Abstract
Background and Purpose
Emerging data suggest that left atrial disease may cause ischemic stroke in the absence of atrial fibrillation or flutter (AF). If true, this condition may provide an etiology for many strokes currently classified as cryptogenic.
Methods
Among 6,741 participants in the Multi-Ethnic Study of Atherosclerosis who were free of clinically apparent cerebrovascular or cardiovascular disease (including AF) at baseline, we examined the association between markers of left atrial abnormality on a standard 12-lead electrocardiogram (ECG)—specifically, P-wave area, duration, and terminal force in lead V1 (PTFV1)—and the subsequent risk of ischemic stroke while accounting for incident AF.
Results
During a median follow-up of 8.5 years, 121 participants (1.8%) suffered a stroke and 541 participants (8.0%) were diagnosed with AF. In Cox proportional hazards models adjusting for potential baseline confounders, PTFV1 was more strongly associated with incident stroke (hazard ratio [HR] per 1-SD increase, 1.21; 95% confidence interval [CI], 1.02–1.44) than with incident AF (HR per 1-SD, 1.11; 95% CI, 1.03–1.21). The association between PTFV1 and stroke was robust in numerous sensitivity analyses accounting for AF, including analyses that excluded those with any incident AF or modeled any incident AF as having been present from baseline.
Conclusions
We found an association between baseline P-wave morphology and incident stroke even after accounting for AF. This association may reflect an atrial cardiopathy that leads to stroke in the absence of AF.
Keywords: Arrhythmia, atrium, electrocardiography, embolism, stroke
Atrial ECG signs have been associated with stroke in the absence of atrial fibrillation (AF),1–3 suggesting that atrial disease may promote embolization without manifesting with AF. We have shown that P-wave area and terminal force are associated with stroke risk,4 but did not control for AF, which may mediate the relationship between P-wave morphology and stroke. Therefore, we examined the association between P-wave morphology and stroke while accounting for incident AF.
Methods
Design
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of risk factors for progression from subclinical to clinical cardiovascular disease.5 Institutional review boards at all participating sites approved this study, and all participants provided written, informed consent.
Participants
MESA enrolled 6,814 men and women 45 to 84 years of age who were free of clinically apparent cerebrovascular or cardiovascular disease, including AF. We excluded 48 participants with missing ECGs, 24 participants receiving warfarin at baseline, and one participant whose baseline ECG showed AF.
Measurements
All sites obtained standard digital 12-lead ECGs at the first and last study visits. All ECGs were obtained using GE MAC 1200 electrocardiograph machines (GE, Milwaukee, WI) calibrated at 10 mm/mV with a speed of 25 mm/s. After initial screening for inadequate quality, all ECGs were centrally interpreted at the Wake Forest Epidemiological Cardiology Research Center for abnormalities including AF. The core ECG laboratory automatically measured P-wave areas, amplitudes, and durations using the 12-SL program (Version 2001, GE Marquette). P-wave terminal force in lead V1 (PTFV1) is the most widely accepted marker of left atrial abnormality; P-wave area and duration may also be useful.6 Based on this and prior work,4 we prespecified predictor variables as PTFV1, mean and maximum P-wave area, and mean and maximum P-wave duration on the baseline 12-lead ECG. Using standard methodology, we defined PTFV1 as the duration (ms) of the downward deflection (terminal portion) of the P-wave in lead V1 multiplied by the absolute value of its amplitude (μV) (Figure 1).7 P-wave areas were a summation of all P-wave amplitudes at respective sampling points multiplied by the sampling interval. P-wave areas were calculated by summing the absolute values of the areas of the upward and downward deflections of the P-wave; in other words, the areas of downward deflections were assigned positive rather than negative values.4 To adjust for technical differences in units of measurement and harmonize the 12-SL program's calculations with other machines, we multiplied P-wave areas by a factor of 19.52.4, 8
Figure 1.
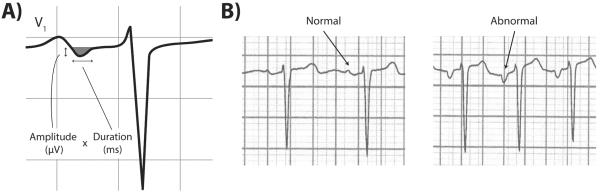
A. Illustration of components of P-wave terminal force (PTFV1), defined as the duration (ms) of the downward deflection (shaded grey area) of the P-wave in lead V1 multiplied by the absolute value of its amplitude (μV).7
B. Representative examples of ECGs with normal and abnormal P-wave terminal force in lead V1 (PTFV1). Note the deep downward deflection of the terminal portion of the P-wave in the ECG on the right, compared with the absence of a deep downward deflection in the normal ECG on the left.
We accounted for incident AF as a potential mediator, and adjusted for the following potential confounders: age, sex, race, hypertension, left ventricular hypertrophy, total cholesterol level, high- and low-density lipoprotein levels, diabetes, chronic obstructive pulmonary disease, chronic kidney disease, body mass index, and smoking status (Supplemental Methods).
Our primary outcome was an adjudicated diagnosis of ischemic stroke, defined as the rapid onset of a neurological deficit that persisted for >24 hours or until death, or was accompanied by report of a clinically relevant infarction on brain imaging, in the absence of a nonvascular cause or brain hemorrhage.
Statistical Analyses
We excluded P-wave measurements >99.9th percentile as outliers. Using separate Cox proportional hazards models for each P-wave predictor variable, we standardized these variables around their means and reported their associations with stroke in 1-SD increments. To explore whether AF mediated associations between P-wave morphology and stroke, we compared models with and without a time-varying covariate for incident AF, and performed multiple sensitivity analyses accounting for AF in other ways (Supplemental Methods).
Results
The 6,741 eligible participants were followed for a median 8.5 years (interquartile range, 7.7–8.6 years). Ischemic stroke occurred in 121 participants (1.8%), and 541 participants (8.0%) were diagnosed with AF. Participants with stroke were older, had more vascular risk factors, and manifested significantly higher PTFV1 (Table 1; Supplemental Table I).
Table 1.
Baseline Characteristics of Participants, Stratified by the Occurrence of Incident Ischemic Stroke
| Characteristic* | Stroke (N = 121) | No Stroke (N = 6,620) | P value |
|---|---|---|---|
| Age, mean (SD), y | 68.2 (9.8) | 62.0 (10.2) | <.001 |
| Female | 58 (47.9) | 3,509 (53.0) | .27 |
| Race/ethnicity: | .13 | ||
| White | 51 (42.1) | 2,525 (38.1) | |
| Black | 35 (28.9) | 1,844 (27.9) | |
| Hispanic | 29 (24.0) | 1,455 (22.0) | |
| Asian | 6 (5.0) | 796 (12.0) | |
| Diabetes status: | <.001 | ||
| Normal | 69 (57.5) | 4,868 (73.8) | |
| Impaired fasting glucose | 18 (15.0) | 911 (13.8) | |
| Untreated diabetes | 5 (4.2) | 172 (2.6) | |
| Treated diabetes | 28 (23.3) | 646 (9.8) | |
| Hypertension | 88 (72.7) | 2,931 (44.3) | <.001 |
| Chronic obstructive pulmonary disease | 10 (8.3) | 740 (11.2) | .31 |
| Chronic kidney disease | 29 (24.0) | 1,105 (16.7) | .03 |
| Current smoker | 18 (14.9) | 864 (13.1) | .56 |
| Left ventricular hypertrophy | 17 (14.1) | 408 (6.2) | <.001 |
| Total cholesterol, mean (SD), mg/dl | 198.0 (33.9) | 194.1 (35.8) | .24 |
| High-density lipoprotein, mean (SD), mg/dl | 47.8 (12.3) | 51.0 (14.8) | .02 |
| Low-density lipoprotein, mean (SD), mg/dl | 121.6 (30.2) | 117.2 (31.5) | .13 |
| Body mass index: | .23 | ||
| <25 kg/mM2 | 25 (20.7) | 1,906 (28.8) | |
| 25–25.99 kg/m2 | 51 (42.1) | 2,591 (39.1) | |
| 30–39.99 kg/m2 | 41 (33.9) | 1,881 (28.4) | |
| >40 kg/m2 | 4 (3.3) | 242 (3.7) | |
| P-wave terminal force in lead V1 (PTFV1): | |||
| Mean (SD), μV*ms | 2,860 (1,996) | 2,171 (1,786) | <.001 |
| ≥95th percentile | 14 (11.6) | 324 (4.9) | .001 |
Abbreviations: SD, standard deviation.
Data are presented as Number (%) of participants unless otherwise specified.
In Cox proportional hazards analysis, PTFV1 was more strongly associated with incident stroke (hazard ratio [HR] per 1-SD increase, 1.21; 95% confidence interval [CI], 1.02–1.44; Table 2) than with incident AF (HR per 1-SD, 1.11; 95% CI, 1.03–1.21). The association between PTFV1 and stroke was similar when we adjusted for incident AF (HR per 1-SD, 1.21; 95% CI, 1.02–1.44) or removed the AF covariate from our model (HR per 1-SD, 1.22; 95% CI, 1.03–1.45). The association held true in numerous sensitivity analyses accounting for AF in other ways, such as excluding those with any incident AF or modeling any incident AF as having been present from baseline (Supplemental Results).
Table 2.
Associations Between P-Wave Morphology and Incident Ischemic Stroke
| Characteristic | HR per 1-SD Increase (95% CI) |
|---|---|
| P-wave terminal force in lead V1 (PTFV1), μV*ms | 1.21 (1.02–1.44) |
| P-wave mean area, μV*ms | 1.16 (0.98–1.39) |
| P-wave maximum area, μV*ms | 1.16 (0.99–1.37) |
| P-wave mean duration, ms | 1.11 (0.92–1.34) |
| P-wave maximum duration, ms | 1.12 (0.93–1.35) |
Abbreviations: CI, confidence interval; HR, hazard ratio; SD, standard deviation.
Discussion
In a prospective cohort study, we found a significant association between PTFV1 and subsequent ischemic stroke. This association persisted in participants without any AF during the study period, suggesting that atrial disease may cause stroke even in the absence of AF. Prior studies have suggested that atrial ECG markers besides AF may be associated with stroke;1, 2, 4, 9 this study represents an important advance because it supports an association between a simple 12-lead ECG measure and stroke, and demonstrates that AF does not mediate this association. Instead, elevated PTFV1 may reflect an underlying atrial cardiopathy that predisposes to embolization even in the absence of AF.
This study has several limitations. First, we may have missed subclinical AF that could have mediated the association between PTFV1 and stroke. However, PTFV1 was less strongly associated with AF than with stroke, which would not be expected if AF were an important mediator. Second, we were unable to examine associations with embolic-appearing or cryptogenic stroke specifically. Third, we lacked power to assess interactions between ethnicity and P-wave morphology in relation to stroke risk.4 Fourth, we lacked imaging of the left atrium. However, P-wave measurements reflect important aspects of atrial disease—such as elevated filling pressures, poor conduction, and fibrosis—that are not necessarily captured by imaging data.6 Further work will be necessary to fully delineate the ECG, imaging, and biomarker characteristics of atrial cardiopathy as it relates to stroke risk. Eventually, a combination of such markers may better identify the risk of left atrial embolization than simply the presence of AF, which can be difficult to screen for and as we have shown may be an imperfect marker of risk.
Supplementary Material
Acknowledgements
None.
Funding Sources: This analysis was supported by grant K23NS082367 (Kamel) from the National Institute of Neurological Disorders and Stroke. Additionally, this research was supported by contracts N01HC95159 through N01HC95169 from the National Heart, Lung, and Blood Institute and by grants UL1RR024156 and UL1RR025005 from the National Center for Research Resources. The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures: The authors report no conflicts of interest involving the work under consideration for publication. Outside the submitted work, Dr. Kamel reports having served on a medical advisory board and serving as a lecturer for Genentech, Inc on the topic of alteplase for the acute treatment of ischemic stroke. Also outside the submitted work, Dr. Nazarian reports serving as a scientific advisor to and principal investigator for research funding awarded to Johns Hopkins University by Biosense Webster, Inc on the topic of imaging and ablation for atrial fibrillation. Also outside the submitted work, Dr. Okin reports serving on a medical advisory board for GE Medical Systems on the topic of new product development. The other authors report no potential conflicts of interest outside the submitted work.
References
- 1.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 2.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todo K, Moriwaki H, Saito K, Naritomi H. Frequent premature atrial contractions in stroke of undetermined etiology. Eur Neurol. 2009;61:285–288. doi: 10.1159/000206853. [DOI] [PubMed] [Google Scholar]
- 4.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Multi-Ethnic Study of Atherosclerosis [Accessed June 2, 2014]; http://www.mesa-nhlbi.org/
- 6.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Circulation. 2009;119:e251–261. doi: 10.1161/CIRCULATIONAHA.108.191097. [DOI] [PubMed] [Google Scholar]
- 7.Soliman EZ, Alonso A, Misialek JR, Jain A, Watson KE, Lloyd-Jones DM, et al. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA) J Electrocardiol. 2013;46:702–706. doi: 10.1016/j.jelectrocard.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewhurst M, Adams P. Regarding article “Ethnic distribution of electrocardiographic predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the atherosclerosis risk in communities study (ARIC)”. Stroke. 2011;42:e19. doi: 10.1161/STROKEAHA.110.592097. author reply e20. [DOI] [PubMed] [Google Scholar]
- 9.Kohsaka S, Sciacca RR, Sugioka K, Sacco RL, Homma S, Di Tullio MR. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke. 2005;36:2481–2483. doi: 10.1161/01.STR.0000185682.09981.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


