical studies have found β-adrenergic responsiveness to be depressed.53 In an interesting study of biopsy samples from HFpEF and HFrEF patients, Hamdani et al.54 found the expression of calcium handling proteins and phosphorylation of myofilament proteins were very similar between the groups (there were no normal controls).β1-adrenergic receptor expression was somewhat reduced in HFpEF; however, GRK2 and GRK5 expression that can suppress stimulatory adrenergic signaling, were far more elevated in HFrEF. Relaxation is also controlled by passive recoil of elastic elements, notably titin, compressed during systole.52 With the termination of active force generation, these molecular springs uncoil quickly and re-extension contributes to the kinetics of force decline. Dilated hearts have depressed recoil,55 as the heart does not contract sufficiently to compress the elastic elements. However, as HFpEF volumes are generally normal, recoil may be less impacted.
Myocardial and Myocyte Stiffening
Passive myocardial stiffness is often observed in HFpEF and is considered an important contributor to disease manifestations. Chamber level analysis has consisted of invasively measured steady-state pressure-volume relations,46, 56 as well as simplified non-invasive estimates57 including the end-diastolic volume at a pressure of 20 mmHg.33 The causes for myocardial stiffening are divided into factors influencing the extracellular space such as fibrosis and infiltrative processes, and those intrinsic to the myocyte itself (Figure 1).
Myocardial fibrosis is a well-established feature of HFrEF and total collagen volume is similarly increased in HFpEF endomyocardial biopsy tissue.58-60 Both collagen type 1 and type III expression and tissue staining are elevated in HFpEF and are coupled to reduced collagenase, metalloproteinase-1, but increased tissue inhibitor of MMP-1 expression, which may further enhance fibrosis.61, 62 In addition to altering matrix turnover, cross-linking of collagen including the formation of advanced glycation end products contribute to fibrosis and stiffening.63, 64 Potential mechanisms for the altered matrix structure include inflammation, diabetes, and neurohumoral stimuli such as the renin-angiotensin-aldosterone system (RAAS). Markers of inflammatory cells are found in HFpEF tissue62 and have been proposed to play an important role in the disease.65, 66 The high prevalence of diabetes in HFpEF suggests a mechanism for fibrosis as well as AGE deposition. However, biopsy studies have found such correlations in HFrEF but not HFpEF.63 RAAS activation stimulates pathological fibrosis in many animal models and has long been presumed a major factor in HFpEF. However, the failure of multiple anti-RAAS clinical HFpEF trials suggests either that other factors and/or mechanisms are more important, or that fibrosis is not as central as assumed. An alternative is myocardial infiltration by amyloid proteins such as transthyretin (wtTTR). This liver synthesized protein is a common form of amyloid whose genetic variations cause hereditary amyloidosis. Recent autopsy data of HF hearts with an EF>40% at time of diagnosis found moderate to severe wtTTR deposition in 5%, with evidence of amyloid deposition in 19%.67 Whether TTR polymorphisms associated with disease68 play a role in HFpEF remains unknown.
While extracellular matrix abnormalities are generally similar between HFrEF and HFpEF, myocyte stiffness differs, being higher in cells from HFpEF. Borbely et al.58 first reported higher passive stiffness in isolated HFpEF myocytes versus controls. This stiffening was normalized by incubation of cells with protein kinase A (PKA), a change also more prominent in myocytes from HFpEF than HFrEF hearts.60 Analogous studies have extended this to protein kinase G (PKG) stimulation as well.69 The protein principally responsible for PKA and PKG responsive cellular stiffening appears to be titin, a macro-molecular spring whose elasticity varies with its isoform and post-translational modifications including phosphorylation and oxidation (reviewed in 70). Titinis synthesized as either the more compliant (fetal) N2BA or stiffer (adult) N2B form.71 Signaling by thyroid hormone, insulin, and Gq-protein coupled receptors to the PI3K-Akt-mTOR pathway enhance N2B expression. The N2BA:N2B ratio generally increases in human HFrEF, but changes with HFpEF remain less certain, with early data suggesting a decline60 and subsequent work finding an increase over normal controls.72 Titin phosphorylation targets two major regions, one in the N2B element (N2Bus) and the other in the in the PEVK (rich in proline, glutamate, valine, and lysine) region. The former is targeted by PKA, PKG, and CamKIIδ73-75 all of which reduce passive stiffness.58, 69, 70, 74 Titin oxidative formation of disulfide bonds in the N2B region, on the other hand, increases stiffness,76 though opposite effects have been reported by S-glutathiolylation of the protein.77
The capacity of PKG to modify titin and lower stiffness has formed the basis for a number of therapeutic interventions that activate this pathway including natriuretic peptides and phosphodiesterase 5A (PDE5A) inhibitors.78, 79 However, human HFpEF myocardial cGMP levels and associated PKG activity have been observed to be very low, far below that in HFrEF or hypertrophy due to aortic stenosis.69 This is consistent with hypophosphorylated titin, and could play an important role in stiffer HFpEF myocytes. The mechanism for depressed PKG activity may involve reduced nitric oxide-dependent cGMP synthesis due to oxidative stress. ROS can interfere with NO-related signaling at multiple nodes, oxidation of soluble guanylate cyclase impairs its responsiveness to NO to generate cGMP,80 NOS can become uncoupled by oxidation resulting in its synthesis of superoxide,81 and NO-ROS interactions thwart downstream signaling. Importantly, the capacity of PDE5A inhibition to augment PKG activity depends upon cyclase generation of cGMP, so this imbalance has clinical implications for treatments.
Resting Systolic Function: Is it “Normal”?
Ejection fraction largely informs us about chamber dilation – since until end-stage HF, stroke volume, (SV, the numerator) is usually maintained while the denominator, end-diastolic volume rises. Preserved EF does not imply systole is normal, and indeed a key set of observations that favored the name change to HFpEF suggested the opposite.19, 20, 82, 83 This has been recently observed using tissue Doppler speckle tracking; HFpEF patients had reduced longitudinal and circumferential strain compared to age- and gender-matched hypertensive patients with diastolic dysfunction but no clinical HF.84 However, the studies employing catheterization with imaging or conductance catheter measurements to derive pressure-volume relations find resting load-independent indexes of systolic function are essentially normal in HFpEF.16, 85 Isolated skinned myocyte data from HFpEF shows similar maximal calcium activated force,15, 54,58 but that is about all we know from human HFpEF tissue. Some measures of systole, such as end-systolic elastance (Ees) a measure of systolic stiffening, was higher in several HFpEF studies,15, 57 thought this seems particularly true in urban populations with a high percent of AA. Rather than implying increased resting contractility, the higher Ees may reflect myocardial hypertrophy, fibrosis, infiltrative disease, and/or titin modifications.
Ventricular-Arterial Coupling
Systolic ejection involves the interaction of time-varying properties of the ventricular pump and the vascular impedance to which it is connected. Vascular stiffening has long been associated with aging and is exacerbated by comorbidities such as hypertension, obesity, diabetes, and chronic kidney disease. To preserve adequate coupling of the heart to arterial system, ventricular systolic stiffening also increases, and this combined ventricular-vascular (VV) stiffening is a feature of HFpEF.15, 48, 86 This limits systolic reserve normally accompanying further rises in Ees, contributes to increased cardiac energy demands to enhance cardiac output,15 and plays a central role in arterial pressure lability with small changes in chamber preload volume. VV coupling is often represented by the ratio of effective arterial elastance (Ea) given by the ratio of end-systolic pressure to stroke volume (Pes/SV) that lumps systemic resistance, pulsatile loading, and heart rate effects into a single “afterload” parameter. VV coupling is then indexed by Ea/Ees ratio that normally ranges 0.5-1.2 to optimize cardiac work and efficiency.87 In HFpEF, Ea and Ees both increase, though similar increases are observed in patients without HF but with hypertension (±LVH).15, 57 When both Ees and Ea are increased, modest changes in LV filling as altered by diuresis or sodium loading (e.g. dietary indiscretions) induce marked swings in blood pressure and thus cardiac work with little change in SV.15
Limitations of Cardiovascular Reserve
The vast majority of HFpEF hemodynamic and myocardial data pertain to resting conditions, but arguably, this syndrome is first and foremost one of limited reserve and exertional intolerance. Multiple mechanisms likely play a role, including depressed systolic augmentation, limited heart rate augmentation (chronotropic incompetence), diastolic filling abnormalities, and reduced peripheral vascular dilation.
Kitzman et al. reported among the first studies of exercise capacity in HFpEF patients and highlighted failure of these patients to increase end-diastolic volume and thus engage the Frank-Starling mechanism.88 However, this study was very limited with 3 of the 7 patients having classic hypertrophic or restrictive cardiomyopathy, diseases known to impair preload reserve. Borlaug et al. studied 17 HFpEF patients versus a similar number of non-HF controls matched for comorbidities (in particular both LVH and hypertension), and also found reduced exercise capacity and peak oxygen consumption in the HFpEF group related to reduced cardiac output reserve.89 However, rather than being from impaired diastolic filling, low CO augmentation was related to a failure to enhance heart rate and peripherally vasodilate.89 Chronotropic incompetence has since been reported by multiple investigators90, 91 and found in large trials.35 This has implications for the use of beta-blockers and sinus node suppressors (If blockers) in the syndrome. The normally rapid heart rate decline after cessation of exercise is delayed in HFpEF, and this behavior is thought due to autonomic dysfunction and an independent risk factor for cardiac death.89, 91,92 Impaired peripheral vasodilation has been documented in exercised HFpEF patients using MRI.93 Borlaug et al examined cardiac systolic reserve in exercising HFpEF subjects and found that in addition to peripheral dilation and HR limitations, contractility increases were also depressed, resulting in VV mismatching.16.
Even if HR were to increase in HFpEF, studies find the ventricular response would likely be abnormal. The normal positive force-frequency was depressed in patients with LVH, many having presented with heart failure symptoms.94 However, in two subsequent HFpEF studies, LV function with incremental pacing increased contractility over controls or showed no difference,46, 95 though reserve was limited due to impaired diastolic filling. However, the normal controls in both studies surprisingly showed no decline in either end-diastolic filling or stroke volume at faster HRs as previously shown94. Instead, they stayed the same or even increased; thus, the HFpEF response was more consistent with normal physiology. Preload reserve limitations were not observed in several HFpEF exercise hemodynamic studies.16, 89 Thus, whether diastolic filling is truly restricted in HFpEF during tachycardia, remains uncertain.
Myocardial Energetics and Skeletal Muscle Metabolism
Among potential mechanisms for limited cardiac systolic reserve with HFpEF are abnormalities of myocardial energetics including adenosine triphosphate (ATP) generation and shuttling between phosphocreatine (PCr) and ATP by the creatine kinase reaction. Smith et al used NMR spectroscopy to assess patients with non-HFrEF (few technically had HFpEF), and found myocardial [ATP] was not significantly reduced in LVH or in LVH+HF compared to controls.96 However, cardiac [PCr] was 30% less in LVH with or without HF, reducing the PCr/ATP ratio in both groups. In addition, creatine kinase flux was 65% lower in LVH+HF versus controls, more than double the decline in LVH alone. Another study examining HFpEF did find a significant decline in PCr/ATP compared with controls.97 In a recent study to evaluate whether skeletal muscle abnormalities contribute to decreased peak exercise oxygen consumption (peak VO2) in HFpEF, Kitzman et al. performed needle biopsies of the vastus lateralis muscle and cardiopulmonary exercise testing to assess muscle fiber type distribution, capillary density, and peak VO2.98 HFpEF patients had reduced type-I oxidative muscle fibers, type I/II fiber ratio, and capillary to fiber ratio compared to healthy controls; the percent of type-II fibers was greater in HFpEF. The type-I fibers and capillary to fiber ratio was significantly associated with peak VO2. Exercise intolerance may also be impaired by endothelial dysfunction and abnormal skeletal muscle metabolism, including reduced mitochondrial volume and enzymes, and muscle atrophy. While the specific defects remain to be identified in HFpEF, several studies have found limited cardiac reserve fails to explain exertional intolerance and have highlighted abnormal skeletal muscle performance as likely contributors.99, 100
Role of Inflammation
Results from LV endomyocardial biopsy69 and analyses of inflammatory cell markers62 suggest increased oxidative stress and depressed NO-signaling resulting in inflammation play a key role in this syndrome.65, 66 The multitude of HFpEF comorbidities maycontribute to a pro-inflammatory state;101 circulating inflammatory cytokines such as interleukin-6, tumor necrosis factor-α, soluble ST2, and pentraxin 3 are elevated in HFpEF.102-105 Systemic inflammation could lead to endothelial dysfunction supported by higher expression of vascular cell adhesion molecules such as VCAM-1, E-selectin, and reactive oxygen species (ROS).62 Increased ROS lowers bioavailable NO and thus reducesc GMP/PKG activation, which can worsen myocyte stiffness as already noted, and also contribute to hypertrophic disease and fibrosis. Transforming growth factor beta signaling may also be increased in HFpEF myocardium,62 though data remain very limited. The complex and cell-specific signaling linked to this cytokine suggests that therapeutic targeting could prove difficult.106, 107
Biomarkers in HFpEF - A Clue to Mechanisms?
Plasma biomarkers consisting of proteins, peptides, and microRNAs, can reflect chronic and acute changes in structure and function of the myocardium, as well has changes in volume status, loading conditions, and vascular tone. A number of these biomarkers are of interest in HFpEF, to aid in diagnosis, prognosis, and to help better understand mechanisms of disease. The natriuretic peptides are perhaps the best characterized biomarkers in HFpEF. B-type natriuretic peptide (BNP) is typically higher in HFpEF than in non-HF patients, but lower than in HFrEF.108, 109 BNP linearly correlates with LV diastolic pressure and with LV diastolic wall stress in HFpEF; the smaller LV cavity size and thicker walls with resultant lower end diastolic wall stress may account for lower BNP levels.110 Biomarkers of extracellular matrix turnover and fibrosis in HFpEF have recently been reviewed, including soluble-ST2, galectin-3; collagen pro-peptides(PICP, PINP, PIINP); collagen telo-peptides (CITP); matrix metalloproteinases (MMP-1,-2,-8-9); tissue inhibitor of MMPs (TIMP-1, TIMP-4); and osteopontin, all of which can be elevated.110 Additional biomarkers including renal biomarkers (cystatin C, urinary albumin), cardiac troponins, and inflammatory markers (discussed previously) have also been noted to be elevated in HFpEF.111 While nearly all of these biomarkers support the diagnosis of HFpEF to some extent, a smaller subset may help predict outcomes, and even fewer may be used to guide therapies (primarily the natriuretic peptides). MicroRNAs as biomarkers for outcome and treatment selection have been described in HFrEF, but to date, no results have been reported in human HFpEF.
Pulmonary Hypertension and the Right Ventricle
Pulmonary hypertension (PH) defined by a mean pulmonary artery (PA) pressure >25 mmHg is commonly associated with HFrEF and harbingers a worse outcome. Data on PH in HFpEF are more limited, but studies are reporting a fairly high prevalence that importantly predicts increased morbidity and mortality.33, 112, 113 Pulmonary artery systolic pressure rises along with pulmonary capillary wedge pressure (PCWP) in patients with both hypertension and HFpEF; however, after adjusting for PCWP, pulmonary systolic pressure is still higher in HFpEF.112 This indicates that PH is due to more than pulmonary venous hypertension (PVH). Distinguishing these factors can be challenging. By definition, pulmonary arterial hypertension (PAH) is differentiated from PVH as the latter has an elevated PCWP> 15 mmHg. Estimation of PCWP by non-invasive methods is not always possible, and PCWP obtained at the time of right heart catheterization is influenced by the patient’s volume status when the procedure is done. Robbins et al. performed a fluid challenge at the time of catheterization to differentiate PAH from PVH, and of 207 patients meeting criteria for PAH, 22% developed elevated PCWP after a fluid bolus and were thus reclassified as overt PVH.114 Borlaug has demonstrated that many HFpEF patients who have normal PCWP at rest display marked increases with supine exercise associated with PAH.8 The implications of such data are that many patients with PH may have an under-recognized component of PVH linked to left-sided HF (including HFpEF), that is more manifest under conditions of exertion or volume loading.115
An additional role of PCWP from LV disease to PAH was revealed by Tedford et al., who studied the inverse relation between total pulmonary vascular compliance (CPa) and resistance (RPa) in patients with varying levels of PAH and PCWP elevation.116 The CPa-RPa relation is hyperbolic with a very tight interdependence between the two properties that is unique to the pulmonary vasculature. This results from having vascular compliance reside with the smaller peripheral vessels where resistance is also regulated; unlike the systemic arteries where the aorta provides most of the compliance but no resistance, and peripheral vessels provide the opposite. The CPa-RPa relation was remarkably invariantbut it did change with a rise in PCWP, with CPa declining at the same RPa. This indicates that PCWP impacts pulmonary arterial pulsatile load and thus RV systolic load, and likely has implications for HFpEF and PH. RV dysfunction is a well-established predictor of poor outcomes in increased mortality in HFrEF, and this may apply to HFpEF in that RV wall thickening was predictive of worse outcomes.33
Renal Dysfunction
Chronic kidney disease occurs in 26-53%of HFpEF and is associated with poor prognosis.30, 117, 118 Beyond baseline impairment, worsening renal function during HFpEF hospital admission predicts higher mortality at 6-months, with a 7-year survival of only 9%.118Albuminuria is an established independent risk factor of mortality in the general population, reflecting glomerular injury, activation of the RAAS system, and systemic inflammation, and has been reported in a third of HFpEF patients.119 During a 2.5 year follow up period, those with albuminuria at all strata of estimated glomerular filtration rate had higher rates of cardiovascular and non-cardiovascular death119. Finally, albuminuria can limit the efficacy of furosemide by binding the compound in tubular fluid, preventing its interaction with ion transporters.
In HFrEF, the mechanism of renal dysfunction is classically related to low cardiac output and decreased renal perfusion. Given that impaired volume homeostasis is a prominent presenting feature of HFpEF, it is no surprise that renal insufficiency is partly to blame, the question is how. Does intrinsic renal dysfunction (as a complication of other comorbidities) lead to myocardial inflammation, fibrosis, and resultant HFpEF? Does HFpEF cause renal dysfunction by triggering RAAS pathway activation, venous congestion,120 and/or from side effects of HF medications? There are intriguing pathways that may link renal and cardiac disease such as transient receptor potential channel-6, a Gq-receptor and ROS activated non-selective cation channel that plays an important role in proteinuria and glomerular dysfunction121 as well as cardiac hypertrophy122 and fibrosis.123 Impaired renal regulation combined with enhanced cardiovascular sensitivity to fluid retention due to VV stiffening and diminished diuretic efficacy can co-conspire to worsen symptoms in the HFpEF patient.
Abdominal Contributions
In many HFpEF patients, fluid retention is less apparent in the periphery, but not infrequently occurs in the abdominal cavity. This may play a significant role in cardiorenal disease in HF beyond vascular congestion, as recently reviewed by Verbrugge et al.124 While this pathophysiology is not unique to HFpEF, it does likely play a role particularly in fluid homeostasis, and is an area deserving attention. The splanchnic vasculature normally contains about 25% of total blood volume in capacitance veins. This capacitance function is impaired in HF, with increased neurohormonal activation resulting in venoconstriction in the setting of long-standing congestion. Splanchnic microcirculation and lymphatic flow are essential to preserve fluid homeostasis, and with HF, increased capillary hydrostatic pressure drives filtration of fluid through to the lymphatic system. Once lymph efflux is maximal, however, interstitial fluid with associated proteins cannot be adequately drained, leading to protein-rich edema and expansion of the interstitial space. Once the splanchnic vasculature and microcirculation can no longer cope with progressive volume overload, intra-abdominal pressure (IAP) increases. Normal IAP is 5-7 mmHg; intra-abdominal hypertension with IAP > 12 mmHg can lead to organ dysfunction. Consequences include abnormal hepatic regulation of renal function, splanchnic bed congestion which creates a false state of “hypovolemia”, and non-occlusive bowel ischemia which may eventually resultant in circulating endotoxin.
Treatment of HFpEF - A brief history of neutral trials
Targeting the RAAS and beta-adrenergic stimulation pathways has long been considered reasonable for HFpEF, the former based on its link to hypertension, fibrosis, and fluid imbalance, and the latter to improve time for diastolic filling. Yet despite their clear success in HFrEF, no clinical trial of these standard therapies has revealed similar mortality benefits, and very few show symptomatic improvement in HFpEF. The major recent neutral trials are summarized in a supplemental Table 2. These include studies of beta blockade (SENIORS125, J-DHF126, and ELANDD127), angiotensin converting enzyme-inhibitors (ACE-I;PEP-CHF128), angiotensin receptor blockers (ARB;I-PRESERVE129), aldosterone antagonists(ALDO-DHF36 and RAAM-PEF130, TOPCAT34), digoxin(DIG-PEF)43, and sildenafil (RELAX)35. Despite broad acceptance of diastolic impairment as a contributor to HFpEF, very few of these studies actually report diastolic analysis or cardiac structural data, making it very difficult to assess the impact of therapy on these behaviors.
A few studies have showed positive signals for potential benefit in HFpEF. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) study evaluated ACE-I in HF patients without demonstrable LV dysfunction, was under-powered for its primary composite endpoint of all-cause mortality and unplanned HF-related hospitalization, but did see some improvements in symptoms, exercise capacity, and fewer heart failure hospitalizations in the first observation year.128 The Effects of Candesartan in Patients with Chronic Heart Failure and Preserved Left-Ventricular Ejection Fraction (CHARM-Preserved) trial demonstrated that compared to placebo, HFpEF patients who received the ARB candesartan had fewer hospital admissions for HF, although there was no mortality benefit from the medication compared to placebo.131 Many HFpEF patients are treated with ACE-I and/or ARB for hypertension, and our clinical outcome data reflects this background therapy.
In 2013, the Effect of Spironolactone on Diastolic Function and Exercise Capacity in Patients with Heart Failure with Preserved Ejection Fraction (ALDO-DHF)study tested the impact of an aldosterone antagonist in HFpEF with the primary endpoints being improved diastolic function and exercise capacity.36 Some measures of diastolic function improved, though maximal exercise capacity, clinical symptoms, and quality of life were not changed. One critique of the study was that patients had early-stage HFpEF without overt signs of volume overload. The larger 2014 Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) also did not meet its primary composite endpoint (cardiovascular mortality, aborted cardiac arrest, or hospitalization for the management of heart failure).34 There was a small, borderline significant decline in hospitalizations. Interestingly, a major interacting factor was where patients were recruited and the criteria used for their entry; Eastern European patients were entered based on HF hospitalization criteria but follow-up course in the placebo arm of this group was surprisingly benign. By contrast, patients in the United States metnatriuretic peptide level entry criteria and had a higher event rate. Spironolactone improved the latter group.
The Effect of Phosphodiesterase 5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure with Preserved Ejection Fraction (RELAX) trial tested a new concept that by blocking PDE5A, cGMP/PKG signaling in HFpEF might be enhanced with associated benefits35. PDE5A hydrolyzes cGMP primarily generated by NO-soluble guanylatecyclase; by blocking the enzyme, drugs such as sildenafil can augment cGMP and thus PKG activity in multiple organs relevant to HF. Experimental studies in mice with pressure-overload,132 cytotoxicity from doxorubicin,133 and myocardial infarction,134-136 have shown benefits from chronic PDE5A inhibition. PDE5A inhibition also enhanced NP-stimulated pulmonary vasodilation in a canine HF model.137 Prior single-center studies had reported benefits of PDE5A inhibition in patients with HFrEF, particularly those with pulmonary hypertension, and in PH patients with preserved EF.138-140 However, RELAX was neutral, reporting no benefit of sildenafil over placebo in the primary endpoint (change in peak oxygen consumption after 24 weeks of therapy) or in any of a myriad of secondary functional and structural endpoints including markers of clinical status. Some argued that choosing exercise capacity as the endpoint caused problems due to the high number of co-morbidities and non-cardiac factors that influence this in HFpEF.141 In addition, the patient population may have played a major role in the neutral findings, as they had relatively mild diastolic dysfunction, the majority lacked LVH, and many had no overt PH and/or RV dysfunction, nor LVH (only 53% met criteria and median LV mass index was essentially normal), or systolic hypertension. This means there likely was little for PKG to impact in the heart as experimental studies showed sildenafil has negligible effect in mild LVH but far more efficacy if applied to severe disease, as only the latter triggers maladaptive signaling that PKG can offset.142 As noted, HFpEF patients have very low myocardial cGMP69, so there would be insufficient cGMP for PDE5a inhibition to modify. NP levels were mildly increased in some patients in RELAX, and minimally elevated in many of the patients, so an alternative cGMP source was not active.
Lessons Learned from Trials to Date
There are a number of potential reasons why these established HFrEF therapies have failed to benefit in HFpEF. First, our fixation on RAAS signaling may indeed be misplaced. It seems unlikely that this neurohormonal stimulation is uninvolved in HFpEF, but it may not be as sustained with less impact gleaned by its blockade. Perhaps HFpEF is less a neurohormonal-driven disease as compared to HFrEF, but rather is an integrative physiology disorder where hemodynamics and the control of blood volume and its distribution are more important. In the case of sildenafil, the question remains whether one needs to stimulate cGMP generation first and then perhaps add in a PDE5a inhibitor. While combining nitrates and PDE5a inhibitors remain relatively counter-indicated, some very low doses of a synthesis stimulator such as a direct sGC activator or natriuretic peptides might still prove effective, particularly if then combined with a blocker of cGMP hydrolysis.
Another important contributing factor is the patient population enrolled in clinical trials. In comparing population-based cohort descriptions to patients enrolled in clinical trials of HFpEF, it appears that the adverse outcome rates in the placebo groups in trials are markedly less than what is observed at the population-study level (see Table 1 compared to Table 2). How do we explain this discrepancy? In comparing the cohorts, patients enrolled in HFpEF therapy trials (irrespective of which treatment arm) have a lower prevalence of hypertension (lower systolic blood pressure), less LVH (when reported), and somewhat less coronary artery disease. Each of these individual morbidities portends increased risk of adverse outcome; and together their lower rates reflect a healthier cohort in the trials. This may reflect the multicenter and often international recruitment in trials versus more local and homogeneous sources in population studies, as well as involvement in a trial itself versus uncontrolled longitudinal observations. It argues for improving our capture of the truly at risk HFpEF group, something we are not presenting doing. It also suggests that more intensive clinical engagement, as accompanies being a participant even in the placebo arm, is rather effective.
Table 2.
Clinical characteristics of HFpEF in Negative Clinical Trials to Date
| First Author/Trial | Japanese-DHF126 | ELANDD127 | I-PRESERVE129 | DIG-PEF43 | ALDO-DHF36 | RAAM-PEF130 | RELAX35 | TOPCAT34, 117 |
|---|---|---|---|---|---|---|---|---|
| Intervention | Carvedilol | Nebivolol | Irbesartan | Digoxin | Spironolactone | Eplerenone | Sildenafil | Spironolactone |
| Sample size | 245 | 116 | 4128 | 988 | 422 | 44 | 216 | 3445 |
| Inclusion Criteria | LVEF > 40%; | LVEF > 45%; diastolic dysfunction by Doppler echo; NYHA class II-III | LVEF > 45%; NYHA II-IV; hospitalization for HF within past 6mo | LVEF > 45%; clinical signs/sx of HF; normal sinus rhythm | LVEF > 50%; NYHA II-III, evidence of diastolic dysfunction | LVEF > 50%, NYHA II-III; elevated BNP | LVEF >50; elevated NT-pro BNP; reduced exercise capacity | LVEF > 45%; controlled HTN (SBP <140mmHg or <160mmHg if on 3+ medication; serum potassium < 5.0mmol; history of hospitalization for HF in past 12 months or elevated BNP/NT-proBNP |
| Primary Endpoint | Composite CV death and unplanned hospitalization for HF | Change in 6MWT | Composite CV death from any cause or hospitalization for CV cause | Combined HF hospitalization or HF mortality | Changes in diastolic function (E/e’) and max exercise capacity (peak Vo2) | Change in 6MWT | Change in peak oxygen consumption | Composite of death from CV causes, aborted cardiac arrest, or hospitalization for HF |
| Outcome | Negative | Negative | Negative | Negative | Improved diastolic function; did not improve exercise capacity | Negative | Negative | Negative; lower hospitalization for HF in spironolactone group |
| One yr survival (%) | Placebo 90*; treatment 90* | Placebo, 90*; treatment 90* | Placebo 77; treatment 77 | Placebo 100; treatment >99 | 6 month survival: Placebo, 100; treatment 97 | Placebo >90; treatment >90 | ||
| Patient Characteristics (means or %) | ||||||||
| Age, yr | 73 | 67 | 72 | 67 | 67 | 72 | 68 | 69 |
| Women, % | 43 | 65 | 59 | 42 | 52 | 5 | 43 | 52 |
| Caucasian, % | 94 | 86 | 90 | 89 | ||||
| African American, % | 2 | |||||||
| NYHA Class, % | I (18), II (69), III (11), IV (2) | II (77), III (21) | II (21), III (77), IV (3) | I (19), II (59), III (20), IV (1) | II (85), III (15) | II (67), III (33) | II (49), III (51) | I (3), II (63), III (33), IV (0.4) |
| Comorbidities | ||||||||
| Hypertension, % | 80 | 86 | 89 | 62 | 92 | 100 | 80 | 91 |
| CAD, % | 28 | 17 | 38 | 50 | 43 | 67 | 42 | 59 |
| Diabetes, % | 28 | 21 | 28 | 27 | 61 | 62 | 42 | 32 |
| CKD, % | 31 | 48 | 56 | 39 | ||||
| Left ventricular hypertrophy, % | 48 | |||||||
| Vital signs | ||||||||
| SBP, mmHg | 134 | 134 | 137 | 135 | 130 | 124 (median) | 129 | |
| DBP, mmHg | 75 | 81 | 79 | 79 | 71 | 76 | ||
| Body mass index, kg/m2 | 24 | 30 | 29.7 | 29 | 30 | 33 (median) | 32 | |
| Admission Data | ||||||||
| BNP, pg/mL | 219 | 255 | 234 (median) | |||||
| N-terminal pro-BNP, pg/mL | 360 | 179 (median) | 757 (median) | 950 (median) | ||||
| Serum creatinine, mg/dl | 1.0 | 1.0 | 1.6 | 1.3 | 1.1 | |||
| LV mass (g), or LVMI (g/m2) | 126 g/m2 | 108 g/m2 | 49 g/m2.7 | 77 g/m2 | ||||
| Medications | ||||||||
| Diuretic, % | 63 | 49 | 82 | 82 | 55 | 95 | 88 | 82 |
| ACE-inhibitor, % | 24 | 75 (ACE-I or ARB) | 26 | 86 | 78 | 95 (ACE-I or ARB) | 65 (ACE-I or ARB) | 65 |
| ARB, % | 51 | 20 | ||||||
| Beta-blocker, % | 59 | 69 | 76 | 77 | 78 | |||
| Digoxin, % | 19 | 14 | ||||||
| Aldosterone antagonist, % | 21 | 15 | 12 | |||||
| Statin, % | 46 | 32 | 53 | 63 | 53 |
Japanese-DHF: Japanese Diastolic Heart Failure; ELANDD: Effects of Nebivolol on Clinical Symptoms, Exercise Capacity, andLeft ventricular Function in Diastolic Dysfunction; I-PRESERVE: Irbesartan in Heart Failure with Preserved Ejection Fraction; DIG-PEF: Digitalis Intervention Group-Preserved Ejection Fraction; ALDO-DHF: Effect of Spironolactone on Diastolic Function and Exercise Capacity in Heart Failure with Preserved Ejection Fraction; RAAM-PEF: Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction; RELAX: Spironolactone for Heart Failure with Preserved Ejection Fraction; TOPCAT: Treatment of Preserved Cardiac Function with an Aldosterone Antagonist; LVEF: left ventricular ejection fraction; HF: heart failure; CV: cardiovascular; NYHA: New York Heart Association; 6MWT: six minute walk test; HTN: hypertension; SBP: systolic blood pressure: BNP: B-type natriuretic peptide; NT-proBNP: N-terminal pro-B-type natriuretic peptide; CAD: coronary artery disease; CKD: chronic kidney disease; ACE-inhibitor: angiotensin converting enzyme-inhibitor; ARB: angiotensin receptor blocker; LVMI: Left ventricular mass index (LV mass/body surface area).
Estimated survival based on Kaplan-Meier curves
Finally, HFpEF is a simple enough label to apply to a patient, but the result is often profoundly heterogeneous, and differences among nations and medical practices can make it nearly impossible to create meaningful clinical trials. The different constellations of co-morbidities also raises the bar very high for a therapeutic home run, as these may play a greater role in symptoms and treatment responses than generally assumed. An approach to this was recently suggested by Sanjiv Shah, who described the concept of “matchmaking” HFpEF patients to clinical trials.143 Subgroups involving major features such as hypertension/LVH, or PH, etc. may respond differentially to a given therapy, and better population selection for clinical trials could yield more promising results.
New Therapeutic Avenues for HFpEF
HMG-Co-A Reductase Inhibitors
The use of HMG-Co-A reductase inhibitors, or statins, has yet to be tested in a large-scale trial. Observational reports of statin therapy in HFpEF have shown mixed findings for effects on diastolic parameters, though meta-analyses of 11 studies, mostly retrospective, suggests a significant benefit on survival 98, 144, 145. This is speculated to involve pleomorphic anti-inflammatory effects. Definitive trials have yet to be performed and may prove difficult given existing wide-spread use of statins in many HFpEF patients.
Ivabradine
The neutral results of β-blocker trials in HFpEF led investigators to pursue therapies targeting the sinus-node, including the inward “funny” (If) channel blocker, ivabradine which slows sinus rate but has no impact on contractility or the peripheral vasculature, unlike β-blockade.144, 146 Experimental data in mice with obesity and diabetes147 found reduced aortic stiffness and fibrosis and improvement in LV function from 4 weeks of ivabradine therapy.147, 148 Kosmala et al. recently published findings from a 7-day randomized clinical trial of ivabradine versus placebo in 61 HFpEF patients.149 Patients had improved peak oxygen consumption, exercise capacity, and decreased exercise-induced E/E’ ratio (index of diastolic pressure). There were no adverse events. Using a fairly homogenous cohort of patients with early stage HFpEF may have helped this particular study.149 However, heart rate lowering seems unlikely to benefit all HFpEF patients, particularly those with resting bradycardia and/or chronotropic incompetence, where further blunting a HR increase could worsen cardiac output reserve and thus exercise capacity. Also, patients with advanced diastolic disease with restrictive physiology are unlikely to benefit, since filling occurs early and rapidly in these patients anyway, and heart rate becomes a primary determinant of cardiac output. Larger-scale, multi-center studies will be needed to test the utility of this approach.
Neprilysin inhibitor (LCZ696)
Neprilysin is a zinc-dependent metalloprotease that degrades biologically active NPs, including ANP, BNP, and C-type NP. It does not affect the biologically inactive NT-proBNP.143 Natriuretic peptides can promote myocardial relaxation, reduce hypertrophy, and are coupled to integral to diuresis, natriuresis, and modest vasodilation.150 Clinical data for all of these effects are less well documented, but benefits have been observed. A recent randomized clinical trial compared LCZ696,151 which combines a neprilysin inhibitor pro-drug AHU377 and the AT1 receptor blocker – valsartan, to valsartan alone in 266 HFpEF patients.150 LCZ696 led to a greater decline in NT-proBNP; however, cardiac structure and function, and symptom composite metrics were similar between groups. Patients receiving LCZ696 had a greater reduction in blood pressure (~6 mmHg) by 12 weeks and fall in NT-proBNP remained significant after adjusting for this blood pressure change. Adverse effects were similar between the groups; overall, LCZ696 was well tolerated. The findings of this phase-2 study are promising and a large, multi-center study is underway comparing LCZ696 to enalapril (PARADIGM-HF).
Exercise Therapy
Exercise intolerance is a major complaint of all HF patients. It is an independent predictor of morbidity and mortality and is increasingly a leading outcome in pharmacologic trials of HFpEF. Exercise training has been used to improve outcomes in HFrEF, particularly in patients with ischemic disease, and is being viewed as a potential therapy for HFpEF.152 Exercise training provides cardioprotection against ischemia-reperfusion injury (see excellent recent review by Powers153), in part by suppressing ROS-mediated cellular damage, decreasing cytosolic free calcium, and reducing inflammatory changes from leukocyte infiltrationand mitochondrial damage. Cardioprotection from exercise training is biphasic; the first phase is rapid in onset and short in duration (onset at 30 min, lasting 3 hours), and involves activation of the endogenous antioxidant enzyme superoxide dismutase in mitochondria of ventricular myocytes. The second phase is longer-lasting (9 days), with multiple proposed mechanisms of benefit, including improved coronary circulation, stimulation of cytosolic antioxidants, increased heat shock proteins, increase in sarcolemmal- and mitochondrial- ATP-sensitive K channels, increase in cycolooxygenase-2, increased NO signaling, and altered mitochondrial phenotype (increased antioxidant capacity). Many of these same mechanisms have been implicated in the development of HF, including HFpEF.
Kitzman et al. reported findings from the first randomized, controlled study of exercise training in older patients with HFpEF over a 16-week period.154 The primary outcome of peak exercise oxygen uptake significantly improved in the exercise therapy group compared to controls. Improvements were also noted in exercise time, 6-minute walk distance, ventilatory anaerobic threshold, and peak power output, as well as the physical component of the quality of life score. Interestingly, exercise training did not appear to improve endothelial function or arterial stiffness in a study of exercise training in HFpEF evaluating flow-mediated arterial dilation and carotid artery stiffness.155 These initial studies of exercise training in HFpEF are promising and suggest that exercise training should be considered part of the treatment algorithm, along with pharmacologic agents, for the management of HFpEF. Effective translation in a population that is notably sedentary and often morbidly obese, will undoubtedly pose challenges, however.
Targeting Neural Reflex Arcs: Renal Denervation and Nerve Stimulation
Long-standing, resistant hypertension is common in HFpEF patients and alternatives to traditional pharmacological therapy are being sought. Renal sympathetic denervation is an example, and early results in small, non-placebo controlled studies raised substantial optimism that this would be effective.156, 157 However, the 2014 SYMPLICITY-HTN 3 Trial which studied 553 patients in a 2:1 randomization between active denervation or sham procedure, found no significant difference in the primary end-point of reduced systolic pressure at 6 months.158 This was strikingly different from the prior SYMPLICITY HTN-2 trial found significant blood pressure decline along with reduced LV mass and improved diastolic function in the active treatment arm, but also lacked a true placebo control159. The reasons for the discrepancies between the trials are being debated, but certainly the unbridled enthusiasm that had first met this therapy has been tempered.
Additional strategies to modulate autonomic tone include vagal nerve stimulators160 and carotid baroreceptor stimulators,161 both of which are emerging as promising therapies with pleomorphic effects. Among the proposed mechanisms of vagal nerve stimulation are anti-inflammatory effects, increased NO signaling, anti-cytokine effects, improved baroreflex sensitivity and RAAS inhibition162. The INNOVATE-HF study will test vagal nerve stimulation (CardioFit system, BioControl, Israel) in HFrEF patients162, but interest is already there for HFpEF as well. While still largely in experimental stages, spinal cord stimulators is another approach that has shown some utility in HF patients.163 A HFrEF study (Defeat-HF, NCT01112579) has completed enrollment with results due in 2015. Lastly, endovascular cardiac plexus stimulation may offer an alternative way to increase contractility without increasing heart rate.164
Pumps, Devices, Monitors
Device therapy has made enormous inroads into HFrEF with pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization therapy. The role of each in HFpEF is undefined; some patients with symptomatic chronotropic incompetence receive pacemakers, and those with a history of sudden death receive a defibrillator. Dyssynchrony in HFpEF can occur though it seems more rare than with HFrEF, and the efficacy of cardiac resynchronization therapy has not yet been demonstrated in HFpEF. If anything, inducing dyssynchrony on purpose by single-site ventricular pacing was found to benefit a group of HFpEF patients with severe concentric LVH and end-systolic cavity obliteration.165, 166 The rationale was that such patients have excessive contraction and generating dyssynchrony increases end-systolic volume at rest, building back in some reserve capacity during exercise.
Another type of technology relates to monitor systems that provide physiological information167 and these too may prove valuable for helping stabilize HFpEF patients and reduce their hospitalization rates. Some of the monitor data comes from existing therapy devices, such as CRT systems that also provide intrathoracic impedance measures via the RV lead,168 or monitor heart rate variability, and patient activity level. These are limited to patients receiving the therapy. Alternatively, devices that purely work as monitors have been developed, and typically assess some pressure measure correlated with central vascular volume, with the goal of identifying critical fluid overload and symptoms before aggressive intervention is needed. These include right ventricular pressure monitors,169 pulmonary artery pressure sensors (CardioMEMS Heart Sensor),168 and left atrial pressure monitors (sensor system implanted transvenously into the atrial septum, oriented towards the left atrium).170 Drug delivery systems such as furosemide pumps might be linked to hemodynamic sensors as an innovative way to treat HF patients “real time”, particularly targeting those patients who have a narrow range of filling pressure and fluid status tolerance - a common situation in HFpEF.
Miscellaneous Clinical Trials
Several other studies are currently underway examining the role of activation of the nitric-oxide soluble guanylatecyclase pathway. These are stimulated by appreciation for the hemodynamic sensitivity of HFpEF patients to vaso/venodilators, and their potential to stimulate a PKG-signaling pathway which is otherwise deficient. These trials are generally small and many single center or involving small consortiums. They are examining the potential value of inorganic nitrite (NCT NCT01932606), isosorbide dinitrate combined with hydralazine (NCT01516346), an oral soluble guanylate cyclase stimulator BAY1021189 (dose-ranging study called SOCRATES PRESERVED, sponsored by Bayer, NCT01951638), and a trial of udenafil, another PDE5A inhibitor (NCT01599117). There are also several ongoing trials of renal denervation (RDT-PEF, NCT01840059, and RESPECT-HF, NCT02041130), as well as a trial of acute HF management in HFpEF, evaluating diuretic strategy with and without low-dose dopamine (ROPA-DOP, NCT01901809)
Concluding Thoughts
HFpEF remains among the more challenging clinical presentations to diagnose and manage. Lack of a clear and consistent mechanism among the many patients that fall into a HFpEF definition, variations in the co-morbidities that modify its presentation and course, and the long list of failed therapies, make it a poster child for “Unmet Medical Needs.” Addressing this need is all the more important given the devastating morbidity and mortality and stress on the global health care system that the syndrome exacts. We are making progress, but it has been extraordinarily slow, and some reassessment of our concepts and perhaps some paradigm changes are in order.
First, we need to recognize that the “face” of HFpEF varies. There are marked differences in HFpEF among different populations around the world based on medical practices, urban versus rural living, racial sub-groups, etc. It is increasingly a disease of younger individuals affecting men and women equally. In many locations, obesity is a very common feature, and we need to understand much more how this impacts the syndrome.
Second, we need to better sub-classify HFpEF patients. Clinical trials and our overall approach would likely be improved by identifying patients based on dominant mechanisms of disease and symptom severity; the grab-bag diagnosis of HFpEF does not tell us very much. For example, patients with substantial diastolic dysfunction with or without structural heart disease may behave differently from those with marked systolic hypertension and ventricular-vascular mis-coupling, or those with substantial inflammatory conditions, or chronotropic incompetence, etc.. Some sense of the severity of the defect would be helpful. The presence of diastolic abnormalities and HF symptoms does not mean that the former is necessarily causal.
Third, we need more myocardial tissue. Not only biopsy pieces, but muscle that can be used to study live beating cells – so we can better identify what has happened and why? We recognize this is non-trivial, since these hearts are rarely ever replaced with a transplant - though if the heart is central enough to the disease and patients appear to be presenting at younger ages, perhaps this will change. The recent spread of integrative pathophysiology studies in humans is welcome, and more are needed.
Fourth, we need to improve experimental models, if possible. Animal models are typically designed to be monothematic on purpose, and while useful, efforts to combine common co-morbidities such as obesity, hypertension, and diabetes or some other pro-inflammatory state, would be welcome. Appreciation that aortic banding or high fat diet fed rodents is not HFpEF despite having some diastolic dysfunction and a preserved EF is important. Still, there is great value in chopping up the puzzle, and experimental efforts are revealing novel signaling cascades and therapies worth trying even from models that capture one or two dimensions of the disease. However, caveat emptor.
Fifth, we need to consider therapies outside of the traditional HFrEF-box. The failure of many clinical anti-RAAS trials and beta-blocker trials sends a message about what types of pathways and mechanisms are involved and we should listen to them. We have barked up this tree for a few decades; it is time to move on. HFpEF is truly a systems physiology disease, and treatments that integrate multiple targets – such as neuro-modulators or pleomorphic drugs – may prove most effective. We may soon have full feedback control systems that sense drug requirements and deliver them automatically; this could be a game changer. We call the disease HFpEF, but more and more data show skeletal muscle abnormalities are critical, and we need to start focusing on why and what this can mean for effective therapy.
The hope is that as we better focus on each of these issues, and gain new insights into how HFpEF works as a disease, we should finally be able to move it off the “un-met need” shelf where it has remained for some time, and onto one with our successful heart failure managements.
Supplementary Material
Acknowledgments
Supported by: NIH T32-HL07227 (KS), HL114910, HL077180, and HL119012, and Fondation Leducq (DAK).
Abbreviations
- HFpEF
heart failure and preserved ejection fraction
- HFrEF
heart failure and reduced ejection fraction
- DHF
diastolic heart failure
- HF
heart failure
- LV
left ventricular
- EF
ejection fraction
- AA
African American
- LVH
left ventricular hypertrophy
- GRK2/GRK5
g-protein receptor kinase 2/5
- RAAS
renin-angiotensin-aldosterone system
- TTR
transthyretin
- PI3K
phosphoinositol 3 kinase
- mTOR
mammalian target of rapamycin
- PKA
protein kinase A
- PKG
protein kinase G
- CamKIId
calcium-calmodulin activated kinase Iid
- cGMP
cyclic guanosine monophosphate
- PDE5A
phosphodiesterase type 5A
- NO
nitric oxide
- ROS
reactive oxygen species
- Ees
end-systolicelastance
- SV
stroke volume
- Ea
effective arterial elastance
- Pes
end-systolic pressure
- VV
ventricular–vascular
- CK
creatine kinase
- PCR
phsophocreatine
- VO2
oxygen consumption
- BNP
B–type natriuretic peptide
- PCWP
pulmonary capillary wedge pressure
- PVH
pulmonary venous hypertension
- PAH
pulmonary arterial hypertension
- Cpa
pulmonary arterial compliance
- Rpa
pulmonary arterial resistance
- IAP
intra–abdominal pressure
- ACE-I
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, Mark DB, Schulman KA, Gottdiener JS. Costs for heart failure with normal vs reduced ejection fraction. Arch Intern Med. 2006;166:112–118. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]
- 6.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Aging and diastolic dysfunction: Molecular pathways. Circulation Research. 2104 doi: 10.1161/CIRCRESAHA.115.302929. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 10.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR. Heart failure with a normal ejection fraction: Is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 11.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part i: Diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 12.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: Is it really a disorder of diastolic function? Circulation. 2003;107:656–658. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 13.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Kitzman DW. Diastolic dysfunction in the elderly. Genesis and diagnostic and therapeutic implications. Cardiol Clin. 2000;18:597–617. x. doi: 10.1016/s0733-8651(05)70164-8. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban baltimore community: The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 18.Quinones MA, Zile MR, Massie BM, Kass DA. Chronic heart failure: A report from the dartmouth diastole discourses. Congest Heart Fail. 2006;12:162–165. doi: 10.1111/j.1527-5299.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- 19.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: Time for a redefinition? Heart. 2002;87:121–125. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 21.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: With emphasis on regional systolic function. Circulation. 2006;113:296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 22.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: Accf/aha guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 23.Lindenfeld J, Albert NM, Boehmer JP, et al. Hfsa 2010 comprehensive heart failure practice guideline. Journal of cardiac failure. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.How to diagnose diastolic heart failure. European study group on diastolic heart failure. Eur Heart J. 1998;19:990–1003. doi: 10.1053/euhj.1998.1057. [DOI] [PubMed] [Google Scholar]
- 25.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–319. doi: 10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, et al. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 27.McMurray JJ, Adamopoulos S, Anker SD, et al. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 28.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: Prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 29.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the optimize-hf registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 30.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: A report from the acute decompensated heart failure national registry (adhere) database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Klapholz M, Maurer M, Lowe AM, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: Results of the new york heart failure registry. J Am Coll Cardiol. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Shah S. Review: Heart failure with preserved ejection fraction in african americans. Ethn Dis. 2012;22:432–438. [PubMed] [Google Scholar]
- 33.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 35.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 37.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yip GW, Ho PP, Woo KS, Sanderson JE. Comparison of frequencies of left ventricular systolic and diastolic heart failure in chinese living in hong kong. Am J Cardiol. 1999;84:563–567. doi: 10.1016/s0002-9149(99)00378-1. [DOI] [PubMed] [Google Scholar]
- 39.Tiller D, Russ M, Greiser KH, Nuding S, Ebelt H, Kluttig A, Kors JA, Thiery J, Bruegel M, Haerting J, Werdan K. Prevalence of symptomatic heart failure with reduced and with normal ejection fraction in an elderly general population-the carla study. PLoS One. 2013;8:e59225. doi: 10.1371/journal.pone.0059225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis EF, Lamas GA, O’Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in charm. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Hoekstra T, Lesman-Leegte I, van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail. 2011;13:1013–1018. doi: 10.1093/eurjhf/hfr072. [DOI] [PubMed] [Google Scholar]
- 42.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The charm-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: The ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. doi: 10.1093/eurjhf/hft062. [DOI] [PubMed] [Google Scholar]
- 45.Mangla A, Kane J, Beaty E, Richardson D, Powell LH, Calvin JE., Jr Comparison of predictors of heart failure-related hospitalization or death in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol. 2013;112:1907–1912. doi: 10.1016/j.amjcard.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, Luers C, Steendijk P, Hasenfuss G, Tschope C, Pieske B. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J. 2009;30:3027–3036. doi: 10.1093/eurheartj/ehp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: Deciphering the rosetta stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 48.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto K, Redfield MM, Nishimura RA. Analysis of left ventricular diastolic function. Heart. 1996;75:27–35. doi: 10.1136/hrt.75.6_suppl_2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kranias EG, Bers DM. Calcium and cardiomyopathies. Sub-cellular biochemistry. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- 51.Sag CM, Wagner S, Maier LS. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free radical biology & medicine. 2013;63:338–349. doi: 10.1016/j.freeradbiomed.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 52.Helmes M, Lim CC, Liao R, Bharti A, Cui L, Sawyer DB. Titin determines the frank-starling relation in early diastole. J Gen Physiol. 2003;121:97–110. doi: 10.1085/jgp.20028652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norman HS, Oujiri J, Larue SJ, Chapman CB, Margulies KB, Sweitzer NK. Decreased cardiac functional reserve in heart failure with preserved systolic function. J Card Fail. 2011;17:301–308. doi: 10.1016/j.cardfail.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamdani N, Paulus WJ, van Heerebeek L, Borbely A, Boontje NM, Zuidwijk MJ, Bronzwaer JG, Simonides WS, Niessen HW, Stienen GJ, van der Velden J. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur Heart J. 2009;30:1863–1872. doi: 10.1093/eurheartj/ehp189. [DOI] [PubMed] [Google Scholar]
- 55.Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res. 2000;87:235–240. doi: 10.1161/01.res.87.3.235. [DOI] [PubMed] [Google Scholar]
- 56.Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: A pressure-volume loop analysis. J Am Coll Cardiol. 2010;55:1701–1710. doi: 10.1016/j.jacc.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 57.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 59.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kuhl U, Schultheiss HP, Tschope C. Diastolic tissue doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 60.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55:1418–1424. doi: 10.1161/HYPERTENSIONAHA.109.149112. [DOI] [PubMed] [Google Scholar]
- 62.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 63.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 64.Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, Woodiwiss AJ. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res. 2003;57:632–641. doi: 10.1016/s0008-6363(02)00733-2. [DOI] [PubMed] [Google Scholar]
- 65.Glezeva N, Baugh JA. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart failure reviews. 2013 doi: 10.1007/s10741-013-9405-8. [DOI] [PubMed] [Google Scholar]
- 66.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 67.Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, DiSpenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart failure. 2014;2:10. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dungu JN, Anderson LJ, Whelan CJ, Hawkins PN. Cardiac transthyretin amyloidosis. Heart. 2012;98:1546–1554. doi: 10.1136/heartjnl-2012-301924. [DOI] [PubMed] [Google Scholar]
- 69.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase g activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 70.Linke WA, Hamdani N. Gigantic business: Titin properties and function through thick and thin. Circ Res. 2014;114:1052–1068. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 71.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 72.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the stiff n2b titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 73.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase g modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 74.Hamdani N, Krysiak J, Kreusser MM, Neef S, Dos Remedios CG, Maier LS, Kruger M, Backs J, Linke WA. Crucial role for ca2(+)/calmodulin-dependent protein kinase-ii in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res. 2013;112:664–674. doi: 10.1161/CIRCRESAHA.111.300105. [DOI] [PubMed] [Google Scholar]
- 75.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase a phosphorylates titin’s cardiac-specific n2b domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 76.Grutzner A, Garcia-Manyes S, Kotter S, Badilla CL, Fernandez JM, Linke WA. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin n2-b unique sequence. Biophys J. 2009;97:825–834. doi: 10.1016/j.bpj.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alegre-Cebollada J, Kosuri P, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernandez JM. S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding. Cell. 2014;156:1235–1246. doi: 10.1016/j.cell.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Jr, Linke WA, Redfield MM. Sildenafil and b-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–2891. doi: 10.1161/CIRCULATIONAHA.111.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamdani N, Franssen C, Lourenco A, et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–1249. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 80.Tsai EJ, Liu Y, Koitabashi N, Bedja D, Danner T, Jasmin JF, Lisanti MP, Friebe A, Takimoto E, Kass DA. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circ Res. 2012;110:295–303. doi: 10.1161/CIRCRESAHA.111.259242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruch C, Gradaus R, Gunia S, Breithardt G, Wichter T. Doppler tissue analysis of mitral annular velocities: Evidence for systolic abnormalities in patients with diastolic heart failure. J Am Soc Echocardiogr. 2003;16:1031–1036. doi: 10.1016/S0894-7317(03)00634-5. [DOI] [PubMed] [Google Scholar]
- 83.Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG. “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Fail. 2005;7:820–828. doi: 10.1016/j.ejheart.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111:2306–2312. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 86.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin. 2011;29:447–459. doi: 10.1016/j.ccl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 88.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: Failure of the frank-starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 89.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 90.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 92.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 93.Puntawangkoon C, Kitzman DW, Kritchevsky SB, Hamilton CA, Nicklas B, Leng X, Brubaker PH, Hundley WG. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. J Cardiovasc Magn Reson. 2009;11:48. doi: 10.1186/1532-429X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu CP, Ting CT, Lawrence W, Maughan WL, Chang MS, Kass DA. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy. Systolic versus diastolic determinants Circulation. 1993;88:1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- 95.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 96.Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Kitzman DW, Nicklas BJ, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky MJ. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: Role of lean body mass. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD, Scheffold T, Zugck C, Maisch B, Regitz-Zagrosek V, Hasenfuss G, Pieske BM, Wachter R. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–764. doi: 10.1007/s00392-011-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J. Inflammatory markers and incident heart failure risk in older adults: The health abc (health, aging, and body composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? European Journal of Heart Failure. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 104.Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li SY, deFilippi CR. Prognostic utility of st2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clinical Chemistry. 2011;57:874–882. doi: 10.1373/clinchem.2010.159277. [DOI] [PubMed] [Google Scholar]
- 105.Matsubara J, Sugiyama S, Nozaki T, et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. Journal of the American College of Cardiology. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 106.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte tgf-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rainer PP, Hao S, Vanhoutte D, Lee DI, Koitabashi N, Molkentin JD, Kass DA. Cardiomyocyte-specific tgfbeta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.302653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 109.Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, Borlaug BA, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. 2012;164:763–770 e763. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zile MR, Baicu CF. Biomarkers of diastolic dysfunction and myocardial fibrosis: Application to heart failure with a preserved ejection fraction. J Cardiovasc Transl Res. 2013;6:501–515. doi: 10.1007/s12265-013-9472-1. [DOI] [PubMed] [Google Scholar]
- 111.O’Meara E, de Denus S, Rouleau JL, Desai A. Circulating biomarkers in patients with heart failure and preserved ejection fraction. Current heart failure reports. 2013;10:350–358. doi: 10.1007/s11897-013-0160-x. [DOI] [PubMed] [Google Scholar]
- 112.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function in heart failure with preserved ejection fraction: Baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013 doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, Fong PP, Newman JH. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7:116–122. doi: 10.1161/CIRCHEARTFAILURE.113.000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borlaug BA. Invasive assessment of pulmonary hypertension: Time for a more fluid approach? Circ Heart Fail. 2014;7:2–4. doi: 10.1161/CIRCHEARTFAILURE.113.000983. [DOI] [PubMed] [Google Scholar]
- 116.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rusinaru D, Buiciuc O, Houpe D, Tribouilloy C. Renal function and long-term survival after hospital discharge in heart failure with preserved ejection fraction. Int J Cardiol. 2011;147:278–282. doi: 10.1016/j.ijcard.2009.09.529. [DOI] [PubMed] [Google Scholar]
- 119.Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, Shimokawa H. Urinary albumin excretion in heart failure with preserved ejection fraction: An interim analysis of the chart 2 study. Eur J Heart Fail. 2012;14:367–376. doi: 10.1093/eurjhf/hfs001. [DOI] [PubMed] [Google Scholar]
- 120.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions. Journal of the American College of Cardiology. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 121.Dryer SE, Reiser J. Trpc6 channels and their binding partners in podocytes: Role in glomerular filtration and pathophysiology. American journal of physiology Renal physiology. 2010;299:F689–701. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eder P, Molkentin JD. Trpc channels as effectors of cardiac hypertrophy. Circ Res. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 123.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A trpc6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Developmental cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62:485–495. doi: 10.1016/j.jacc.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 125.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (seniors) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 126.Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: The japanese diastolic heart failure study (j-dhf) Eur J Heart Fail. 2013;15:110–118. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 127.Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: Results of the elandd study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 128.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (pep-chf) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 129.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 130.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (raam-pef) J Card Fail. 2011;17:634–642. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The charm-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 132.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic gmp phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 133.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 134.Perez NG, Piaggio MR, Ennis IL, Garciarena CD, Morales C, Escudero EM, Cingolani OH, Chiappe de Cingolani G, Yang XP, Cingolani HE. Phosphodiesterase 5a inhibition induces na+/h+ exchanger blockade and protection against myocardial infarction. Hypertension. 2007;49:1095–1103. doi: 10.1161/HYPERTENSIONAHA.107.087759. [DOI] [PubMed] [Google Scholar]
- 135.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: Expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084–1095. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- 137.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of b-type natriuretic peptide and potentiates b-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–1088. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 138.Lepore JJ, Maroo A, Bigatello LM, Dec GW, Zapol WM, Bloch KD, Semigran MJ. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: Combined administration with inhaled nitric oxide. Chest. 2005;127:1647–1653. doi: 10.1378/chest.127.5.1647. [DOI] [PubMed] [Google Scholar]
- 139.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 140.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 141.Guazzi M, Bandera F, Forfia P. Sildenafil and exercise capacity in heart failure. JAMA. 2013;310:432. doi: 10.1001/jama.2013.7437. [DOI] [PubMed] [Google Scholar]
- 142.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Pressure-overload magnitude-dependence of the anti-hypertrophic efficacy of pde5a inhibition. J Mol Cell Cardiol. 2009;46:560–567. doi: 10.1016/j.yjmcc.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: No laughing matter. J Am Coll Cardiol. 2013;62:1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fukuta H, Little WC. Observational studies of statins in heart failure with preserved systolic function. Heart Fail Clin. 2008;4:209–216. doi: 10.1016/j.hfc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 145.Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011;301:H2198–2206. doi: 10.1152/ajpheart.00781.2011. [DOI] [PubMed] [Google Scholar]
- 146.Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: A preliminary report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 147.Reil JC, Reil GH, Bohm M. Heart rate reduction by i(f)-channel inhibition and its potential role in heart failure with reduced and preserved ejection fraction. Trends Cardiovasc Med. 2009;19:152–157. doi: 10.1016/j.tcm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 148.Reil JC, Hohl M, Reil GH, et al. Heart rate reduction by if-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34:2839–2849. doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of if-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: A randomized trial. J Am Coll Cardiol. 2013;62:1330–1338. doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 150.Solomon SD, Zile M, Pieske B, Voors AA, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong JJ, Lefkowitz M, Packer M, McMurray JJV, Investigators P. The angiotensin receptor neprilysin inhibitor lcz696 in heart failure with preserved ejection fraction: A phase ii randomised-controlled trial. Journal of cardiac failure. 2012;18:883–883. [Google Scholar]
- 151.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of lcz696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (arni) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 152.Edelmann F, Gelbrich G, Dungen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the ex-dhf (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 153.Powers SK, Smuder AJ, Kavazis AN, Quindry JC. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda) 2014;29:27–38. doi: 10.1152/physiol.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bohm M, Ewen S, Linz D, Reil JC, Schirmer SH, Ukena C, Mahfoud F. Therapeutic potential of renal sympathetic denervation in patients with chronic heart failure. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional. Cardiology of the European Society of Cardiology. 2013;9(Suppl R):R122–126. doi: 10.4244/EIJV9SRA21. [DOI] [PubMed] [Google Scholar]
- 157.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: One-year results from the symplicity htn-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 158.Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 159.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 160.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 161.Scheffers IJ, Kroon AA, Schmidli J, et al. Novel baroreflex activation therapy in resistant hypertension: Results of a european multi-center feasibility study. J Am Coll Cardiol. 2010;56:1254–1258. doi: 10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 162.Hauptman PJ, Schwartz PJ, Gold MR, Borggrefe M, Van Veldhuisen DJ, Starling RC, Mann DL. Rationale and study design of the increase of vagal tone in heart failure study: Inovate-hf. American heart journal. 2012;163:954–962 e951. doi: 10.1016/j.ahj.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 163.Lopshire JC, Zipes DP. Spinal cord stimulation for heart failure: Preclinical studies to determine optimal stimulation parameters for clinical efficacy. J Cardiovasc Transl Res. 2014;7:321–329. doi: 10.1007/s12265-014-9547-7. [DOI] [PubMed] [Google Scholar]
- 164.Kobayashi M, Sakurai S, Takaseya T, Shiose A, Kim HI, Fujiki M, Karimov JH, Dessoffy R, Massiello A, Borowski AG, Van Wagoner DR, Jung E, Fukamachi K. Effects of percutaneous stimulation of both sympathetic and parasympathetic cardiac autonomic nerves on cardiac function in dogs. Innovations (Philadelphia, Pa) 2012;7:282–289. doi: 10.1097/IMI.0b013e31826f14ff. [DOI] [PubMed] [Google Scholar]
- 165.Pak PH, Maughan WL, Baughman KL, Kieval RS, Kass DA. Mechanism of acute mechanical benefit from vdd pacing in hypertrophied heart: Similarity of responses in hypertrophic cardiomyopathy and hypertensive heart disease. Circulation. 1998;98:242–248. doi: 10.1161/01.cir.98.3.242. [DOI] [PubMed] [Google Scholar]
- 166.Kass DA, Chen CH, Talbot MW, Rochitte CE, Lima JA, Berger RD, Calkins H. Ventricular pacing with premature excitation for treatment of hypertensive-cardiac hypertrophy with cavity-obliteration. Circulation. 1999;100:807–812. doi: 10.1161/01.cir.100.8.807. [see comments] [DOI] [PubMed] [Google Scholar]
- 167.Abraham WT. Disease management: Remote monitoring in heart failure patients with implantable defibrillators, resynchronization devices, and haemodynamic monitors. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15(Suppl 1):i40–i46. doi: 10.1093/europace/eut105. [DOI] [PubMed] [Google Scholar]
- 168.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 169.Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Jr, Magalski A, Zile MR, Smith AL, Smart FW, O’Shaughnessy MA, Jessup ML, Sparks B, Naftel DL, Stevenson LW. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The compass-hf study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 170.Troughton RW, Ritzema J, Eigler NL, et al. Direct left atrial pressure monitoring in severe heart failure: Long-term sensor performance. J Cardiovasc Transl Res. 2011;4:3–13. doi: 10.1007/s12265-010-9229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.