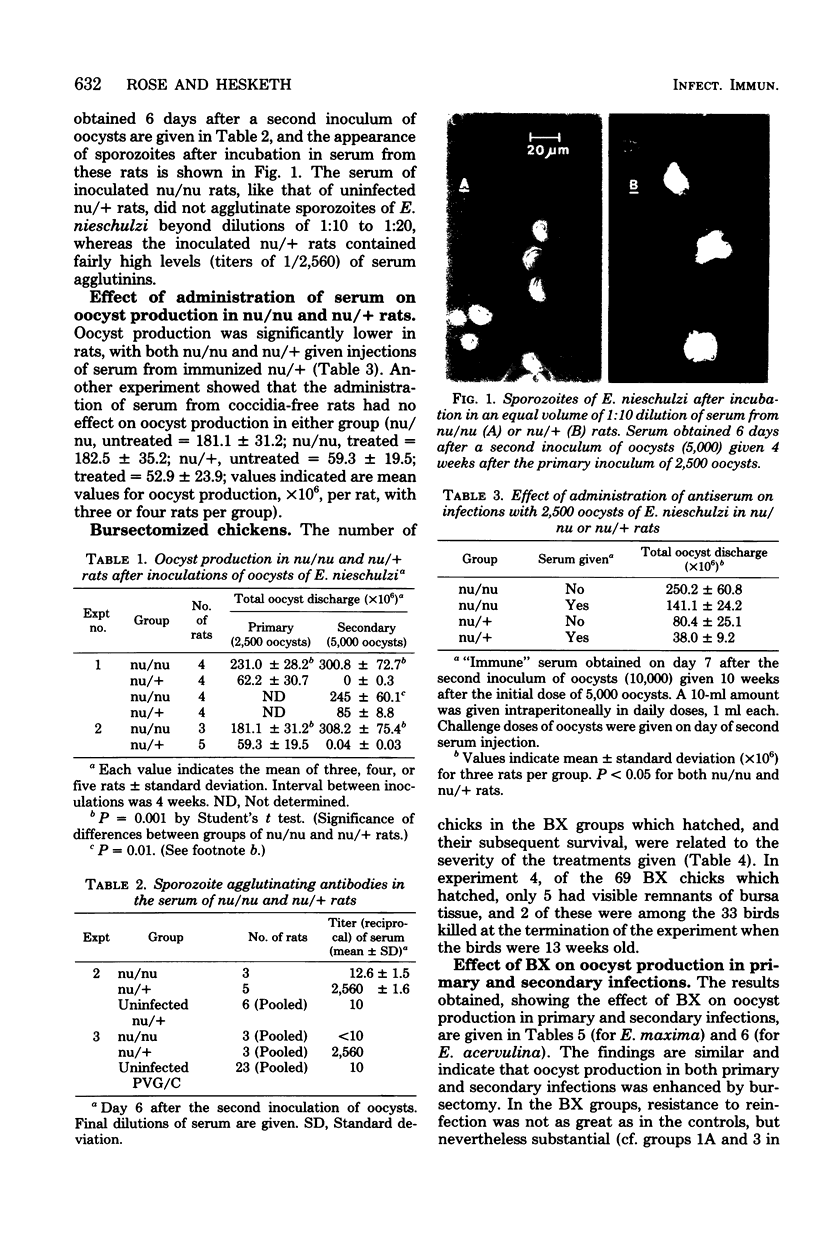
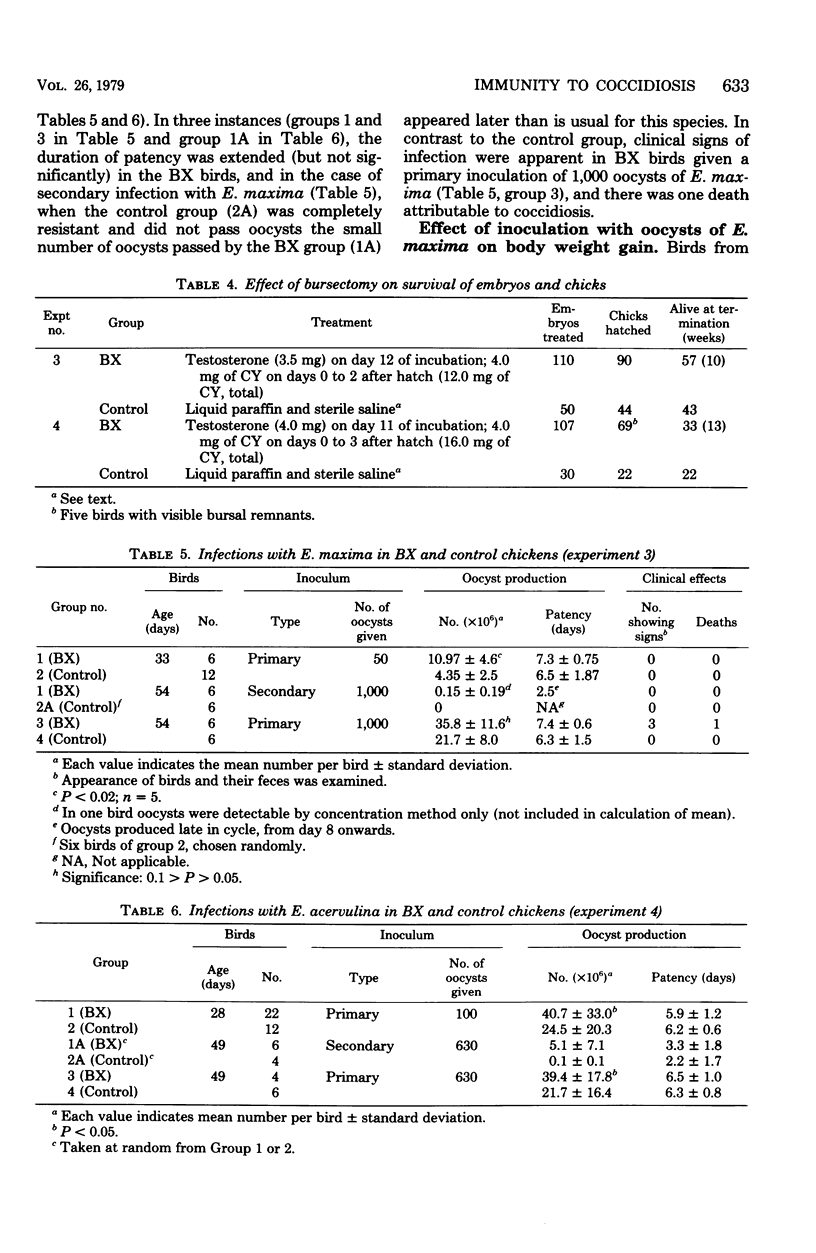
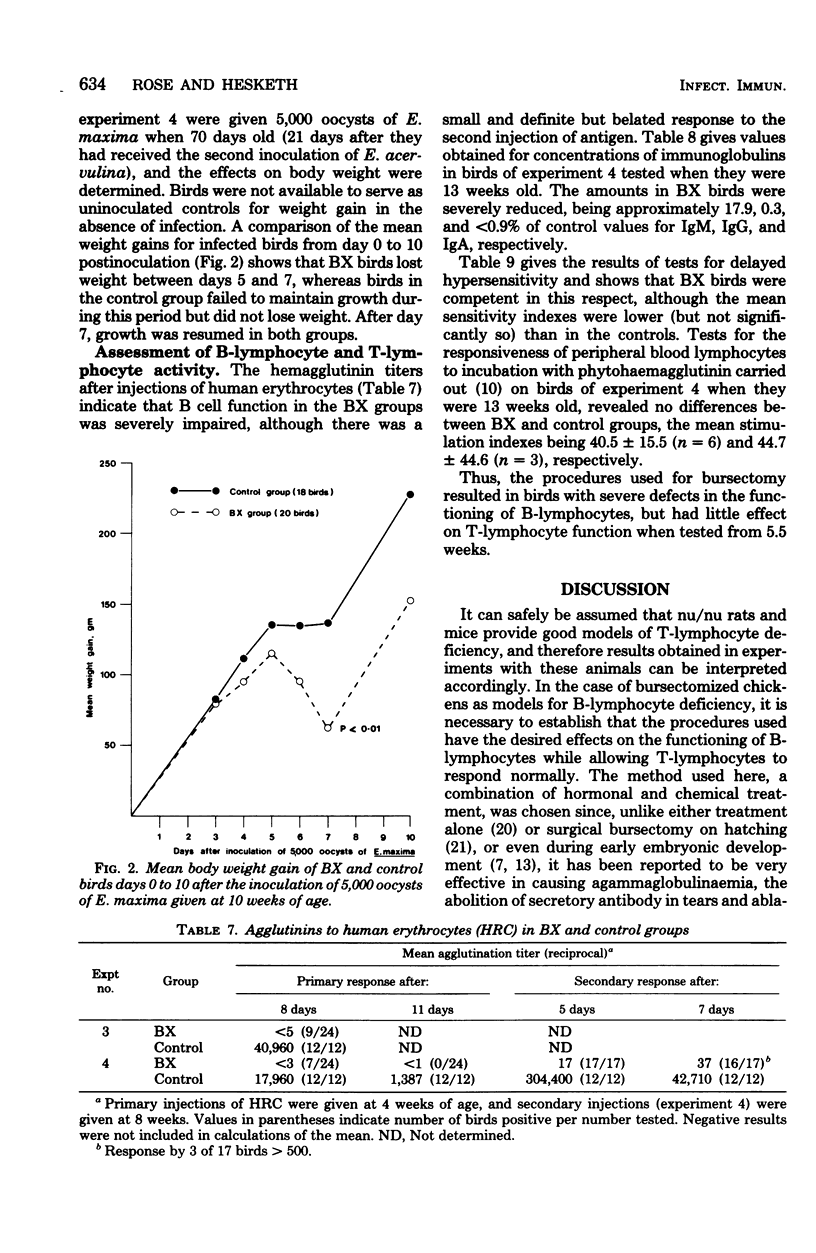
Abstract
Comparisons were made between infections with Eimeria spp. in normal animals and in animals with functional deficiencies in either T-lymphocytes (athymic nude rats) or B-lymphocytes (bursectomized chickens). Approximately three times more oocysts of E. nieschulzi were passed during a primary infection in the nu/nu rats, and in contrast to the nu/+ rats, they were completely susceptible to reinfection. Nu/nu rats did not produce agglutinating antibodies to sporozoites, and injections of serum from immunized nu/+ rats caused a reduction of oocyst production during a primary infection in both nu/nu and nu/+ rats. In chickens, oocyst production in primary infections with E. maxima or with E. acervulina was increased 1.5 to 2-fold in the bursectomised (BX) groups, and the clinical effects of infection with E. maxima were greater than in controls. The BX birds were slightly more susceptible than the controls to challenge inoculations of oocysts, but nevertheless, they were very substantially immune. Tests for the functioning of B- and T-lymphocytes indicated that in the BX birds there was a severe deficiency of B-cell function, but near-normal T-cell function. The results show that T-lymphocytes are essential for immunity and that, although they may function partly as helper cells for immunoglobulin production, their major effect is exerted via some other mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinchilla M., Frenkel J. K. Mediation of immunity to intracellular infection (Toxoplasma and Besnoitia) within somatic cells. Infect Immun. 1978 Mar;19(3):999–1012. doi: 10.1128/iai.19.3.999-1012.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. D., Mims L. H., Strober W. Deficient IgA antibody responses to arsanilic acid bovine serum albumin (BSA) in neonatally thymectomized rabbits. J Immunol. 1971 Jun;106(6):1624–1629. [PubMed] [Google Scholar]
- Davis P. J., Parry S. H., Porter P. The role of secretory IgA in anti-coccidial immunity in the chicken. Immunology. 1978 May;34(5):879–888. [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Porter P. A mechanism for secretory IgA-mediated inhibition of the cell penetration and intracellular development of Eimeria tenella. Immunology. 1979 Mar;36(3):471–477. [PMC free article] [PubMed] [Google Scholar]
- Eskola J., Toivanen P. Effect of in ovo treatment with cyclophosphamide on lymphoid system in chicken. Cell Immunol. 1974 Sep;13(3):459–471. doi: 10.1016/0008-8749(74)90265-2. [DOI] [PubMed] [Google Scholar]
- Festing M. F., May D., Connors T. A., Lovell D., Sparrow S. An athymic nude mutation in the rat. Nature. 1978 Jul 27;274(5669):365–366. doi: 10.1038/274365a0. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons R. C., Garrod E. M., Garnett I. Immunological responses following early embryonic surgical bursectomy. Cell Immunol. 1973 Dec;9(3):377–383. doi: 10.1016/0008-8749(73)90052-x. [DOI] [PubMed] [Google Scholar]
- Kowalski W. J., Malkinson M., Leslie G. A., Small P. A. The secretory immunological system of the fowl. VI. The effect of chemical bursectomy on immunoglobulin concentration in tears. Immunology. 1978 Apr;34(4):663–667. [PMC free article] [PubMed] [Google Scholar]
- LONG P. L. A study of Eimeria maxima Tyzzer, 1929, a coccidium of the fowl (Gallus gallus). Ann Trop Med Parasitol. 1959 Sep;53:325–333. doi: 10.1080/00034983.1959.11685930. [DOI] [PubMed] [Google Scholar]
- Leathem W. D., Burns W. C. Effects of the immune chicken on the endogenous stages of Eimeria tenella. J Parasitol. 1967 Feb;53(1):180–185. [PubMed] [Google Scholar]
- Lee L. F. In vitro assay of mitogen stimulation of avian peripheral lymphocytes. Avian Dis. 1974 Oct-Dec;18(4):602–609. [PubMed] [Google Scholar]
- Lemaitre-Coelho I., Jackson G. D., Vaerman J. P. Relevance of biliary IgA antibodies in rat intestinal immunity. Scand J Immunol. 1978;8(5):459–463. doi: 10.1111/j.1365-3083.1978.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Lerman S. P., Weidanz W. P. The effect of cyclophosphamide on the ontogeny of the humoral immune response in chickens. J Immunol. 1970 Sep;105(3):614–619. [PubMed] [Google Scholar]
- Lerner K. G., Glick B., McDuffie F. C. Role of the bursa of Fabricius in IgG and IgM production in the chicken: evidence for the role of a non-bursal site in the development of humoral immunity. J Immunol. 1971 Aug;107(2):493–503. [PubMed] [Google Scholar]
- Linna T. J., Frommel D., Good R. A. Effects of early cyclophosphamide treatment on the development of lymphoid organs and immunological functions in the chickens. Int Arch Allergy Appl Immunol. 1972;42(1):20–39. doi: 10.1159/000230590. [DOI] [PubMed] [Google Scholar]
- Long P. L., Millard B. J., Joyner L. P., Norton C. C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976 Jul-Sep;6(3):201–217. [PubMed] [Google Scholar]
- Long P. L. Observations on the oocyst production and viability of Eimeria mivati and E. tenella in the chorioallantois of chicken embryos incubated at different temperatures. Z Parasitenkd. 1972;39(1):27–37. doi: 10.1007/BF00329218. [DOI] [PubMed] [Google Scholar]
- Long P. L. Studies on Eimeria mivati in chickens and a comparison with Eimeria acervulina. J Comp Pathol. 1967 Jul;77(3):315–325. doi: 10.1016/0021-9975(67)90041-2. [DOI] [PubMed] [Google Scholar]
- Mesfin G. M., Bellamy J. E. Thymic dependence of immunity to Eimeria falciformis var. pragensis in mice. Infect Immun. 1979 Feb;23(2):460–464. doi: 10.1128/iai.23.2.460-464.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers P., Qualtiere L. F. The efficacy of chemical bursectomy in chickens with congenital leukosis-virus infection. Immunology. 1976 Oct;31(4):527–532. [PMC free article] [PubMed] [Google Scholar]
- Perey D. Y., Biennenstock J. Effects of bursectomy and thymectomy on ontogeny of fowl IgA, IgG, and IgM. J Immunol. 1973 Aug;111(2):633–637. [PubMed] [Google Scholar]
- Pritchard H., Riddaway J., Micklem H. S. Immune responses in congenitally thymus-less mice. II. Quantitative studies of serum immunoglobulins, the antibody response to sheep erythrocytes, and the effect of thymus allografting. Clin Exp Immunol. 1973 Jan;13(1):125–138. [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidiosis: stages of the life-cycle of Eimeria maxima which induce, and are affected by, the response of the host. Parasitology. 1976 Aug;73(1):25–57. doi: 10.1017/s0031182000051295. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Immunity to coccidiosis: maternal transfer in Eimeria maxima infections. Parasitology. 1972 Oct;65(2):273–282. doi: 10.1017/s0031182000045054. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Immunity to coccidiosis: protective effect of transferred serum in Eimeria maxima infections. Parasitology. 1971 Feb;62(1):11–25. doi: 10.1017/s0031182000071249. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Infections with Eimeria maxima and Eimeria acervulina in the fowl: effect of previous infection with the heterologous organism on oocyst production. Parasitology. 1975 Apr;70(2):263–271. doi: 10.1017/s0031182000049726. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Long P. L. Resistance to Eimeria infections in the chicken: the effects of thymectomy, bursectomy, whole body irradiation and cortisone treatment. Parasitology. 1970 Apr;60(2):291–299. doi: 10.1017/s0031182000078124. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Ogilvie B. M., Hesketh P., Festing M. F. Failure of nude (athymic) rats to become resistant to reinfection with the intestinal coccidian parasite Eimeria nieschulzi or the nematode Nippostrongylus brasiliensis. Parasite Immunol. 1979 Summer;1(2):125–132. doi: 10.1111/j.1365-3024.1979.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Szenberg A. Functional and morphological observations on the effect of cyclophosphamide on the immune response of the chicken. Aust J Exp Biol Med Sci. 1974 Dec;52(6):873–885. doi: 10.1038/icb.1974.87. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. Characteristics of soluble T-cell derived factors(s) which can induce non-immune murine macrophages to exert anti-Toxoplasma activity. Z Immunitatsforsch Immunobiol. 1978 Jun;154(3):226–242. [PubMed] [Google Scholar]
- Sharma J. M., Lee L. F. Suppressive effect of cyclophosphamide on the T-cell system in chickens. Infect Immun. 1977 Jul;17(1):227–230. doi: 10.1128/iai.17.1.227-230.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley M. W. Enzyme variation in Eimeria species of the chicken. Parasitology. 1975 Dec;71(3):369–376. doi: 10.1017/s0031182000047144. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Wagenbach G. E. Purification of Eimeria tenella sporozoites with glass bead columns. J Parasitol. 1969 Aug;55(4):833–838. [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. T and B cells in the transfer of immunity against Trichinella spiralis in mice. Immunology. 1979 May;37(1):103–109. [PMC free article] [PubMed] [Google Scholar]