Abstract
In this study we investigated the effects of fucoidan on the proliferation of fibroblasts and the reconstruction of a skin equivalent (SE). Fucoidan significantly stimulated the proliferation of CCD-25Sk human fibroblasts and Western blot analysis demonstrated that fucoidan markedly increased the expression of cyclin D1 and decreased the expression of p27. Fucoidan was used to reconstruct SE. Immunohistochemical staining showed that the addition of fucoidan to dermal equivalents increased expression of proliferating cell nuclear antigen (PCNA) and p63. In addition, expression of α6-integrin was significantly increased by fucoidan, whereas expression of β1-integrin, type 1 collagen, elastin, fibronectin did not markedly change. These results suggest that fucoidan has positive effects on epidermal reconstruction and will therefore be beneficial in the reconstruction of SE.
Keywords: Fibroblasts, Fucoidan, Proliferation, Skin equivalent
INTRODUCTION
The European Union has banned the use of animal experiments for cosmetic constituents since March 2009, and, therefore, cosmetics that have been tested on animals cannot be marketed. Furthermore, the use of animal studies for drug development has also decreased because of animal protection regulations. Therefore, alternative testing models, such as reconstructed skin equivalents (SEs), have been developed to test cosmetic and pharmaceutical ingredients that may cause skin irritation [1]. Current SE models, however, are insufficient and have higher permeability than real human skin; therefore improved SEs need to be developed [2].
Fucoidans are sulfated polysaccharides that contain high levels of L-fucose and are mainly found in brown seaweeds [3,4]. Recently, fucoidan has been shown to exhibit many biologic activities such as anti-coagulant [5], anti-tumor [6], anti-inflammatory [7], anti-viral [8], and anti-oxidant effects [9]. It was also reported that fucoidan blocked UVB-induced expression of matrix metalloproteinase (MMP)-1 and thus increased the synthesis of type I procollagen [10]. Fucoidan was also shown to increase fibroblast proliferation in the presence of transforming growth factor-β1 (TGF-β1), indicating that fucoidan may aid in wound healing [11]. Furthermore, sulfated polysaccharides, such as chondroitin sulfate, are major constituents of human dermis [12]. These findings all indicated that fucoidan could be beneficial for the reconstruction of SE, and we therefore investigated the effects of fucoidan on fibroblast proliferation and the reconstruction of SE.
Cell cycle progression is important for cell proliferation [13]. D-type cyclins, including cyclin D1, cyclin D2, and cyclin D3, are expressed in most proliferating cells and regulate cell cycle progression [14,15]. On the other hand, p27Kip1 (p27) is a cyclin-dependent kinase (CDK) inhibitor that binds to the cyclin D/CDK complex and inhibits CDK4, thereby decreasing cell proliferation [16,17]. To further investigate the mechanisms by which fucoidan elicits its effects, we also examined the expression of these cell cycle-related proteins.
METHODS
Reagents
Fucoidan from Fucus vesiculosus, Dulbecco's Modified Eagle's Medium (DMEM) powder, nutrient mixture F-12 Ham (F-12) powder, sodium bicarbonate, HEPES, formaldehyde, insulin, L-ascorbic acid, isoproterenol, and hydrocortisone were obtained from Sigma-Aldrich, Inc. (St Louis, MO, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). DMEM/F-12 in a 3:1 mixture, antibiotic-antimycotic (penicillin, streptomycin), trypsin-EDTA, and sodium hydroxide were purchased from WelGENE (Daegu, South Korea). Recombinant human epidermal growth factor (EGF) was obtained from Invitrogen Co. (Gibco, Camarillo, CA, USA). Antibodies against cyclin D1 (sc-718), p27 (sc-528), and actin (sc-1616) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
CCD-25Sk human fibroblasts were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and HaCaT human keratinocytes [18] were purchased from Cell Lines Service (Eppelheim, Germany). The cells were grown in DMEM supplemented with 10% FBS, 50 µg/mL streptomycin, and 50 µg/mL penicillin in a 5% CO2 incubator at 37℃.
Crystal violet assay for cell viability
Cell viability was assessed using the crystal violet staining assay [19]. CCD-25Sk cells were treated with fucoidan for 24 h. The culture medium was removed and the cells were stained with 0.1% crystal violet in 10% ethanol for 5 min at room temperature and then rinsed four times with distilled water. The crystal violet retained by adherent cells was extracted with 95% ethanol and the absorbance at 590 nm was determined using an ELISA reader (VERSAMax; Molecular Devices, Sunnyvale, CA, USA).
MTT assay for cell proliferation
CCD-25Sk cells were seeded into 6-well plates (5×104 cells/well) and cultured for 24 h before incubation with various concentrations of fucoidan (0~50 µg/ml) in DMEM containing 10% FBS at 37℃ and 5% CO2 for 4 days. After addition of an MTT solution (5 mg/ml; 50 µl/well) and incubation for 4 h, the supernatants were removed and the formazan crystals were solubilized in 1 ml dimethylsulfoxide. Optical density was determined at 540 nm using a VERSAMax.
Western blotting
CCD-25Sk cells were lysed in cell lysis buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 5% β-mercaptoethanol, 2 mM phenylmethylsulfonyl fluoride, protease inhibitors [Complete™; Roche, Mannheim, Germany], 1 mM Na3VO4, 50 mM NaF, and 10 mM EDTA). Proteins in the total cell lysates were separated by SDS-polyacrylamide gel electrophoresis using 20 µg of protein per lane, blotted onto polyvinylidene fluoride (PVDF) membranes, and blocked with 5% dried milk in Tris-buffered saline containing 0.5% Tween 20. Blots were incubated with the appropriate primary antibodies at a dilution of 1:1000 and then with horseradish peroxidase-conjugated secondary antibody. Bound antibody was identified using an enhanced chemiluminescence testing kit (Thermo Scientific Inc., Bremen, Germany). All immunoblot images were obtained using a LAS-1000 lumino-image analyzer (Fuji Film, Tokyo, Japan).
Preparation of dermal equivalents
Dermal equivalents were prepared as described previously, with some modifications [20,21]. Briefly, type I collagen extracted from rat tail tendons was dissolved by stirring in 1/1000 glacial acetic acid and incubating at 4℃ for 48 h. Dermal equivalents were prepared by mixing one volume of 10× reconstitution buffer (0.05 N NaOH, 0.26 mM NaHCO3, 200 mM HEPES), one volume of 10× medium (DMEM: F-12=3:1), and eight volumes of 1% type I collagen solution. All dermal equivalents contained 3×105 CCD-25Sk cells. Three milliliters of this mixture was transferred to 24-mm transwell inserts with a 3.0-µm pore polycarbonate membrane (Corning, Inc., USA) and placed in the incubator at 37℃ for gelation.
Reconstruction of skin equivalents
HaCaT cells were seeded onto dermal equivalents at a density of 1×106 cells and cultured submerged for 1 day and at the air-liquid interface for 12 days. The growth medium was DMEM/F-12 supplemented with 5% FBS, 0.4 µg/ml hydrocortisone, 1 M isoproterenol, 25 µg/ml ascorbic acid, and 5 µg/ml insulin. Epidermal growth factor (EGF) was added to the growth medium at 1 ng/ml for the submerged culture and 10 ng/ml for the air-liquid interface culture. The culture medium was refreshed three times a week.
Histology and immunohistochemistry
After 13 days, SEs were fixed in 10% formaldehyde for 1 day and processed for conventional paraffin embedment. Four- to six-micrometer-thick sections were prepared. For morphologic analysis, sections were stained with hematoxylin and eosin (H&E). For immunohistochemical analysis, sections were processed using the avidin-biotin-peroxidase complex technique (DAKO, Glostrup, Denmark). Antibodies specific for p63, proliferating cell nuclear antigen (PCNA), α6-integrin, and β1-integrin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); antibodies specific for type I collagen (No. 20111), elastin (No. 25011), and fibronectin (No. 24911) were from Novotec (Lyon, France). Image analysis was performed using MetaMorph Offline version 7.7.0.0 image analysis software (Molecular Devices, Downington, PA).
Statistics
The statistical significance of intergroup differences was assessed by analysis of variance (ANOVA) and Student's t-test. p-values<0.01 were considered statistically significant.
RESULTS
Fucoidan did not show cytotoxicity against CCD-25Sk cells
To examine the cytotoxic effects of fucoidan, CCD-25Sk cells were treated with fucoidan at concentrations of 0~50 µg/ml for 24 h. Cell viability was measured by crystal violet staining. Fucoidan did not show cytotoxic effects against CCD-25Sk cells (data not shown).
Effects of fucoidan on CCD-25Sk cell proliferation
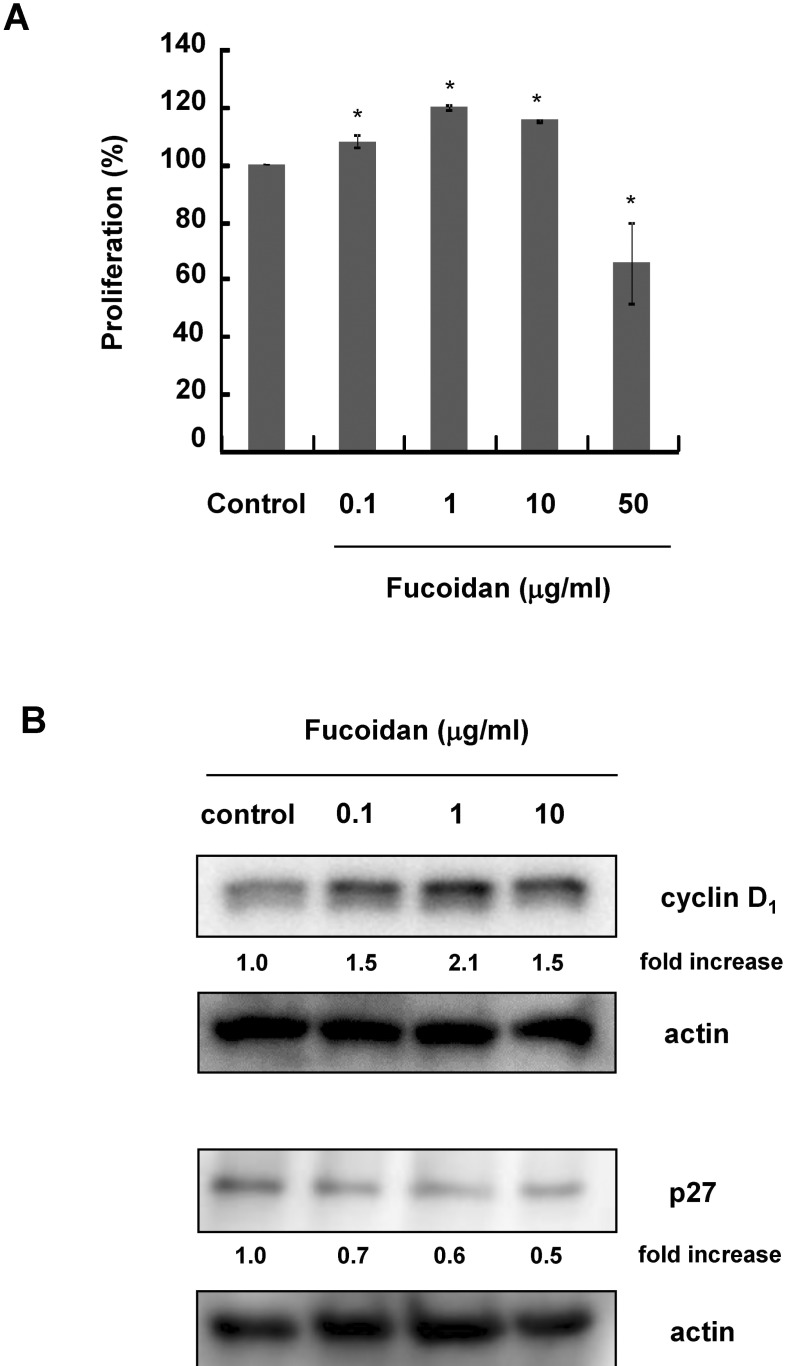
CCD-25Sk cells were cultured with 0~50 µg/ml fucoidan for 3 days. Cell proliferation was examined by MTT assay. Fucoidan significantly increased the proliferation of CCD-25Sk cells at concentrations up to 10 µg/ml (Fig. 1A). Proliferation increased by about 20% when cells were treated with 1 µg/ml, but proliferation was inhibited by 50 µg/ml fucoidan (Fig. 1A). To investigate the mechanism by which fucoidan promotes proliferation, we examined changes in the expression of cell cycle-related proteins by Western blot analysis. As shown in Figure 1B, fucoidan increased the expression of cyclin D1 and decreased the expression of p27.
Fig. 1.
Effects of fucoidan on CCD-25Sk cell proliferation. Cells were treated with 0~50 µg/ml fucoidan for 3 days. (A) Cell proliferation was measured using the MTT assay. Data represent the means±S.D. of triplicate assays expressed as percentages of the control. *p<0.01 (B) Whole cell lysates were subjected to Western blot analysis with antibodies against phospho-specific ERK, cyclin D1, and p27. Equal protein loading was confirmed using an antiactin antibody. Fold increases over the control were determined by densitometric analysis and are shown below in each lane.
Effects of fucoidan on skin equivalent
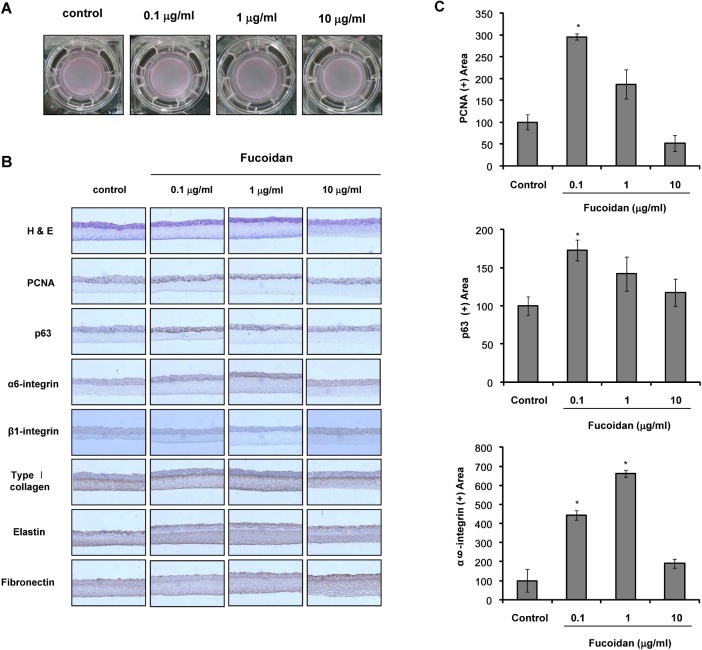
Because 50 µg/ml fucoidan showed anti-proliferative effects on cultured cells, fucoidan was used at concentrations up to 10 µg/ml in the reconstruction of SE. SE constructed with fucoidan contained more cells expressing PCNA, a nuclear marker of proliferating cells, and p63, a putative keratinocyte stem cell marker, than the control SE (Fig. 2B, C). Expression of α6-integrin was significantly increased by fucoidan (Fig. 2B, C), whereas expression of β1-integrin was not altered. We also examined extracellular matrix (ECM) formation and found that expression of type 1 collagen, elastin, and fibronectin was not changed by the addition of fucoidan.
Fig. 2.
Effects of fucoidan in the SE reconstruction. (A) Photographs of skin equivalents (SEs) in 24-mm transwell culture after 12 days of SE reconstruction. (B) SE sections were stained for H&E, PCNA, p63, α6-integrin, β1-integrin, type I collagen, elastin, and fibronectin. Each experiment was repeated independently three times, and representative results are shown. Original magnification, ×400. (C) PCNA, p63, and α6-integrin intensities were measured as described in 'Materials and Methods.' *p<0.01 compared to the control model.
DISCUSSION
In the present study, we showed that fucoidan stimulated the proliferation of CCD-25Sk human fibroblast cells. A previous study similarly reported that fucoidan stimulated fibroblast proliferation in the presence of TGF-β1; however, that study reported that fucoidan alone inhibited fibroblast proliferation by approximately 55% at 100 µg/ml [11]. Fucoidan also inhibited CHO-K1 cell proliferation at concentrations greater than 100 µg/ml [22]. Thus, fucoidan seems to have anti-proliferative properties at these concentrations. The reason why a high concentration of fucoidan inhibited the proliferation of fibroblasts remains to be investigated. However, a great deal of evidence indicates that high concentrations of many substances inhibit cell proliferation. For example, a high glucose concentration induced growth inhibition of osteoblast-like cells [23] and (-)-epigallocatechin-3-gallate (EGCG) increased the proliferation of keratinocytes at low concentrations of 0.1~1 µM, but significantly inhibited the proliferation of keratinocytes at a high concentration of EGCG (100 µM) [24]. In accordance with these studies, our results indicated that 50 µg/ml fucoidan inhibited fibroblast proliferation, without a cytotoxic effect, whereas low concentrations (0.1~10 µg/ml) of fucoidan significantly increased cell proliferation. Thus, fucoidan may have dual effects on cell proliferation depending on the concentration.
To further confirm the proliferative effects of fucoidan, we examined the expression of cell-cycle-related molecules. Cyclin D1 forms a complex with CDK4 or CDK6 and promotes cell cycle G1/S transition [14,15]. The CDK inhibitor p27, on the other hand, inhibits cell cycle progression. Therefore, decreased expression of p27 may contribute to cell proliferation [16,17]. Our results showed that fucoidan treatment decreased expression of p27 and increased expression of cyclin D1. These results suggest that fucoidan stimulates cell proliferation through cell cycle regulation.
SE models are three-dimensional systems in which the effect of certain substances can be examined on the tissue level, rather than on the cellular level [20]. In the present study, we investigated the effects of fucoidan on the construction of SE. p63 is regarded as a putative epithelial stem cell marker [25]. Our results showed that the addition of fucoidan increased p63 expression in SE, as compared to the control (Fig. 2B, C), indicating that fucoidan may contribute to the maintenance of epidermal stemness. It was also reported that p63 is an indispensable factor for the proliferation and differentiation of keratinocytes [26]. These results indicate that fucoidan may promote the proliferation of epidermal cells in SE. Therefore, we examined the expression of PCNA, a proliferating cell marker, by immunohistochemical staining and showed that PCNA expression was increased by the addition of fucoidan (Fig. 2B, C). These findings suggest that fucoidan increased epidermal stemness, as well as the proliferative potential of the SE model, by providing a favorable microenvironment.
It has been reported that normal human skin shows higher integrin expression than standard SE models [27]. Therefore, integrin expression should be enhanced in SEs to a level similar to human skin. Expression of α6-integrin is typically found along the basement membrane [28]. Our results showed that α6-integrin expression in the basal region increased in SE containing fucoidan, whereas β1-integrin expression did not change. Therefore, fucoidan may contribute to reconstruction of an improved SE.
In summary, this study demonstrated that fucoidan promoted CCD-25Sk human fibroblast cell proliferation as confirmed by increased expression of cyclin D1 and decreased expression of p27. Furthermore, fucoidan increased expression of PCNA, p63, and α6-integrin in our SE model. Therefore, fucoidan may be a useful component in the reconstruction of SE.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (Grant No. A103017).
ABBREVIATIONS
- CDK
cyclin-dependent kinase
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- PCNA
proliferating cell nuclear antigen
- PVDF
polyvinylide fluoride
- p27
p27Kip1
- SE
skin equivalent
- TGF-β1
transforming growth factor-β1
References
- 1.Régnier M, Asselineau D, Lenoir MC. Human epidermis reconstructed on dermal substrates in vitro: an alternative to animals in skin pharmacology. Skin Pharmacol. 1990;3:70–85. doi: 10.1159/000210854. [DOI] [PubMed] [Google Scholar]
- 2.Augustin C, Damour O. Pharmacotoxicological applications of an equivalent dermis: three measurements of cytotoxicity. Cell Biol Toxicol. 1995;11:167–171. doi: 10.1007/BF00756519. [DOI] [PubMed] [Google Scholar]
- 3.Senthilkumar K, Manivasagan P, Venkatesan J, Kim SK. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int J Biol Macromol. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Ko EJ, Joo HG. Fucoidan enhances the survival and sustains the number of splenic dendritic cells in mouse endotoxemia. Korean J Physiol Pharmacol. 2011;15:89–94. doi: 10.4196/kjpp.2011.15.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Zhang Q, Chen L, Ren S, Xu P, Tang Y, Luo D. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thromb Res. 2010;125:419–426. doi: 10.1016/j.thromres.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Ale MT, Maruyama H, Tamauchi H, Mikkelsen JD, Meyer AS. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int J Biol Macromol. 2011;49:331–336. doi: 10.1016/j.ijbiomac.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Ko CI, Jee Y, Jeong Y, Kim M, Kim JS, Jeon YJ. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr Polym. 2013;92:84–89. doi: 10.1016/j.carbpol.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K, Lee JB, Nakano T, Hayashi T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013;15:302–309. doi: 10.1016/j.micinf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010;46:6–12. doi: 10.1016/j.ijbiomac.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Moon HJ, Lee SH, Ku MJ, Yu BC, Jeon MJ, Jeong SH, Stonik VA, Zvyagintseva TN, Ermakova SP, Lee YH. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur J Dermatol. 2009;19:129–134. doi: 10.1684/ejd.2008.0611. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary R, Rerek M, Wood EJ. Fucoidan modulates the effect of transforming growth factor (TGF)-beta1 on fibroblast proliferation and wound repopulation in in vitro models of dermal wound repair. Biol Pharm Bull. 2004;27:266–270. doi: 10.1248/bpb.27.266. [DOI] [PubMed] [Google Scholar]
- 12.Mezzano V, Cabrera D, Vial C, Brandan E. Constitutively activated dystrophic muscle fibroblasts show a paradoxical response to TGF-beta and CTGF/CCN2. J Cell Commun Signal. 2007;1:205–217. doi: 10.1007/s12079-008-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YS, Lee KH, Park JW. Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha. Korean J Physiol Pharmacol. 2010;14:91–97. doi: 10.4196/kjpp.2010.14.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prietzsch H, Brock J, Kleine HD, Liebe S, Jaster R. Interferon-alpha inhibits cell cycle progression by Ba/F3 cells through the antagonisation of interleukin-3 effects on key regulators of G(1)/S transition. Cell Signal. 2002;14:751–759. doi: 10.1016/s0898-6568(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 15.Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–110. [PubMed] [Google Scholar]
- 16.Tang PP, Hsieh SC, Wang FF. Modulation of caspase activation and p27(Kip1) degradation in the p53-induced apoptosis in IW32 erythroleukemia cells. Cell Signal. 2002;14:961–968. doi: 10.1016/s0898-6568(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Wu J, Ho PY, Chen LC, Chen CT, Liang YC, Cheng CK, Lee WS. Anti-angiogenic action of 5,5-diphenyl-2-thiohydantoin-N10 (DPTH-N10) Cancer Lett. 2008;271:294–305. doi: 10.1016/j.canlet.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong YM, Lee JE, Kim SY, Yun HY, Baek KJ, Kwon NS, Kim DS. Enhanced effects of citrate on UVB-induced apoptosis of B16 melanoma cells. Pharmazie. 2009;64:829–833. [PubMed] [Google Scholar]
- 20.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auger FA, López Valle CA, Guignard R, Tremblay N, Noël B, Goulet F, Germain L. Skin equivalent produced with human collagen. In Vitro Cell Dev Biol Anim. 1995;31:432–439. doi: 10.1007/BF02634255. [DOI] [PubMed] [Google Scholar]
- 22.Nobre LT, Vidal AA, Almeida-Lima J, Oliveira RM, Paredes-Gamero EJ, Medeiros VP, Trindade ES, Franco CR, Nader HB, Rocha HA. Fucan effect on CHO cell proliferation and migration. Carbohydr Polym. 2013;98:224–232. doi: 10.1016/j.carbpol.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Terada M, Inaba M, Yano Y, Hasuma T, Nishizawa Y, Morii H, Otani S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone. 1998;22:17–23. doi: 10.1016/s8756-3282(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 24.Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003;17:1913–1915. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]
- 25.Blanpain C, Fuchs E. p63: revving up epithelial stem-cell potential. Nat Cell Biol. 2007;9:731–733. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]
- 26.Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185–3197. doi: 10.1101/gad.1463206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wha Kim S, Lee IW, Cho HJ, Cho KH, Han Kim K, Chung JH, Song PI, Chan Park K. Fibroblasts and ascorbate regulate epidermalization in reconstructed human epidermis. J Dermatol Sci. 2002;30:215–223. doi: 10.1016/s0923-1811(02)00108-1. [DOI] [PubMed] [Google Scholar]
- 28.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]