Abstract
Most known osteoporosis medicines are effective for bone resorption, and so there is an increasing demand for medicines that stimulate bone formation. Watercress (N. officinale R. Br.) is widely used as a salad green and herbal remedy. This study analyzed a watercress extract using ultra-performance liquid chromatography/mass spectrometry, and identified a rutin as one of its major constituents. Osteogenic-related assays were used to compare the effects of watercress containing rutin (WCR) and rutin alone on the proliferation and differentiation of human osteoblast-like MG-63 cells. The reported data are expressed as percentages relative to the control value (medium alone; assigned as 100%). WCR increased cell proliferation to 125.0±4.0% (mean±SD), as assessed using a cell viability assay, and increased the activity of alkaline phosphatase, an early differentiation marker, to 222.3±33.8%. In addition, WCR increased the expression of collagen type I, another early differentiation marker, to 149.2±2.8%, and increased the degree of mineralization, a marker of the late process of differentiation, to 122.9±3.9%. Rutin alone also increased the activity of ALP (to 154.4±12.2%), the expression of collagen type I (to 126.6±6.2%), and the degree of mineralization (to 112.3±5.0%). Daidzein, which is reported to stimulate bone formation, was used as a positive control; the effects of WCR on proliferation and differentiation were significantly greater than those of daidzein. These results indicate that WCR and rutin can both induce bone formation via the differentiation of MG-63 cells. This is the first study demonstrating the effectiveness of either WCR or rutin as an osteoblast stimulant.
Keywords: Differentiation, Osteoblast, Proliferation, Rutin, Watercress
INTRODUCTION
Osteoporosis is a skeletal disorder characterized by compromised bone strength, which can lead to an increased risk of fracture [1]. Many countries are experiencing acute increases in the number of elderly people, which has resulted in osteoporosis becoming a major and growing health problem. Osteoporosis will occur when bone resorption by osteoclasts exceeds bone formation by osteoblasts [2,3].
Almost all of the therapies currently recommended for the treatment of osteoporosis act mainly to inhibit osteoclast-mediated bone resorption. The only medicines that are known to stimulate bone formation are parathyroid hormone analogues such as teriparatide and Preotact. However, these medicines are more expensive than other medicines, such as bisphosphonates, which are used as primary agents against osteoclast-mediated bone loss for osteoporosis [4]. It has been suggested that stimulating bone formation via actions on osteoblasts is an important therapeutic approach for osteoporosis, and since many of the modern pharmacological therapies are expensive and are accompanied by unwanted side effects, there is an increasing focus on alternative therapies such as foods or herbal remedies. There is thus a great need for research into functional food ingredients that stimulate bone formation as preventive or therapeutic agents [5].
Watercress (Nasturtium officinale R. Br.) is a perennial aquatic herb from the Brassicaceae family, which is native to Europe and is applied widely in local recipes. Fresh raw watercress leaves, which are used as salad greens [6], contain high levels of vitamin C, vitamin E, and members of the B-group vitamins, as well as many minerals, such as Ca, P, K, Fe, Mg, Zn, traces of Se, β-carotene, and glucosinolates [7]. Watercress leaves are widely used as home remedies with depurative, diuretic, expectorant, hypoglycemic, antidontalgic, stimulant, and stomachic properties, but also to treat hypertension and cardiovascular diseases [8]. Anticarcinogenic and antioxidative activities have also been reported [9]. However, there have been no studies published on the use of watercress as an antiosteoporotic activity.
Quercetin, the major representative of the flavonoid, occurs mainly as a glycoside, rutin (quercetin-3-O-rutinoside), which is plentiful in vegetables and fruits such as onions, tomatoes, apples, and berries [10]. Rutin, a dietary glycoside, is converted into quercetin in the large intestine by the glycosidases of the intestinal bacteria [11]. It has been reported that quercetin increases the ALP activity in MG-63 cells without any significant cytotoxic effects [12]. In contrast, quercetin inhibits the proliferation, differentiation, and mineralization of osteoblasts in rat calvarial osteoblast-like cells [13]. Rutin is known for its anti-inflammatory properties and vasoconstrictive effects on peripheral blood vessels [14], and is known to inhibit ovariectomy-induced osteopenia in female rats [15]. In addition, rutin reportedly increased the proliferation and osteoblastic differentiation of mouse bone marrow mesenchymal stem cells, and also the ALP activity, degree of mineralization, and expression of prominent osteoblastic differentiation markers [16]. However, the proliferative and differentiative effects of rutin on human osteoblast-like MG-63 cells have not been investigated in detail previously.
The present study used ultra-performance liquid chromatography (UPLC)/mass spectrometry (MS) to characterize the chemical constituents of watercress, and identified a rutin as one of its major constituents. This study thus clarified the roles of watercress containing rutin (WCR) and rutin alone in bone formation by investigating the proliferative and differentiative effects of WCR and rutin on osteoblasts by measuring alkaline phosphatase (ALP) activity, collagen type I synthesis, and bone mineralization in WCR- or rutin-treated human osteoblast-like MG-63 cells in vitro.
METHODS
Plant material
The leaves of N. officinale R. Br. were purchased at the Garak Market in Seoul, Korea, washed with distilled water, chopped, and then dried for 3 days. Three grams of the dried leaves was milled and extracted three times with 30 ml of 70% aqueous ethanol (HPLC grade, Fisher Scientific, Fair Lawn, NJ, USA) under continuous agitation at room temperature for 3 days. After centrifugation of the extract at 800×g for 1 hr, the supernatant was filtered with a 0.45-µm syringe filter. The solution was concentrated under reduced pressure in a rotary evaporator and lyophilized. The final lyophilized material weighed 314 mg and was stored at 4℃ until use.
UPLC/MS analysis
The constituents of WCR were analyzed using the Ultimate 3000 Rapid Separation LC system (Thermo Scientific, Waltham, MA, USA) with Orbitrap Q, an executive mass spectrometer (Thermo Scientific). Separation was achieved on an Acquity UPLC BEH C18 column (2.1×150 mm, 1.7-µm particle size, Waters, Milford, MA, USA) maintained at 40℃ with mobile phases that consisted of 0.1% formic acid (Fisher Scientific) in distilled water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). Elution was performed at a flow rate of 0.3 ml/min with the following linear gradient: 0 min, 5% solvent B; 30 min, 70% solvent B; 31 min, 100% solvent B; 32 min, 100% solvent B; 33 min, 5% solvent B; and 40 min, 5% solvent B. Peaks were detected on a photodiode array (PDA) range of 200~400 nm. For liquid chromatography/tandom MS analysis, heated electrospray ionization was achieved at 4 kV with a capillary temperature of 350℃ in the positive mode. The precursor ions were detected at a resolution of 70,000 at m/z of 200 within m/z range of 150~1000. The precursor ions were fragmented at a normalized collision energy of 20, and the fragmented ions were detected at a resolution of 35,000 at m/z of 200. MS and MS/MS spectra were analyzed using Xcalibur software (version 2.1, Thermo Scientific). Compounds were identified with accurate mass (mass tolerance 5 ppm) using online mass spectrum databases (METLIN, Massbank, ReSpect) and the literature.
Cell culture
Human osteoblast-like MG-63 cells were purchased from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured to confluence in Dulbecco's modified Eagle's medium (DMEM; Corning, VA, USA) containing 10% fetal bovine serum (Gibco, NY, USA) and 1% penicillin-streptomycin (Gibco) at 37℃ and 5% CO2.
Cell viability assay
Water-soluble tetrazolium-8, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8; Dojindo, Kumamoto, Japan) was used to assay cell viability using the Tominaga method [17]. Briefly, MG-63 cells were seeded at a density of 2×104 cells/well in 96-well plates and preincubated overnight to allow the cells to adhere to the plates. The cells were treated with WCR or rutin (Sigma, St Louis, MO, USA) dissolved in DMEM at various concentrations or in DMEM alone (control), and incubated for 72 hr. WST-8 reagents (10 µl/well) were added to the 96-well plates and incubated for 3 hr. The absorbance at 450 nm was measured using a microplate reader (FlexStation3, Molecular Devices, Sunnyvale, CA, USA).
Measurement of ALP activity
ALP activity was measured by analyzing the rate of p-nitrophenyl phosphate (Sigma) hydrolysis [18]. MG-63 cells were seeded at a density of 2×104 cells/well in 24-well plates and preincubated overnight to allow them to adhere to the plates. The cells were treated with samples dissolved in DMEM at various concentrations or in DMEM alone (control) and incubated for 72 hr. The medium was removed and 500µl of 0.1% Triton X-100 (Sigma) was added to each well. The cells were frozen at -70℃ and melted at 37℃ to break the cell membrane, centrifuged for 10 min at 14,000×g and 4℃, and the supernatant was removed and used for measuring ALP activity. The absorbance at 405 nm was measured using a microplate reader (FlexStation3, Molecular Devices). Protein levels were quantified using the bicinchoninic acid assay (iNtRON Biotechnology, Seoul, Korea).
Measurement of collagen content
Collagen production was evaluated using a Sirius-red-based colorimetric microassay [19]. MG-63 cells were seeded at a density of 3×104 cells/well in 24-well plates and preincubated overnight to allow them to adhere to the plates. The cells were treated with samples dissolved in DMEM (which is used in mineralization assays) at various concentrations or in DMEM alone (control). The media were replaced on the third day with fresh media and samples. After removing the medium and washing the cells briefly with phosphate-buffered saline (PBS), 1 ml of Bouin's fluid (Sigma) was added for 1 hr to fix the cells. Bouin's fluid was then removed by suction, and the cells were washed three times with distilled water. The plates were air dried before adding 1 ml of Sirius red dye (Sigma) dissolved in picric acid (Sigma) at a concentration of 100 mg/100 ml for 1 hr. The cells were stained under mild shaking on a microplate shaker, and the dye solution was removed by suction. Any nonbound dye was removed by washing with 0.01 M hydrochloric acid. The stained material was dissolved in 300 µl of 0.1 M sodium hydroxide for 30 min at room temperature on a microplate shaker. The absorbance at 550 nm was measured using a microplate reader (FlexStation3, Molecular Devices) relative to 0.1 M sodium hydroxide as a blank.
Mineralization assay
MG-63 cells were seeded at a density of 3×104 cells/well in 24-well plates and preincubated overnight to allow them to adhere to the plates. The cells were then treated with samples dissolved at various concentrations in DMEM containing 50 µg/ml ascorbic acid (Sigma), 10 mM β-glycerophosphate (Sigma), and 10-7 M dexamethasone (Sigma), or in DMEM alone (control). The media were refreshed every 3~4 days. After removing the medium and washing the cells with PBS, 70% ethanol was added and incubated at 4℃ for 1 hr to fix the cells. The cells were washed with distilled water, dyed with 40 mM Alizarin red solution (pH 4.2; Sigma) for 10 min, and then washed five times with distilled water to reduce nonspecific staining. PBS was then added and allowed to remain for 15 min. After removing the PBS, 10 mM sodium phosphate (pH 7.0) containing 10% (w/v) cetylpyridinium chloride (Sigma) was added and allowed to remain for 15 min. The absorbance at 562 nm was measured using a microplate reader (FlexStation3, Molecular Devices).
Statistical analysis
The data are expressed as mean±standard deviation (SD) values of triplicate measurements. Student's t test was used to assess the significance of any differences, and the cutoff for statistical significance was set at p<0.05.
RESULTS
UPLC/MS analysis
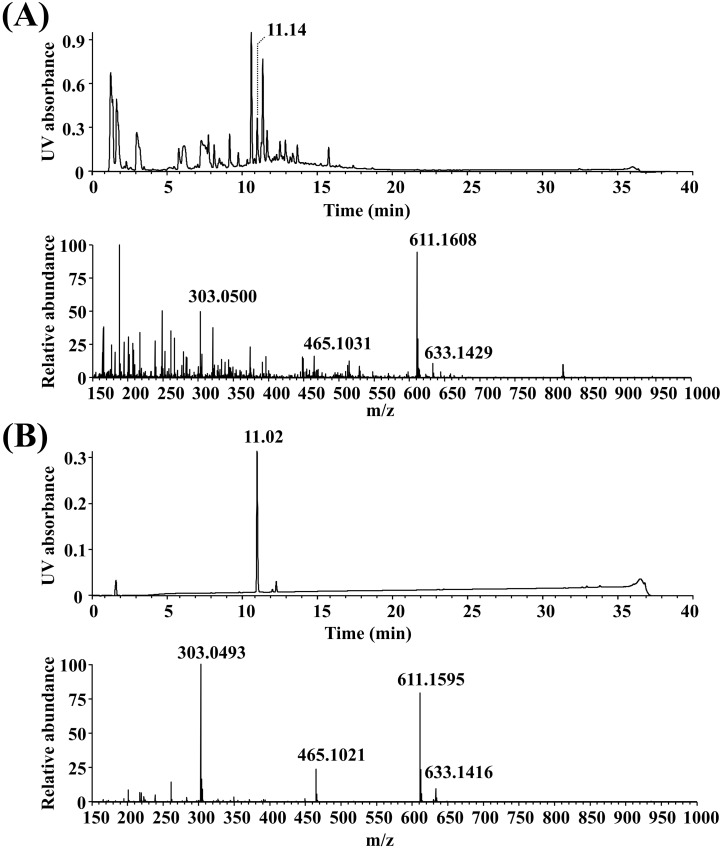
Watercress extract was analyzed by UPLC/MS with a PDA detector at 200~400 nm in the positive ionization mode (Fig. 1A). More than 20 chemical constituents were identified, one of which was rutin, which represented about 3.2% of the total constituents based on a comparison of the findings with the retention time and mass data of commercially available rutin (Fig. 1B). Namely, in the mass spectra of WCR and rutin, the peaks at m/z=611.1608 and 611.1595 corresponded to the [M+H]+ ion, respectively. Molecular ion fragmentations and adduct peaks were respectively present at m/z=633.1429 and 633.1416 (corresponding to the [M+Na]+ ion), m/z=465.1031 and 465.1021 (rhamnose loss), and m/z=303.0500 and 303.0493 (quercetin).
Fig. 1.
PDA chromatogram (upper) of MS positive ion electrospray (lower) analyses of (A) WCR and (B) rutin.
Effects on cell viability
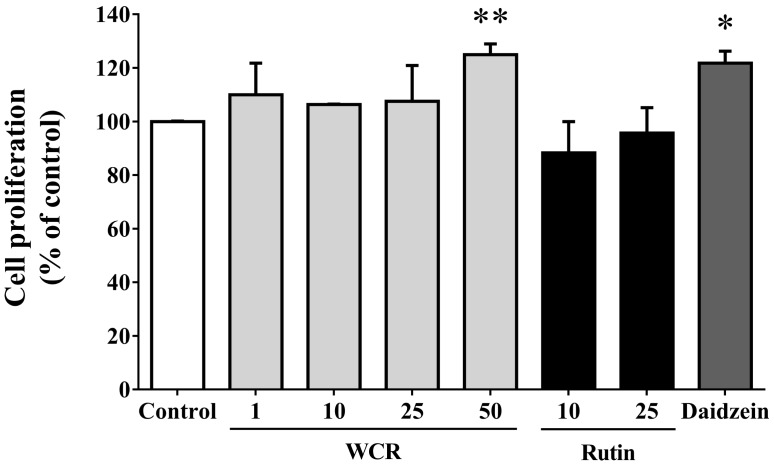
The effects of WCR or rutin on osteoblast proliferation were explored by adding samples at various concentrations to the culture medium for 72 hr and using the cell-proliferation WST-8 assay. Fig. 2 shows the effects of WCR and rutin on the viability of MG-63 cells. WCR and rutin did not exert any significant cytotoxic effects on the MG-63 cells at the tested concentrations after 72 hr of incubation; however, WCR did have a stimulatory effect on MG-63 cell proliferation at all tested concentrations from 1 to 50 µg/ml. Daidzein was used as a positive control, and at 10 µg/ml it increased the cell viability to 121.8±4.5% (p<0.05). WCR at 50 µg/ml caused a significant increase in cell viability over the control level of 125.0±4.0% (p<0.01); the control level corresponded to that for the medium alone, and was assigned as 100%.
Fig. 2.
Effects of WCR and rutin at various concentrations (µg/ml) on cell proliferation in MG-63 cells. The data are expressed as percentages relative to the control value (assigned a value of 100%), and are mean and SD values of three independent samples. *p<0.05, **p<0.01, significantly different from the control group.
Effects on ALP activity
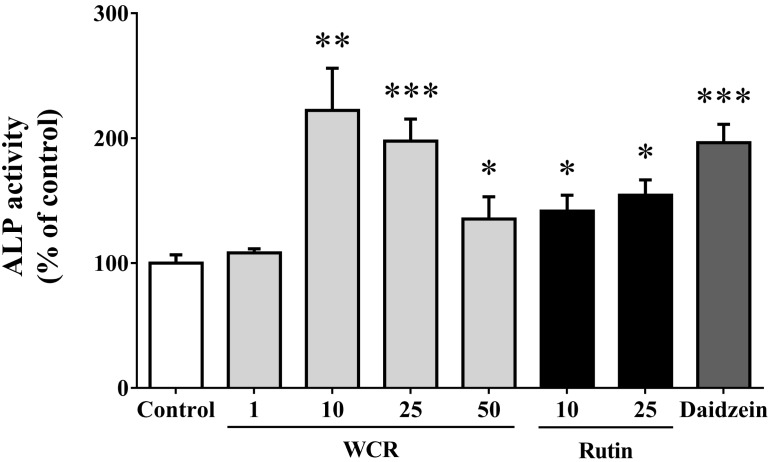
The effects of WCR or rutin on osteoblast differentiation were assessed by measuring the ALP activity, which is an enzyme that is involved in the maturation step of osteoblasts and one of the major promoters of early osteoblast differentiation. Fig. 3 shows the effects of WCR and rutin on the differentiation of MG-63 cells (i.e., ALP activity). WCR at concentrations of 10 and 25 µg/ml also induced significant increases in ALP activity (p<0.01 and <0.001); at 10 µg/ml it was 222.3±33.8% of the control level. Rutin at 25 µg/ml induced significant increases in ALP activity (154.4±12.2%, p<0.05) relative to the control level. The ALP activity of MG-63 cells exposed to 10 µg/ml daidzein as a positive control was increased to 196.4±14.7% (p<0.001).
Fig. 3.
Stimulation of ALP activity in MG-63 cells by WCR and rutin at various concentrations (µg/ml). The data are expressed as percentages relative to the control value (assigned a value of 100%), and are mean and SD values of three independent samples. *p<0.05, **p<0.01, ***p<0.001, significantly different from the control group.
Effects on collagenogenesis
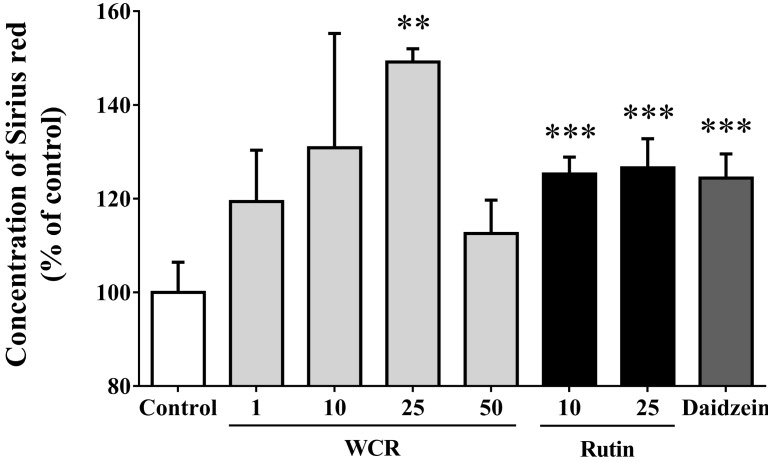
The effects of WCR and rutin on the synthesis of collagen type I, which is involved in the process of early osteoblast differentiation, were examined using Sirius red staining (Fig. 4). The WCR- or rutin-induced increase in collagen content was significant at a concentration of 25 µg/ml (p<0.01, p<0.01). The maximum increases for all concentrations used in this study, to 149.2±2.8% and 126.6±6.2%, occurred on the 5th day of culture when WCR and rutin were present at a concentration of 25 µg/ml, respectively. Daidzein (10 µg/ml) as a positive control increased the collagen content to 124.4±5.2% (p<0.001).
Fig. 4.
Effects of WCR and rutin at various concentrations (µg/ml) on the collagen content of MG-63 cells after 5 days. The data are percentages relative to the control value (assigned a value of 100%), and are mean and SD values of three independent samples. **p<0.01, ***p<0.001, significantly different from the control group.
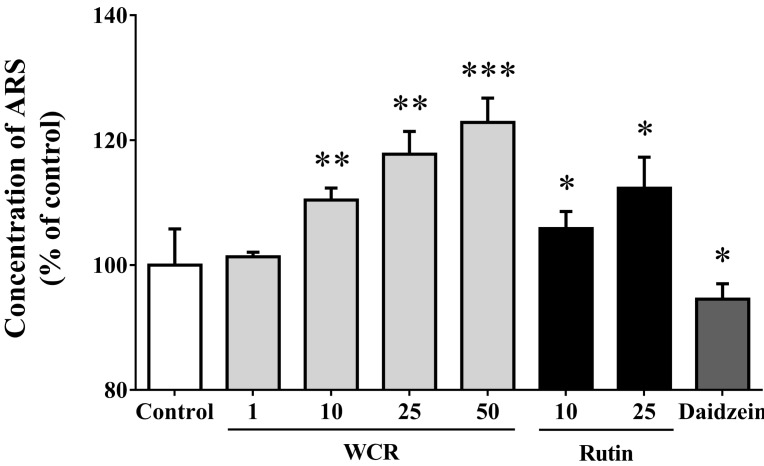
Effects on mineralization
The effect of WCR or rutin on mineralization, an important process in late osteoblast differentiation, was examined by measuring the calcium deposition in MG-63 cells using Alizarin red staining (Fig. 5). The increase in calcium content was significant when WCR and rutin were present at concentrations of 50 and 25 µg/ml, respectively. The maximum increase for all of the tested concentrations, to 122.9±3.9% and 112.3±5.0% of control levels, occurred on the 7th day of culture at an WCR and rutin concentration of 50 µg/ml (p<0.001) and 25 µg/ml (p<0.05), respectively. Daidzein (10 µg/ml) had almost no effect on mineralization (p<0.05).
Fig. 5.
Effects of WCR and rutin at various concentrations (µg/ml) on mineralization of the extracellular matrix in MG-63 cells after 7 days. The data are expressed as percentages relative to the control value (assigned a value of 100%), and are mean and SD values of three independent samples. ARS, Alizarin red staining. *p<0.05, **p<0.01, ***p<0.001, significantly different from the control group.
DISCUSSION
In this study we investigated the stimulatory effects of WCR and rutin on the proliferation and differentiation of human osteoblast-like MG-63 cells in vitro by measuring cell viability, ALP activity, collagenogenesis, and mineralization.
The chemical constituents of watercress extract were analyzed using UPLC/MS and MS/MS data (data not shown), and rutin was identified from the recently developed online resource METLIN, Massbank, and ReSpect, and by comparison with standard commercially available rutin. None of the other compounds matched with previously reported compounds from watercress [7,8,20,21,22,23] or online resources.
The results indicate that WCR had no cytotoxic effects on MG-63 cells when it was present at concentrations of 1~50 µg/ml, and significantly increased cell viability after 3 days. WCR and rutin also induced cell differentiation, as evidenced by an increase in ALP activity over control levels in MG-63 cells. ALP is one of the major enzymes expressed during the early maturation of osteoblasts [24]. ALP activity increased after treatment with WCR or rutin for 3 days, indicating that WCR effectively induced the differentiation of early-stage osteoblasts. Collagenogenesis in MG-63 cells was greatly increased as compared with the control level after 5 days of WCR or rutin treatment. Osteoblasts synthesize collagen and noncollagenous proteins to make woven bone. One-third of the bone is matrix phase, and 90% of that is collagen [25]. Since collagen is a major matrix material of bone, increases in collagen content reflect the process of osteoblastic maturation. Similarly to collagenogenesis, mineralization of MG-63 cells was stimulated by treatment with WCR or rutin, with the degree of mineralization increasing in proportion with the WCR or rutin concentration. Mineralization occurs to make mature bone in the late stage of osteoblastic differentiation [24]. Two-thirds of bone is the mineral phase, and comprises hydroxyapatite crystals and amorphous calcium phosphate deposited on the bone matrix [26]. Since bone formation is completed by mineralized bone nodules, elevation of mineralization by WCR and rutin demonstrates that these compounds have a bone-forming effect. It has been reported that the bisphosphonate alendronate acts an inhibitor of osteoclast-mediated bone resorption; however, it was also shown to exert a significant stimulatory effect on mineralization in MG-63 cells, increasing it to 118~124% relative to control levels [27], similar to the effects of WCR and rutin on mineralization observed in the present study. These results suggest that both WCR and rutin stimulate bone mineralization.
The present findings demonstrate the stimulatory effects of WCR and rutin on differentiation in MG-63 cells in terms of cell viability, ALP activity, collagenogenesis, and mineralization, indicating that both WCR and rutin could induce osteoblast differentiation in MG-63 cells. Moreover, these proliferative and differentiative effects of WCR were significantly greater than the effects of daidzein in the positive control group. Members of the Leguminosae family are rich in daidzein, a natural isoflavone and a structural estrogen analog that is known to prompt bone formation and prevent bone resorption. Many studies have demonstrated that daidzein stimulates the activity of ALP and mineralization of mouse-derived MC3T3-E1 osteoblast cells [28,29,30], and that it does not affect the proliferation of MC3T3-E1 cells [31,32]. In the present investigation, daidzein had a significant effect on MG-63 cell proliferation and differentiation, but not on mineralization. These discrepant findings may be attributable to differences between human- and mouse-derived cells.
Certain plant herbs, such as cabbage and broccoli, are known to affect osteoporosis; however, they act only by increasing the mineral density [33]. In addition, Maca, Lepidium meyenii is the plant in the Brassicaceae family that possesses the ability to increase bone mineral density [34]. However, the watercress used in the present stimulated cellular proliferation and differentiation. Previous studies have explored the properties of plants that affect osteoblast activity. For example, Acanthus ilicifolius L. [35], a chrysoeriol isolated from the leaves of Eurya ciliate [36], and triterpene from the leaves of Acanthopanax koreanum [37] were found to stimulate cell proliferation and to increase the levels of ALP and collagen type I, and the degree of mineralization. Moreover, Eucommiae cortex leaves [38] and Scutellariae radix extract [39] have dual functions, stimulating osteoblasts and suppressing osteoclasts.
The present study found that rutin stimulated the differentiation of MG-63 cells, but its effects did not exceed those of WCR. These results suggest that although rutin is one of the principal components of watercress, it is not solely responsible for the proliferative and differentiative effects of WCR on MG-63 cells. Further research is required to analyze the constituents of WCR.
In conclusion, the present results demonstrate that WCR and rutin stimulate the proliferation and differentiation of osteoblastic MG-63 cells. This suggests that watercress is beneficial in stimulating the osteoblastic activity that results in bone formation. To our knowledge, this is the first report of the use of watercress or rutin as an osteoblast stimulant on human osteoblast-like MG-63 cells. Further studies are required to (1) identify other constituents of WCR and their effects on osteoblasts, (2) investigate their effects on bone resorption by osteoclasts, and (3) determine the in vivo bioavailability of WCR in animal models to evaluate its potential as a bone health supplement. These studies are currently underway in our laboratory.
ACKNOWLEDGEMENTS
This study was supported by the Rural Development Administration (PJ0098432013), Republic of Korea and a Chung-Ang University Excellent Student Scholarship.
ABBREVIATIONS
- ALP
alkaline phosphatase
- DMEM
Dulbecco's modified Eagle's medium
- MS
mass spectrometry
- PBS
phosphatebuffered saline
- PDA
photodiode array
- UPLC
ultra-performance liquid chromatography
- WCR
watercress containing rutin
- WST-8
water-soluble tetrazolium-8, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt
References
- 1.Bhutani G, Gupta MC. Emerging therapies for the treatment of osteoporosis. J Midlife Health. 2013;4:147–152. doi: 10.4103/0976-7800.118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau RY, Guo X. A Review on current osteoporosis research: with special focus on disuse bone loss. J Osteoporos. 2011;2011:293808. doi: 10.4061/2011/293808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roux S. New treatment targets in osteoporosis. Joint Bone Spine. 2010;77:222–228. doi: 10.1016/j.jbspin.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 5.Bakhsh A, Mustapha NM, Mohamed S. Catechin-rich oil palm leaf extract enhances bone calcium content of estrogen-deficient rats. Nutrition. 2013;29:667–672. doi: 10.1016/j.nut.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Gonçalves EM, Cruz RMS, Abreu M, Brandão TRS, Silva CLM. Biochemical and colour changes of watercress (Nasturtium officinale R. Br.) during freezing and frozen storage. J Food Eng. 2009;93:32–39. [Google Scholar]
- 7.Zahradníková H, Petříková K. Nematocid effects of watercress (Nasturtium officinale R. Br.) Acta Univ Agric Silvic Mendelianae Brun. 2013;61:233–236. [Google Scholar]
- 8.Amiri H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat Prod Res. 2012;26:109–115. doi: 10.1080/14786419.2010.534998. [DOI] [PubMed] [Google Scholar]
- 9.Ozen T. Investigation of antioxidant properties of Nasturtium officinale (watercress) leaf extracts. Acta Pol Pharm. 2009;66:187–193. [PubMed] [Google Scholar]
- 10.Manach C, Morand C, Crespy V, Demigné C, Texier O, Régérat F, Rémésy C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 11.Tamura G, Gold C, Ferro-Luzzi A, Ames BN. Fecalase: a model for activation of dietary glycosides to mutagens by intestinal flora. Proc Natl Acad Sci U S A. 1980;77:4961–4965. doi: 10.1073/pnas.77.8.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prouillet C, Mazière JC, Mazière C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67:1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Notoya M, Tsukamoto Y, Nishimura H, Woo JT, Nagai K, Lee IS, Hagiwara H. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur J Pharmacol. 2004;485:89–96. doi: 10.1016/j.ejphar.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 14.La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V, Martín Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 15.Horcajada-Molteni MN, Crespy V, Coxam V, Davicco MJ, Rémésy C, Barlet JP. Rutin inhibits ovariectomy-induced osteopenia in rats. J Bone Miner Res. 2000;15:2251–2258. doi: 10.1359/jbmr.2000.15.11.2251. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava S, Bankar R, Roy P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine. 2013;20:683–690. doi: 10.1016/j.phymed.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun. 1999;36:47–50. [Google Scholar]
- 18.Rivkin RB, Swift E. Characterization of alkaline phosphatase and organic phosphorous utilization in the oceanic dinoflagellate Pyrocystis noctiluca. Mar Biol. 1980;61:1–8. [Google Scholar]
- 19.Tullberg-Reinert H, Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol. 1999;112:271–276. doi: 10.1007/s004180050447. [DOI] [PubMed] [Google Scholar]
- 20.Boyd LA, McCann MJ, Hashim Y, Bennett RN, Gill CI, Rowland IR. Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr Cancer. 2006;55:232–241. doi: 10.1207/s15327914nc5502_15. [DOI] [PubMed] [Google Scholar]
- 21.Spence RMM, Tucknott OG, Baker EA, Holloway PJ. Compounds associated with the surface lipid layer of watercress. Phytochemistry. 1983;22:1753–1756. [Google Scholar]
- 22.Park NI, Kim JK, Park WT, Cho JW, Lim YP, Park SU. An efficient protocol for genetic transformation of watercress (Nasturtium officinale) using Agrobacterium rhizogenes. Mol Biol Rep. 2011;38:4947–4953. doi: 10.1007/s11033-010-0638-5. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Sánchez A, Gil-Izquierdo A, Gil MI, Ferreres F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J Agric Food Chem. 2008;56:2330–2340. doi: 10.1021/jf072975+. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Jung EY, Bae SH, Kwon KH, Kim JM, Suh HJ. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by yeast hydrolysate. Phytother Res. 2011;25:716–723. doi: 10.1002/ptr.3328. [DOI] [PubMed] [Google Scholar]
- 25.Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal NC, Posner AS. Hydroxyapatite: mechanism of formation and properties. Calcif Tissue Res. 1973;13:235–243. doi: 10.1007/BF02015413. [DOI] [PubMed] [Google Scholar]
- 27.Xiong Y, Yang HJ, Feng J, Shi ZL, Wu LD. Effects of alendronate on the proliferation and osteogenic differentiation of MG-63 cells. J Int Med Res. 2009;37:407–416. doi: 10.1177/147323000903700216. [DOI] [PubMed] [Google Scholar]
- 28.Ge Y, Chen D, Xie L, Zhang R. Enhancing effect of daidzein on the differentiation and mineralization in mouse osteoblast-like MC3T3-E1 cells. Yakugaku Zasshi. 2006;126:651–656. doi: 10.1248/yakushi.126.651. [DOI] [PubMed] [Google Scholar]
- 29.Gao YH, Yamaguchi M. Anabolic effect of daidzein on cortical bone in tissue culture: comparison with genistein effect. Mol Cell Biochem. 1999;194:93–98. doi: 10.1023/a:1006949912266. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto E, Yamaguchi M. Stimulatory effect of daidzein in osteoblastic MC3T3-E1 cells. Biochem Pharmacol. 2000;59:471–475. doi: 10.1016/s0006-2952(99)00351-2. [DOI] [PubMed] [Google Scholar]
- 31.Lee YS, Chen X, Anderson JJ. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutr Res. 2001;21:1287–1298. [Google Scholar]
- 32.Kanno S, Hirano S, Kayama F. Effects of phytoestrogens and environmental estrogens on osteoblastic differentiation in MC3T3-E1 cells. Toxicology. 2004;196:137–145. doi: 10.1016/j.tox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Michaelsen KF, Astrup AV, Mosekilde L, Richelsen B, Schroll M, Sørensen OH. The importance of nutrition for the prevention of osteoporosis. Ugeskr Laeger. 1994;156:958–960. 963–965. [PubMed] [Google Scholar]
- 34.Zhang Y, Yu L, Ao M, Jin W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J Ethnopharmacol. 2006;105:274–279. doi: 10.1016/j.jep.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Van Kiem P, Quang TH, Huong TT, Nhung le TH, Cuong NX, Van Minh C, Choi EM, Kim YH. Chemical constituents of Acanthus ilicifolius L. and effect on osteoblastic MC3T3E1 cells. Arch Pharm Res. 2008;31:823–829. doi: 10.1007/s12272-001-1232-3. [DOI] [PubMed] [Google Scholar]
- 36.Tai BH, Cuong NM, Huong TT, Choi EM, Kim JA, Kim YH. Chrysoeriol isolated from the leaves of Eurya ciliata stimulates proliferation and differentiation of osteoblastic MC3T3-E1 cells. J Asian Nat Prod Res. 2009;11:817–823. doi: 10.1080/10286020903117317. [DOI] [PubMed] [Google Scholar]
- 37.Park SH, Nhiem NX, Kiem PV, Choi EM, Kim JA, Kim YH. A new norlupane triterpene from the leaves of Acanthopanax koreanum increases the differentiation of osteoblastic MC3T3-e1 cells. Arch Pharm Res. 2010;33:75–80. doi: 10.1007/s12272-010-2228-4. [DOI] [PubMed] [Google Scholar]
- 38.Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH, Kim C. Effects of Eucommiae cortex on osteoblast-like cell proliferation and osteoclast inhibition. Arch Pharm Res. 2003;26:929–936. doi: 10.1007/BF02980202. [DOI] [PubMed] [Google Scholar]
- 39.Shin JM, Park CK, Shin EJ, Jo TH, Hwang IK. Effects of Scutellaria radix extract on osteoblast differentiation and osteoclast formation. Korean J Food Sci Technol. 2008;40:674–679. [Google Scholar]