Abstract
Traumatic injury is often associated with hemorrhagic shock. Liposome-encapsulated hemoglobin (LEH) is being developed as an artificial oxygen carrier to address post-hemorrhage oxygen and volume deficit. Here, we report a new composition of LEH based on the use of a PEG-non-phospholipid, hexadecylcarbamoylmethylhexadecanoate-PEG2K (HDAS-PEG2K) for modifying the surface of LEH particles. LEH was manufactured by high-pressure homogenization method using dipalmitoylphosphatidylcholine (~38 mol%), cholesterol (~38 mol%), HDAS (~20 mol%), and highly-purified stroma-free human hemoglobin. HDAS-PEG2K was post-inserted into the resultant LEH to generate HDAS-PEG2K-LEH. We investigated HDAS-PEG2K-LEH in mice models for the potential immune response. At the same time the preparation was tested in a rat model to study the effect of repeated HDAS-PEG2K-LEH injection over 4 weeks. We found that HDAS-PEG2K modification substantially reduced the circulating levels of anaphylotoxins C3a and C5a, as well as plasma levels of thromboxane B2 in mice. Repeated injections of HDAS-PEG2K-LEH in rats did not appear to alter its clearance profile after 4 weeks of treatment. No antibody response against human hemoglobin and PEG was detected in rat plasma. Histological observations of lung, liver, spleen, and kidney were inconspicuous between saline-treated rats and HDAS-PEG2K-LEH-treated rats. Immunohistochemical staining for rat heme oxygenase-1 (HO-1) did not show induced expression of HO-1 in these organs. These results suggest that the new surface modification of LEH is immune neutral and does not adversely affect histology even after repeated administration.
Keywords: Oxygen delivery, Liposome-encapsulated hemoglobin, Complement
It is estimated that hemorrhage is responsible for 30–40% of trauma-associated mortality, and approximately half of these casualties occur before hospital care could be provided [1]. Lack of timely resuscitation, excessive bleeding, oxygen deficit in critical organs, and coagulopathy associated with hemorrhage and trauma are the major reasons of these trauma-related deaths. Massive transfusion of blood and/or crystalloids, together with fresh frozen plasma, is an instinctive approach of resuscitative intervention. However, adequate availability of safe blood remains a major world-wide concern in transfusion medicine. The issues related to inventory management, possibility of known and unknown infections, requirements of pre-transfusion processing and cross-matching of blood, and limited shelf-life are the factors that have provided thrust to the search for safe, shelf-stable, and efficacious oxygen carrying fluids. Moreover, an oxygen-carrying fluid mimicking safety and efficacy profile of natural red blood cells (RBCs) is the goal. Therefore, an ideal artificially-assembled oxygen carrier would be hemoglobin (Hb) in an encapsulated form, such as in liposome-encapsulated Hb (LEH) [2–7]. By mimicking the membrane enclosed cellular structure of red blood cells, LEH eliminates the toxicity associated with free, whether modified or unmodified, Hb [3, 6, 8].
One goal of LEH formulation is to enable its prolonged circulation after administration. Stealth technology based on the use of poly(ethylene) glycol (PEG)-linked phosphatidylethanolamine (PEs) is employed for this purpose [9]. PEG prolongs circulation persistence of LEH by evading the opsonization and subsequent elimination process through mononuclear phagocyte system (MPS) [10]. In a recent study, we have demonstrated that stealth liposomes formulated with novel synthetic hexadecylcarbamoylmethylhexadecanoate-conjugated PEG2000 (HDAS-PEG2K) maintained longer circulation and lowered complement activation better than the liposomes modified with commonly used stealth phospholipid distearoylphosphatidylethanolamine(DSPE)-PEG2K [11]. As a non-phospholipid alternative to PEG-PEs, HDAS-PEG2K does not contain phosphoryl domain which has been implicated in a few in vivo complications associated with phospholipids in general [12–15]. In case of LEH, the problems could be magnified many fold, because of the large amounts of product infused during resuscitation. In this article, we report immunologic evaluation and consequences of resuscitation with LEH constituted of HDAS-PEG2K as the stealth lipid in rodent models.
MATERIALS AND METHODS
Unless otherwise mentioned, all chemicals were obtained from Sigma–Aldrich (St. Louis, MO) and/or various suppliers represented by VWR Scientific (West Chester, PA). HDAS and HDAS-PEG2K were synthesized in-house using methods described elsewhere [11]. Phospholipids were purchased from Lipoid (Ludwigshafen, Germany), Avanti Polar Lipids (Alabaster, AL), or NOF Corporation (Tokyo, Japan). High-purity cholesterol (Cho) was obtained from Calbiochem (Gibbstown, NJ). Outdated RBC units were sourced from Sylvan Goldman Center, Oklahoma Blood Institute (Oklahoma City, OK). Stroma-free Hb (SFH) was isolated from RBC units and characterized by essentially following methods described previously [16]. For in vivo work, mice and rats were purchased from Harlan (Indianapolis, IN, USA). All animal work was performed according to the National Institutes of Health Animal Use and Care Guidelines and was approved by the Institutional Animal Care Committee of the University of Oklahoma Health Science Center.
LEH preparation
A homogenization method of LEH preparation based on loading of SFH in pre-formed empty pro-liposomes was employed. The technical details of the method have been described elsewhere [16]. Strict aseptic conditions were maintained throughout the manufacturing in a laminar flow environment. The pro-liposomes consisted of lyophilized mixture of dipalmitoylphosphatidylcholine (DPPC, ~38 mol%), Cho (~38 mol%), HDAS (~20 mol%), HDAS-PEG2K (0.3 mol%), and vitamin E (~2.4 mol%). Briefly, the pro-liposome powder was gently mixed with SFH and homogenized by high-pressure homogenization in an Emulsiflex-C3 homogenizer at 20K psi for 4 cycles, maintaining the processing temperature at about 20°C. The extravesicular Hb was separated from LEH by tangential-flow filtration through 50 nm hollow fiber filter using PBS (pH 7.4) as diluting solvent [17, 18]. The purified LEH was PEGylated with HDAS-PEG2K using post-insertion method [11]. Briefly, a dilute (1.2 mg/mL) aqueous solution HDAS-PEG2K was slowly injected into a dilute dispersion of LEH and allowed to insert overnight at 4°C with constant stirring, followed by 2 h at room temperature. The dilute preparation of PEGylated LEH was further subjected to tangential-flow filtration (50 nm filter, PBS as wash-fluid) to eliminate remnants of free Hb and unincorporated HDAS-PEG2K. Finally, LEH was concentrated to the desired batch volume and stored at 4°C.
The LEH preparations were characterized for Hb content, metHb, size, oxygen affinity (p50), and lipid concentration. The phospholipid concentration was determined by the method of Stewart [19]. Oxygen affinity (p50) was measured by a Hemox-analyzer (TCS Scientific Corp., New Hope, PA). The amount of encapsulated Hb was determined by monitoring absorbance of the LEH lysate at 540 nm [20]. MetHb content was measured in SFH as well as in LEH [21]. Particle size was determined by dynamic light scattering using a Brookhaven particle size analyzer equipped with Particle Solutions v.1.2 (Brookhaven Instruments Corp., Holtsville, NY). For estimation of inserted HDAS-PEG2k within the liposomes, we employed indirect enzyme-linked immunosorbent assay (ELISA) as described below. The presence of PEG on the surface of LEH was also visualized by confocal microscopy as described elsewhere [11]. To document the formation and integrity of liposome using HDAS and HDAS-PEG2K, we performed scanning electron microscopy (SEM) at imaging core facility of Oklahoma Medical Research Foundation (OK, USA).
TXB2 determination
Female six to eight week old CF1 mice (25–30 g) were randomized in three groups (n = 3 per group). One group of mice was kept as control to obtain the required baseline values. Appropriate liposome formulations were injected intravenously (80 mg/kg body weight in 0.1–0.2 mL). Further blood samples were collected at 60 min post-liposome injection to obtain plasma in EDTA-containing tubes. TXB2 levels were estimated by ELISA kit (Cayman Chemical Company, Ann Arbor, MI).
Detection of serum C3a and C5a
BALB/c mice (6 groups, 6 mice/group) were infused with HDAS-PEG2K-LEH, non-PEGylated LEH, free SFH, saline, and liposomes modified with DSPE-PEG2K. The amount of infusion was maintained at approximately 10% of total blood volume, calculated as 6% of body weight. After 1 h of infusion, blood was collected into pre-cooled EDTA tubes containing FUT-175 as protease inhibitor. ELISA was performed on separated plasma according to the manufacturer’s protocol (BD Pharmingen, San Jose, CA).
HDAS-PEG2K-LEH reinjection in rats
Male Sprague Dawley rats weighing 250–300 g were recruited to study the effect of prior exposure to HDAS-PEG2K-LEH on elimination kinetics from blood. The rats were randomized in two groups (n = 6 each) for HDAS-PEG2K-LEH and saline. HDAS-PEG2K LEH was intravenously infused (10 ml/kg) every 7th day for 21 days. The rats in saline group were treated with equal volume of saline. On 28th day, LEH was administered in both groups and time-based blood samples were collected up to 24 h after injection. The blood samples were preserved for estimation of circulating PEG and human Hb using ELISA methods. Finally, the rats were euthanized for immunohistochemical detection of rat hemoxygenase-1 (HO-1) and presence of human Hb in the rat lung, liver, spleen, and kidney by a method described by Sakai et al [22].
Neoantigen formation
For determination of antibody formation against human Hb or HDAS-PEG in rats injected with HDAS-PEG2K LEH, we employed direct ELISA method on sera obtained on weeks 0 through 3. Briefly, the wells of Immulon 4 strips (Thermo, Milford, MA) were coated overnight with probe-sonicated LEH, diluted 1:1,000,000 with 0.1 M NaHCO3/Na2CO3, pH 8.0. The wells were washed three times with 0.05% Tween 80, followed by washing twice with deionized water. The nonspecific sites were blocked with 0.25% bovine serum albumin for 30 min. The wells were washed and incubated overnight with rat sera obtained from various weeks of LEH injection. The wells were again washed and incubated for 4 h with FITC-labeled mouse anti-rat IgG (2,000× dilution). After washing the wells, the plate was read at 450 nm. Rabbit anti-human Hb and rabbit anti-PEG antibody were used as positive controls.
ELISA for PEG and human Hb
We determined the amount of PEG and human Hb in LEH and in plasma samples by employing ELISA using anti-PEG antibody (Epitomics, Burlingame, CA) and anti-Human Hb antibody (Abcam, Cambridge, MA), respectively. The processes of sample coating (diluted 1:1,000,000), washing, blocking of non-specific sites, and probing with primary antibodies (2,000× dilution) and HRP-conjugated anti-rabbit IgG secondary antibody (1,000× dilution, Cell signaling, Danvers, MA) were similar to the one described above.
Histopathological and immunohistochemical (IHC) examination
Rat liver, lung, spleen, and kidney were fixed in 10% buffered formalin immediately after removal. The paraffin sections were stained with hematoxylin & eosin (H&E) for histopathological examination. IHC was performed to detect rat HO-1. Briefly, 2 µm thick paraffin sections were mounted on 3-aminopropyl triethoxysilane-coated glass slides. The sections were treated with 0.05% H2O2 in methanol for 10 min at room temperature to block the endogenous peroxidase activity. For antigen retrieval, the sections were treated with proteinase K (0.4 mg/ml; DakoCytomation California Inc., Carpinteria, CA) for 10 min at room temperature. After blocking the non-specific binding with 5% normal goat serum, they were incubated with goat polyclonal antibody against rat HO-1 (2 µg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The slides were washed and incubated with biotinylated link universal antiserum, followed by streptavidin-HRP conjugate. The slides were rinsed and their color was developed using 3,3-diaminobenzidine hydrochloride as a chromogen. Finally, the sections were rinsed in distilled water, counterstained with Mayer’s hematoxylin solution, and mounted with DPX mounting medium for microscopic evaluation (Olympus microscope 1X701 equipped with DP70 camera).
Data Analysis
All average values are given as ± standard error of mean. Wherever applicable, the data were statistically analyzed by student t-test or analysis of variance using GraphPad Prism software for Windows (La Jolla, CA). For multiple comparisons, Bonferroni's post hoc test was applied. The acceptable probability for significance was set at p < 0.05. The plasma profile for human Hb and PEG was analyzed by method of residuals.
RESULTS AND DISCUSSION
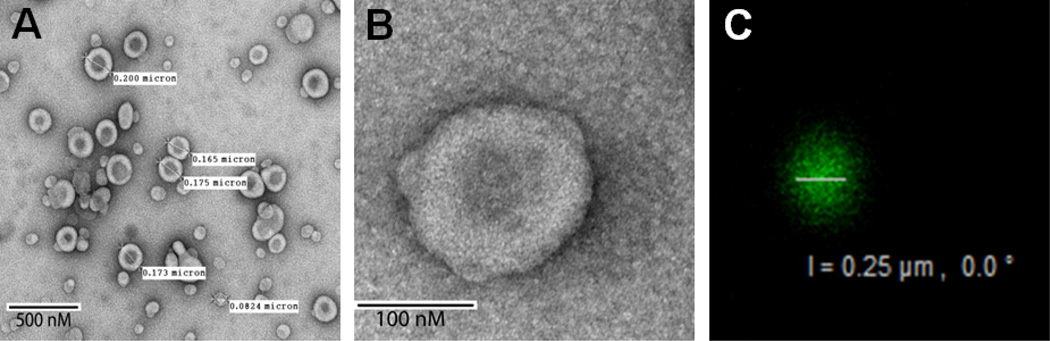
Development of non-phospholipid amphiphiles for liposome preparation is of considerable interest because of biological reactivity, relative instability and the costs associated with extraction and synthesis of phospholipids [15, 23, 24]. The phosphoryl moiety of phospholipids is implicated in many hypersensitivity reactions observed with liposomes [25, 26]. We recently reported a novel non-phospholipid PEG-based lipopolymer, HDAS-PEG2K, and used it to modify pre-formed liposomes for prolonging the circulation of liposomes [11]. Here, we report the evaluation of post-inserted HDAS-PEG2K in a composition of LEH. The advantages of post-insertion of PEG-lipopolymer have been discussed elsewhere [11]. The characteristics of resultant LEH are described in Table 1. The narrow polydispersity of LEH suggests that the liposomes had homogeneous size distribution. SEM images showed that the particles were spherical in shape and maintained typical features of liposome structure (Fig. 1a and 1b). To confirm the presence of post-inserted HDAS-PEG2K on the liposome surface, LEH was labeled with anti-PEG antibody and observed by confocal microscopy using Alexa Fluor® 488 Dye-conjugated anti-IgG antibody. The green fluorescence emanating from the liposome surface clearly shows the presence of surface PEG (Fig 1c). As expected, plain liposomes without HDAS-PEG2K did not show any green fluorescence (not shown). We estimated that only approximately 35% of total HDAS-PEG2K ended up as being associated with LEH surface after post-insertion. We have shown earlier that PEG-lipopolymers exist in dynamic equilibrium and are desorbed upon dilution [11]. In the resultant LEH, the molar ratio of DPPC: HDAS-PEG2K was 13:2.
Table 1.
LEH characteristics
| Parameter | Value |
|---|---|
| Size (nm) | 164 |
| Polydispersity | 0.164 |
| Methemoglobin | <4% |
| Hemoglobin in LEH (g/dl) | 12.4 |
| Osmolality (mOsmol/kg) | 358 |
| Phospholipid concentration (mg/ml) | 11.43 |
| p50 (mm Hg) | 30.46 |
Figure 1.
(A and B) SEM of LEH surface-modified with HDAS-PEG2K. (C) A confocal micrograph of HDAS-PEG2K-LEH showing the presence of PEG on the surface of the liposome. HDAS-PEG2K-LEH was labeled with FITC-labeled anti-PEG antibody.
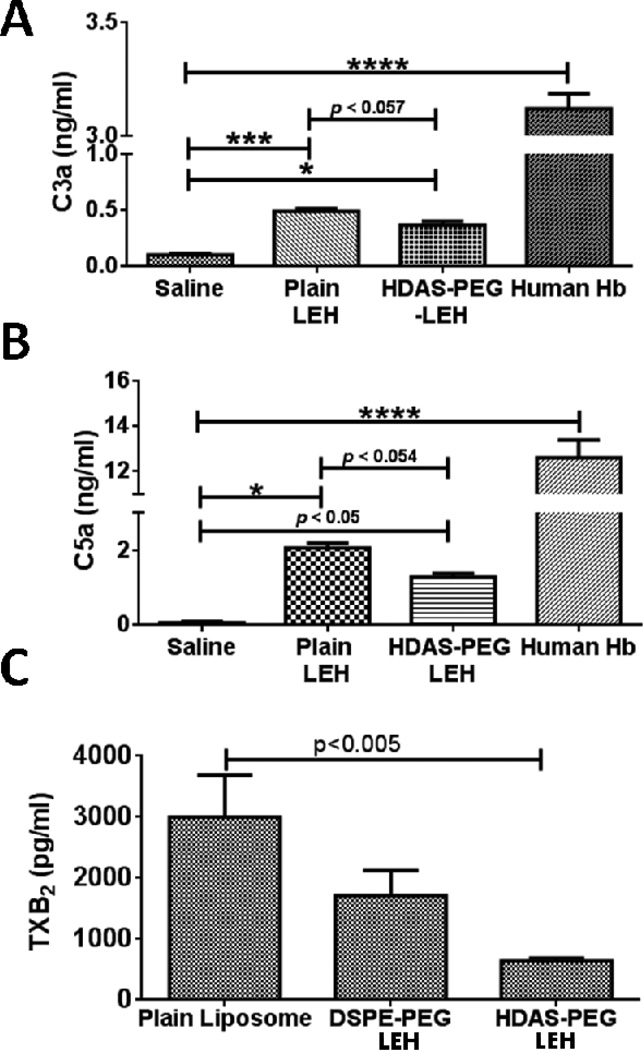
Complement proteins play an important role in liposome clearance from circulation [27–29], and the anionic nature of phospholipids plays a role in this phenomenon [14, 29, 30]. They normally circulate as inactive precursors and are activated by protease-mediated cleavage. The activated complement proteins such as anaphylotoxins C3a and C5a are responsible for opsonization of foreign bodies, enhanced phagocytosis, mast cell degranulation, and other immune responses. Liposome surface is known to activate complement proteins in vivo. A complement-dependent rapid, but transient, decrease in circulating platelets immediately following liposome injection has been noted [31, 32]. Recently, we reported that the liposome-mediated platelet activation could be partially addressed by reduction in anionic phospholipid content and PEGylation [12]. Therefore, we first examined the effect of HDAS-PEG2K modification on the levels of anaphylotoxins (C3a and C5a) by LEH in mice. Plain LEH preparation without any modification was taken as a control, whereas free human Hb was administered for maximum complement activation. As shown in Fig. 2a and 2b, plain LEH significantly increased the circulating levels of C3a and C5a. In comparison, the surface modification of LEH with HDAS-PEG2K substantially reduced these levels. Free human Hb (10% of blood volume) activated complement to a considerable extent which was similar in magnitude to that shown by pure endotoxin administration (not shown). These results clearly suggest that not only encapsulation eliminates complement activation by free Hb, but post-insertion of HDAS-PEG2K further reduces complement activation induced by plain LEH particles. Another indication of intravascular complement activation is the production of TXA2. Anaphylotoxins C3a and C5a activate polymorphonuclear leukocytes, resulting in thrombosane A2 (TXA2) release in circulation. TXA2 is very unstable and is hydrolyzed rapidly to biologically inactive thromboxane B2 (TXB2). Therefore, we determined TXB2 in mice plasma because it is an established and direct marker of TXA2 production [33]. As shown in Fig. 2c, liposomes that were post-inserted with HDAS-PEG2K resulted in negligible increase in the levels of TXB2 as compared to both plain liposomes and DSPE-PEG2K-modified liposomes. We speculate that the novel HDAS-PEG2K, which does not contain phospholipid moiety, is the reason behind the lack of complement activation because the anionic phosphoryl group of PEG-PE has been attributed as a cause for liposome-induced complement activation [34, 35].
Figure 2.
(A) and (B) are the levels of complement proteins C3a and C5a, respectively. The complement proteins were estimated by ELISA in BALB/c mice serum (n = 3/group). Treatment with human Hb was taken as a positive control. (C) Plasma TxB2 concentrations (mean ± standard error of mean) measured in CF1 mice after 60 min of plain liposome, DSPE-PEG2K-LEH, and HDAS-PEG2K-LEH infusion.
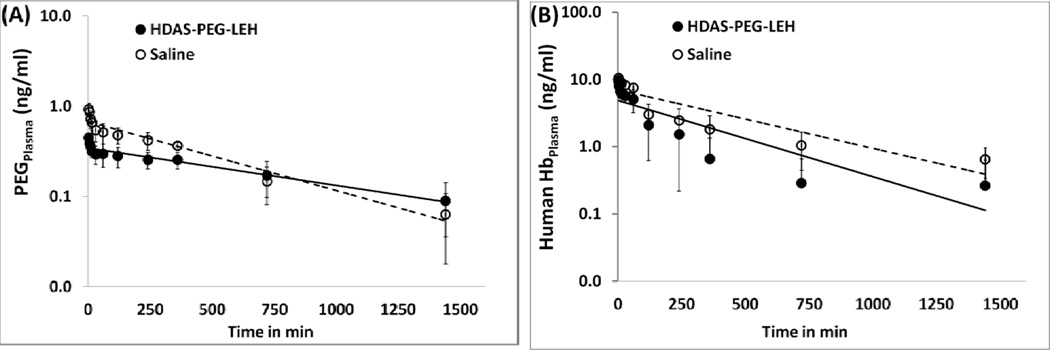
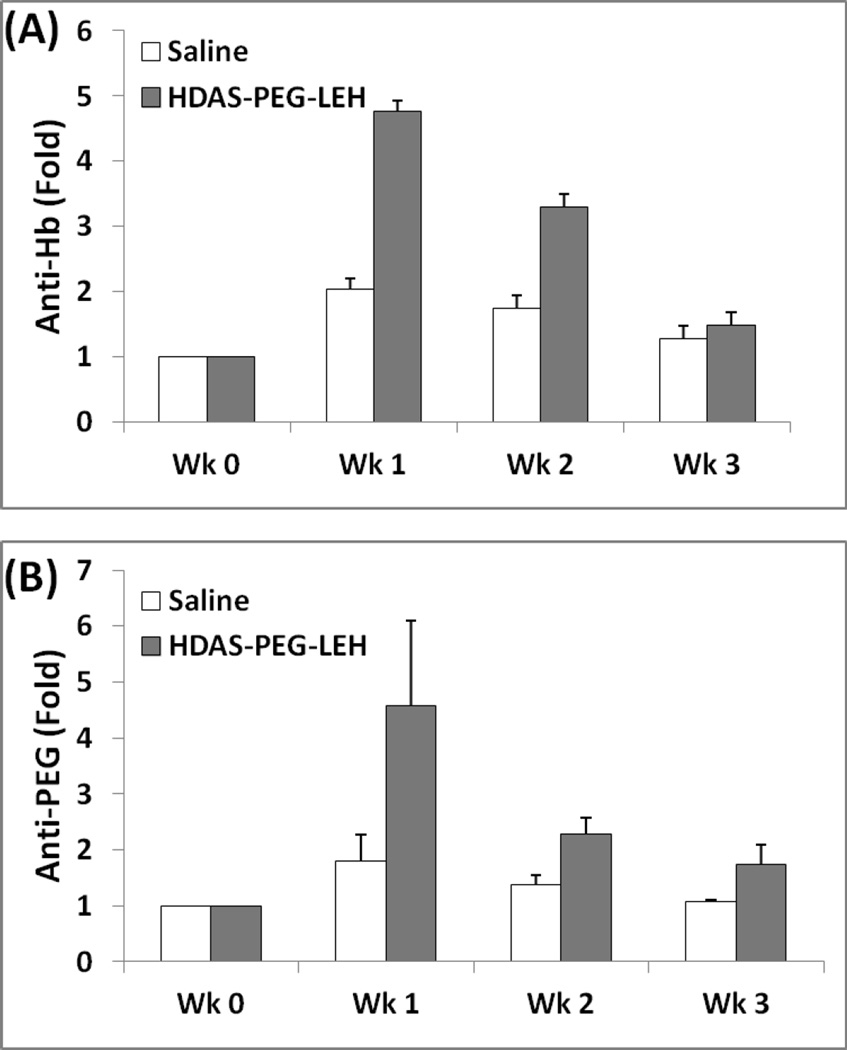
Next, we investigated if the rats with prior exposure to HDAS-PEG2K-LEH will handle clearance of subsequent injection of LEH in a different manner. The rats received HDAS-PEG2K-LEH (treatment) or saline (control) for every 7 days. The last injection on 28th day in both the groups was HDAS-PEG2K-LEH. Immediately after the last i.v. administration, blood samples were collected up to 24 h for assay of circulating human Hb and PEG. Fig. 3 shows the profile of HDAS-PEG2K-LEH in two groups as evidenced by either PEG or human Hb in rat plasma. The terminal half-life of HDAS-PEG2K-LEH appears to be about 4 h in rats that were pre-exposed to HDAS-PEG2K-LEH and 5.8 h in unexposed rats. However, the clearance profile picture was not much different between the two groups. We also determined if repeated HDAS-PEG2K-LEH administrations resulted in any antibody response over four weeks of treatment (Fig. 4). The antibody response against HDAS-PEG2K-LEH was determined by sampling serum and performing ELISA for anti-PEG antibody and anti-human Hb antibody. The first dose of HDAS-PEG2K-LEH administration induced maximum level of immune response, but the response subsided to control levels at the conclusion of the study on fourth week. The subdued antibody response against Hb and PEG could be related to the reduced complement activation by HDAS-PEG2K-modified LEH. Antibodies against exogenous antigens activate complement components which serve as effectors of cell damage [36]. The processes of antigen-antibody immune complex formation and fixation of complement components occur simultaneously [37]. The absence of antibody response and complement activation by HDAS-PEG2K-LEH is an improvement, because liposomes by themselves have been shown to serve as immune adjuvant [38]. PEG-modification of liposomes has been known to prevent their antibody-mediated elimination from circulation [39]. However, PEG itself can induce a strong IgM response as demonstrated by Ishida et al [40]. The effect of LEH on broader immune response has been studied by Kawaguchi’s group. They showed that administration of their LEH preparation did not change lymphocyte composition and response in immunodeficient mice reconstituted with human hematopoietic stem cells [41]. More recently, the same groups reported that LEH overload in wild-type mice did not alter the cellularity of dendritic cells (DCs) and macrophages in the recipient spleen remained unchanged from control mice receiving RBCs or saline. However, DCs from LEH-administered mice produced higher levels of interleukin-2 and activated helper T-cells in response to antigens, compared to the control mice [42]. Together, these observations suggest that LEH is an immune tolerant hemoglobin-based oxygen carrier whose properties could be further improved by surface modification with novel non-phospholipid amphiphiles such as HDAS-PEG2K.
Figure 3.
Clearance profile of HDAS-PEG2K-LEH in plasma with respect to (A) PEG and (B) human Hb. The rats were administered saline or HDAS-PEG2K-LEH every week for 3 weeks. On fourth week (28th day), all the rats were injected HDAS-PEG2K-LEH. Blood was collected to estimate the amount of PEG and human Hb by ELISA.
Figure 4.
(A) Anti-PEG and (B) anti-human Hb antibody response in rats injected with HDAS-PEG2K-LEH or saline over 4 weeks (0, 7, 14, 21 days). Rat serum analyzed by ELISA. One group received weekly injections of HDAS-PEG2K-LEH at a dose rate of 10 ml/kg, whereas the other group was given equal volume of saline (control).
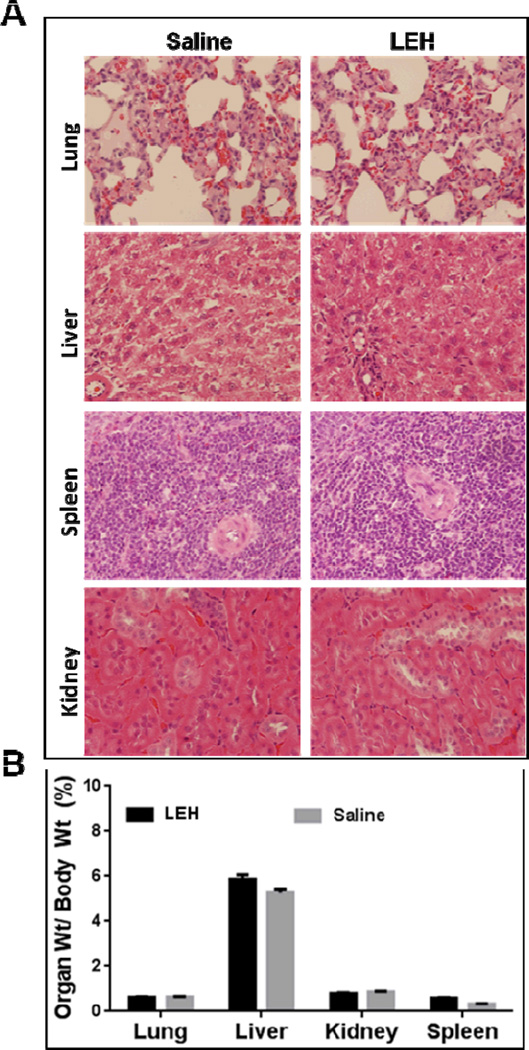
In clinical setting, LEH would be administered in large volumes because of the nature of indication. Therefore, it is important to evaluate its effect on various organs. Histologically, we could not find any significant pathological changes lung, liver, kidney, and spleen after of HDAS-PEG2K-LEH in rats (Fig. 5a), and the weights of these organs were more or less same in the treatment and control groups (Fig. 5b). There was no HDAS-PEG2K-LEH accumulation in the red pulp zone in spleen or in liver macrophages. In the kidneys also, the mesangial cells in the renal glomerulus did not entrap any LEH. Earlier, a first generation of LEH without PEG-modification was reported to aggregate in the plasma and showed a slight accumulation in the kidneys [8]. Sakai, et al also reported accumulation of Hb vesicles in liver, spleen, and kidney after daily repeat injections; however, after 14 days the accumulated Hb vesicles almost disappeared [22]. In lung, there were no pulmonary infarcts observed after HDAS-PEG2K-LEH administration, suggesting an absence of classic secondary pulmonary embolic injury associated with trapping of LEH particles. These morphologic findings demonstrate that there are no long-term effects of HDAS-PEG2K-LEH administration manifested in histology. The absence of any residual LEH in liver and spleen suggest that within a few days of administration the accumulated LEH is cleared from these organs.
Figure 5.
(A) Rat organ weights (lung, liver, kidney, and spleen) after HDAS-PEG2K-LEH and saline administration for 4 weeks (0, 7, 14, 21 days) at a dose rate of 10 ml/kg. The values are mean ± SEM. (B) Histology of lung, liver, spleen, and kidney. One group received repeated weekly injections of HDAS-PEG2K-LEH for 4 weeks (10 ml/Kg), whereas the other group was given equal volume of saline (control).
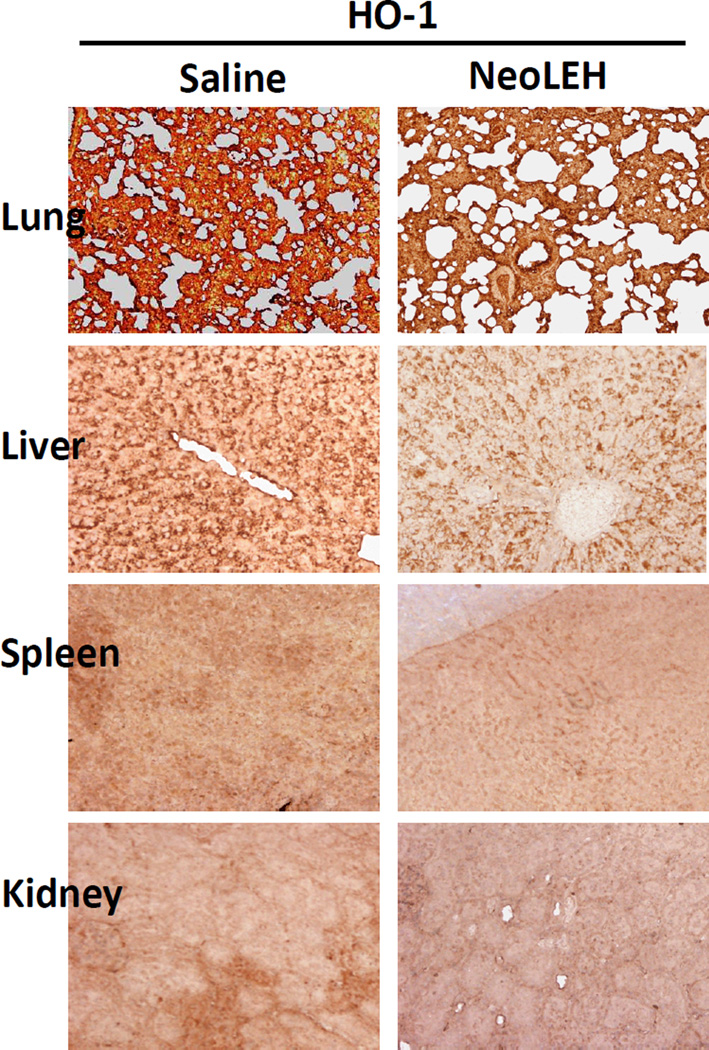
The heme released from Hb is subject to metabolism by the inducible form of HO-1, especially in spleen [43] and liver Kupffer cells [44]. In order to investigate the effect of repeated administration of human Hb-based LEH in rats, we performed IHC examination for the induction of HO-1 expression in various organs. Fig. 6 shows the IHC for the residual HO-1 in various organs from the two groups of rats after 28 days. In our study, we could not observe substantial induction of HO-1 expression in lung, spleen, liver, and kidney of rats pre-exposed to HDAS-PEG2K-LEH. Earlier, Sakai et al had reported that liver showed positive IHC for HO-1 only after 14 days of daily repeated injections, whereas in spleen the presence of HO-1 was confirmed both after 1 and 14 days of injections [22]. This discrepancy could be explained by the fact that, unlike in Sakai’s study, the rats in our experiments were not given LEH on daily basis. That hemoglobin-induced HO-1 expression in Kupffer cells is transient and returns to baseline within 2 d, has been shown in mice injected with hemoglobin-based oxygen carrier [45]. Also, hemoglobin-induced HO-1 expression could be tissue-selective. Damle et al found that in rats injected with a hemoglobin-based oxygen carrier both the heart and lung demonstrated a dose-dependent induction of HO-1, but brain tissue did not show any significant amount of HO-1 [46]. Similarly, a bovine hemoglobin based oxygen carrier HBOC-200 (Oxyglobin) could not induce HO-1 in rat aortic endothelial cells [47]. Moreover, considering that isoflurane (an anesthetic used in our study) has been recently found to induce HO-1 in rat liver [48], more work is needed for appropriate conclusion in this regard.
Figure 6.
Immunohistochemical staining for HO-1 in rat lung, liver, spleen, and kidney. The treatment groups are the same as defined in figures 3–5. The tissues were stained (brown-color) with antibody against rat HO-1.
CONCLUSION
Overall, the results described in this study suggest that administration of HDAS-PEG2K-LEH does not significantly influence the handling and clearance of its subsequent dosing in rats. The repeated injections of LEH in rats were well tolerated and there were no signs of apparent toxicity in the major organs. The surface modification of LEH with HDAS-PEG2K reduced complement activation observed in case of unmodified LEH. As such, this new composition of LEH containing HDAS and HDAS-PEG2K warrants further investigation.
ACKNOWLEDGEMENT
The work was supported by a grant from National Heart, Lung & Blood Institute [R01HL104286].
Footnotes
Presented at the 4th International Symposium on Artificial Oxygen Carriers, September 28, 2013 Yokohama, Japan.
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Awasthi V. Pharmaceutical aspects of hemoglobin-based oxygen carriers. Curr Drug Deliv. 2005;2:133–142. doi: 10.2174/1567201053586029. [DOI] [PubMed] [Google Scholar]
- 3.Phillips WT, Klipper RW, Awasthi VD, Rudolph AS, Cliff R, Kwasiborski V, et al. Polyethylene glycol-modified liposome-encapsulated hemoglobin: a long circulating red cell substitute. J Pharmacol Exp Ther. 1999;288:665–670. [PubMed] [Google Scholar]
- 4.Takaori M, Fukui A. Treatment of massive hemorrhage with liposome encapsulated human hemoglobin (NRC) and hydroxyethyl starch (HES) in beagles. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:643–653. doi: 10.3109/10731199609118889. [DOI] [PubMed] [Google Scholar]
- 5.Usuba A, Motoki R, Sakaguchi K, Suzuki K, Kamitani T. Effect of neo red cells on hemodynamics and blood gas transport in canine hemorrhagic shock and its safety for vital organs. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:503–516. doi: 10.3109/10731199409117878. [DOI] [PubMed] [Google Scholar]
- 6.Sakai H, Hamada K, Takeoka S, Nishide H, Tsuchida E. Physical properties of hemoglobin vesicles as red cell substitutes. Biotechnol Prog. 1996;12:119–125. doi: 10.1021/bp950068w. [DOI] [PubMed] [Google Scholar]
- 7.Farmer MC, Johnson SA, Beissinger RL, Gossage JL, Lynn AB, Carter KA. Liposome-encapsulated hemoglobin: a synthetic red cell. Adv Exp Med Biol. 1988;238:161–170. doi: 10.1007/978-1-4684-7908-9_13. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph AS, Spielberg H, Spargo BJ, Kossovsky N. Histopathologic study following administration of liposome-encapsulated hemoglobin in the normovolemic rat. J Biomed Mater Res. 1995;29:189–196. doi: 10.1002/jbm.820290208. [DOI] [PubMed] [Google Scholar]
- 9.Seifert DB, Phillips JA. The production of monoclonal antibody in growth-arrested hybridomas cultivated in suspension and immobilized modes. Biotechnol Prog. 1999;15:655–666. doi: 10.1021/bp990068m. [DOI] [PubMed] [Google Scholar]
- 10.Torchilinl VP, Papisov MI. Why do Polyethylene Glycol-Coated Liposomes Circulate So Long?: Molecular Mechanism of Liposome Steric Protection with Polyethylene Glycol: Role of Polymer Chain Flexibility. Journal of Liposome Research. 1994;4:725–739. [Google Scholar]
- 11.Nag OK, Yadav VR, Hedrick A, Awasthi V. Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. Int J Pharm. 2013;446:119–129. doi: 10.1016/j.ijpharm.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthi V, Goins B, Phillips WT. Insertion of poly (ethylene glycol)-lipid reduces the liposome-encapsulated hemoglobin-induced thrombocytopenic reaction. Am J Pharmacol Toxicol. 2007;2:98. [Google Scholar]
- 13.Zimmerman GA, Prescott SM, McIntyre TM. Oxidatively fragmented phospholipids as inflammatory mediators: the dark side of polyunsaturated lipids. J Nutr. 1995;125:1661S–1665S. doi: 10.1093/jn/125.suppl_6.1661S. [DOI] [PubMed] [Google Scholar]
- 14.Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. FASEB J. 2006;20:2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- 15.Bastiat G, Oliger P, Karlsson G, Edwards K, Lafleur M. Development of non-phospholipid liposomes containing a high cholesterol concentration. Langmuir. 2007;23:7695–7699. doi: 10.1021/la700824m. [DOI] [PubMed] [Google Scholar]
- 16.Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids Surf B Biointerfaces. 2010;75:573–583. doi: 10.1016/j.colsurfb.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: effect of postinserted poly(ethylene glycol)-distearoylphosphatidylethanolamine on distribution and circulation kinetics. J Pharmacol Exp Ther. 2004;309:241–248. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- 18.Awasthi VD, Garcia D, Klipper R, Phillips WT, Goins BA. Kinetics of liposome-encapsulated hemoglobin after 25% hypovolemic exchange transfusion. Int J Pharm. 2004;283:53–62. doi: 10.1016/j.ijpharm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 20.Tomita S, Enoki Y, Santa M, Yoshida H, Yasumitsu Y. A simple spectrophotometric method for determination of met-hemoglobin in dilute solution. J Nara Med Assoc. 1968;19:6. [Google Scholar]
- 21.Matsuoka T. Determination of methemoglobin and carboxyhemoglobin in blood by rapid colorimetry. Biol Pharm Bull. 1997;20:1208–1211. doi: 10.1248/bpb.20.1208. [DOI] [PubMed] [Google Scholar]
- 22.Sakai H, Masada Y, Horinouchi H, Ikeda E, Sou K, Takeoka S, et al. Physiological capacity of the reticuloendothelial system for the degradation of hemoglobin vesicles (artificial oxygen carriers) after massive intravenous doses by daily repeated infusions for 14 days. J Pharmacol Exp Ther. 2004;311:874–884. doi: 10.1124/jpet.104.073049. [DOI] [PubMed] [Google Scholar]
- 23.Mathur R, Capasso P. Nonphospholipid liposomes: Properties and potential use in flavor encapsulation (Chapter 17) In: Ho C-T, Tan C-T, Tong C-H, editors. Flavor Technology. American Chemical Society; 1997. pp. 219–230. (ACS Symposium Series). [Google Scholar]
- 24.Gupta RK, Varanelli CL, Griffin P, Wallach DF, Siber GR. Adjuvant properties of non-phospholipid liposomes (Novasomes) in experimental animals for human vaccine antigens. Vaccine. 1996;14:219–225. doi: 10.1016/0264-410x(95)00182-z. [DOI] [PubMed] [Google Scholar]
- 25.Bonte F, Juliano RL. Interactions of liposomes with serum proteins. Chem Phys Lipids. 1986;40:359–372. doi: 10.1016/0009-3084(86)90079-4. [DOI] [PubMed] [Google Scholar]
- 26.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–4241. [PubMed] [Google Scholar]
- 27.Bradley AJ, Devine DV. The complement system in liposome clearance: Can complement deposition be inhibited? Adv Drug Deliv Rev. 1998;32:19–29. doi: 10.1016/s0169-409x(97)00129-4. [DOI] [PubMed] [Google Scholar]
- 28.Szebeni J, Baranyi L, Savay S, Milosevits J, Bodo M, Bunger R, et al. The interaction of liposomes with the complement system: in vitro and in vivo assays. Methods Enzymol. 2003;373:136–154. doi: 10.1016/S0076-6879(03)73010-9. [DOI] [PubMed] [Google Scholar]
- 29.Szebeni J, Wassef NM, Hartman KR, Rudolph AS, Alving CR. Complement activation in vitro by the red cell substitute, liposome-encapsulated hemoglobin: mechanism of activation and inhibition by soluble complement receptor type 1. Transfusion. 1997;37:150–159. doi: 10.1046/j.1537-2995.1997.37297203517.x. [DOI] [PubMed] [Google Scholar]
- 30.Szebeni J, Spielberg H, Cliff RO, Wassef NM, Rudolph AS, Alving CR. Complement activation and thromboxane secretion by liposome-encapsulated hemoglobin in rats in vivo: inhibition by soluble complement receptor type 1. Artificial Cells Blood Substitutes & Immobilization Biotechnology. 1997;25:347–355. doi: 10.3109/10731199709118925. [DOI] [PubMed] [Google Scholar]
- 31.Phillips WT, Klipper R, Fresne D, Rudolph AS, Javors M, Goins B. Platelet reactivity with liposome-encapsulated hemoglobin in the rat. Exp Hematol. 1997;25:1347–1356. [PubMed] [Google Scholar]
- 32.Loughrey HC, Bally MB, Reinish LW, Cullis PR. The binding of phosphatidylglycerol liposomes to rat platelets is mediated by complement. Thromb Haemost. 1990;64:172–176. [PubMed] [Google Scholar]
- 33.Szebeni J, Wassef NM, Spielberg H, Rudolph AS, Alving CR. Complement activation in rats by liposomes and liposome-encapsulated hemoglobin: evidence for anti-lipid antibodies and alternative pathway activation. Biochem Biophys Res Commun. 1994;205:255–263. doi: 10.1006/bbrc.1994.2658. [DOI] [PubMed] [Google Scholar]
- 34.Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J Liposome Res. 2002;12:165–172. doi: 10.1081/lpr-120004790. [DOI] [PubMed] [Google Scholar]
- 35.van den Hoven JM, Nemes R, Metselaar JM, Nuijen B, Beijnen JH, Storm G, et al. Complement activation by PEGylated liposomes containing prednisolone. Eur J Pharm Sci. 49:265–271. doi: 10.1016/j.ejps.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Basta M. Ambivalent effect of immunoglobulins on the complement system: activation versus inhibition. Mol Immunol. 2008;45:4073–4079. doi: 10.1016/j.molimm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Vivanco F, Munoz E, Vidarte L, Pastor C. The covalent interaction of C3 with IgG immune complexes. Mol Immunol. 1999;36:843–852. doi: 10.1016/s0161-5890(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 38.Phillips NC, Emili A. Enhanced antibody response to liposome-associated protein antigens: preferential stimulation of IgG2a/b production. Vaccine. 1992;10:151–158. doi: 10.1016/0264-410x(92)90004-4. [DOI] [PubMed] [Google Scholar]
- 39.Li WM, Mayer LD, Bally MB. Prevention of antibody-mediated elimination of ligand-targeted liposomes by using poly(ethylene glycol)-modified lipids. J Pharmacol Exp Ther. 2002;300:976–983. doi: 10.1124/jpet.300.3.976. [DOI] [PubMed] [Google Scholar]
- 40.Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi AT, Kametani Y, Kato S, Furuya H, Tamaoki K, Habu S. Effects of liposome-encapsulated hemoglobin on human immune system: evaluation in immunodeficient mice reconstituted with human cord blood stem cells. Artif Organs. 2009;33:169–176. doi: 10.1111/j.1525-1594.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 42.Kawaguchi AT, Aokawa J, Yamada Y, Yoshiba F, Kato S, Kametani Y. Effect of liposome-encapsulated hemoglobin on antigen-presenting cells in mice. Artif Organs. 36:194–201. doi: 10.1111/j.1525-1594.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 43.Braggins PE, Trakshel GM, Kutty RK, Maines MD. Characterization of two heme oxygenase isoforms in rat spleen: comparison with the hematin-induced and constitutive isoforms of the liver. Biochem Biophys Res Commun. 1986;141:528–533. doi: 10.1016/s0006-291x(86)80205-4. [DOI] [PubMed] [Google Scholar]
- 44.Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, et al. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babu AN, Damle SS, Moore EE, Ao L, Song Y, Johnson JL, et al. Hemoglobin-based oxygen carrier induces hepatic heme oxygenase 1 expression in Kupffer cells. Surgery. 2007;142:289–294. doi: 10.1016/j.surg.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Damle SS, Moore EE, Babu AN, Meng X, Fullerton DA, Banerjee A. Hemoglobin-based oxygen carrier induces heme oxygenase-1 in the heart and lung but not brain. J Am Coll Surg. 2009;208:592–598. doi: 10.1016/j.jamcollsurg.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Rempf C, Ritter A, Schrepfer S, Freitag M, Standl T, Gottschalk A. Failed induction of heme oxygenase 1 in endothelial cells exposed to the hemoglobin based oxygen carrier Oxyglobin. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:34–44. doi: 10.1080/10731190701857769. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, et al. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg. 2007;245:931–942. doi: 10.1097/01.sla.0000256891.45790.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]