Summary
The Aggregatibactor actinomycetemcomitans cytolethal distending toxin (Cdt) induces G2 arrest and apoptosis in lymphocytes; these toxic effects are due to the active subunit, CdtB, which functions as a phosphatidylinositol-3,4,5-triphosphate (PIP3) phosphatase. We now extend our investigation and demonstrate that Cdt is able to perturb human macrophage function. THP-1- and monocyte-derived macrophages were found not to be susceptible to Cdt-induced apoptosis. Nonetheless, the toxin was capable of binding to macrophages and perturbing PI-3K signaling resulting in decreased PIP3 levels and reduced phosphorylation of Akt and GSK3β; these changes were accompanied by concomitant alterations in kinase activity. Exposure of monocytes and macrophages to Cdt resulted in pro-inflammatory cytokine production including increased expression and release of IL-1β, TNFα and IL-6. Furthermore, treatment of cells with either TLR-2, -3 or -4 agonists in the presence of Cdt resulted in an augmented pro-inflammatory response relative to agonist alone. GSK3β inhibitors blocked the Cdt-induced pro-inflammatory cytokine response suggesting a pivotal role for PI-3K blockade, concomitant decrease in GSK3β phosphorylation and increased kinase activity. Collectively, these studies provide new insight into the virulence potential of Cdt in mediating the pathogenesis of disease caused by Cdt-producing organisms.
Introduction
The innate immune system is the first line of host defense against microbial pathogens. Toll-like receptors (TLRs) trigger innate immune reactions in response to microbial products leading to the production of a number of pro-inflammatory cytokines. These responses, by and large, are considered to be protective, however, under conditions of excessive or prolonged cytokine production can lead to inflammation and associated tissue damage. Phosphatidylinositol-3-kinase (PI-3K), a lipid kinase, phosphorylates phosophatidylinositol-4,5-diphosphate (PI-4,5-P2) generating phosphatidylinositol-3,4,5-triphosphate (PIP3). Generation of PIP3 at the plasmalemma promotes the recruitment and phosphorylation of Akt and a number of downstream targets. The PI-3K signaling cascade has been implicated as a regulator of the pro-inflammatory response, in general, and TLR signaling, in particular, with contradictory roles. TLR-associated activation of PI-3K is a common feature in macrophages [reviewed in (Hazeki et al., 2007)]; however, this means of activation is moderate at best demonstrating low levels of activation compared to that commonly observed with other types of PI-3K stimulation. In contrast, the PI-3K signaling pathway is also proposed to be a negative feedback regulator of TLR-mediated activation that serves to suppress excessive macrophage responses by inhibiting pro-inflammatory cytokine production and augmenting anti-inflammatory responses (Antoniv and Ivashkiv, 2011;Fang et al., 2004;Hochdorfer et al., 2011;Lee et al., 2007;Pengal et al., 2006;Tachado et al., 2008). A key component of this inhibitory effect involves the downstream phosphorylation and inactivation of GSK3β which in turn regulates the amount of active transcription factors, CREB and the p65 subunit of NF-κB, that interact with the common co-activator, CBP, leading to altered cytokine synthesis.
Over the course of our investigations, we have demonstrated that Aggregatibactor actinomycetemcomitans, a putative pathogen implicated in the etiology and pathogenesis of localized juvenile periodontitis, produces an immunotoxin that affects human T-cell responses (Shenker et al., 1982;Shenker et al., 1990;Shenker et al., 1999). This toxin was shown to interfere with normal cell cycle progression resulting in G2 arrest as well as induction of apoptosis (Shenker et al., 1995). Moreover, we have shown that the toxin is the product of the cytolethal distending toxin (Cdt) operon (Shenker et al., 1999). Cdts are a family of heat-labile protein cytotoxins produced by several different bacterial species including diarrheal disease-causing enteropathogens such as some Escherichia coli isolates, Campylobacter jejuni, Shigella species, Haemophilus ducreyi and A. actinomycetemcomitans (Comayras et al., 1997;Mayer et al., 1999;Okuda et al., 1995;Okuda et al., 1997;Pickett et al., 1994;Pickett et al., 1996;Pickett and Whitehouse, 1999;Shenker et al., 1999). The Cdt operon contains three genes: cdtA, cdtB and cdtC which specify three polypeptides with molecular masses of 18, 32 and 20kDa, respectively. The three subunits form a heterotrimeric holotoxin that functions as an AB2 toxin.
Our studies have focused on Cdt subunit function, events critical to toxin-cell interaction, fate of Cdt-treated-cells and mode of action by which Cdt induces G2 arrest and apoptosis. In particular, we have demonstrated that Cdt subunits A and C comprise the binding unit which involves recognition and binding by CdtC to cholesterol in the context of lipid rafts (Boesze-Battaglia et al., 2006;Boesze-Battaglia et al., 2009). Other studies have also implicated glycans and glycoproteins on target cells as a site for toxin binding (Eshraghi et al., 2010;Nesic et al., 2004). The active subunit, CdtB, functions as a PIP3 phosphatase mimicking the tumor suppressors, PTEN and SHIP, and is able to block the PI3K/PIP3/Akt signaling cascade (Shenker et al., 2007;Shenker et al., 2010). We now demonstrate that human macrophages are not susceptible to Cdt-induced apoptosis. However, macrophages treated with Cdt exhibit blockade of PI-3K signaling with a concomitant increase in GSK3β kinase activity leading to the production of pro-inflammatory cytokines. We propose that in addition to its ability to function as an immunotoxin capable of perturbing PI-3K signaling, cell growth and survival, Cdt also represents a potent virulence factor capable of blocking PI-3K signaling in macrophages leading to a pro-inflammatory cytokine response.
Results
Cdt interacts with macrophages and alters PI-3K signaling
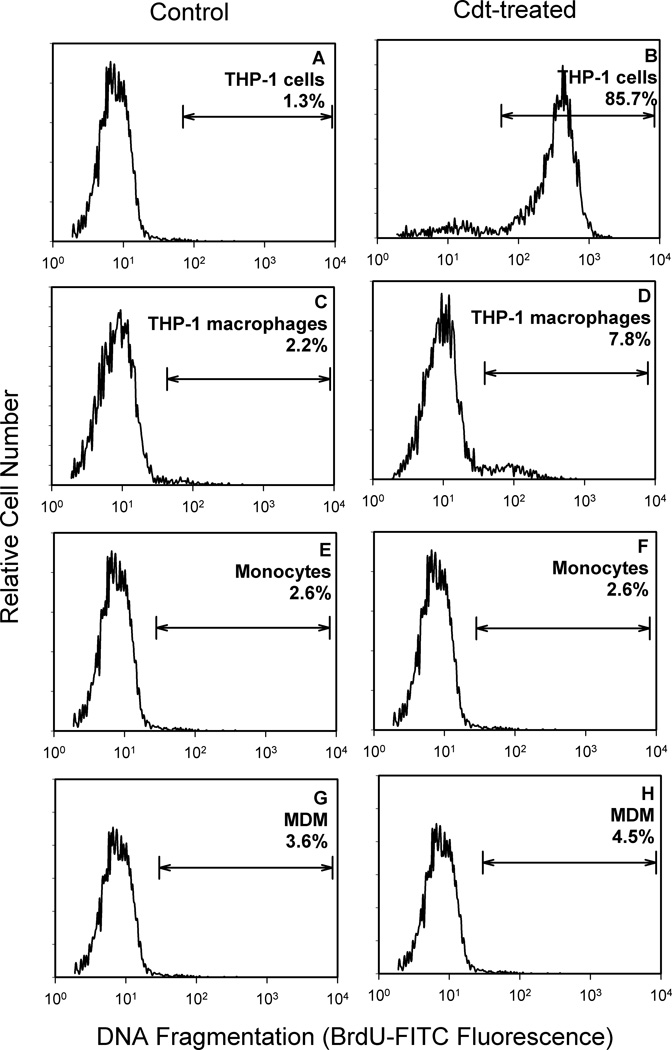
THP-1 cells and human monocytes as well as macrophages derived from either the THP-1 cell line or monocytes (monocyte-derived macrophages; MDM) were initially tested for susceptibility to Cdt-induced apoptosis. As shown in Figure 1, cells were incubated in medium alone or with Cdt for 48 hr and then assessed for DNA fragmentation using the TUNEL assay. Cdt (10 ng/ml) induced DNA fragmentation in 85.7% of THP-1 cells compared with 1.3% in the untreated control cells (Fig 1A,B). However, when THP-1 cells were treated with PMA and allowed to differentiate into macrophages for 72 hr, they no longer were susceptible to Cdt as exposure to toxin (1 µg/ml) failed to induce significant apoptosis (7.8%; Fig. 1C,D). It should be noted that differentiated THP-1 cells exposed to 2.5 µg/ml Cdt for up to 72 hr also failed to become apoptotic (data not shown). Neither human monocytes or MDM were susceptible to Cdt-induced apoptosis as cells treated with Cdt (500 ng/ml) for 48 hr resulted in DNA fragmentation in 2.6% and 4.5% cells, respectively. DNA fragmentation in control monocytes and MDM was 2.6% and 3.6%, respectively (Fig. 1E–H).
Figure 1.
Effect of Cdt on THP-1, monocyte and macrophage DNA fragmentation. THP-1 cells (panels A and B), THP-1-derived macrophages (panels C and D), monocytes (panels E and F) and MDM (panels G and H) were exposed to medium (panels A, C, E, G) or treated with Cdt (panels A, C, F, H) for 48 hr. THP-1 cells were exposed to 10 ng/ml Cdt and THP-1-derived macrophages to 1 µg/ml of toxin; monocytes and MDM were treated with 500 ng/ml Cdt. The cells were then analyzed by flow cytometry for the presence of DNA fragmentation as described in Methods and Materials. Results are plotted as relative cell number versus DNA fragmentation (BrDU-FITC fluorescence). The line represents the analytical gate used to assess cells exhibiting DNA fragmentation and the numbers are the percentage of positive cells. Results are representative of three experiments.
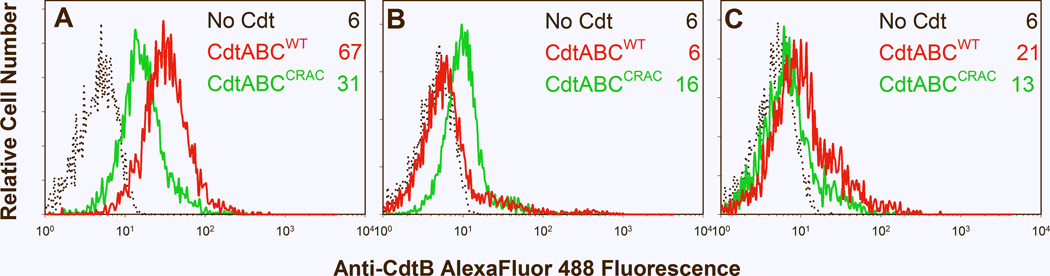
We next determined if Cdt was able to associate with differentiated THP-1 cells and deliver the active subunit, CdtB, to intracellular compartments. In this regard, we have previously demonstrated that Cdt subunits A and C were responsible for cell association and further, that CdtC contains a cholesterol recognition/interaction amino acid consensus sequence (CRAC) critical for toxin binding and subsequent internalization of CdtB (Boesze-Battaglia et al., 2009). Internalization of the active subunit, CdtB, was assessed by flow cytometry following permeabilization and intracellular immunofluorescence staining for CdtB. As shown in Figure 2A, cells treated with Cdt contained detectable levels of intracellular CdtB; mean channel fluorescence (MCF) was 67 compared to background fluorescence of 6 observed in untreated cells exposed to medium alone. It should be noted that incubation of cells with toxin at 5°C prevents internalization of CdtB (MCF=6; Fig. 2B). We have previously demonstrated that Cdt containing a mutation in the CRAC site, CdtABCY71P, was unable to bind to lymphocytes and deliver CdtB to the cell (Boesze-Battaglia et al., 2009). Similarly, CdtABCY71P exhibited reduced capacity to deliver CdtB to THP-1-derived macrophages as these cells exhibit a MCF of 31 (Fig. 2A). We have previously demonstrated that following association with Jurkat cells, CdtB is rapidly internalized and further, that this subunit is minimally detected on the cell surface. For control purposes, Cdt-treated THP-1 derived macrophages were also assessed for surface associated CdtB (Fig. 2C). The MCFfor surface CdtB was 21demonstrating that most of the immunofluorescence associated with CdtB results from staining of the internalized subunit following permeabilization; cells exposed to CdtABCY71P and stained without permeabilization exhibited a MCF of 13 (Fig 2C).
Figure 2.
Immunofluorescence analysis of internalization of CdtB. THP-1 derived macrophages were exposed to media alone (black dotted line), CdtWT (red line) or CdtABCY71P (green line) for 1 hr and then analyzed by immunofluorescence and flow cytometry for the presence of CdtB following fixation, permeabilization and staining with anti-CdtB mAb conjugated to AlexaFluor 488. Panel A shows results of cells incubated at 37°C, fixed, permeabilized and stained for CdtB. Panel B shows results of cells incubated at 5°C and treated as described above. Panel C shows fluorescence associated with CdtB in non-permeabilized cells incubated at 37°C. Fluorescence is plotted versus relative cell number. Numbers represent the mean channel fluorescence (MCF); at least 10,000 cells were analyzed per sample. Results are representative of three experiments. The MCF for untreated cells (autofluorescent) was 4.
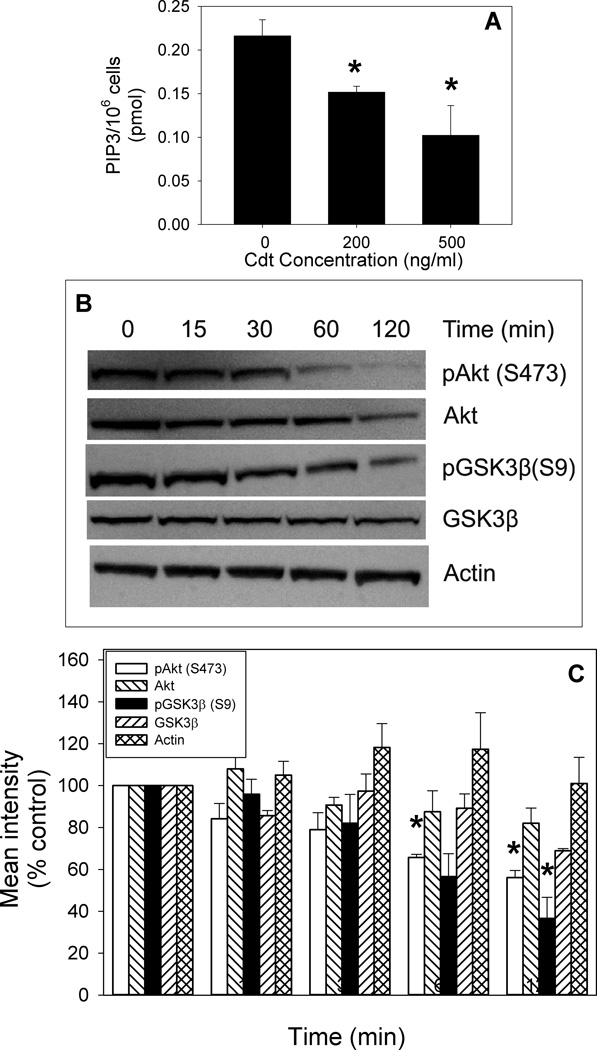
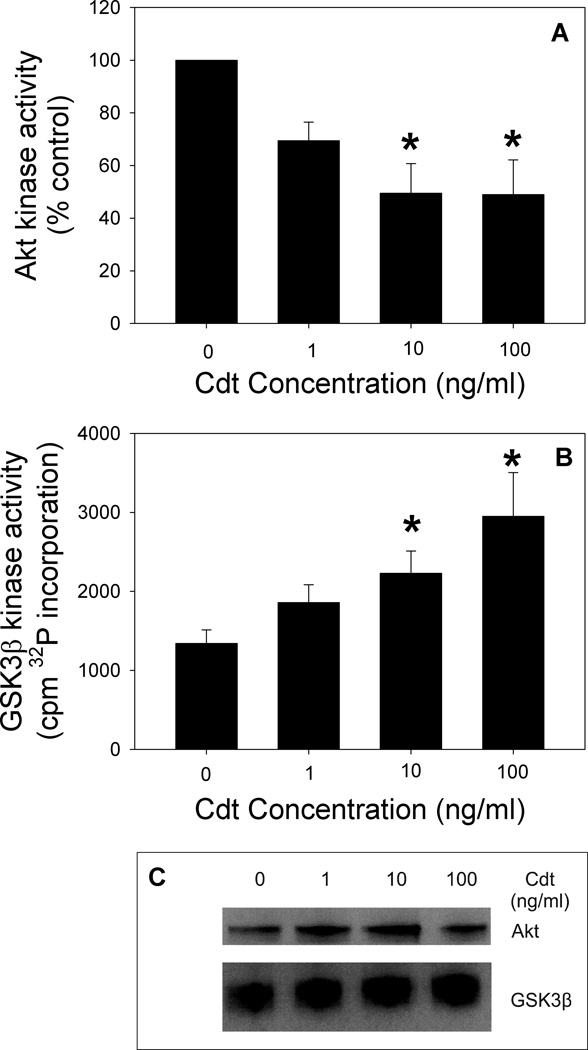
As Cdt is capable of binding to macrophages leading to internalization of CdtB, we next determined if Cdt-treated macrophages exhibit perturbations in PI-3K signaling consistent with CdtB’s functioning as a PIP3 phosphatase. In Fig 3A we demonstrate that monocytes treated with 200 and 500 ng/ml Cdt for 2 hr exhibit reduced levels of PIP3 to 0.15±0.006 and 0.10±0.03 pmol/106 cells; this compares with 0.22±0.02 pmol/106 cells in untreated cells. THP-1-derived macrophages were treated with Cdt (50 ng/ml) for 0–120 min and then assessed by Western blot for changes in pAkt and pGSK3β. As shown in Fig ure 3B and C, baseline levels of pAkt(S473) were reduced in Cdt-treated cells in a time-dependent manner; pAkt levels were significantly reduced to 65 ± 1.5% and 56 ± 3.4% of control values within 60 and 120 min of exposure to toxin, respectively. Reduction in Akt phosphorylation was accompanied by a concomitant decrease in its kinase activity which was reduced to 70 ± 7% of control values in the presence of 1 ng/ml Cdt after a two hr exposure (Fig. 4A); kinase activity was further reduced to 49 ± 11.3% in the presence of 10 ng/ml. A downstream target of pAkt is GSK3β as phosphorylation of this kinase is dependent upon Akt activity. GSK3β phosphorylation was reduced to 57±11% and significantly reduced to 37 ±10% of control levels at 1 and 2 hr, respectively (Fig. 3). In contrast to Akt, dephosphorylation of GSK3β is associated with an increase in kinase activity. Indeed, Cdt-treated macrophages exhibited a 1.4-fold increase in GSK3β kinase activity in the presence of 1 ng/ml Cdt after 2 hr exposure; activity continued to increase by 1.7 and 2.2-fold in cells exposed to 10 ng/ml and 100 ng/ml of toxin, respectively (Fig. 4B).
Figure 3.
Effect of Cdt on downstream components of the PI-3K signaling pathway. Monocytes were treated with Cdt and then assessed for levels of PIP3 (panel A). Results are the mean ± S.E. for three experiments. The effect of Cdt on Akt and GSK3β phosphorylation is shown in panels B and C. THP-1 derived macrophages were exposed to 50 ng/ml of Cdt for 0–120 min then harvested, solubilized and fractionated by SDS-PAGE. Gels were analyzed by Western blot for the presence of Akt, pAkt(S473), GSK3β and pGSK3β(S9) followed by digital scanning densitometry; actin is shown as a loading control. A representative Western blot is shown in panel B and the mean ± SEM is shown for three experiments in panel C. Asterisks indicate p<0.05 when compared to untreated cells.
Figure 4.
Analysis of the effect of Cdt on Akt and GSK3β kinase activity. THP-1 derived macrophages were treated with 1.0, 10 and 100 ng/ml Cdt for 2 hr. Total Akt was immunoprecipitated from solubilized cells and kinase activity measured by monitoring GSK3β phosphorylation in vitro. Western blots were analyzed by digital scanning densitometry and values expressed as a percentage of control; results are plotted as the mean ± SEM of three experiments (panel A). GSK3β was immunoprecipitated and kinase activity was measured in similarly treated cells by monitoring the utilization 32P-ATP to phosphorylate a synthetic substrate. Data are plotted as 32P (cpm) incorporation versus Cdt concentration; results represent the mean ±S.E. for three experiments. Asterisks indicate p<0.05 when compared to untreated cells. Panel C shows the recovery of Akt and GSK3β from representative immunoprecipitates assessed by Western blot.
Cdt induces a pro-inflammatory response from macrophages
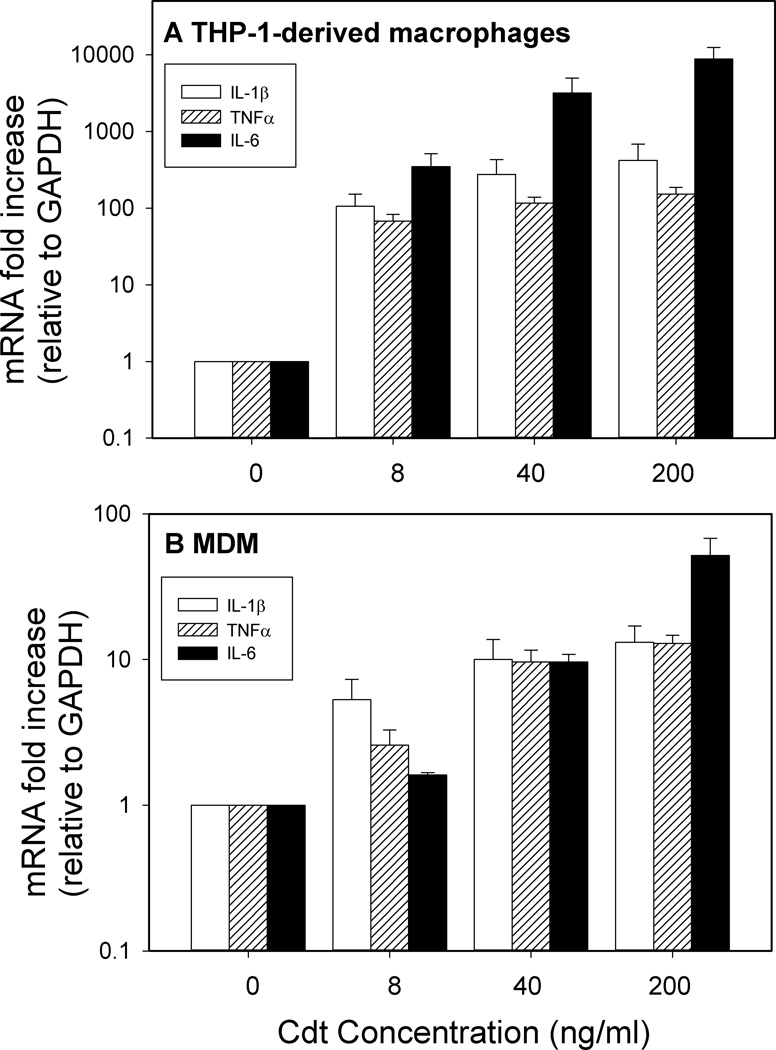
Although macrophages are resistant to Cdt-induced apoptosis, it is clear that the toxin is able to associate with these cells, deliver CdtB and perturb the PI-3K signaling pathway in a manner consistent with the action of a PIP3 phosphatase. While the PI-3K signaling pathway is not considered to be directly involved in signal pathways leading to cytokine synthesis and release, it is becoming clear that this pathway plays a regulatory role that can influence cytokine production from macrophages at rest or following activation by TLR agonists (Guha and Mackman, 2002;Hazeki et al., 2007;Molnarfi et al., 2007). Therefore, we next assessed Cdt for its ability to induce macrophages to express mRNA for three pro-inflammatory cytokines: IL-1β, TNFα and IL-6. Differentiated THP-1 cells were treated with varying amounts of Cdt (0–200 ng/ml) for 2 hr and cytokine mRNA levels were assessed by quantitative real-time PCR (Fig. 5A). Exposure to the lowest dose of Cdt (8 ng/ml) resulted in >80-fold increase in mRNA levels for IL-1β, TNFα and IL-6. Exposure to 200 ng/ml Cdt resulted in further increases of >120-fold for IL-1β and TNFα along with >24,000 fold increase for IL-6. MDM also exhibited >10-fold increases in the levels of mRNA for each of these cytokines when treated with 8–200 ng/ml of Cdt (Fig. 5B)
Figure 5.
Effect of Cdt on IL-1β, TNFα and IL-6 gene expression. THP-1-derived macrophages (panel A) and MDM (panel B) were treated with varying concentrations of Cdt for 2 hr. Total RNA was collected and analyzed for IL-1β (open bars), TNFα (striped bars) and IL-6 (solid bars) gene expression using RT-PCR as described in Materials and Methods. Results are the mean ± SEM for three experiments.
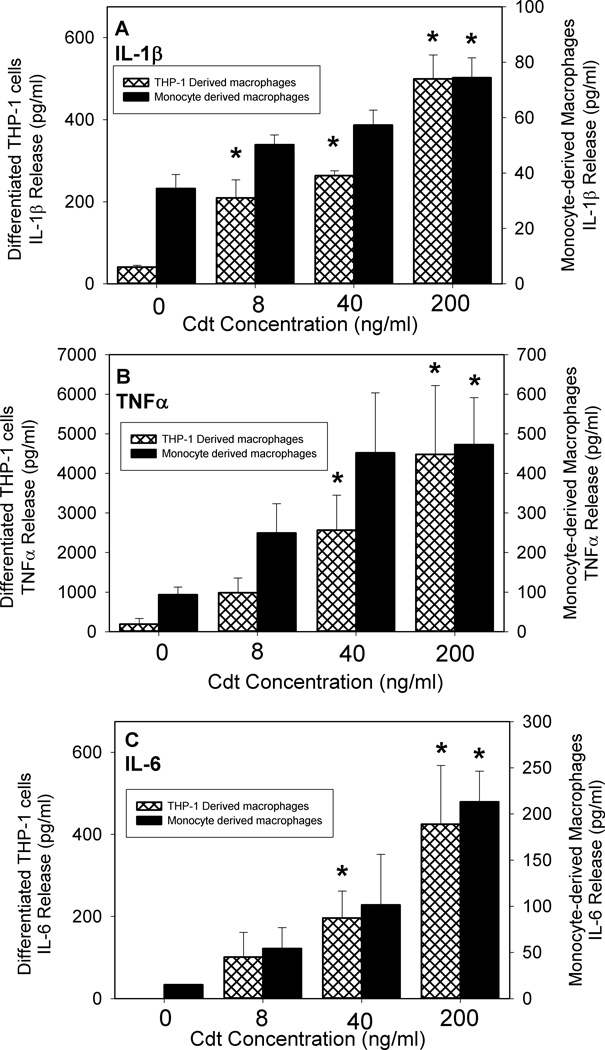
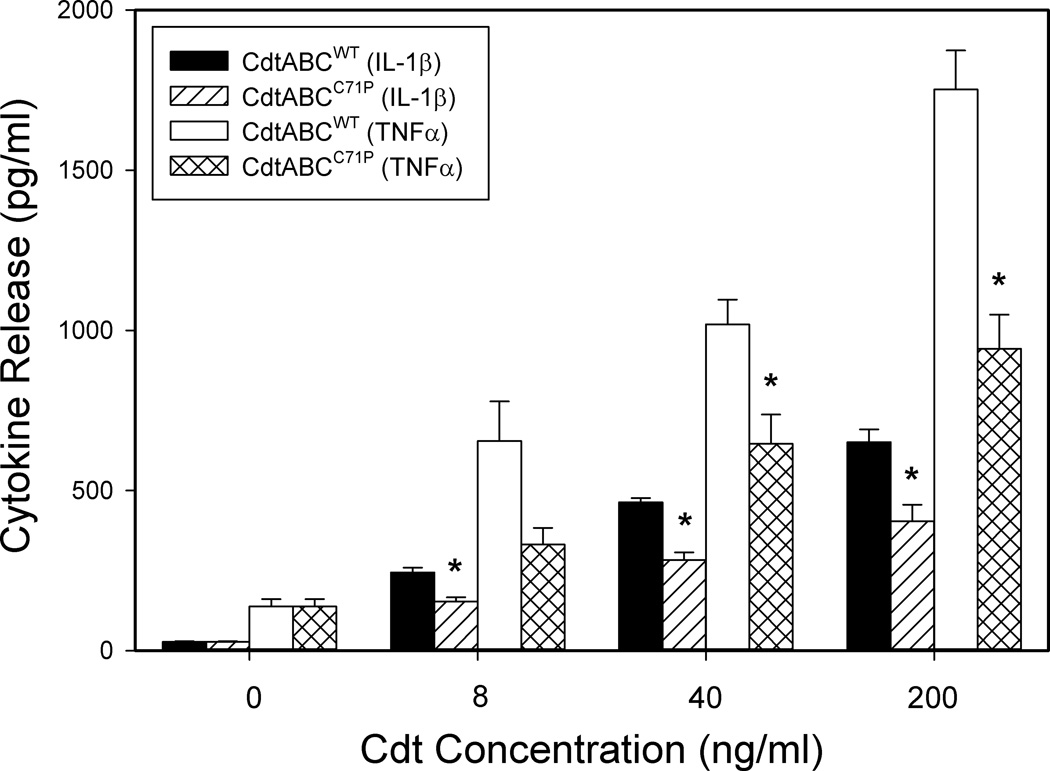
In the next series of experiments we determined if increased expression of cytokine mRNA was accompanied by increased cytokine release. As shown in Fig. 6A, untreated THP-1 derived-macrophages released low levels of IL-1β (41.1 ± 4.2 pg/ml) and TNFα (171.1 ± 17); IL-6 was undetectable in supernatants. In contrast, macrophages were induced to release cytokines within 5 hr of exposure to Cdt. IL-1β release was dose-dependent as exposure to 8 ng/ml Cdt resulted in release of 209 ± 44 pg/ml; increasing the dose of toxin to 40 and 200 ng/ml induced 264 ± 12 and 499 ± 58 pg/ml of cytokine, respectively. Likewise, the toxin induced dose-dependent release of both TNFα and IL-6 (Figs. 6B and C). Cells exposed to 8 ng/ml Cdt induced 983 ± 372 pg/ml TNFα and 101 ± 60 pg/ml IL-6; increasing the dose of toxin to 200 ng/ml resulted in 4482 ± 1738 and 425 ± 142 pg/ml of TNFα and IL-6, respectively. It is also noteworthy that toxin containing the CRAC mutation, CdtABCY71P, not only exhibited reduced ability to deliver CdtB, but also decreased ability to induce cytokine release (Fig. 7). Cells exposed to 8, 40 and 200 ng/ml CdtABCY71P secreted 153 ± 13, 283 ± 23 and 404 ± 51 pg/ml IL-1β, respectively; this compares to 244 ± 15, 463 ± 13 and 651 ±39 pg/ml IL-1β from cells treated with CdtWT. Similar results were observed for TNFα: cells exposed to CdtABCY71P released 331± 52 (8 pg/ml), 645±91 (40 pg/ml) and 942±106 compared to 655±123, 1019±77 and 1752±122 pg/ml in cells treated with 8, 40 and 200 ng/ml CdtWT, respectively (Fig. 7).
Figure 6.
Cdt induces macrophages to release pro-inflammatory cytokines. THP-1-derived macrophages (hatched bars) and MDM (solid bars) were treated with Cdt (0–200 ng/ml) and the supernatants analyzed 5 hr (THP-1) or 24 hr (MDM) later by ELISA for IL-1β (panel A), TNFα (panel B) and IL-6 (panel C). Results are the mean ± SEM for three experiments each performed in triplicate; asterisks indicate p<0.05 when compared to untreated cells.
Figure 7.
Effect of CdtC CRAC mutant on cytokine release. THP-1-derived macrophages were treated with CdtABCWT or CdtABCY71P for 5 hr. Supernatants were analyzed by ELISA for IL-1β and TNFα. Results are the mean ± SEM for three experiments each performed in triplicate; asterisk indicates statistical significance (p<0.05) when compared to CdtABCWT at the same concentration.
In addition to THP-1-derived cells, Cdt was also assessed for its ability to induce cytokine release from MDM (Fig6A–C). Twenty-four hr exposure to Cdt induced MDM release of IL-1β in a dose-dependent manner (0–200 ng/ml); 50 ± 5 pg IL-1β was released in the presence of 8 ng/ml of toxin and increased to 74 ± 7 pg/ml in the presence of 200 ng/ml while control cells released 34 ± 5 pg/ml. Similarly, exposure to 8 ng/ml Cdt induced the release of both TNFα and IL-6, 249 ± 74 and 54 ± 22 pg/ml respectively; in the presence of 200 ng/ml, these values increased to 473 ± 118 and 213 ± 33 pg/ml, respectively. Control cells released 93 pg/ml (TNFα) and 5 (IL-6) pg/ml.
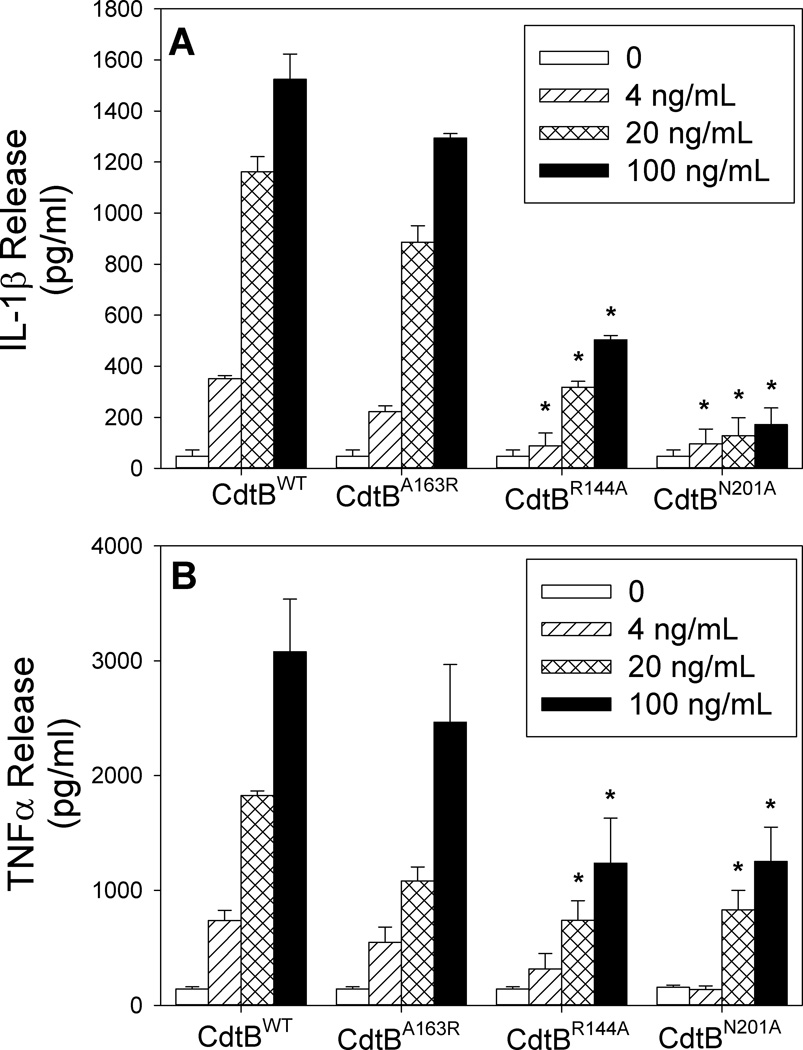
To further demonstrate that the Cdt-induced pro-inflammatory cytokine response was dependent upon CdtB associated PIP3 phosphatase activity, we employed toxin containing CdtB mutants in addition to wildtype toxin. Three CdtB mutants involving amino acid substitutions in residues that correspond to the putative active site and likely to be involved in substrate binding were employed: CdtBA163R, CdtBR144A and CdtBN201A. It should be noted that we have previously shown that CdtBA163R retains PIP3 phosphatase activity while the other two mutants exhibit significantly reduced phosphatase activity (Shenker, BJ, unpublished). As shown in Figure 8, toxin containing the CdtBA163R mutation induced both IL-1β and TNFα release from THP-1-derived macrophages in a dose-dependent manner. In the presence of 20 ng/ml CdtBA163R, cells released 223 ± 23 pg/ml IL-1β and 547 ± 134 pg/ml TNFα; this compares to 352 ± 12 and 738 ± 89 pg/ml for CdtBWT, respectively. Cytokine release in the presence of 100 ng/ml CdtBA163R resulted in 1294 ± 17 (IL-1β) and 2465 ± 502 (TNFα) compared to 1524 ± 99 (IL-1β) and 3080 ± 458 (TNFα) pg/ml for cells exposed to CdtBWT. In contrast, cells exposed to the CdtB mutants, CdtBR144A and CdtBN201A, released lower amounts of cytokines. For example, exposure to 100 ng/ml Cdt resulted in 503 ± 16 (CdtBR144A) and 171 ±66 (CdtBN201A) pg/ml IL-1β; exposure to these mutants resulted in reduced TNFα production as well: 1237 ± 391 (CdtBR144A) and 1252 ± 298 pg/ml (CdtBN201A).
Figure 8.
Analysis of CdtB mutants for their ability to induce cytokine release. THP-1-derived macrophages were treated for 5 hr with 50 ng/ml each of CdtA and CdtC along with varying amounts of CdtBWT, CdtBA163R, CdtBR144A or CdtBN201 mutants. Supernatants were then analyzed for IL-1β (panel A) and TNFα (panel B). Results are the mean ± SEM for three experiments each performed in triplicate; asterisk indicates statistical significance (p<0.05) when compared to CdtBWT at the same concentration.
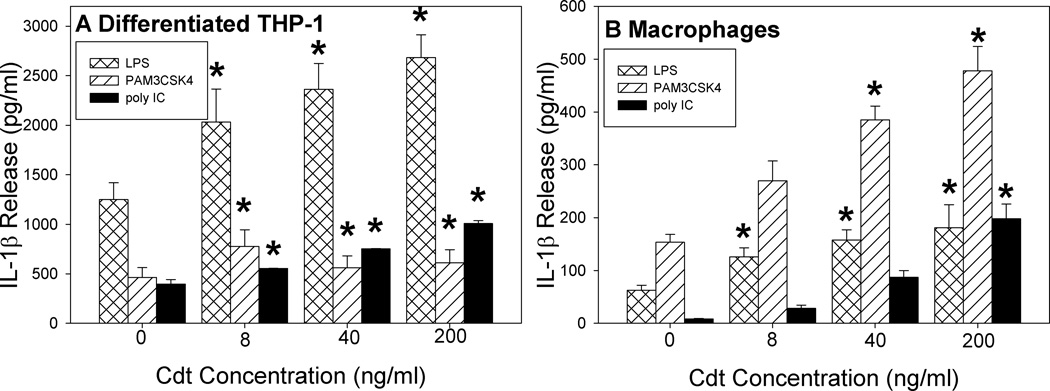
As discussed above, the PI-3K signaling pathway has been implicated as a regulator of the pro-inflammatory response of macrophages to TLR agonists. Therefore, we next assessed Cdt for its ability to augment the pro-inflammatory cytokine response induced by three TLR agonists: LPS (TLR4), PAM3CSK4 (TLR2) and poly I:C (TLR3). As shown in Figure 9, exposure of THP-1 derived macrophages cells to varying amounts of Cdt (0–200 ng/ml) augmented LPS-induced release of IL-1β in a dose-dependent manner; cells treated with 8 ng/ml toxin released 2032 ± 332 pg/ml versus 1248 ± 170 pg in cells treated with LPS alone. These values increased to 2362 ± 261 and 2683 ± 230 pg/ml in the presence of 40 and 200 ng/ml Cdt, respectively. THP-1 derived macrophages activated by PAM3CSK4 showed similar effects. The maximum effect of the toxin on PAM3CSK4-induced IL-1β release was observed with 8 ng/ml Cdt resulting in 776 ± 168 pg/ml versus 464 ± 100 pg/ml in cells exposed to PAM3CSK4 alone; these values remained elevated in the presence of 200 ng/ml Cdt at 610 ± 133 pg/ml. Likewise, the toxin enhanced IL-1β release induced by the intracellular TLR2 agonist poly I:C; cells exposed to agonist alone secreted 396 ± 47 pg/ml of cytokine versus 752 ± 4 and 1009 ± 28 pg/ml in the presence of 40 and 200 ng/ml Cdt, respectively. The dose-dependent effect of Cdt on TLR agonist-induced cytokine release from MDM was also assessed and found to be significantly increased in the presence of toxin. LPS-induced IL-1β secretion was maximally increased in the presence of 200 ng/ml Cdt resulting in a 2.9-fold increase over LPS control, 3.1-fold increase over PAM3CSK4 control and a 23-fold increase over poly I:C control. Similar results were observed for TNFα with both MDM and THP-1-derived macrophages (data not shown).
Figure 9.
Cdt augments macrophage response to TLR agonist stimulation. THP-1-derived macrophages (panel A) and MDM (panel B) were pre-treated with varying amounts of Cdt for 1 hr; cells were then exposed to LPS, PAM3CSK4 or poly I:C for 5 hr (THP-1-derived macrophages) or 24 hr (MDM). Supernatants were analyzed by ELISA for IL-1β. Results are the mean ± SEM for three experiments each performed in triplicate; asterisks indicate p<0.05 when compared to untreated cells.
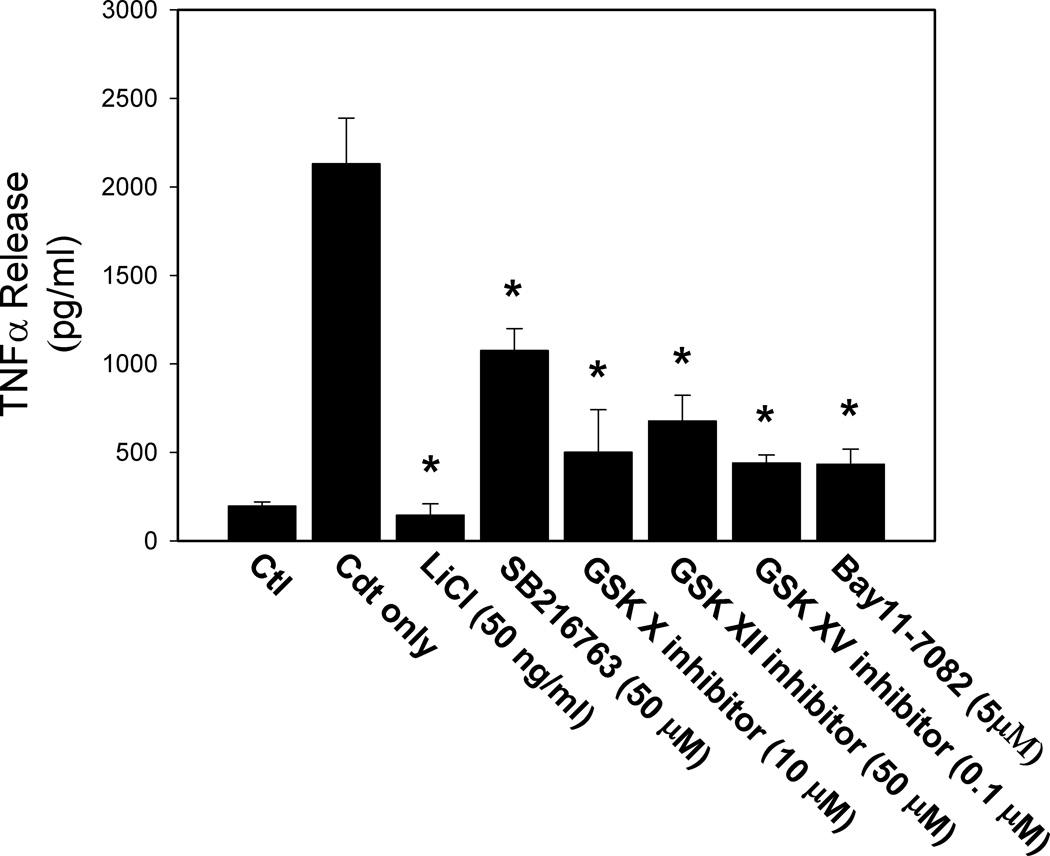
In the last series of experiments we addressed the mechanism by which Cdt-mediated perturbation of the PI-3K/PIP3/Akt signaling cascade leads to synthesis and release of pro-inflammatory cytokines. As discussed earlier, there is evidence supporting the notion that the PI-3K pathway is a negative feedback regulator of the synthesis and release of pro-inflammatory cytokines by macrophages. A key component of this inhibitory effect involves the downstream inactivation of GSK3β (i.e., phosphorylation); it has been proposed that inactivation of the kinase influences the amount of active transcription factors, CREB and p65 subunit of NF-κB, which interact with the common coactivator CBP leading to altered cytokine synthesis (Guha and Mackman, 2002;Hazeki et al., 2007). Likewise, it has been suggested that inactivation of the PI-3K signaling pathway results in decreased phosphorylation of GSK3β leading to increased kinase activity, and in turn, kinase-dependent changes in NF-κB-dependent expression of pro-inflammatory cytokines. We have demonstrated that Cdt-treated macrophages exhibit reduced levels of pGSK3β and increased kinase activity. Therefore, we extended this observation and employed five inhibitors of GSK3β. As shown in Figure 10, all inhibitors reduced levels of Cdt-induced TNFα release. Cells treated with Cdt alone released 2131±258 pg/ml TNFα compared to 197±24 pg/ml in untreated control cells. TNFα release was reduced in cells pre-treated with inhibitor to 146±64 (LiCl), 1075±125 (SB216763), 501±240 (GSK X inhibitor), 678±144 (GSK XII inhibitor) and to 440±46 (GSK XV inhibitor). We also employed the inhibitor Bay 11–7062 to block downstream activation of NF-κB. As shown in Figure 10, cytokine release from cells treated with Bay 11–7062 (50 µM) exhibited reduced levels of TNFα (433 ± 85 pg/ml).
Figure 10.
Effect of GSK3β and NF-κβ inhibitors on Cdt-induced cytokine release. THP-1 derived macrophages were pre-exposed to LiCl, SB216763, GSK X inhibitor, GSK XII inhibitor, GSK XV inhibitor or Bay11–7082 (50 µM) for 30 min followed by the addition of Cdt (50 ng/ml). Twenty-four hr later supernatants were harvested and analyzed for TNFα by ELISA. Results are the mean ± SEM of three experiments. Asterisk indicates statistical significance (p<0.05) when compared to cells exposed to Cdt alone.
Discussion
The Cdts are a family of heat-labile protein cytotoxins produced by several different bacterial species which were originally characterized by their ability to cause progressive cellular distension and finally death in some cell lines [reviewed in (Guerra et al., 2011)]. It should be noted that the gross cellular changes associated with Cdt activity are clearly different from those caused by other known toxins that induce rapid morphological alterations culminating in cell death (Aragon et al., 1997;Cope et al., 1997;Lally et al., 1997). More recently, it has become clear that exposure to Cdt induces proliferating cells to undergo irreversible G2 arrest which eventually culminates in cell death resulting from activation of the apoptotic cascade (Cortes-Bratti et al., 2001;Gelfanova et al., 1999;Ohara et al., 2004;Shenker et al., 2001). Indeed, we have demonstrated that activated lymphocytes and lymphocytic cell lines exhibit heightened sensitivity to both Cdt-induced G2 arrest and apoptosis leading us to propose that the toxin functions as an immunotoxin (Shenker et al., 2000;Shenker et al., 2001;Shenker et al., 2004;Shenker et al., 2005;Shenker et al., 2006).
We now demonstrate that macrophages, both MDM and THP-1 derived-macrophages, are resistant to Cdt-induced cell death as exposure to high concentrations of toxin for extended periods of time did not result in apoptosis. This observation is consistent with our previous studies in which we demonstrated that non-activated lymphocytes, i.e., cells that are not proliferating, are also not susceptible to Cdt-induced apoptosis (Shenker et al., 2001). It should be noted that undifferentiated, proliferating THP-1 cells are susceptible to both Cdt-induced cell cycle arrest (data not shown) and apoptosis. Our results are different from those of Rabin et al (Rabin et al., 2009) who previously reported that macrophages-derived from the U937 myeloid leukemia cell line were susceptible to Cdt-induced apoptosis. It is possible that the discrepancy between our observations and Rabin et al (Rabin et al., 2009) represents subtle differences between macrophages-derived from THP-1 versus U937 leukemic cell lines. Indeed, Baird et al (Baird et al., 2005) have reported differential susceptibility to apoptotic stimuli between these cells. Moreover, Rabin et al (Rabin et al., 2009) also reported that Cdt-treated U937-derived macrophages failed to exhibit caspase-3 activation, thus it is possible that they were studying an entirely different biologic process. In addition to cell lines, we have extended our study to include both primary human blood monocytes and MDM which we also have found to be resistant to Cdt-induced apoptosis. It is particularly noteworthy and relevant to our observations those reported by Busca et al (Busca et al., 2011) who demonstrated that MDM and THP-1 derived macrophages also exhibit heightened resistance to Vpr, a potent apoptosis inducing agent derived from HIV. Thus, the ability of macrophages to resist Cdt-induced apoptosis is not unique and likely a consequence of maturation changes that also lead to protection from other stimuli as well.
In this study, we also demonstrate that Cdt is capable of interacting with macrophages in a manner similar to lymphocytes as it associates with cells and delivers the active subunit, CdtB, to intracellular compartments; these interactions are dependent upon a competent cholesterol recognition sequence, CRAC site, within the CdtC subunit. Although macrophages are resistant to Cdt-induced apoptosis, we now demonstrate that MDM and THP-1-derived macrophages are susceptible to Cdt-induced production of inflammatory cytokines including IL-1β, TNFα and IL-6. Moreover, the toxin is capable of augmenting cytokine production by macrophages in response to TLR-2,-3 and -4 agonists. Similar cytokine responses were also observed from undifferentiated monocytes (data not shown). It should be noted that Cdt preparations are highly purified and it is unlikely that these effects are the result of a contaminant unrelated to toxin. Nonetheless, the CdtABCY71P mutant with reduced binding capability also exhibited reduced ability to stimulate cytokine production and serves as a control for this possibility. In contrast to our observations, Xu et al (Xu et al., 2004) failed to observe an increase in IL-1β production from Cdt-treated monocyte-derived dendritic cells. At this point we can only speculate that this contradictory observation may relate to differences in differentiation pathways.
Previously, we demonstrated that the active Cdt subunit, CdtB, was capable of functioning as a PIP3 phosphatase leading to perturbation of the PI-3K signaling pathway; this activity appears to be critical to toxin-induced G2 arrest in lymphocytes and inhibition of mast cell degranulation (Shenker et al., 2007;Shenker et al., 2011;Shenker et al., 2010). In our current study, we also demonstrate that Cdt-treated macrophages exhibit perturbations in the PI-3K signaling pathway consistent with the action of a PIP3 phosphatase: decreased levels of PIP3 and reduced phosphorylation of both Akt and GSK3β. These changes in phosphorylation status were accompanied by altered kinase activity; Akt activity was decreased while GSK3β kinase activity increased. We propose that the Cdt-induced pro-inflammatory cytokine response was the result of CdtB’s lipid phosphatase activity; this conclusion is drawn from two results. First, we employed toxin containing three CdtB containing mutants with altered enzymatic activity: CdtBA163R mutant retains PIP3 phosphatase activity comparable to CdtBWT and induces macrophages to produce cytokines at levels comparable to the wildtype toxin. In contrast, the CdtBR144A and CdtBN201A mutants both have significantly reduced phosphatase activity and, likewise, exhibit reduced ability to induce macrophages to release IL-1β, TNFα and IL-6. The second line of evidence in support of PIP3 phoshatase activity being critical to Cdt-induced cytokine release is derived from the use of several GSK3β inhibitors. It is well documented that inactivation of PI-3K signaling leads to decreased PIP3 and pAkt levels resulting in decreased phosporylation of GSK3β and concomitant increases in its kinase activity. In addition to demonstrating that Cdt-treated cells exhibit increased GSK3β activity, we also demonstrate that the GSK3β inhibitors suppressed Cdt-induced cytokine release.
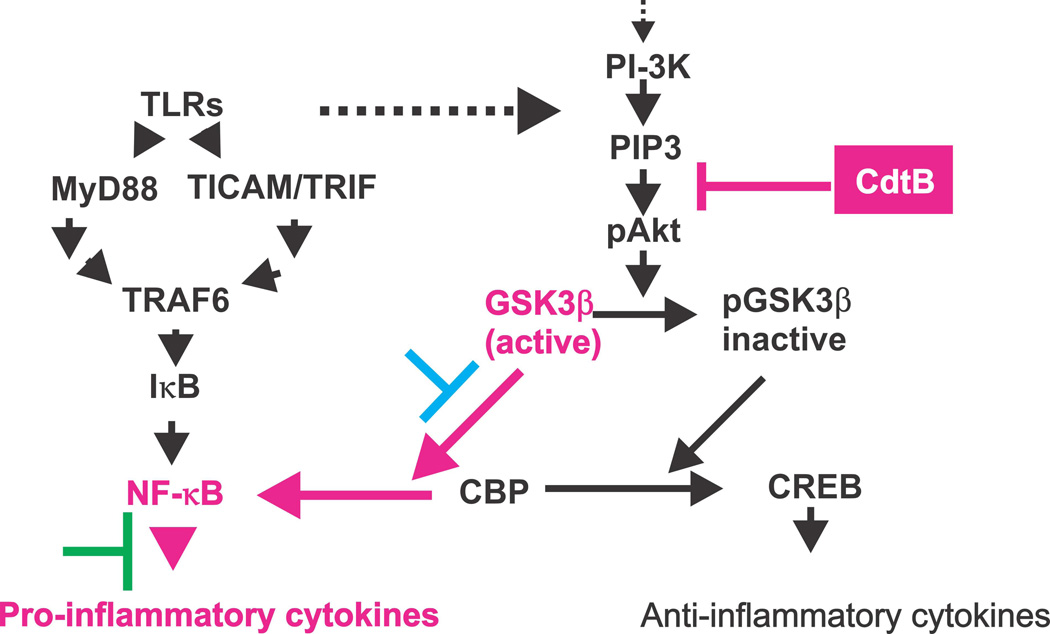
The studies described in this report provide critical insight into the molecular mechanism by which Cdt acts to promote a pro-inflammatory response as well as advance our understanding of the regulatory role of the PI-3K pathway in governing these responses (summarized in Fig. 11). In this regard, a relationship between TLR-signaling and the PI-3K pathway in macrophages was first established by Arbibe et al (Arbibe et al., 2000) who demonstrated that TLR-2 signaling involves interaction with components of the PI-3K cascade. Over the last several years it has also become increasingly clear that the PI-3K signaling pathway plays a regulatory role that governs cytokine production (Antoniv and Ivashkiv, 2011;Arbibe et al., 2000;Brown et al., 2010;Fang et al., 2004;Guha and Mackman, 2002;Hazeki et al., 2007;molnarfi et al., 2007). Specifically, activation of the pathway reduces the release of pro-inflammatory cytokines, likely serving to dampen inflammatory responses. In contrast, inactivation of PI-3K signaling leads to elevated production of pro-inflammatory cytokines and concomitant chronic inflammation. Additionally, it has recently been shown that the activation of the downstream PI-3K targets, GSK3β and mTORC1 are most likely involved in regulating NF-κB activation and, thereby, the pro-inflammatory response (Ohtani et al., 2008;Wang et al., 2008;Weichhart et al., 2008). Indeed, we demonstrate that in addition to GSK3β inhibitors discussed above, Bay11–7082, a NF-κB inhibitor, also blocks the pro-inflammatory effects of Cdt. Of particular relevance to our study are the findings of Fang et al (Fang et al., 2004) who studied the role of SHIP, a PIP3 phosphatase, on macrophage cytokine production. Indeed, they demonstrated that SHIP expression in deficient cells could reverse Akt activation and in turn, lead to increased cytokine production in response to LPS. Likewise, Schabbauer et al (Fang et al., 2004;Schabbauer et al., 2010)demonstrated that the absence of another PIP3 phosphatase, PTEN, lead to PI-3K activation and decreased inflammation in vivo. Our studies with the bacterial-derived PIP3 phosphatase, CdtB, provides further support for the role of PI-3K signaling in regulating IL-1β, TNFα and IL-6 production as exposure to the toxin blocked PI-3K signaling and resulted in increased cytokine production by macrophages at rest as well as in the presence of TLR agonists. Moreover, we demonstrate that this regulatory control involves a signaling pathway common to multiple TLRs (TLR-2, TLR-3 and TLR-4).
Figure 11.
Schematic summary indicating the mechanism by which Cdt, specifically CdtB, affects the PI-3K signaling pathway and the production of pro-inflammatory cytokines. The effects of CdtB on PI-3K signaling is shown in red. Exposure to the toxin results in degradation of PIP3 and reduced phosphorylation of both GSK3β and Akt and a increase in kinase activity of the former. GSK3β influences pro-inflammatory cytokine expression by modifying NF-κβ activation by influencing p65 subunit association with the common co-activator, CBP. The blue and green bars indicate the ability of GSK3β (blue) and NF-κβ (green) inhibitors to block the pro-inflammatory effects of Cdt.
These studies provide crucial insight into the molecular mechanism by which Cdt acts to perturb host responses and thereby promote disease associated with Cdt-producing organisms. A critical question that will need to be addressed in future studies is whether these pro-inflammatory in vitro effects are the result of biologically relevant quantities of Cdt? To date there are no estimates of the Cdt load in infected tissues; however, it is important to note that our observations occur at Cdt concentrations that are several orders of magnitude lower than those reported for the induction of apoptosis and/or cell cycle arrest in a number of cell types in vitro (Cortes-Bratti et al., 2001;Elwell et al., 2001;Nesic et al., 2004). Furthermore, our observations are consistent with the pathogenesis of inflammatory disorders that result from infection by Cdt-producing organisms such as A. actinomycetemcomitans. For example, periodontal disease is a bacterial-induced inflammatory disorder leading to the destruction of the tooth-supporting tissues. A common finding of both experimental and clinical investigations is the presence of elevated levels of pro-inflammatory cytokines in and around the damaged periodontium (Andrukhov et al., 2011;Bezerra et al., 2012;Fredman et al., 2011;Hajishengallis et al., 2012). Moreover, it has been suggested that the inflammatory destruction of tissue generates both degraded proteins and hemin which serve to fuel the nutritional needs of the bacteria and thereby promote bacterial growth and survival and further exacerbate inflammation (Hajishengallis et al., 2012)
In conclusion, our observations clearly demonstrate that Cdt is capable of interacting with macrophages, promote a pro-inflammatory cytokine response and thereby may contribute to persistent infection and inflammation. These effects are mediated at the molecular level by the action of the active Cdt subunit, CdtB, functioning as a PIP3 phosphatase leading to blockade of the PI-3K signaling pathway. In contrast, the acquired immune system is also affected by Cdt via effects on PI-3K signaling; specifically, treatment of activated lymphocytes with Cdt results in cell cycle arrest (G2) and apoptosis. This effect would likely prevent a productive immune response against the toxin, and possibly to microorganism(s) as well, leading to conditions that sustain infection and further promote inflammation. We anticipate that future studies will provide further insight into these in vitro relationships and the in vivo interactions that ultimately contribute to the pathogenesis of disease involving Cdt-producing bacteria.
Experimental Procedures
Cells and Reagents
The human acute monocytic leukemia cell line, THP-1, was obtained from ATCC; cells were maintained in RPMI 1640 containing 10% FBS, 1mM sodium pyruvate, 20 µM 2-mercaptoethanol and 2% penicillin-streptomycin at 37°C with 5% CO2 in a humidified incubator. THP-1 cells were differentiated into macrophages by incubating cells in the presence of 50 ng/ml PMA for 48 hr at which time the cells were washed and incubated an additional 24 hr in medium prior to use.
Human peripheral blood mononuclear cells (HPBMC) were obtained from venous blood of healthy donors as previously described (Shenker et al., 2004). Monocytes were further purified from HPBMC by positive selection using anti-CD14 mAb according to the manufacturers directions (Dynabeads® FlowComp™ Human CD14; Invitrogen). Anti-CD14 mAb-bead complexes were removed from the positively selected monocytes using a release buffer (Invitrogen). Monocytes were routinely found to be >96% pure as determined by immunofluorescent staining and analyzed by flow cytometry. Monocyte-derived macrophages (MDM) were generated by incubating monocytes in DMEM containing 10% fetal bovine serum and 2% penicillin/streptomycin for 6–8 days.
THP-1 derived macrophages and MDM were treated with recombinant Cdt holotoxin (both wildtype and mutant) or with individual Cdt subunits (including CdtBWT and CdtB mutants) which were expressed and purified as previously described (20,24). In some experiments, cells were also exposed to TLR agonists: LPS (E. coli 0111:B4; Calbiochem), PAM3CSK4 (Invivogen) or poly I:C (Invivogen). Two GSK3β inhibitors were employed: LiCl (Sigma-Aldrich) and SB216763 (Enzo) as well the NF-κB inhibitor, Bay-ll-7062 (Invivogen).
Analysis of DNA fragmentation and immunofluorescence
THP-1 cells and THP-1 derived macrophages (5 × 105) were prepared as described above and then treated with Cdt for 48 hr. Cells were harvested by replacing medium with ice cold PBS containing 5 mM EDTA. Likewise, monocytes or MDM (1 × 106) were incubated with Cdt for 48 hr; cells were collected following trypsinization. DNA fragmentation was measured using the TUNEL assay [In Situ Cell Death Detection Kit; (Boehringer Mannheim;)] (Shenker et al., 2006). Cells were harvested and re-suspended in 1 ml of freshly prepared 4% formaldehyde and permeabilized in 0.1% Triton X-100 for 2 min at 4°C. The cells were then washed with PBS and incubated in a solution containing FITC labeled nucleotide and terminal deoxynucleotidyl transferase (TdT) according to the manufacturers specifications. FITC fluorescence was assessed by flow cytometry using an argon laser at 488 nm to excite the fluorochrome; emission was measured through a 530/30 nm bandpass filter.
Intracellular CdtB was monitored in cells (1 × 106) incubated for 1 hr in the presence of medium or 2 µg/ml of CdtABCwt or CdtABCY71P at 37° or 5°C. Intracellular CdtB was detected following fixation with 2% formaldehyde for 30 min, permeabilization with 0.1% Triton X-100 in 0.1% sodium citrate and stained with anti-CdtB mAb conjugated to AlexaFluor 488 (Molecular Probes).
Measurement of cytokine mRNA by RT-PCR
THP-1 derived macrophages or MDM (1 × 106) were prepared and treated as described above for 2 hr. RNA was extracted from cells using a commercially available kit (RNeasy; Qiagen). RT-PCR was employed to measure relative mRNA levels for IL-1β, TNFα and IL-6. Briefly, cDNA synthesis was performed using a commercially available kit and according to the manufacturers specifications (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems). RT-PCR was performed on an ABI Fast Real-Time PCR System using TaqMan sequence specific probes (Invitrogen). The fold increase in mRNA was calculated according to the method described by Livak and Schmittgen (Livak and Schmittgen, 2001).
Measurement of cytokine production
Cytokine production was measured in THP-1 derived macrophages (2 × 105 cells) and MDM (1 × 106 cells) incubated for 5 hr and 24 hr, respectively. Culture supernatants were collected and analyzed by ELISA for IL-1β (Quantikine Elisa Kit; R and D Systems), TNFα (Peprotech) and IL-6 (Peprotech) using commercially available kits according to the manufacturers instructions. In each instance, the amount of cytokine present in the supernatant was determined using a standard curve.
Analysis of Akt and GSK3β phosphorylation status and kinase activity
Replicate wells of THP-1 derived macrophages (1 × 106 cells/well) were incubated as described with Cdt. The cells were washed and treated with 20 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholate and protease and phosphatase inhibitors (Pierce); replicate wells were pooled and protein concentration determined. Samples (30 µg) were separated on 12% SDS-PAGE and then transferred to nitrocellulose. The membrane was blocked with BLOTTO and then incubated with one of the following primary antibodies for 18 hr at 4°C (Shenker et al., 1999): anti-pAkt (S473) and anti pGSK (S9) (Cell Signaling). Membranes were washed, incubated with goat anti-mouse immunoglobulin [1:1000 dilution; (Southern Biotech)]conjugated to horseradish peroxidase. The Western blots were developed using chemiluminescence and analyzed by digital densitometry (Kodak Image Systems) as previously described (Shenker et al., 2010b).
Akt and GSK3β specific kinase activity was assessed in control and Cdt-treated cells. THP-1-derived macrophages (2 × 106) were treated with Cdt as described; replicate cultures pooled and solubilized. Akt activity was measured using a commercially available assay kit (Cell Signaling Technology). Briefly, pAkt was immunoprecipitated and the enzyme was assessed for its ability to phosphorylate GSK3β in vitro; activity was monitored by Western blot as described above. GSK3β kinase activity was also measured using a commercially available kit (Sigma Chemical Co). The kinase was immunoprecipitated and activity determined by following the utilization of 32P-ATP to phosphorylate a synthetic substrate.
Measurement of cellular PIP3 content
Cells (1 × 106/ml) were incubated in the presence of medium or Cdt for 0–240 min. Replicate cultures (0.5–1 × 107 cells) were pooled and harvested. The cell pellet was treated with cold 0.5 TCA for 5 min, centrifuged and the pellet washed twice with 5% TCA containing 1 mM EDTA. Neutral lipids were extracted twice with methanol:chloroform (2:1) at RT. Acidic lipids were extracted with methanol:chloroform:12M HCl (80:40:1) for 15 min at RT; the samples were centrifuged for 5 min and the supernatant recovered. The supernatant was then treated with 0.75 ml chloroform and 0.1 M HCl and centrifuged to separate organic and aqueous phases; the organic phase was collected and dried. The dried lipids were resuspended in 120 µl 50 mM HEPES buffer (pH 7.4) containing 150 mM NaCl and 1.5% sodium cholate, and left overnight at 4°C. PI-3,4,5-P3 levels were then determined using commercially available competitive ELISA according to the manufacturers directions (Echelon).
Statistical analysis
Mean ± standard error of the mean were calculated for replicate experiments. Significance was determined using a Student’s t-test; a p-value of less than 0.05 was considered to be statistically significant.
Acknowledgments
We thank the SDM flow cytometry core facility for its outstanding technical expertise. This work was supported by the National Institutes of Health grants DE06014, DE023071 and DE022465.
References
- Andrukhov O, Ulm C, Reischl H, Nguyen P, Matejka M, Rausch-Fan X. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontology. 2011;82:885–892. doi: 10.1902/jop.2010.100425. [DOI] [PubMed] [Google Scholar]
- Antoniv T, Ivashkiv L. Interleukin-10-induced gene expression and suppressive function are selectively modulated by the PI3K-Akt-GSK3 pathway. Immunol. 2011;132:567–577. doi: 10.1111/j.1365-2567.2010.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon V, Chao K, Dreyfus LA. Effect of cytolethal distending toxin on F-Actin assembly and cell division in chinese hamster ovary cells. Infection & Immunity. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbibe L, Mira J-P, Teusch N, Kline L, Guha M, Mackman N, et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nature Immunology. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- Baird S, Reid L, Hampton M, Gieseg S. OxLDL induced cell death is inhibited by the macrophage synthesized pterin, 7,8-dihydroneopterin, in U937 cells but not THP-1 cells. Biochim Biophys Acta. 2005;1745:361–369. doi: 10.1016/j.bbamcr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Bezerra B, Andriankaja O, Kang J, Pacios S, Bae H, Li Y, et al. A. actinomycetemcomitans-induced periodontal disease promotes systemic and local responses in rat periodontium. J Clin Perio. 2012;39:333–341. doi: 10.1111/j.1600-051X.2011.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Besack D, McKay TL, Zekavat A, Otis L, Jordan-Sciutto K, et al. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Cellular Microbiology. 2006;8:823–836. doi: 10.1111/j.1462-5822.2005.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Brown A, Walker L, Besack D, Zekavat A, Wrenn S, et al. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J Biol Chem. 2009;284:10650–10658. doi: 10.1074/jbc.M809094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Wang H, Hajishengallis H, Martin M. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2010;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busca A, Saxena M, Kumar A. Critical role for anti-apoptotic Bcl-xL and Mcl-1 in human macrophage survival and cellular IAP1/2 (cIAP1/2) in resistance to HIV-Vpr-induced apoptosis. Journal Biological Chemistry. 2011;287(18):15118–15133. doi: 10.1074/jbc.M111.312660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comayras C, Tasca C, Peres SY, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infection & Immunity. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope L, Lumbley SLJ, Klesney-Tait J, Stevens M, Johnson L, Purven M, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad SCi. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Bratti X, Karlsson C, Lagergard T, Thelastam M, Frisan T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J Biol Chem. 2001;276:5296–5302. doi: 10.1074/jbc.M008527200. [DOI] [PubMed] [Google Scholar]
- Elwell CA, Chao K, Patel K, Dreyfus LA. Escherichia coli CdtB Mediates Cytolethal Distending Toxin Cell cycle Arrest. Infect Immun. 2001;69:3418–3422. doi: 10.1128/IAI.69.5.3418-3422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi A, Maldonado-Arocho F, Gargi A, Cardwell M, Prouty M, Blanke S, et al. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. J Bio Chem. 2010;285(24):18199–18207. doi: 10.1074/jbc.M110.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Pengal R, Cao X, Ganesan L, Wewers M, Marsh C, et al. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J Immunol. 2004;173:360–366. doi: 10.4049/jimmunol.173.1.360. [DOI] [PubMed] [Google Scholar]
- Fredman G, Oh S, Ayilavarapu S, Hasturk H, Serhan C, Van Dyke T. Impaired phagocytosis iin localized aggressive periodontitis: rescue by resolvin E1. PLoS ONE. 2011;6(9):e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfanova V, Hansen E, Spinola S. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins. 2011;3:172–190. doi: 10.3390/toxins3030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau R, Curtis M. The keystone-pathogen hypothesis. Nature Reviews. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- Hochdorfer T, Kuhny M, Zorn C, Hendriks R, Vanhaesebroeck B, Bohnacker T, et al. Activation of the PI3K pathway increases TL-induced TNF-α and IL-6 but reduces IL-1β production in mast cells. Cell Signalling. 2011;23:866–875. doi: 10.1016/j.cellsig.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Lally ET, Kieba I, Sato A, Green C, Rosenbloom J, Korostoff J, et al. RTX toxins recognize a β2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- Lee J, Nauseef W, Moeenrezakhanlou A, Sly L, Noubir S, Leidal K, et al. Monocyte P110α phosphatidylinositol 3-kinase regulates phagocytosis, the phagocyte oxidase, and cytokine production. J Leukocyte Bio. 2007;81:1548–1561. doi: 10.1189/jlb.0906564. [DOI] [PubMed] [Google Scholar]
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the −ΔΔT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bueno L, Hansen E, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnarfi N, Gruaz L, Dayer J, Burger D. Opposite regulation of IL-1β and secreted IL-1 receptor antagonist production by phophatidylinositide- kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J Immunol. 2007;178:446–454. doi: 10.4049/jimmunol.178.1.446. [DOI] [PubMed] [Google Scholar]
- Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004;429:429–433. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- Ohara M, Hayashi T, Kusonoki Y, Miyauchi M, Takata T, Sugai M. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infect Immun. 2004;72:871–879. doi: 10.1128/IAI.72.2.871-879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infection & Immunity. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Pengal R, Ganesan L, Wei G, Fang J, Ostrowski M, Tridandapani S. Lipopolysaccharide-induced production of interleukin-10 is promoted by serine/threonine kinase Akt. Mol Immunol. 2006;43:1557–1564. doi: 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infection & Immunity. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtBgene. Infection & Immunity. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Whitehouse CA. The cytolethal distending toxin family. Trends in Microbiology. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- Rabin S, Flitton J, Demuth DR. Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces aoptosis in nonproliferating macrophages by a phosphatase-independent mechanism. Infect Immun. 2009;77:3161–3169. doi: 10.1128/IAI.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabbauer G, Matt U, Gunl P, Warszawska J, Furtner T, Hainzl E, et al. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Ali H, Boesze-Battaglia K. PIP3 regulation as promising targeted therapy of mast cell mediated diseases. Current Pharm Design. 2011;17:3815–3822. doi: 10.2174/138161211798357926. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Besack D, McKay TL, Pankoski L, Zekavat A, Demuth DR. Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt): evidence that the holotoxin is composed of three subunits: CdtA, CdtB, and CdtC. J Immunol. 2004;172:410–417. doi: 10.4049/jimmunol.172.1.410. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Besack D, McKay TL, Pankoski L, Zekavat A, Demuth DR. Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin requires three subunits for maximum activity. J Immunol. 2005;174:2228–2234. doi: 10.4049/jimmunol.174.4.2228. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Demuth DR, Zekavat A. Exposure of lymphocytes to high doses of Actinoibacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infect Immun. 2006;74:2080–2092. doi: 10.1128/IAI.74.4.2080-2092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Dlakic M, Walker L, Besack D, Jaffe E, Labelle E, et al. A novel mode of action for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol (3,4,5) tri-phosphate phospatase activity. J Immunol. 2007;178:5099–5108. doi: 10.4049/jimmunol.178.8.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Hoffmaster RH, McKay TL, Demuth DR. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T-cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Hoffmaster RH, Zekavat A, Yamguchi N, Lally ET, Demuth DR. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J Immunol. 2001;167:435–441. doi: 10.4049/jimmunol.167.1.435. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, McArthur WP, Tsai CC. Immune Suppression Induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982;128:148–154. [PubMed] [Google Scholar]
- Shenker BJ, McKay TL, Datar S, Miller M, Chowhan R, Demuth DR. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- Shenker BJ, Vitale L, King C. Induction of human T cells that coexpress CD4 and CD8 by an immunomodulatory protein produced by Actinobacillus actinomycetemcomitans. Cell Immunol. 1995;164:36–46. doi: 10.1006/cimm.1995.1140. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Vitale LA, Welham DA. Immune suppression induced by Actinobacillus actinomycetemcomitans: Effects on immunoglobulin production by human B cells. Infect Immun. 1990;58:3856–3862. doi: 10.1128/iai.58.12.3856-3862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B, Boesze-Battaglia K, Zekavat A, Walker L, Besack D, Ali H. Inhibition of mast cell degranulation by a chimeric toxin containing a novel phosphatidylinositol-3,4,5-triphosphate phosphatase. Molec Immunol. 2010;48:203–210. doi: 10.1016/j.molimm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachado S, Li X, Swan K, Patel N, Koziel H. Constitutive activation of phsphatidylinositol 3-kinase signaling pathway down regulates TLR4-mediated tumor necrosis factor-α release in alveolar macrophages from asymptomatic HIV positive persons in vitro. J Biol Chem. 2008;283:33191–33198. doi: 10.1074/jbc.M805067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Garcia C, Rehani K, Cekic C, Alard P, Kinane D, et al. IFN-β production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-β. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier K, et al. The TSC0mTOR signaling pathway regulates theinnate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Xu T, Lundqvist A, Ahmed H, Eriksson K, Yang Y, Lagergard T. Interactions of Haemoophilus ducreyi and purified cytolethal distending toxin with human monocyte-derived dendritic cells, macrophages and CD4+T cells. Microbes and Infection. 2004;6:1171–1181. doi: 10.1016/j.micinf.2004.07.003. [DOI] [PubMed] [Google Scholar]