Summary
Neutrophil (polymorphonuclear leucocytes; PMN) transmigration across mucosal surfaces contributes to dysfunction of epithelial barrier properties, a characteristic underlying many mucosal inflammatory diseases. Using Salmonella enterica serovar Typhimurium (S. Typhimurium) as a prototypic proinflammatory insult, we have previously reported that the eicosanoid hepoxilin A3 (HXA3), an endogenous product of 12-lipoxygenase (12-LOX) activity, is secreted from the apical surface of the intestinal epithelium to establish a chemotactic gradient that guides PMN across the epithelial surface. Since little is known regarding the molecular mechanisms that regulate 12-LOX during S. Typhimurium infection, we investigated this pathway. We found that expression of phospholipid glutathione peroxidase (GPX4), which is known to have an inhibitory effect on 12-LOX activity, is significantly decreased at both the mRNA and protein level during infection with S. Typhimurium. Moreover, employing intestinal epithelial cell monolayers expressing siRNA against GPX4 mRNA, S. Typhimurium-induced PMN migration was significantly increased compared with the non-specific siRNA control cells. Conversely, in cells engineered to overexpress GPX4, S. Typhimurium-induced PMN migration was significantly decreased, which is consistent with the finding that partial depletion of GPX4 by RNAi resulted in a significant increase in HXA3 secretion during S. Typhimurium infection. Mechanistically, although we found Salmonella entry not to be required for the induced decrease in GPX4, the secreted effector, SipA, which is known to induce epithelial responses leading to stimulation of HXA3, governed the decrease in GPX4 in a process that does not lead to an overall increase in the levels of ROS. Taken together, these results suggest that S. Typhimurium induces apical secretion of HXA3 by decreasing the expression of phospholipid GPX, which in turn leads to an increase in 12-LOX activity, and hence HXA3 synthesis.
Introduction
Bacterial pathogens continually confront epithelial barriers of the body, such as those of the gastrointestinal, respiratory and reproductive tracts. Although mucosal surfaces are generally impermeable to most foreign entities, many microorganisms have developed sophisticated strategies to breach or alter this barrier (Viswanathan et al., 2009). Overall, microbial pathogens have evolved the capacity to intricately interface with host cells using a diversity of approaches that typically involve the exchange of biochemical signals; the net result of which is often the triggering of a host proinflammatory response. At the forefront of this inflammatory response is the infiltration of neutrophils (polymorphonuclear leucocytes; PMN) to the site of bacterial insult (Kolaczkowska and Kubes, 2013). PMN represent a class of crucial white cells needed to defend the host from such pathogenic injury, and thus, the accumulation of PMN at inflamed sites represents a characteristic feature of the innate host response. However, the mechanisms by which PMN eradicate offending bacteria are non-specific and can lead to tissue damage which, if excessive, contributes to the pathology of the disease.
Despite the critical nature of PMN transepithelial migration in pathogen elimination and disease pathophysiology, the process that controls PMN recruitment across mucosal surfaces is not well understood. Using Salmonella enterica serovar Typhimurium (S. Typhimurium) as a prototypical enteric pathogen that orchestrates the movement of PMNs from the bloodstream to luminal sites of the inflammatory insult (Day et al., 1978), we have begun to dissect the molecular mechanisms that govern PMN movement across the intestinal epithelium. Our prior studies (McCormick et al., 1993; 1995a,b; 1998,,,), in addition to the contribution of others (Kucharzik et al., 2005), have championed the concept that pathogen stimulation of epithelial cells prompts the polarized release of distinct PMN chemoattractants. More specifically, such studies have shown that the chemokine IL-8 is released from the basolateral surface of epithelial cells, where it facilitates PMN escape from the bloodstream and eventual accumulation within the submucosal space (Sansonetti et al., 1999). In concert, the potent PMN chemoattractant, hepoxilin A3 (HXA3), is secreted from the apical surface of the epithelial barrier where it establishes a chemotactic gradient, which guides PMN from the submucosa across the epithelium, constituting the final step in the process of PMN recruitment to the mucosal lumen (Mrsny et al., 2004).
HXA3 is a hydroxy epoxide metabolite formed from the intermediate 12S-hydroperoxyeicosa-5Z,8Z,10E,14Z-tetraenoic acid (12S-HpETE), the main product of 12S-lipoxygenase activity (Pace-Asciak et al., 1999). Hepoxilin synthase activity, responsible for the conversion of 12-S-HETE to HXA3, has been confirmed for human platelet 12-lipoxygenase (12-LOX), human epidermal LOX type 3 and murine and porcine 12/15-LOX (Nigam et al., 2004). Although a crucial role for HXA3 in PMN-mediated events in both intestinal and pulmonary inflammation has been demonstrated (Mrsny et al., 2004), the molecular mechanisms by which 12-LOX expression and activity are regulated during S. Typhimurium infection have yet to be elucidated.
Members of the glutathione peroxidase (GPX) family of oxido-reductase enzymes play an important role in the regulation of 12-LOX activity (Schneider et al., 2010). These proteins are known to catalyse the removal of hydroperoxides, utilizing glutathione as a reducing agent. The central function of glutathione peroxidases is in detoxification, where these enzymes reduce reactive oxygen species (ROS), generated during cellular metabolism and inflammatory responses, to water and oxygen (Fridovich, 1978). In humans, four family members of these selenium-dependent enzymes have been identified (GPX1–4) (Brigelius-Flohe et al., 1997), and all members are able to reduce hydroperoxides, alkyl peroxides, and fatty acid hydroperoxides. However, GPX4 (also called PHGPX or phospholipid GPX) is the only member demonstrated to interact with cell membranes and reduce membrane phospholipid hydroperoxides. Herein, we test the hypothesis that Salmonella Typhimurium regulates 12-LOX activity by modulating the levels and activity of the glutathione peroxidases.
Results
Salmonella Typhimurium infection decreases the expression of glutathione peroxidase 1 and 4 (GPX1 and 4) in T84 intestinal epithelial cells
It has previously been established that glutathione peroxidases, specifically GPX4, function as negative regulators of 12-LOX, decreasing 12-LOX activity and lipid peroxidation (Schneider et al., 2010). We therefore sought to investigate the dynamics of 12-LOX expression in relation to GPX expression during S. Typhimurium infection. Infection of T84 intestinal epithelial cells with wild type S. Typhimurium results in an approximately twofold increase in 12-LOX expression at the message (mRNA) level (Fig. 1A) and a significant increase at the protein level (Fig. 1D). We next examined the effects of S. Typhimurium infection on the expression levels of the glutathione peroxidases over a three-hour time-course. RT-PCR analysis revealed that S. Typhimurium infection results in a significant time-dependent decrease in both GPX1 and GPX4 mRNA expression (Fig. 1B and C). Such a decrease in GPX mRNA expression also correlated with a reduction in protein expression for both GPX1 and GPX4 (Fig. 1E and F) respectively. We also examined the expression of GPX2 (the gut-specific GPX) but found no significant changes during S. Typhimurium infection (data not shown).
Figure 1.
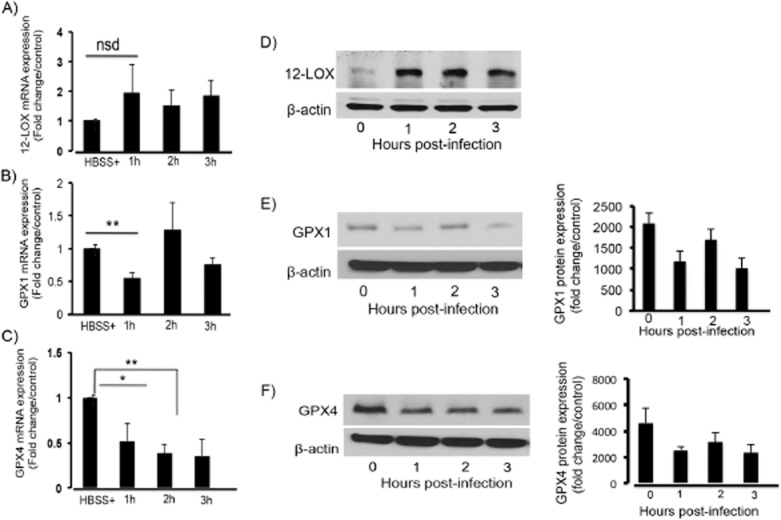
Salmonella Typhimurium infection decreases mRNA and protein expression of GPX 1 and GPX 4. T84 intestinal epithelial cells were infected apically with S. Typhimurium SL1344 for the indicated time points. RNA was extracted, reverse transcribed to cDNA and used for RT-PCR for (A) 12-LOX, (B) GPX1 and (C) GPX4. Results indicate that infection of cells with S. Typhimurium results in a significant decrease in the mRNA expression of GPX1 and GPX4 and an increase in 12-LOX mRNA expression. Gene expression was normalized against the 18S ribosomal subunit. Results are expressed as fold change relative to control. Whole cell lysates were also extracted, and the proteins normalized and Western blotted for (D) 12S-LOX, (E) GPX1 and (F) GPX4. Results indicate that infection of cells with S. Typhimurium results in a decrease in GPX1 and GPX4 protein expression and an increase in 12-LOX protein expression. Right panels are the densitometry following protein quantification of GPX 1 and GPX 4. Data shown are a representation of one of n = 3 (The symbols ‘*’ and ‘**’ indicate statistical significance, using the Student's t-test; P < 0.05, n = 3).
To determine if the decreased expression of either GPX1 or GPX4 during infection with S. Typhimurium was accompanied by a reduction in total GPX cellular activity, polarized intestinal cell monolayers were infected with wild-type S. Typhimurium. Following a three-hour time-course, infected lysates were extracted and total cell GPX activity was determined. As shown in Fig. 2, S. Typhimurium infection resulted in an approximately 20% decrease in total cell GPX activity as early as 2 h post infection (Fig. 2). We also observed an early increase in total GPX activity. Since bacterial infection has been shown to induce cellular oxidative stress, which triggers the induction of cellular anti-oxidant machinery (Rodenburg et al., 2007), this could account for the 20% increase in total cell GPX activity following the first hour of infection with S. Typhimurium (Fig. 2).
Figure 2.
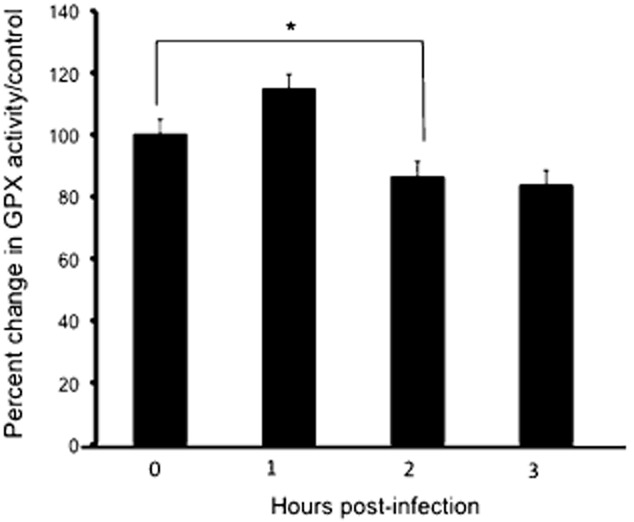
S. Typhimurium infection decreases total cell GPX activity. T84 intestinal epithelial cells maintained in 6 well plates were infected with S. Typhimurium SL1344 or left uninfected for the indicated time points. Cells were lysed with a GPX assay lysis buffer, and the cell lysates normalized and used in a GPX activity assay (Cayman Chemical Company). Results show that infection induces ∼ 20% decrease in total cell GPX activity 2 and 3 h after infection. Total GPX activity increases by ∼ 20% after the first hour of infection (The asterisk ‘*’ indicates statistical significance, using the Student's t-test; P < 0.05, n = 3).
Inhibition of total cell GPX activity with the pan-GPX inhibitor sodium iodoacetate augments S. Typhimurium-induced transepithelial migration of PMNs
We previously reported that during S. Typhimurium infection inhibition of 12-LOX activity using baicalein (a 12-LOX inhibitor) significantly diminished HXA3 bioactivity and attenuated PMN transpeithelial migration (Mrsny et al., 2004). Since GPX negatively regulates lipoxygenases, we reasoned that inhibition of GPX during S. Typhimurium infection might promote an increase in 12-LOX activity, which may in turn lead to an increase in HXA3 synthesis and PMN transepithelial migration. We therefore examined the effect of inhibition of whole cell GPX activity on S. Typhimurium-induced PMN transepithelial migration. We found that infection of cells with wild type S. Typhimurium in the presence of iodoacetate (a pan GPX inhibitor) significantly enhanced transepithelial migration of PMNs (by ∼ 80%) as compared with the untreated but infected control cell monolayers (Fig. 3A). However, GPX inhibition alone, in the absence of Salmonella infection, did not induce any significant change in PMN transepithelial migration.
Figure 3.
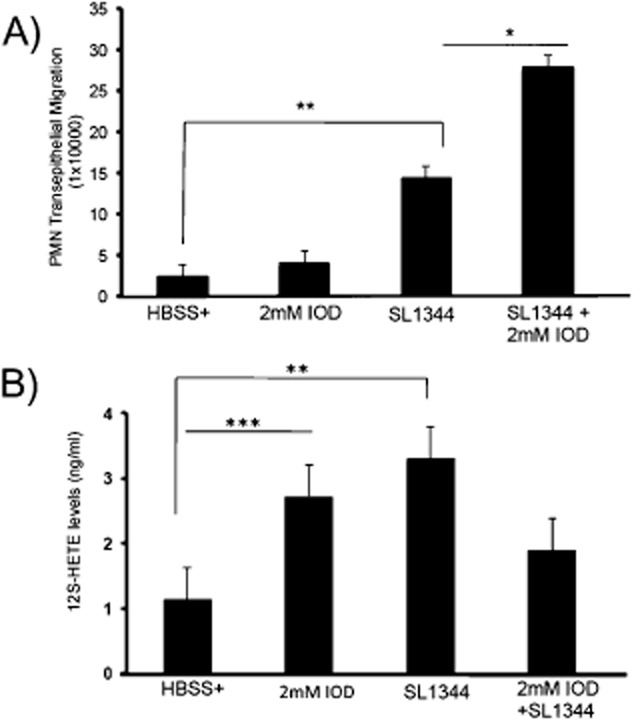
Inhibition of total cell GPX activity significantly augments transepithelial migration of PMNs. Intestinal epithelial cells maintained on transwells were treated with 2 mM of sodium iodoacetate (IOD; a pan GPX inhibitor) or HBSS+ (negative control) for 10 min, and then infected with S. Typhimurium SL1344 in the absence or presence of IOD, or treated with IOD alone or HBSS+. PMN transmigration was then performed and we show that inhibition of total cell GPX with IOD resulted in a significant increase in SL1344-induced PMN transmigration compared with SL1344 infection in the absence of IOD. (B). Cells were treated as above, supernatants were collected 4 h after infection, and lipids in the supernatants were semi-purified by column chromatography and eluted with methanol. Samples were dried, resuspended in 300 μl of HBSS+ and used to carry out 12S-HETE ELISA. Infection of cells with S. Typhimurium and IOD treatment resulted in a significant increase in 12S-HETE levels compared with uninfected cells (The symbols ‘*’, ‘**’ and ‘***’ indicate statistical significance, using the Student's t-test; P < 0.05, n = 3).
Since this result indicates that suppression of GPX augments S. Typimurium-induced PMN transepithelial migration, we next considered whether the 12-LOX pathway was concurrently activated. As an indirect measurement of 12-LOX activity, we performed an ELISA assay to quantify the amount of 12S-HETE secreted during S. Typhimurium infection. 12S-HETE is a downstream metabolite of lipid peroxidation by 12-LOX. As shown in Fig. 3B, infection of cells with S. Typhimurium or treatment of cells with the GPX inhibitor sodium iodoacetate itself produced a significant increase in 12S-HETE, indicating that both S. Typhimurium infection and GPX inhibition activates the 12-LOX pathway. However, infection of the cells with S. Typhmurium in the presence of iodocatetate only caused a modest increase in 12S-HETE levels (Fig. 3B) and was lower than either iodocatate treatment or S. Typhimurium infection alone.
Partial knockdown of GPX4 with siRNA results in a significant increase in PMN transmigration during S. Typhimurium infection
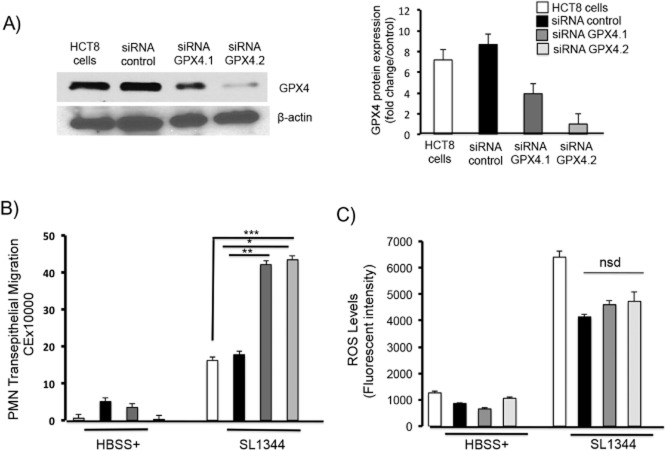
To identify the GPX isoform(s) critical for driving the signalling cascade that prompts the ability of S. Typhimurium to induce PMN transepithelial migration, we initially focused on GPX4. GPX4 has been reported to play a pivotal role in regulating 12-LOX activity by decreasing the cellular lipid hydroperoxide tone (Zafiriou et al., 2007). Moreover, GPX4 localizes to the lipid bilayer of cell membranes, thus placing the enzyme in close proximity to the substrates of 12-LOX catalysis (namely, arachidonic acid; Thomas et al., 1990).
To examine the role played by GPX4 in regulating 12-LOX activity and PMN transmigration during S. Typhimurium infection, we used an RNA interference (RNAi)-based strategy to generate two siRNAs against GPX4 (see Experimental procedures). To knockdown GPX4, we used HCT8 cells because unlike T84 intestinal epithelial cells, HCT8 cells are easily transfectable. As shown in Fig. 4A, each of the partial knockdowns against GPX4 significantly reduced the GPX4 protein expression level compared with that of the vector transfected control cells. Moreover, infection of the GPX4 knockdown cells with S. Typhimurium markedly enhanced the ability of the pathogen to induce PMN transmigration as compared with infection of the vector control cells (Fig. 4B). Since GPX plays a critical role in maintaining cellular reactive oxygen species (ROS) levels within homeostatic limits, it is possible that suppression of GPX4 itself may produce a burst in oxidative stress during infection that might impact the cellular inflammatory response in general. However, we found no significant difference in ROS production between the vector control transfected cells and the GPX4 knockdown cells either with or without infection (Fig. 4C).
Figure 4.
Partial knockdown of GPX4 with siRNA results in a significant increase in PMN transmigration during S. Typhmurium infection.
A. HCT8 intestinal epithelial cells were stably transfected with two GPX4 siRNAs, which we generated and Western blotted for GPX4 expression. Results indicate that both GPX4 siRNAs induced a significant decrease in GPX4 expression relative to untransfected cells or vector control cells. Densitometry is indicated on the right hand side.
B. The knockdown cells and control cells were maintained on transwells, then infected with S. Typhimurium SL1344 or left uninfected for 1h before being used for a PMN transmigration assay. Partial knockdown of GPX4 using siRNA resulted in a significant increase in transepithelial migration of neutrophils compared non-transfected and vector control cells.
C. Salmonella infection induces a significant increase in reactive oxygen species (ROS) production in both non-transfected and vector control and in GPX4 knockdown cells. The levels of ROS production in control and GPX4 knockdown cells following SL1344 infection were not significantly different (The symbols ‘*’, ‘**’ and ‘***’ indicate statistical significance, using the Student's t-test; P < 0.05, n = 3). Key: white bars = untransfected cells, black bars = control siRNA, dark grey bars = siRNA GPX4.2, light grey bars = siRNA GPX4.1.
Thus far, we have shown that infection of the GPX4 knockdown cells with S. Typhimurium significantly enhances the ability of this organism to induce PMN transepithelial migration. Conversely, to determine whether overexpression of GPX4 reduces the ability of S. Typhimurium to induce PMN transmigration, we stably transfected HCT8 cells with the pCMV6-GPX4 plasmid or the pCMV6 empty vector plasmid (purchased from OriGene). As shown in Fig. 5A, overexpression of GPX4 resulted in approximately a 25% increase in baseline GPX4 protein expression compared with the vector control-transfected cells (Fig. 5A, right panel), and this correlated with a significant decrease in the ability of S. Typhimurium to induce PMN transmigration (Fig. 5B). One unforeseen observation was that transfection of cells with the vector control itself resulted in a modest increase in GPX4 protein expression. Moreover, in keeping with the modest increase in GPX4 expression exhibited by the vector control cells, there was also a correlative decrease in the ability of S. Typhimurium to induce PMN transepithelial migration. We presume that the extra energy required for the cell to maintain the plasmid yields an increase in the baseline levels of ROS. Regardless, these results suggest that the ability of S. Typhimurium to induce PMN transepithelial migration is inversely related to/dependent on the amount of intracellular GPX4.
Figure 5.
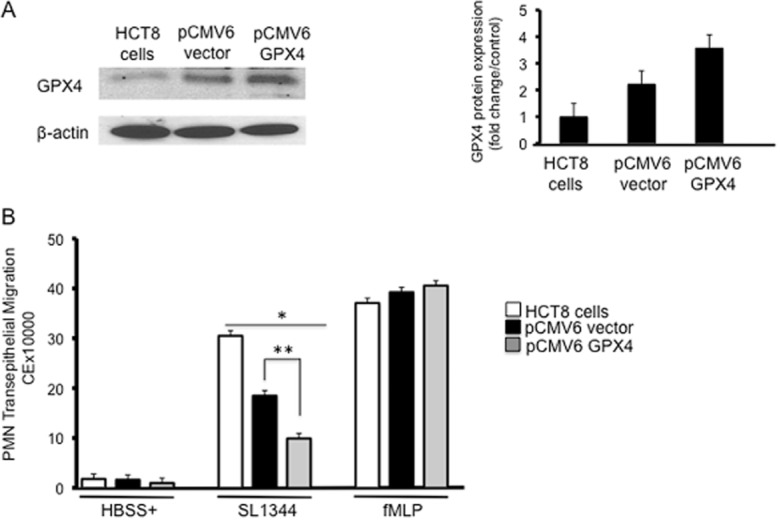
Overexpression of GPX4 decreases PMN transmigration relative to control cells.
A. HCT8 cells were stably transfected with either empty vector pCMV6 or pCMV6-GPX4 and Western blotted for the expression of GPX4. Overexpression of GPX4 resulted in ∼ 30% increase in GPX4 protein expression, and pCMV6 vector also increased GPX4 expression relative to untransfected cells but less so than pCMV6-GPX4 transfected cells (densitometry on right panel).
B. The cells were infected with S. Typhimurium SL1344, followed by PMN transmigration. Cells overexpressing GPX4 show a significant decrease in PMN transmigration compared with untransfected and pCMV6 control vector transfected cells. pCMV6 vector expressing cells which also express relatively higher levels of GPX4 compared with control cells show a significant decrease in PMN transmigration. fMLP was used as a positive control and GPX4 overexpression had no effects on fMLP-induced PMN transmigration (The symbols ‘*’ and ‘**’ indicate statistical significance, using the Student's t-test; P < 0.05, n = 3). Key: white bars = untransfected cells, black bars = pCMV6 vector, grey bars = pCMV6-GPX4
Bacterial entry is not required but the SipA effector is sufficient for decreased GPX expression during S. Typhimurium infection
To begin to probe the molecular mechanism by which S. Typhimurium induces the decreased expression of GPX4, we first examined whether bacterial cell entry was required. T84 intestinal epithelial cells monolayers were infected with either wild-type S. Typhimurium (SL1344) or the invasion deficient mutant (HilA mutant – VV341) over a three-hour time-course, cell lysates were then harvested and immunoblotted for GPX4 protein expression. As shown in Fig. 6A, we observed that the isogenic invasion-defective strain, VV341, induced a decrease in GPX4 protein expression to the same extent as infection wild type S. Typhimurium. In addition, we inhibited bacterial cell entry by treating polarized monolayers of T84 cells with 5 μg ml−1 Cytochalasin D, an inhibitor that prevents actin polymerization (Casella et al., 1981; Gewirtz et al., 1999), and followed GPX4 protein expression over a three-hour time-course by Western blot analysis. Consistent with the genetic-based approach, we determined that pharmacological prevention of Salmonella entry also failed to inhibit the induced decrease of GPX4 protein expression (Fig. 6B). Taken together, these data suggest that bacterial entry is not required for the S. Typhimurium induced decrease in GPX4 protein levels.
Figure 6.
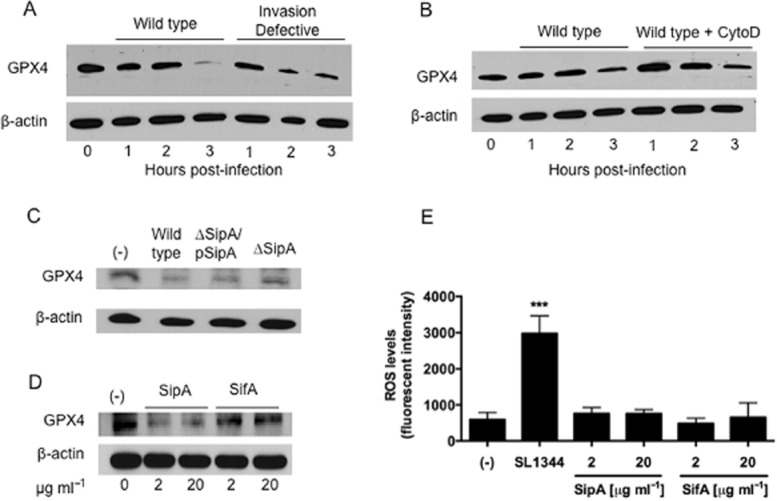
S. Typhimurium-induced decreased GPX4 expression is through the secreted effector SipA and does not require bacterial entry.
A. HCT-8 intestinal epithelial cells were infected apically with wild-type S. Typhimurium SL1344 or the isogenic invasion-defective strain VV341 for the indicated time points. Whole cell lysates were then extracted and the proteins normalized and Western blotted for GPX4.
B. HCT-8 cells were treated with 5 μg ml−1 of cytochalasin D or HBSS+ (negative control) for 45 min, infected with wild-type SL1344 for the indicated time points, and then analysed for GPX4 expression as above.
C. HCT-8 cells were infected with wild-type SL1344, the isogenic SipA-deficient strain EE633 (ΔSipA) or the SipA-complemented strain AJK63 (ΔSipA/pSipA) for 1 h, and then analysed for GPX4 expression.
D. HCT-8 cells were exposed on their apical surface to exogenous S. Typhimurium effectors SipA-HA or GST-SifA for 1 h, and then analysed for GPX4 expression.
E. SipA does not induce an increase in overall levels of ROS. The levels of ROS production in untreated cells and cells exposed to SipA or SifA for 90 min were not significantly different (The symbol ‘***’ indicates statistical significance, using the Student's t-test; P < 0.01).
Our prior results indicate that the secretion of SipA (a Salmonella Type III effector protein) and the ability of SipA to induce epithelial cell responses that lead to stimulation of HXA3 are not coupled to its direct delivery into epithelial cells from bacterial cells (Gewirtz et al., 1999; Lee et al., 2000; Mrsny et al., 2004; Wall et al., 2007; Srikanth et al., 2010). These results are consistent with our observation that infection of the apical surface of polarized intestinal epithelial cells with a S. Typhimurium SipA mutant strain (ΔSipA) is less able to induce a decrease in GPX4 expression compared with its isogenic wild type parent. However, complementation of the SipA mutant with a plasmid expressing SipA (ΔSipA/pSipA) rescued the ability of the organism to induce a decrease in the expression of GPX4 to the same extent as the wild type strain (Fig. 6C).
Furthermore, since our data suggest that an extracellular form of SipA is an important intermediate that mediates the epithelial cell responses triggered by S. Typhimurium (Lee et al., 2000; Srikanth et al., 2010), we hypothesized that purified SipA added to the apical surface of polarized T84 cell monolayers might be sufficient to trigger the decreased protein expression of GPX4. As shown in Fig. 6D, exposure of the apical surface of epithelial cells to 20 μg ml−1 recombinant SipA-HA epitope-tagged protein elicits a decrease in GPX4 protein expression that is equivalent to that induced by wild-type S. Typhimurium. This effect was found be dose-dependent and specific given that similar exposure to 20 μg ml−1 of an irrelevant Type III secretion system S. Typhimurium effector protein GST-SifA failed to induce a decrease in GPX4 protein expression (Fig. 6D). Such exogenous addition of SipA, however, did not induce an increase in overall levels of ROS (Fig. 6E), suggesting that additional bacterial factors are required to induce ROS production and that GPX4 may be a direct target of SipA. However, the precise mechanism(s) remain unknown.
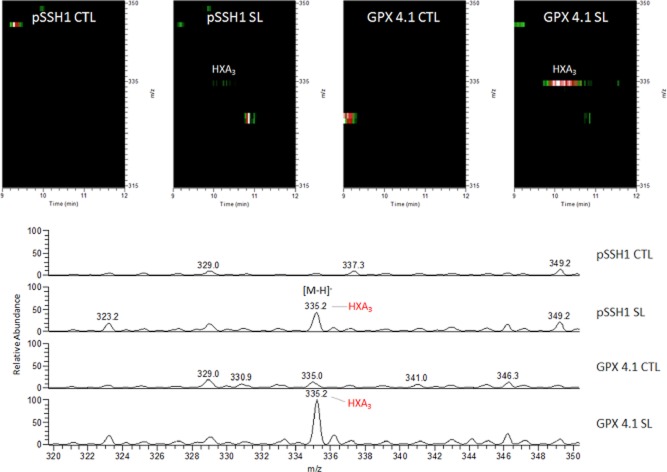
Partial knockdown of GPX4 results in a significant increase in HXA3 secretion during S. Typhimurium infection
Based on the results thus far, we speculate that S. Typhimurium might be regulating 12-LOX activity, and hence HXA3 secretion and PMN transmigration, by decreasing cellular levels of GPX4. Thus, we next sought to determine the levels of HXA3 under conditions of GPX4 suppression. Using Liquid Chromatography/Mass Spectrometry (LC/MS) and Liquid chromatography mass spectrometry mass spectrometry (LC/MS/MS), we quantified HXA3 levels of semi-purified lipid samples from supernatants of uninfected cells or cells infected with S. Typhimurium. As shown in Fig. 7A, our LC/MS/MS analysis was able to detect HXA3 (shown on the extraction ion maps). We also demonstrated that infection with S. Typhimurium resulted in a significant increase in HXA3 secretion (Fig. 7B). We further showed that lipid extracts from GPX4 knockdown cells secreted significantly higher amounts of HXA3 compared with vector control cells (Fig. 6B). These results indicate that partial knockdown of GPX4 induces an activation of the 12-LOX pathway and HXA3 secretion during S. Typhimurium infection.
Figure 7.
Hepoxilin A3 secretion is increased in Salmonella infected GPX knockdown cells. Extracted ion maps (A) depict the lipid ions detected in the mass-to-charge ratio (m/z) range from 315 to 350 m/z and time range from 9 to 12 min. Colours represent the relative abundance of the detected lipid signals: white equals 100%, red 80%, green 60%. Mass spectra (B) of the lipid ions detected in the m/z range from 333.5 to 335.5 m/z and time range ∼ 9.5 to 10.5 min. Mass spectra were normalized to the largest peak present across the samples. pSSH1 CTL: vector control transfected cells uninfected; pSSH1 SL: vector control transfected cells infected with Salmonella; GPX 4.1 CTL: GPX 4.1 knockdown cells control uninfected; GPX 4.1 SL: GPX 4.1 knockdown cells infected with Salmonella. LC/MS/MS analyses were done in negative ion mode with a mass accuracy of ± 0.1–0.2 atomic mass units (amu). Hepoxilin A3 (HXA3) identification and quantification was done as described in Experimental procedures. Data shown are a representation of one of n = 3.
Discussion
A major consequence of acute infection of the intestinal epithelium is induction of a pro-inflammatory response that results in the overzealous recruitment and accumulation of PMN at the site of infection (Day et al., 1978). In this role, PMN are signalled to navigate to the site of infection and are charged with the task of pathogen eradication (Kolaczkowska and Kubes, 2013). This is achieved through a series of highly co-ordinated responses culminating in ingestion (phagocytosis) and killing of invading microbes. Such functions provide the first line of host defence and are usually performed without injury to host tissues. However, under pathological circumstances, PMN recruitment lacking resolution contributes directly to intestinal injury and dysfunction. Therefore, one of the key challenges in treating active states of inflammation lies in attenuating the inappropriate influx of PMN without compromising the ability of the patient to fight normal infections.
It is becoming increasingly evident that 12-LOX can be regulated at sites of mucosal inflammation. Shannon et al. initially observed that in the healthy colonic mucosal epithelium cells do not express 12-LOX, whereas in patients with inflammatory bowel disease the colonic tissue is not only actively involved in the disease but also expresses 12-LOX in mucosal epithelial cells and displays an increase in 12-LOX enzymatic activity (Shannon et al., 1993). Not only was this study the first to document that 12-LOX participates in colonic epithelial function, it also provided the first in situ evidence for selective increase in epithelial 12-LOX in inflammatory disease. Moreover, our previous studies have shown that inhibition of the 12-LOX pathway, which is required for the synthesis of HXA3, dramatically reduces PMN-mediated tissue trauma associated with enteric infection (Mrsny et al., 2004). Here, we show that S. Typhimurium governs 12-LOX activity by modulating the levels of glutathione peroxidases, especially GPX4. Based on these findings, we propose that S. Typhimurium infection triggers an initial increase in reactive oxygen species (ROS) (Fig. 4C), including lipid hydroperoxide, which consequently induces a reduction in the activity and expression of GPX4 (Huang et al., 2002). We further demonstrated that the secreted S. Typhimurium effector protein SipA was sufficient to induce decreased GPX4 protein expression (Fig. 6D) consistent with its previously identified function of stimulating processes that result in apical release of HXA3 (Mrsny et al., 2004; Pazos et al., 2008; Wall et al., 2007). As GPX4 inversely regulates the activity of 12/15 LOX, its reduced expression results in a corresponding increase in 12-LOX activity and apical HXA3 synthesis/secretion, which ultimately culminates in PMN transmigration (modelled in Fig. 8).
Figure 8.
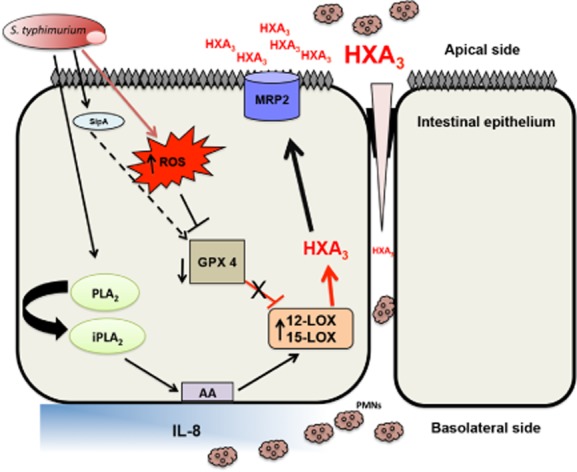
Working model of S. Typhimurium regulation of 12-LOX expression/activity and HXA3 synthesis during infection. Infection of intestinal epithelial cells with Salmonella Typhimurium induces an increase in reactive oxygen species (ROS) production, which induces a decrease in GPX4 expression and total cell GPX activity. Treatment of intestinal epithelial cells with the S. Typhimurium effector protein SipA also directly decreased GPX4 expression (dotted lines) through mechanisms yet to be elucidated. The decrease in GPX4 expression and activity results in the accumulation of phospholipid hydroperoxides thereby activating the 12-LOX pathway. Furthermore, S. Typhimurium infection also induces an increase in the expression of 12-LOX. These two events (decrease in GPX activity/expression and increase in 12-LOX expression) culminate in the activation of the 12-LOX pathway of arachidonic acid metabolism leading to the synthesis of HXA3. Synthesized HXA3 is then effluxed across the intestinal epithelial cell membrane by MRP2. Once in the lumen, HXA3 induces transepithelial migration of neutrophils. PLA2 is involved in the release of arachidonic acid from the cell membrane. AA = arachidonic acid, ROS = reactive oxygen species, PLA2 = phospholipase A2, HXA3 = hepoxilin A3, IL-8 = interleuken 8, MRP2 = multidrug resistant associated protein 2.
While all GPXs have the capacity to reduce hydroperoxides and fatty acid peroxides, only GPX4 has the ability to reduce phospholipid hydroperoxides and membrane phospholipids (Thomas et al., 1990). GPX4 has also been shown to play a regulatory role in arachidonic and linoleic metabolism by controlling the activities of 12-LOX and cycloxygenase (COX) (Huang et al., 1999). For example, since 12-LOX and COX require basal levels of lipid hydroperoxides for their activities, modulation of GPX4 directly influences the cellular lipid hydroperoxide tone, and hence, synthesis of their downstream metabolites, namely HXA3 and prostaglandins respectively. Finally, analogous to our studies, neuron-specific GPX4 knockout mice have been observed to develop reactive astrogliosis (neuroinflammation – notably inflammation of astrocytes) and increase lipid peroxidation as a result of increased activity of 12/15-LOX (Seiler et al., 2008). Inhibition of 12-LOX reversed the phenotype, and is in keeping with the role of GPX4 in controlling 12-LOX induced inflammation (Seiler et al., 2008).
In the case of S. Typhimurium, this enteric pathogen promoted a decrease in mRNA and protein expression of both GPX1 and GPX4, which may account for the burst in oxidative stress and reactive oxygen species observed during infection (Fig. C). These results are consistent with the findings of Mertha and colleagues who observed a substantial reduction in the enterocyte activities of GPX, catalase, glutathione reductase, and glyceraldehyde-6-phosphate dehydrogenase (all of which are anti-oxidant enzymes) using a rat model of S. Typhimurium infection (Mehta et al., 1998a). This group further noted that S. Typhimurium-induced intestinal infection is accompanied by an increased generation of ROS and lipid peroxidation at the site of the enterocyte membrane (Mehta et al., 1998a, b), which is presumably the result of a decrease in cellular anti-oxidant enzymes, most notably the glutathione peroxidases. Reinforcing this line of investigation, we provide new information demonstrating that S. Typhimurium manipulates the host cellular anti-oxidant machinery (specifically GPX4) to modulate 12-LOX activity, HXA3 synthesis, and ultimately PMN transmigration.
A key question emanating from these observations is the mechanism by which Salmonella induces the decreased GPX expression. Reactive oxygen species and certain cytokines, notably IL-4 and IL-13, are known to have an inverse relationship with regards to GPX4 expression (Schnurr et al., 1999; Huang et al., 2002). For example, treatment with IL-4, which increased levels of lipid hydroperoxides, accounted for a significant decrease in GPX4 activity/expression. IL-4, in particular, is produced during Salmonella infection and plays an important role in Salmonella pathology (Everest et al., 1997). However, whether it also plays a role in decreasing the activity and expression of GPX4 during Salmonella infection remains to be determined. Furthermore, it is also not known whether transcription factors that regulate GPX4 expression are altered during Salmonella infection.
We observed that bacterial entry is not required for the S. Typhimurium-induced decrease in GPX4 protein levels, but rather, recombinant SipA exposure to the apical surface of model intestinal epithelial cells was sufficient to elicit a decrease in GPX4 protein expression (Fig. 6). These results are consistent with our previous studies that show that the secretion of SipA and the ability of SipA to induce epithelial cells responses that lead to the synthesis of HXA3 are not coupled to its direct delivery into epithelial cells (Gewirtz et al., 1999; Lee et al., 2000; Mrsny et al., 2004; Wall et al., 2007; Srikanth et al., 2010). It is notable that such exogenous addition of SipA did not induce an increase in overall levels of ROS. Collectively, these observations suggest that additional Salmonella factors are required to stimulate ROS production, and that GPX4 might be a direct target of SipA. To our knowledge, this is the first report to show that GPX4 is a target of a bacterial type III secreted effector. In keeping with this concept, other studies have shown that the Salmonella type III effector protein, SlrP, functions as an E3 ubiqutin ligase for the mammalian antioxidant enzyme thioredoxin (Bernal-Bayard and Ramos-Morales, 2009). Analogous to SipA, SlrP was found to directly interact with thioredoxin, an interaction that resulted in a significant decrease in the activity of thioredoxin. Furthermore, both over expression of SlrP and Salmonella infection of Hela cells resulted in significant decrease in thioredoxin activity and induction of cell death (Bernal-Bayard and Ramos-Morales, 2009). Whether SipA functions to decrease GPX4 expression, and possibly activity, through similar mechanisms remains unknown and requires investigation.
We envisage that the central mechanism governing the ability of S. Typhimurium to evoke enteritis is by suppressing the expression of GPX4. These results are consistent with previous reports that have documented members of the GPX protein family, including GPX4, to have protective effects against pathogenic insults (Esworthy et al., 2001; 2003,). In addition, more recent studies indicate that GPX1 is protective against Influenza A virus-induced lung inflammation (Yatmaz et al., 2013), and thus underscores the critical role GPXs play in protection against pathogen-induced inflammation. GPXs have also been implicated in the development of murine ileocolitis (Esworthy et al., 2001; 2003; 2012,,). Indeed, double GPX1/2 knockout mice develop spontaneous colitis, which is characterized by infiltration of neutrophils and formation of crypt abscesses, (Esworthy et al., 2001; 2003,), consistent with the pathological features of S. Typhimurium-induced enteritis.
Finally, we also noted that both chemical depletion of all GPX activity (iodoacetate treatment) as well as GPX4 specific knockdown resulted in a significant increase in the release and synthesis of 12-LOX metabolites during S. Typhimurium infection (i.e. 12S-HETE and HXA3; Figs 3 and 7). However, infection of the cells with S. Typhimurium in the presence of iodocatetate (a pan GPX inhibitor) only caused a modest increase in 12S-HETE levels (Fig. 3B), which was lower than either iodoacetate treatment or S. Typhimurium infection alone. While counterintuitive, this result is consistent with studies showing GPX catalyses the reduction of 12S-HpETE to 12S-HETE (Shankaranarayanan et al., 2003).
In sum, we have found that S. Typhimurium regulates HXA3 synthesis and neutrophil transmigration by decreasing the expression and activity of GPX4, an enzyme that functions to downregulate the activity of 12-LOX. To our knowledge, this is the first study to show that bacteria can manipulate the host anti-oxidant system by decreasing the expression and activity of the GPX. Prior studies have only reported that infection increases oxidative stress and anti-oxidant enzymes activity as a response to ROS production. While only GPX1 and 2 have previously been linked to intestinal inflammatory pathologies, the identification of GPX4 adds a new player of the antioxidant protein family, important in understanding the pathophysiology gastrointestinal diseases. Thus, members of the cellular anti-oxidant machinery, specifically GPX4, may serve as potential therapeutic targets for the treatment of inflammatory disorders.
Experimental procedures
Reagents and antibodies
GPX1, GPX4 and 12-LOX antibodies were purchased from Abcam and R&D Systems. The pan GPX inhibitor (Sodium iodoactate), the mouse anti-β-actin antibody, N-formylmethionyl-leucyl-phenylalanine (fMLP) and cytochalasin D were purchased from Sigma (St Loius, MO). 12S-HETE ELISA kit was purchased from Enzo Life Sciences while the Glutathione Peroxidase Assay kit was purchased from Cayman Chemical Company (Ann Arbor, MI). GPX1, GPX4 and 12-LOX probes for RT-PCR were purchased from Qiagen while the GPX4 over expressing plasmid (Cat. No. SC324086, Gene Accession No. NM_002085.1) and pCMV6-A carrier vector (Cat. No. PS10020) were purchased from OriGene. Unless otherwise indicated all secondary antibodies used for Western blotting were purchased from Santa Cruz.
Cell culture
The human colon cancer-derived HCT8 (passage number 40–56) intestinal epithelial cell line was grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS and 100 units/ml Penicillin/Streptomycin. The human colon cancer-derived T84 (passage 57–77) intestinal epithelial cell line was grown in DMEM and Ham's F-12 medium (Invitrogen) supplemented with 7.5% FBS, 15 mM Hepes, 14 mM NaHCO3 and 100 units/ml Penicillin/Streptomycin. Both cell lines were maintained in tissue culture treated flasks (Costar, Cambridge, MA 3151) using the protocol described by McCormick et al. (1993). Briefly, monolayers were grown on polycarbonate filters (Costar 3412) and used 6–8 days after plating. Inverted monolayers (Costar 3421) were used for PMN transmigration assays. All transformed cell lines were HCT8-derived and were maintained in HCT8 cell media supplemented with 1 mg/ml G418 (Neomycin) for selection.
Bacterial strains and growth conditions
The S. Typhimurium strains used in this study were wild-type SL1344, the HilA mutant strain VV341, the SipA mutant strain EE633 (ΔSipA) and the corresponding SipA-complemented strain AJK63 (ΔSipA/pSipA) (Lee et al., 2000). S. Typhimurium cultures were grown in Luria Bertani broth (LB; Becton Dickinson, Sparks, MD) aerobically with agitation at 37°C for ∼ 6 h to reach stationary phase of growth (McCormick et al., 1993). Once stationary phase was reached, bacteria were diluted 1:1000 into fresh LB and incubated overnight (∼ 18 h) at 37°C without agitation. Bacteria from these cultures are in the late logarithmic phase of growth and correlate to 5–7 × 108 bacteria ml−1.
PMN transepithelial migration assay
The PMN transepithelial migration assay was performed as previously described with some modifications (Parkos et al., 1992; McCormick et al., 1993). Briefly, inverted polarized HCT8 cell monolayers seeded on 0.33 cm2 filters were apically infected with S. Typhimurium at a multiplicity of infection (moi) of 100:1 at 37°C for 1 h. Following infection, cells were washed and transferred to a new 24-well plate containing 1 ml HBSS+ in the bottom chamber (apical side). One hundred microlitres of HBSS+ was added on the basolateral surface of the monolayers followed by 20 μl of prepared PMN (1 × 106 PMN). The monolayers were incubated at 37°C for 2 h, after which the monolayers and non-migrating PMN were gently removed, leaving only those PMN that had migrated through the monolayer. As a positive control for PMN transmigration, imposed gradients of the potent chemoattractant fMLP were generated. Transmigration was quantified by assaying for the PMN azurophilic granule marker myeloperoxidase (MPO) as previously described (Parkos et al., 1992).
Purification of SipA-HA fusion protein
SipA-HA recombinant fusion was purified as previously described (Lee et al., 2000).
Purification of SifA-GST fusion protein
Escherichia coli BL21 (Fisher Scientific) expressing SifA-GST recombinant fusion protein was grown to an OD of 1.0 at 37°C and then induced with 1 mM IPTG for 12 h. Cell pellets were resuspended in 25 mM Tris pH 8, 150 mM NaCl, 3 mM DTT and 1 mM PMSF. The resuspensions were then lysed by sonication and clarified by centrifugation at 4°C for 1 h at 14 000 rpm. The supernatant was applied to a glutathione sepharose column with glutathione sepharose 4B affinity media. Unbound supernatant was washed free from the column using PBS. To elute the bound protein, reduced glutathione was added to the column. The elution was reapplied to the column and collected again. Protein purification was verified via SDS/PAGE and Western blotting. All materials used in the purification process were part of a GST Bulk Kit purchased from GE Healthcare.
Protein extraction for Western blotting
To generate whole cell lysates, cells were lysed in accordance with Kohler et al. (2007). Once lysed, the cells were passed through a 25-gauge needle five times prior to centrifuging at 12 000 rpm for 5 min. Following cell lysis, the proteins were normalized, separated by SDS-PAGE gels and immunoblotted with the respective antibodies accordingly.
Messenger RNA extraction, cDNA synthesis and real-time PCR
T84 intestinal epithelial cells maintained on membrane filters were infected apically over a time-course before RNA was extracted using an RNAeasy Mini Kit (Qiagen, Valencia, CA, USA) according to manufacturer's instructions. Briefly, cells were scrapped with RNA lysis buffer and homogenized by spinning through a Qiashredder. The homogenized lysate was then mixed with one volume of 70% ethanol then transferred to a RNeasy spin column placed in a 2 ml collection tube (all supplied with the kit). Following a series of washes, the mRNA was eluted with RNase free water from the columns and subsequently quantified using a DN 1000-Spectrophotometer (Nanodrop) to determine the RNA concentration. One microgram of the RNA was used to generate complementary DNA (cDNA) using a first strand cDNA synthesis kit from Applied Biosystems. Briefly, the master mix was prepared to which 1 μg of RNA was added and loaded in a C1000 Thermo Cycler PCR machine (BIORAD). cDNA was generated using the following cycles: 42°C for 15 min, 99°C for 5 min and 5°C for 5 min. The generated cDNA together with the Gene specific primers (probes) were then used for RT-PCR. The samples were prepared and loaded in a 96-well plate, and RNA quantified by RT-PCR using the following cycles: 95°C for 10 min followed by 40 cycles of (95°C, 15 s; and 60°C, 30 s). Results are expressed as fold change relative to control. The 18S ribosomal subunit was used as an internal control.
Glutathione peroxidase activity assay
The GPX activity assay for total cell GPX was performed according to manufacturer's instructions using an activity assay kit (Glutathione Peroxidase Assay Kit, Cayman Chemical, Item Number 703102). Briefly, control and infected cells maintained on transwells in 6-well plates were washed and lysed with GPX activity lysis buffer. Lysates were normalized for protein prior to being tested in the activity assay. One hundred microlitres of the sample buffer and 50 μl of the co-substrate mixture were transferred into a well on a 96-well plate. Twenty microlitres of the sample, positive control (GPX) and standards were added followed by addition of 20 μl of cumene hydroperoxide to initiate the reaction. The plates were briefly shaken before reading the absorbance at 340 nm using a plate reader. A standard curve was used to determine the GPX concentration per sample.
Generation of small interfering RNAs (siRNA) for suppression of GPX4 expression
The plasmid used to generate siRNAs was constructed using the pSUPER vector (Oligoengine, Seattle, WA), in the method described by Brummelkamp et al. (2002). Oligonucleotides were designed by incorporating a 19 nt sequence (in italics) from the human gene encoding GPX4 (GenBank Accession number NM_002085.3); a short spacer region; and the reverse complement 19 nt sequence (in italics). The GPX4 sequence (F) and its reverse complement (R), flanked by HindIII and BglII restriction sites, respectively, were: F: 5′-AGCT GCAAGGGCATCCTGGGAAA-3′, and R: 5′-GATC TTTCCCAGGATGCCCTTGC-3′. As a random control cells were transfected with the empty pSuper vector (pSSH1). Oligonucleotides were annealed, yielding double-stranded DNAs with overhanging restriction sites, and ligated into digested pSUPER. Constructs were transformed into competent E. coli DH5α by standard methods and plated on LB plates with ampicillin (50 μg ml−1). Plasmids were extracted (QIAprep Spin Mini-prep Kit, Qiagen, Valencia, CA) and sequenced for confirmation. Once confirmed, bulk plasmid was prepared for transfection using Qiagen Plasmid Midi Kit (Qiagen, Valencia, CA).
Transfection of HCT8 intestinal epithelial cells
HCT8 cells (kind gift of Cheleste Thorpe, Tufts University School of Medicine) are a polarizing intestinal human transformed cell line and were used for this protocol because of their high transfection efficiency. HCT8 cells were transfected with the modified pSUPER using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) per manufacturer's instructions. Briefly, 4 μg of plasmid was diluted into Opti-MEM reduced serum medium (Invitrogen, Carlsbad, CA). Separately, Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was diluted into Opti-MEM reduced serum medium and incubated at room temperature for 5 min. Following incubation, the two mixtures were combined and incubated at room temperature for 20 min. This cocktail was added to HCT8 cells with RPMI 1640, and the cells were incubated in RPMI with 8% v/v FBS without selection. Cells were passaged into fresh media with selection added the next day (neomycin-G418, 1 mg/ml; Sigma-Aldrich, St Louis, MO). Cells underwent two additional cycles of growth/passage in G418 prior to use. This protocol was used to transfect both the siRNA GPX4 plasmid and the GPX4 overexpressing plasmid.
Reactive oxygen species (ROS) measurements
The DCFDA Cellular ROS detection Assay Kit (Abcam-Ab113851) was used in accordance with the manufacturer's instructions. Briefly, 2500 cells/well were seeded overnight in a 96-well plate. The following day, cells were washed with HBSS+ before 100 μl of freshly prepared DCFDA was added and incubated for 45 min at room temperature. Stained cells were washed with freshly prepared 1× buffer (supplied as 10×) before cells were infected with S. Typhimurium or left uninfected for 90 min. Following infection, unattached bacteria were washed away with PBS before being incubated for another 30 min with 100 μl PBS at 37°C. Plates were then read using a fluorescent plate reader (FluoSTAR OPTIMA – BMG Labtech) with the excitation wavelength set at 485 nm and emission wavelength at 535 nm. Results are expressed as fluorescent intensity of samples minus blank (DCFDA-unstained cells).
In addition, ROS production was quantified using CellROX Green Reagent (Molecular Probes) in accordance with the manufacturer's instructions. Briefly, cells grown to near-confluency in a 96-well plate were infected with S. Typhimurium, or exposed to SipA-HA or GST-SifA, or left untreated for 90 min at 37°C. Unattached bacteria or effector proteins were then washed away with PBS, after which the cells were stained with 5 μM CellROX Green Reagent for 30 min at 37°C. Plates were then washed with PBS, 100 μl PBS were added to the cells, and the plates were read as described above. Results are expressed as fluorescent intensity of samples minus blank (CellROX Green Reagent-unstained cells).
Extraction of lipids and ELISA assays
Polarized T84 monolayers seeded on transwells in 100 mm tissue culture dishes were apically infected with 5 ml of bacterial suspensions at 37°C for 90 min at an moi of approximately 100:1. After infection, non-adhering bacteria were removed by washing with HBSS+. Following washing, 2 ml HBSS+ was added to the apical side of the filters for 2 h at 37°C, after which the apical HBSS+ was collected. This was repeated for a total of two collections over 4 h. The apical HBSS+ samples were acidified to pH 3-4 and passed through a Discovery DSC-18 column (Supelco) primed with HPLC grade methanol and dH2O. The column was then washed with dH2O and eluted with HPLC grade methanol. After elution, the methanol was dried off using compressed nitrogen gas and the samples were resuspended into ELISA assay buffer. ELISAs were carried out for 12-S-HETE as per manufacturer's protocol (Enzo Life Sciences, Farmingdale, NY, USA).
Hepoxilin A3 (HXA3) analysis by LC-MS/MS
The collection of lipids secreted from non-infected or Salmonella-infected human intestinal epithelial cells, and the isolation of HXA3 by solid-phase extraction columns was done as described by Mrsny et al. (2004). All samples were processed using silanized glassware. In brief, lipid fractions suspended in methanol were dried under a stream of nitrogen and resuspended in 50 μl of solvent A (LC/MS water-acetonitrile-formic acid [63:37:0.02; v/v/v]) for MS analysis. HXA3 analysis was done by LC-MS/MS following the method from Deems et al. (2007) except that a Vydac TP C18 column (1.0 × 150 mm; part no. 218TP5115, Grace, MD) was used for reverse-phase liquid chromatography. Samples were injected at a volume of 20 μl at a flow rate of 50 μl min−1 at 24°C. HXA3 was eluted via gradient with solvent B (acetonitrile-isopropyl alcohol [50:50; v/v]) as described by Deems et al. (2007). All MS analyses were performed using a Thermo Electron LTQ linear ion trap, operating in negative ion mode with a spray voltage of 5 kV. The data acquisition method was set up to perform one MS scan (m/z 300–2000) followed by one MS/MS scan of m/z 335.3 with an isolation window of 2.5Da and a collision energy of 35.0%.
All chemicals and organic solvents used were ACS (American Chemical Society) or HPLC/Spectro grade. To identify and quantify HXA3 present in the samples, the chromatographic retention time, mass-to-charge ratio (m/z), and an MS/MS fragmentation pattern of a HXA3 standard (Cat. No. HE-008, Enzo Life Sciences, NY) were used. MS data processing and analysis were done using the software XCalibur 2.0.7 (Thermo Fisher Scientific Inc., MA).
Statistical analysis
All data are presented as mean ± SE. for n independent experiments. Statistical significance was evaluated by one-tailed paired Student's t-test [asterisk corresponds to P < 0.05 (n ≥ 3 individual experiment)].
Acknowledgments
The authors thank the University of Massachusetts Medical School Proteomics and Mass Spectrometry Facility, and Dr Scott Schafer and Karin Green for their assistance. Dr Regino Mercado-Lubo and Samir Patel, University of Massachusets Medical School, are thanked for purifying Salmonella effector proteins. B.A.M. is supported by the National Institutes of Health Grants DK56754 and DK33506, as well as the Crohn's and Colitis Foundation of America.
References
- Bernal-Bayard J, Ramos-Morales F. Salmonella type III secretion effector SlrP is an E3 ubiquitin ligase for mammalian thioredoxin. J Biol Chem. 2009;284:27587–27595. doi: 10.1074/jbc.M109.010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Friedrichs B, Maurer S, Schultz M, Streicher R. Interleukin 1-induced nuclear factor kappa B activation is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem J. 1997;328:199–203. doi: 10.1042/bj3280199. (Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Casella JF, Flanagan MD, Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- Day DW, Mandal BK, Morson BC. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978;2:117–131. doi: 10.1111/j.1365-2559.1978.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Deems R, Buczynski MW, Bowers-Gentry R, Harkewicz R, Dennis EA. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 2007;432:59–82. doi: 10.1016/S0076-6879(07)32003-X. [DOI] [PubMed] [Google Scholar]
- Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- Esworthy RS, Binder SW, Doroshow JH, Chu FF. Microflora trigger colitis in mice deficient in selenium-dependent glutathione peroxidase and induce Gpx2 gene expression. Biol Chem. 2003;384:597–607. doi: 10.1515/BC.2003.067. [DOI] [PubMed] [Google Scholar]
- Esworthy RS, Kim BW, Rivas GE, Leto TL, Doroshow JH, Chu FF. Analysis of candidate colitis genes in the Gdac1 locus of mice deficient in glutathione peroxidase-1 and −2. PLoS ONE. 2012;7:e44262. doi: 10.1371/journal.pone.0044262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everest P, Allen J, Papakonstantinopoulou A, Mastroeni P, Roberts M, Dougan G. Salmonella Typhimurium infections in mice deficient in interleukin-4 production: role of IL-4 in infection-associated pathology. J Immunol. 1997;159:1820–1827. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Siber AM, Madara JL, McCormick BA. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect Immun. 1999;67:608–617. doi: 10.1128/iai.67.2.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Chen CJ, Suzuki H, Yamamoto S, Chang WC. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases. Prostaglandins Other Lipid Mediat. 1999;58:65–75. doi: 10.1016/s0090-6980(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Huang HS, Chang WC, Chen CJ. Involvement of reactive oxygen species in arsenite-induced downregulation of phospholipid hydroperoxide glutathione peroxidase in human epidermoid carcinoma A431 cells. Free Radic Biol Med. 2002;33:864–873. doi: 10.1016/s0891-5849(02)00983-8. [DOI] [PubMed] [Google Scholar]
- Kohler H, Sakaguchi T, Hurley BP, Kase BA, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol. 2007;293:G178–G187. doi: 10.1152/ajpgi.00535.2006. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Hudson JT, 3rd, Lugering A, Abbas JA, Bettini M, Lake JG, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565–1572. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Silva M, Siber AM, Kelly AJ, Galyov E, McCormick BA. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc Natl Acad Sci USA. 2000;97:12283–12288. doi: 10.1073/pnas.97.22.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella Typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella Typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995a;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA, Miller SI, Carnes D, Madara JL. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995b;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella Typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- Mehta A, Singh S, Ganguly NK. Impairment of intestinal mucosal antioxidant defense system during Salmonella Typhimurium infection. Dig Dis Sci. 1998a;43:646–651. doi: 10.1023/a:1018887813713. [DOI] [PubMed] [Google Scholar]
- Mehta A, Singh S, Ganguly NK. Role of reactive oxygen species in Salmonella Typhimurium-induced enterocyte damage. Scand J Gastroenterol. 1998b;33:406–414. doi: 10.1080/00365529850171044. [DOI] [PubMed] [Google Scholar]
- Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam S, Patabhiraman S, Ciccoli R, Ishdorj G, Schwarz K, Petrucev B, et al. The rat leukocyte-type 12-lipoxygenase exhibits an intrinsic hepoxilin A3 synthase activity. J Biol Chem. 2004;279:29023–29030. doi: 10.1074/jbc.M307576200. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak CR, Reynaud D, Demin P, Nigam S. The hepoxilins. A review. Adv Exp Med Biol. 1999;447:123–132. [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992;117:757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Siccardi D, Mumy KL, Bien JD, Louie S, Shi HN, et al. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J Immunol. 2008;181:8044–8052. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg W, Bovee-Oudenhoven IM, Kramer E, van der Meer R, Keijer J. Gene expression response of the rat small intestine following oral Salmonella infection. Physiol Genomics. 2007;30:123–133. doi: 10.1152/physiolgenomics.00190.2006. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun. 1999;67:1471–1480. doi: 10.1128/iai.67.3.1471-1480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Wortmann M, Mandal PK, Arpornchayanon W, Jannasch K, Alves F, et al. Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia. 2010;12:254–263. doi: 10.1593/neo.91782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr K, Borchert A, Kuhn H. Inverse regulation of lipid-peroxidizing and hydroperoxyl lipid-reducing enzymes by interleukins 4 and 13. FASEB J. 1999;13:143–154. doi: 10.1096/fasebj.13.1.143. [DOI] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Shankaranarayanan P, Ciccoli R, Nigam S. Biosynthesis of hepoxilins: evidence for the presence of a hepoxilin synthase activity in rat insulinoma cells. FEBS Lett. 2003;538:107–112. doi: 10.1016/s0014-5793(03)00151-0. [DOI] [PubMed] [Google Scholar]
- Shannon VR, Stenson WF, Holtzman MJ. Induction of epithelial arachidonate 12-lipoxygenase at active sites of inflammatory bowel disease. Am J Physiol. 1993;264:G104–G111. doi: 10.1152/ajpgi.1993.264.1.G104. [DOI] [PubMed] [Google Scholar]
- Srikanth CV, Wall DM, Maldonado-Contreras A, Shi HN, Zhou D, Demma Z, et al. Salmonella pathogenesis and processing of secreted effectors by caspase-3. Science. 2010;330:390–393. doi: 10.1126/science.1194598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990;265:454–461. [PubMed] [Google Scholar]
- Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–119. doi: 10.1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall DM, Nadeau WJ, Pazos MA, Shi HN, Galyov EE, McCormick BA. Identification of the Salmonella enterica serotype typhimurium SipA domain responsible for inducing neutrophil recruitment across the intestinal epithelium. Cell Microbiol. 2007;9:2299–2313. doi: 10.1111/j.1462-5822.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- Yatmaz S, Seow HJ, Gualano RC, Wong ZX, Stambas J, Selemidis S, et al. Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. Am J Respir Cell Mol Biol. 2013;48:17–26. doi: 10.1165/rcmb.2011-0345OC. [DOI] [PubMed] [Google Scholar]
- Zafiriou MP, Deva R, Ciccoli R, Siafaka-Kapadai A, Nigam S. Biological role of hepoxilins: upregulation of phospholipid hydroperoxide glutathione peroxidase as a cellular response to oxidative stress? Prostaglandins Leukot Essent Fatty Acids. 2007;77:209–215. doi: 10.1016/j.plefa.2007.08.007. [DOI] [PubMed] [Google Scholar]