Abstract
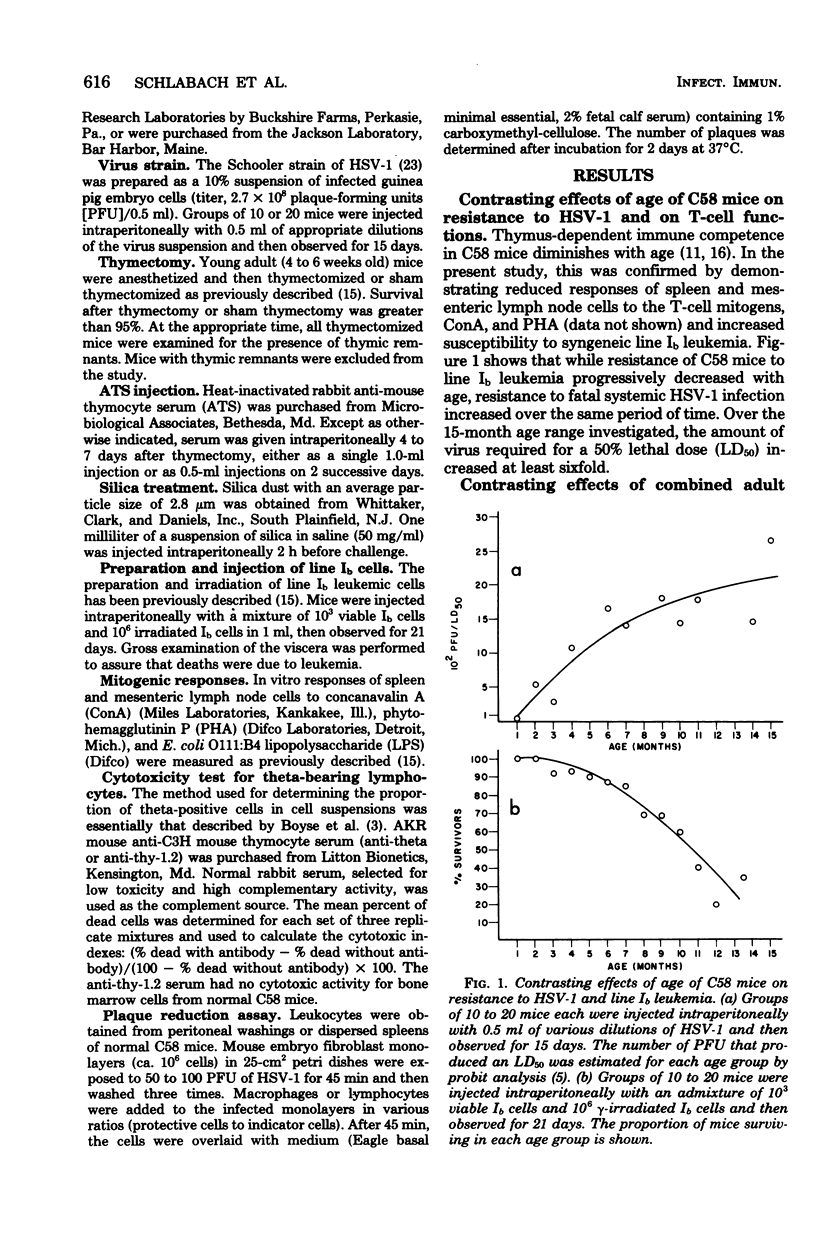
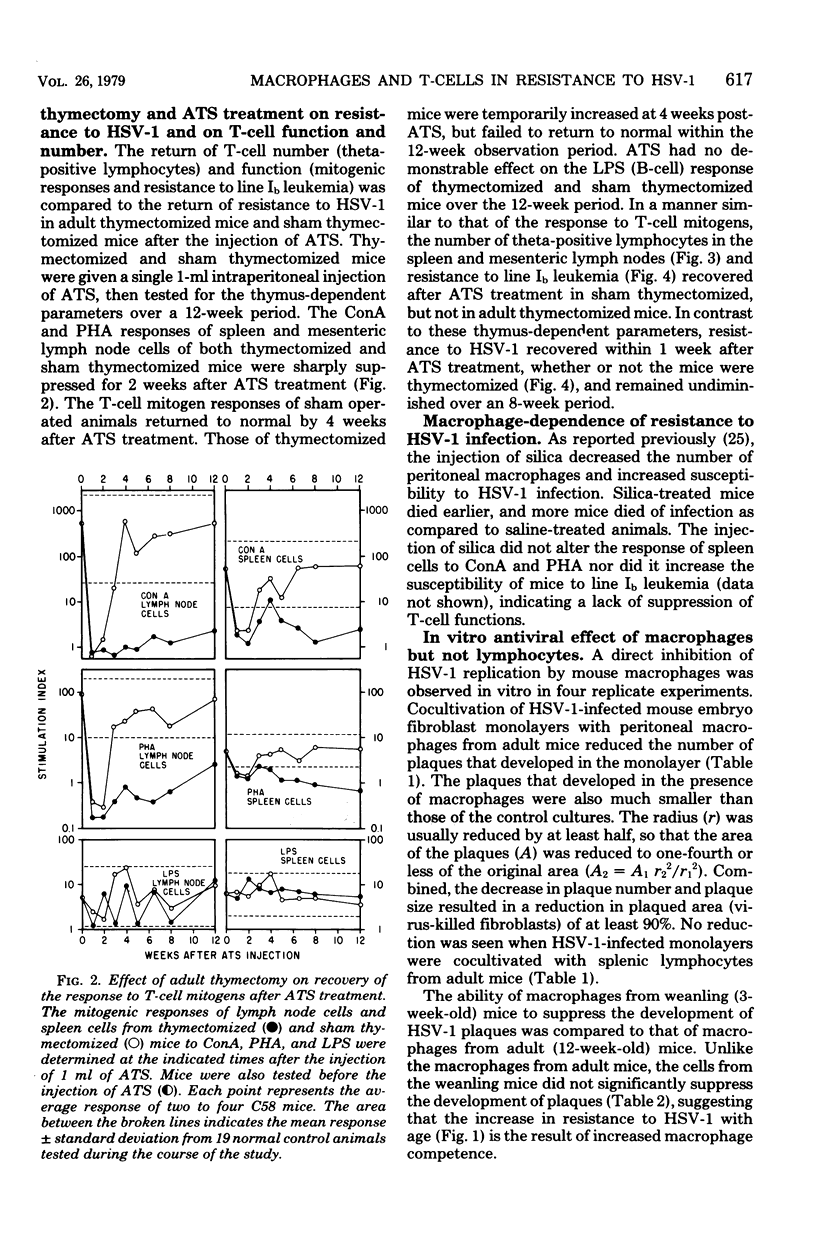
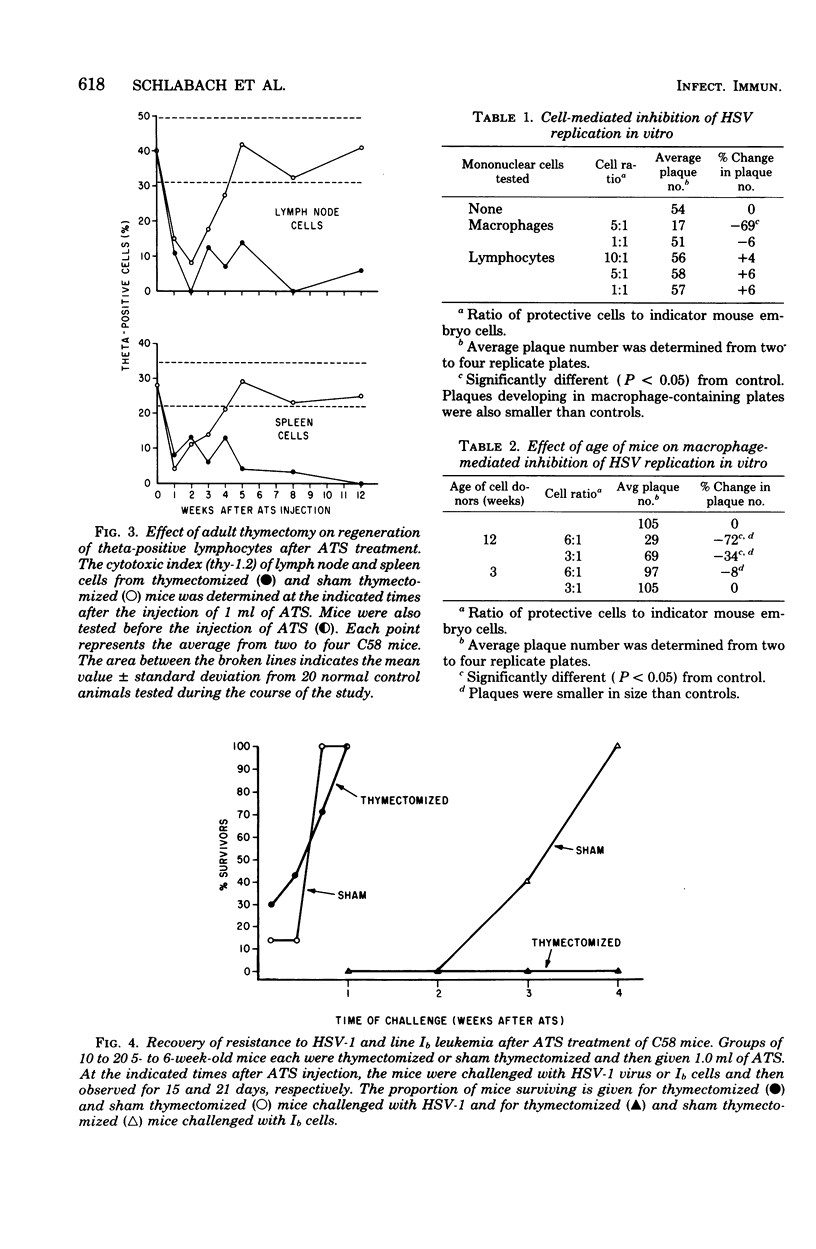
The relative contribution of thymus-derived lymphocytes (T-cells) and of macrophages to resistance to primary infection with herpes simplex virus type 1 (HSV-1) was studied in C58 mice. Resistance was dependent on macrophage competence, but was relatively independent of T-lymphocyte competence. Although aging mice became progressively more deficient in functional T-cells, as demonstrated by a decreasing resistance to transplanted line Ib leukemia and by declining responses to T-cell nitogens (concanavalin A and phytohemagglutinin), their resistance to HSV-1 increased with increasing age. Moreover, in mice that were made selectively deficient in T-cells by the combination of adult thymectomy and treatment with anti-thymocyte serum, resistance to HSV-1 did not correlate spleen and mesenteric lymph nodes. However, selective reduction of macrophages by intraperitoneal injection of silica resulted in enhanced susceptibility to HSV-1. Furthermore, in vitro suppression of HSV-1 plaque formation in mouse embryo fibroblast cells was obtained by cocultivation of infected fibroblast monolayers with peritoneal macrophages, but not with splenic lymphocytes, from adult mice. Macrophages from weanling mice failed to suppress the development of plaques, indicating that the increase in resistance to HSV-1 with age is a result of increased macrophage competence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Immunity and immunopathology in virus infections. Ann Inst Pasteur (Paris) 1972 Oct;123(4):585–608. [PubMed] [Google Scholar]
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Hubbard L., Stockert E., Lamm M. E. Improved complementation in the cytotoxic test. Transplantation. 1970 Nov;10(5):446–449. doi: 10.1097/00007890-197011000-00019. [DOI] [PubMed] [Google Scholar]
- Chare M. J., Boak J. L. Effect of antilymphocyte serum on macrophage activity. Clin Exp Immunol. 1970 May;6(5):655–659. [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen A. Rabbit anti rat lymphocyte serum: in vitro antimacrophage activity of different types of antisera and relationship to immunosuppression. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Jun;81(3):353–358. doi: 10.1111/j.1699-0463.1973.tb02215.x. [DOI] [PubMed] [Google Scholar]
- KILBOURNE E. D., HORSFALL F. L., Jr Studies of herpes simplex virus in newborn mice. J Immunol. 1951 Oct;67(4):321–329. [PubMed] [Google Scholar]
- Lawton J. W., Murphy W. H. Characterization of the blastogenic response of C58 spleen cells: age-dependent changes. Immunology. 1974 Jun;26(6):1093–1099. [PMC free article] [PubMed] [Google Scholar]
- Lukasewycz O. A., Duffey P. S., Murphy W. H. Immune mechanisms in leukemia: protective capacity of the major lymphoid cell compartments. J Immunol. 1976 Apr;116(4):976–981. [PubMed] [Google Scholar]
- Martinez D., Field A. K., Schwam H., Tytell A. A., Hilleman M. R. Failure of thymopoietin, ubiquitin and synthetic serum thymic factor to restore immunocompetence in T-cell deficient mice. Proc Soc Exp Biol Med. 1978 Nov;159(2):195–200. doi: 10.3181/00379727-159-40313. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Role of activated macrophages in resistance of congenitally athymic nude mice to hepatitis induced by herpes simplex virus type 2. Infect Immun. 1978 Mar;19(3):792–798. doi: 10.1128/iai.19.3.792-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R., Tasaki T., Kimura G., Takeya K. Depression of acquired resistance against herpes simplex virus infection in neonatally thymectomized mice. Arch Gesamte Virusforsch. 1967;21(3):459–462. doi: 10.1007/BF01241745. [DOI] [PubMed] [Google Scholar]
- Murphy W. H., Tam M. R., Lanzi R. L., Abell M. R., Kauffman C. Age dependence of immunologically induced central nervous system disease in C58 mice. Cancer Res. 1970 Jun;30(6):1612–1622. [PubMed] [Google Scholar]
- Nahmias A. J., Hirsch M. S., Kramer J. H., Murphy F. A. Effect of antithymocyte serum on herpesvirus hominis (type 1) infection in adult mice. Proc Soc Exp Biol Med. 1969 Nov;132(2):696–698. doi: 10.3181/00379727-132-34290. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Tarro G., Sabin A. B. Virus-specific, labile, nonvirion antigen in herpesvirus-infected cells. Proc Natl Acad Sci U S A. 1970 Mar;65(3):753–760. doi: 10.1073/pnas.65.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]


