Abstract
Rationale
The α4β2 nicotinic acetylcholine receptor partial agonist varenicline has been reported to reduce drinking among both heavy-drinking smokers and primary alcoholics, and this effect may be related to varenicline-mediated reduction of alcohol craving. Among smokers, varenicline has been reported to modulate cigarette cue-elicited brain activation in several reward-related areas.
Objectives
This pilot study tested varenicline’s effects on drinking, alcohol craving, and alcohol cue-elicited activation of reward-related brain areas among non-treatment-seeking alcohol-dependent individuals.
Methods
Thirty-five such individuals (mean age = 30, 57% male, 76% heavy drinking days in the past month, 15 smokers) were randomized to either varenicline (titrated to 2 mg) or placebo for 14 days, and were administered an alcohol cue reactivity fMRI task on day 14. A priori regions of interest (ROIs) were bilateral and medial orbitofrontal cortex (OFC), right ventral striatum (VS), and medial prefrontal cortex (mPFC).
Results
Despite good medication adherence, varenicline did not reduce heavy drinking days or other drinking parameters. It did, however, increase self-reported control over alcohol-related thoughts and reduced cue-elicited activation bilaterally in the OFC, but not in other brain areas.
Conclusions
These data indicate that varenicline reduces alcohol craving and some of the neural substrates of alcohol cue reactivity. However, varenicline effects on drinking mediated by cue-elicited brain activation and craving might be best observed among treatment-seekers motivated to reduce their alcohol consumption.
Keywords: Varenicline, alcohol, fMRI, neuroimaging, craving
Introduction
Several groups have reported that the α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist varenicline (Chantix) (Pfizer, New York, NY), a medication initially approved by the Food and Drug Administration for smoking cessation, reduces alcohol consumption among heavy-drinking smokers (Fucito, et al., 2011, McKee, et al., 2009, Mitchell, et al., 2012). Recently, a multisite randomized controlled trial reported that treatment-seeking alcoholics who received varenicline for 13 weeks demonstrated reductions in several drinking parameters, including percent heavy drinking days and drinks per drinking day (Litten, et al., 2013). Interestingly, although this trial was not designed to evaluate the issue, smoking status appeared not to moderate these effects, suggesting that varenicline’s influence on drinking may not be limited to individuals with comorbid alcohol and nicotine dependence. Among smokers, varenicline is believed to reduce nicotine intake via saturation of nAChRs normally bound by nicotine, thereby reducing cigarette craving (Ray, et al., 2013). Some data suggest that it also reduces alcohol craving (Fucito, et al., 2011, Litten, et al., 2013), but other mechanisms have been suggested (Childs, et al., 2012, Kamens, et al., 2010a), and the neurobiological mechanism of action of varenicline for reducing alcohol craving is less clear than for its nicotine effects.
Craving for all drugs of abuse is thought to be mediated, in part, by neurotransmission along the mesolimbic and mesocortical dopamine pathways, which project from the ventral tegmental area (VTA) and substantia nigra in the midbrain to the ventral striatum (VS), orbitofrontal cortex (OFC), and medial prefrontal cortex (mPFC). Drug-related cues potently elicit craving, and as addiction progresses, drug-induced dopamine release in these areas diminishes, while cue-elicited dopamine release increases (Volkow, et al., 2011). Midbrain dopaminergic projection neurons express α4β2 nAChRs, and nicotine acutely elicits dopamine release through agonism of these receptors (Di Chiara, 2000). Thus, partial agonism of midbrain nAChRs may influence alcohol craving and consumption through modulation of dopamine release. Administration of the nonselective nAChR antagonist mecamylamine in the VTA has been shown to block alcohol-induced potentiation of dopamine release in VS (Tizabi, et al., 2002) and attenuate alcohol intake and preference among alcohol-preferring rats (Ericson, et al., 1998). Further, systemically administered varenicline reduces alcohol seeking, voluntary alcohol intake, and cue-induced reinstatement of drinking among animals chronically exposed to alcohol (Kamens, et al., 2010b, Steensland, et al., 2007, Wouda, et al., 2011), consistent with preclinical data suggesting that its partial agonist property engenders weak dopamine release (Rollema, et al., 2007) that may satiate craving while blocking reward-seeking behavior. In humans, positron emission tomography indicates that a single 0.5 mg dose of varenicline completely saturates midbrain α4β2 nAChRs (Lotfipour, et al., 2012). Thus, varenicline may reduce alcohol craving through nAChrR-mediated attenuation of alcohol cue-elicited dopamine release.
Lending further support to this putative neurobiological mechanism, a recent functional magnetic resonance imaging (fMRI) study of heavy smokers reported that varenicline, relative to placebo, reduced cigarette cue-elicited activation of several mesolimbic and mesocortical areas, including VS and OFC (Franklin, et al., 2011). Among alcoholics, alcohol cues elicit increased activation in the same brain areas (see Schacht, et al., 2013a), and previous studies have indicated that naltrexone, a medication that reduces alcohol craving through opioid-mediated attenuation of dopamine transmission, also reduces cue-elicited activation of these areas (Lukas, et al., 2013, Myrick, et al., 2008). Given these findings, in the current pilot study, we hypothesized that varenicline, relative to placebo, would reduce alcohol craving and alcohol cue-elicited activation of reward-related brain areas among individuals with alcohol dependence.
Materials and methods
Subjects
Subjects were recruited via media advertisements and administered a brief phone screen to assess inclusion/exclusion criteria. Forty individuals met these criteria and participated in the study. Subjects were required to be between ages 21 and 60, to report consuming at least 20 drinks per week, to meet DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, revised 4th edition) (American Psychiatric Association, 2000) diagnostic criteria for Alcohol Dependence, as assessed by the Structured Clinical Interview for DSM-IV (SCID) (First, et al., 2002), and to not currently be seeking treatment for their drinking. Exclusion criteria were: current DSM-IV diagnosis of dependence on any substance except nicotine; use of any psychoactive medication or substance except nicotine or marijuana in the past 30 days, as evidenced by self-report and urine drug screen (UDS); current DSM-IV Axis I diagnosis or suicidal/homicidal ideation; history of significant alcohol-related medical illness; or liver enzymes ≥ 2.5 times normal. Although recent marijuana use was not exclusionary, all subjects were required to have a negative UDS for Δ9-tetrahydocannabinol (cutoff: 50 ng/mL) before beginning the study.
Procedure
The Medical University of South Carolina Institutional Review Board for Human Research approved all procedures, and all subjects provided informed consent before participation, for which they were compensated. The study comprised four visits across 14 days: a screening visit; a randomization visit (study day 1); a mid-point assessment (day 7); and the fMRI scan session (day 14). At the screening visit, a trained interviewer administered the SCID and the Timeline Follow-back (TLFB) (Sobell and Sobell, 1992) to assess past-month drinking. Subjects then provided a urine sample for UDS and completed the Alcohol Dependence Scale (ADS) (Skinner and Allen, 1982) and Obsessive-Compulsive Drinking Scale (OCDS) (Anton, et al., 1996). Eligible subjects returned for the randomization visit, at which they were urn-randomized, on the basis of gender, smoking status, and family history of alcoholism (defined as having one or more first-degree biological relatives who subjects reported had a problem with drinking), to identical-appearing varenicline or placebo. Both investigators and subjects were blind to medication group assignment.
Subjects were provided eleven 0.5 mg capsules on study day 1, and were instructed to take one capsule on days 1–3 and two capsules on days 4–7. When they returned to the laboratory on day 7, subjects were provided fourteen 1 mg capsules, and were instructed to take one capsule twice daily on days 8–14 (2 mg/day). To allow assessment of medication adherence, both active and placebo capsules were over-encapsulated with 100 mg riboflavin. Urinary riboflavin was assessed at baseline and on day 14. Subjects were given no explicit instructions regarding their drinking behavior for days 1–12 but were instructed to abstain from drinking for 24 hours before the scan session (i.e., on day 13). Subjects were also given no explicit instructions regarding smoking, but were asked to keep a smoking log to record the number of cigarettes they smoked each day. On days 7 and 14, the TLFB and OCDS were administered again to assess past-week drinking and alcohol craving. At these visits, subjects were also administered a modified version of the SAFTEE checklist (Levine and Schooler, 1986) that assessed the presence or absence of 18 possible side effects: nausea, vomiting, diarrhea, abdominal pain, increased/decreased appetite, headache, dizziness, fatigue, anxiety, insomnia, somnolence, depression, itching, rash, increased/decreased libido, and vivid dreams. During debriefing after the scan session, three subjects, all in the placebo group, revealed violations of the experimental protocol, and were excluded from all analyses: one reported that he worked as a professional study subject; one reported past-week cocaine use, and one reported that he had stopped drinking to the levels required for study inclusion before medication randomization.
Alcohol cue reactivity task
At the scan session, subjects were breathalyzed and administered the Clinical Institute Withdrawal Assessment for Alcohol-Revised (CIWA-Ar) (Sullivan, et al., 1989). No subject had a breath alcohol level > 0 or a CIWA-Ar score > 3. Subjects who smoked were permitted to do so until one hour before the scan. After subjects were positioned in the scanner, a high-resolution anatomical image was acquired. Subjects were then given a sip (10 mL) of 80-proof liquor mixed with fruit juice immediately before a 720-s-long alcohol cue reactivity task was administered. This task, previously described in greater detail in Schacht et al. (2011), consisted of 24 pseudorandomly interspersed blocks of alcoholic beverage images (ALC), non-alcoholic beverage images (BEV), blurred versions of both of these types of images that served as visual controls, and a fixation cross. Each 24-s-long block was composed of five individual pictures, each displayed for 4.8 s, and was followed by a 6-s washout period intended to allow the hemodynamic response from the previous block to decline before the next was presented. Images were selected from a normative set (Stritzke, et al., 2004), supplemented with images from advertisements, and matched for intensity, color, and complexity. ALC blocks were equally distributed between images of beer, wine, and liquor.
Image acquisition and pre-processing
Functional images were acquired with a 3T Siemens (Erlangen, Germany) TIM Trio scanner, using a gradient echo, echo-planar imaging scan sequence. Image acquisition parameters were: repetition/echo time = 2200/30 ms; 328 volumes; flip angle = 90°; field of view = 208 mm; matrix = 64 × 64; voxel size = 3.25 × 3.25 mm; 36 contiguous 3.0-mm-thick transverse slices. Using FSL v. 4.1.7 (Oxford Centre for Functional MRI of the Brain, Oxford England) (Smith, et al., 2004), images were realigned to the first volume, spatially smoothed with an 8-mm anisotropic Gaussian kernel, resampled to 2-mm isotropic voxel size, spatially whitened with a global autoregressive filter, and high-pass filtered (period = 240 s) to eliminate low-frequency noise in the blood-oxygen-level-dependent (BOLD) signal. To eliminate task-correlated motion, for each subject, the six motion parameters from the realignment were regressed out of the images. Two subjects, both in the varenicline group, had more than 2 mm of translational or 2° of rotational movement during the scan and were excluded from the imaging analysis. Finally, the preprocessed images were registered with a 12-degree-of-freedom affine transformation, first to the subject’s high-resolution anatomical image and subsequently to the Montreal Neurological Institute (MNI) 152-subject-average template.
Regions of interest (ROIs)
ROIs were defined by the arithmetic means of the MNI coordinates of regions in which two or more previous studies had reported a main effect of medication or an interaction between medication and an individual difference variable (i.e., genotype, clinical presentation) on alcohol cue-elicited activation. Six such studies were identified (Hermann, et al., 2006, Lukas, et al., 2013, Myrick, et al., 2008, 2010, Schacht, et al., 2013b, 2013c). Regions from the recent study that reported varenicline effects on cigarette cue-elicited activation (Franklin, et al., 2011) were also included. This process yielded five ROIs, defined as a 6-mm-radius sphere with its center at these MNI coordinates: left OFC, [−22 42 −12]; right OFC, [32 34 −11]; medial OFC, [0 43 −16]; right VS, [7 10 −6]; and medial PFC, [3 53 13] (see Figure 1). Using the transformation matrices previously calculated, ROIs were reverse-registered to each subject’s anatomical image, and the average timecourse of the BOLD signal was extracted from the preprocessed images across all the voxels in each ROI.
Figure 1.
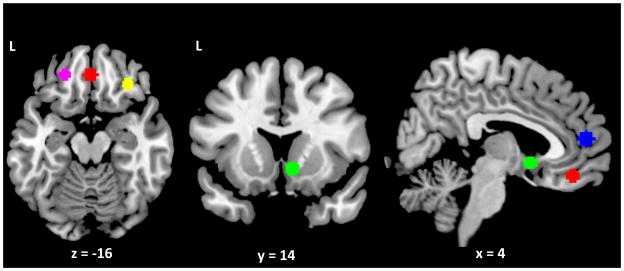
Regions of interest: left (purple), medial (red) and right (yellow) orbitofrontal cortex; right ventral striatum (green); and medial prefrontal cortex (blue).
Statistical analysis
Drinking and craving data were analyzed with linear mixed models, using the MIXED function in SPSS v. 19 (IBM, Armonk, NY). For the drinking data, medication group, week of assessment (week 1 or 2), and their interaction were entered as predictors of the percentage of drinking days on which subjects drank heavily during the treatment period (%HDD; i.e., ≥ 5 standard drinks in one day for men, or ≥ 4 for women), with week of assessment as a within-subjects repeated measure and baseline %HDD, cigarette smoking, age and family history of alcoholism covaried. %HDD was chosen as the drinking outcome because it was the primary endpoint on which varenicline demonstrated an effect in the recent (Litten, et al., 2013) trial among treatment-seeking alcoholics. Secondary drinking outcomes were the percentage of days during the treatment period on which subjects were abstinent from alcohol and the number of standard drinks consumed per drinking day. These variables excluded study day 13, on which subjects were instructed not to drink. Among smokers, the number of cigarettes smoked per day was widely distributed (M = 9.8, SD = 6.5, range = 1–20); therefore, smoking was modeled as a continuous variable (average number of cigarettes per day in the month before randomization). For the craving data, the same models were used to predict overall OCDS score and, in an exploratory fashion, the three empirically derived OCDS factor scores (Resistance/Control Impairment [RCI], Obsession, and Interference) (Roberts, et al., 1999), but with the baseline scores on these factors covaried instead of %HDD. Overall OCDS score was calculated as the sum of these three factor scores. To isolate the processes specific to control over alcohol-related thoughts, the drinking quantity and frequency items (items 7 and 8) were removed from the RCI factor. Secondarily, medication effects on cigarettes per day during treatment were analyzed with a mixed model that included medication, week of assessment, and their interaction, with baseline cigarettes per day, age, and family history covaried. For the drinking, craving, and smoking analyses, an alpha level of p ≤ .05 was considered statistically significant.
For the imaging data, BOLD timecourses from the ROIs described above were entered as the dependent variables in each of five hierarchical linear models (HLMs), using HLM v. 7.0 (Scientific Software International, Inc., Skokie, IL). The order, onset, and duration of presentation of the cue reactivity task stimuli were entered as random first-level (i.e., within-subjects) independent variables, and medication group was entered as a random second-level (i.e., between-subjects) independent variable, with age, family history of alcoholism, cigarettes per day, and pre-treatment %HDD entered as random second-level covariates. These models yielded individual estimates of ALC – BEV activation in each ROI for each subject, as well as second-level effects of medication. To examine whether drinking or craving was associated with ALC – BEV activation, HLMs with the cue reactivity stimuli at the first level and either %HDD during the study or each of the week 2 OCDS factor scores at the second level, covarying for cigarettes per day, were run in each medication group. Statistics reported for the HLMs are t contrasts for the effects of the second-level variables with robust standard errors. For the imaging analyses, Bonferroni correction was implemented to account for the five different ROIs tested; thus, an alpha level of p ≤ .01 was considered statistically significant.
Results
Baseline data, medication adherence, and side effects
Table 1 presents baseline demographic and behavioral data for the 35 study completers with usable scan data. As expected given the urn randomization procedure, the medication groups were well matched on gender, smoking status, and family history of alcoholism, although the varenicline group was significantly younger than the placebo group. There was a trend towards better adherence with varenicline than placebo; 17/18 varenicline subjects, but only 12/17 placebo subjects, had urinary riboflavin > 1500 ng/mL on day 14 (χ2(1, N = 35) = 3.50, p = 0.06). Results reported below did not differ when non-adherent subjects were removed from the analysis. There were no significant differences between medication groups in the presence of any side effects at day 7, but at day 14, significantly more subjects in the varenicline group (4/18) reported headache than in the placebo group (0/17) (χ2(1, N = 35) = 4.27, p = 0.04). There were no significant differences between medication groups in the presence of any other side effects at day 14.
Table 1.
Demographic and baseline data.
| Varenicline | Placebo | Test for difference | |
|---|---|---|---|
| N (M/F) | 11/7 | 9/8 | χ2(1, N = 35) = 0.24, p = 0.63 |
| Age | 26.8 (4.3) | 33.7 (12.0) | t(19.8) = −2.25, p = 0.04* |
| Education, years | 14.2 (1.7) | 15.3 (2.1) | t(33) = −1.77, p = 0.09 |
| Alcohol Dependence Scale | 11.0 (5.2) | 12.0 (6.8) | t(33) = −0.49, p = 0.63 |
| Family history positive, N | 9 | 11 | χ2(1, N = 35) = 0.77, p = 0.38 |
| Drinking days (past month) | 21.1 (6.3) | 22.9 (4.6) | t(33) = −0.94, p = 0.35 |
| Drinks per drinking day (past month) | 8.6 (6.9) | 6.8 (1.8) | t(33) = 1.04, p = 0.31 |
| Percent heavy drinking days (past month) | 75.5 (23.2) | 76.2 (18.5) | t(33) = −0.10, p = 0.92 |
| Cigarette smokers, N | 7 | 8 | χ2(1, N = 35) = 0.24, p = 0.63 |
| Cigarettes per day (past month) | 10.6 (7.5) | 9.1 (5.9) | t(13) = 0.44, p = 0.67 |
Equal variances not assumed
Varenicline effects on drinking and alcohol craving
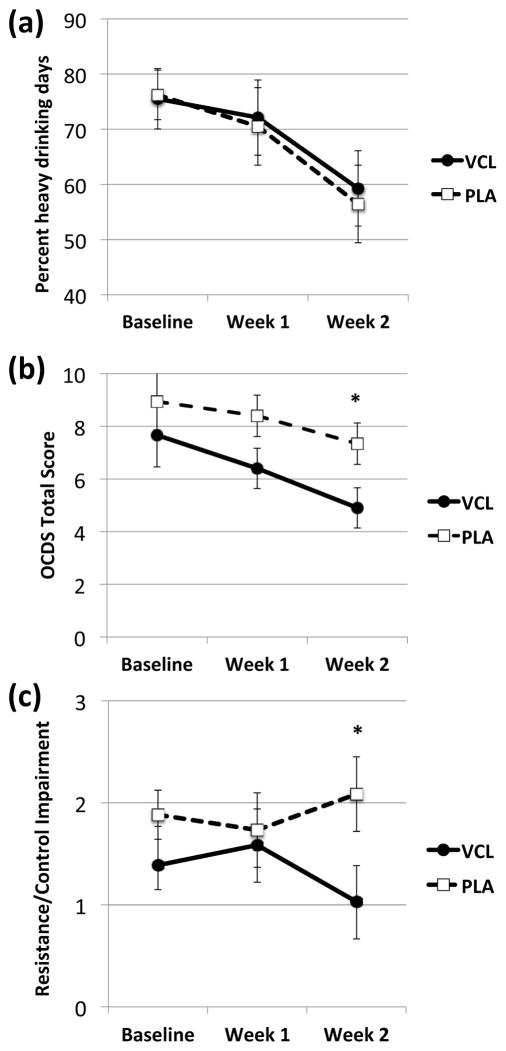
Pre-treatment %HDD predicted %HDD during treatment (F(1, 29) = 6.63, p = 0.02), but varenicline, relative to placebo, did not reduce %HDD over both weeks of treatment (F(1, 29) = 0.07, p = 0.80) or interact with time in reducing it more rapidly between weeks 1 and 2 (F(1, 33) = 0.01, p = 0.91) (see Figure 2a). Varenicline also had no effect on drinks per drinking day (main effect: F(1, 29) = 0.03, p = 0.87; interaction with time: F(1, 33) = 0.17, p = 0.68) or percent days abstinent (main effect: F(1, 29) = 0.53, p = 0.47; interaction with time: F(1, 33) = 0.25, p = 0.62). Similarly, pre-treatment smoking predicted cigarettes per day during treatment (F(1, 10) = 8.92, p = 0.01), but varenicline, relative to placebo, did not reduce smoking overall (F(1, 10) = 1.68, p = 0.23) or reduce it more rapidly between weeks 1 and 2 (F(1, 13) = 0.002, p = 0.96).
Figure 2.
(a) Percent heavy drinking days (%HDD); (b) Obsessive Compulsive Drinking Scale (OCDS) total scores; and (c) OCDS Resistance/Control Impairment (RCI) factor scores at baseline and during each week of treatment in each medication group. Figures are estimated marginal means (± standard errors) from linear mixed models that included medication, cigarettes per day, time, and the interaction of medication and time, covarying for baseline score, age, and family history of alcoholism. There was no significant difference between the varenicline (VCL) and placebo (PLA) groups in %HDD, but the VCL group had lower overall OCDS scores (p = 0.04), an effect partially driven by a greater decline in RCI scores between weeks 1 and 2 (p = 0.04). * p < 0.05 for simple effect of medication.
Varenicline did significantly reduce OCDS scores at both weeks 1 and 2 relative to placebo (F(1, 29 = 4.43, p = 0.04); simple effects analysis indicated the varenicline group displayed a trend towards lower scores at week 1 (F(1, 38.46 = 3.08, p = 0.09) and significantly lower scores at week 2 (F(1, 38.46) = 4.60, p = 0.04) (see Figure 2b). Exploratory follow-up analysis of the OCDS factors suggested that this effect was partially driven by changes in the RCI factor, such that varenicline significantly reduced RCI scores between weeks 1 and 2 (i.e., increased control over alcohol-related thoughts), while placebo did not affect this factor (F(1, 29 = 5.63, p = 0.02) (see Figure 2c). Simple effects analysis indicated that the varenicline group had significantly lower scores at week 2 (F(1, 37.68) = 3.99, p = 0.05). For the Interference factor, there was a trend for varenicline to reduce scores at both weeks 1 and 2 (F(1, 29) = 3.77, p = 0.06), and simple effects analysis indicated that the varenicline group had significantly lower scores at week 1 (F(1, 49.22) = 4.22, p = 0.05). For the Obsession factor, there was no significant main effect of medication, nor an interaction between medication and time. Smoking status did not significantly affect any of the OCDS factors.
Varenicline effects on alcohol cue-elicited activation
Among subjects who received placebo, there was significant ALC – BEV activation in left OFC and right VS, with a trend for significance in right OFC (see Table 2). Varenicline, relative to placebo, significantly reduced ALC – BEV activation in the left (t(29) = −3.58, p = 0.001) and right (t(29) = −3.32, p = 0.002) OFC, (see Figure 3). However, varenicline did not significantly moderate ALC – BEV activation of the medial OFC (t(29) = −1.65, p = 0.11), right VS (t(29) = −1.71, p = 0.10), or mPFC (t(29) = −0.02, p = 0.99). Heavier smoking was associated with greater ALC – BEV activation in left OFC (t(29) = 2.69, p = 0.01) and right VS (t(29) = 2.83, p = 0.008), with a trend for this relationship in right OFC (t(29) = 2.18, p = 0.04). Older individuals had less ALC – BEV activation in right (t(28) = −2.69, p = 0.01) and medial (t(28) = −3.46, p = 0.002) OFC, and family history positive individuals had less ALC – BEV activation in medial OFC (t(28) = −2.62, p = 0.01), but there were no other significant effects of covariates on ROI activation.
Table 2.
Alcohol cue-elicited activation in each region of interest in the placebo group.
| Region of interest | ALC – BEV contrast | p value |
|---|---|---|
| Left OFC | t(16) = 3.13 | 0.007 |
| Right OFC | t(16) = 2.51 | 0.02 |
| Medial OFC | t(16) = 1.34 | 0.20 |
| Right VS | t(16) = 2.95 | 0.009 |
| Medial PFC | t(16) = 1.23 | 0.24 |
Figure 3.
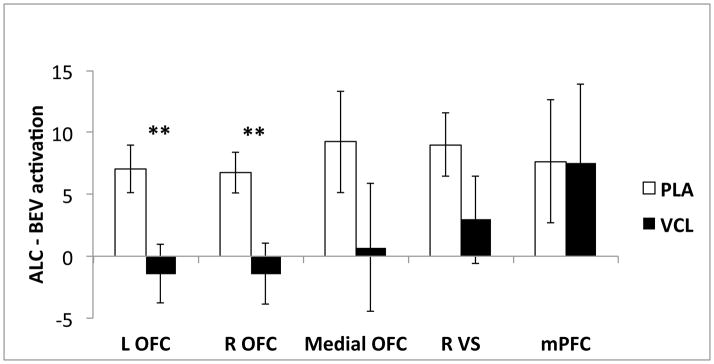
Mean alcohol cue-elicited activation (ALC – BEV) in each medication group. Figures are means (arbitrary units ± standard errors) from hierarchical linear models that included medication, cigarettes per day, age, family history of alcoholism, and baseline percent heavy drinking days. Varenicline (VCL), relative to placebo (PLA), significantly reduced ALC – BEV activation in left and right orbitofrontal cortex (OFC), but there were no significant between-groups differences in ALC – BEV activation of medial OFC, right ventral striatum (VS), or medial prefrontal cortex (mPFC). ** p < 0.005.
Relationships between alcohol cue-elicited activation, drinking, and alcohol craving
There were no significant relationships between ALC-BEV activation in any ROI and %HDD during treatment or the OCDS factor scores (all p values > 0.10) in either medication group.
Discussion
In this small pilot study of varenicline among individuals with alcohol dependence who were not seeking to reduce their drinking or cigarette smoking, two weeks of varenicline treatment did not reduce drinking or smoking, but did decrease alcohol craving and alcohol cue-elicited activation of bilateral orbitofrontal cortex. However, varenicline did not reduce cue-elicited activation of other regions (i.e., medial OFC, VS, mPFC) in which previous studies had reported that medications had attenuated such activation. Further, cue-elicited activation in these ROIs was not related to drinking or alcohol craving during the treatment period. Thus, considered together, these data present a mixed picture regarding the potential neurobiological mechanism of action for varenicline in alcoholism.
In contrast to previous reports of varenicline effects on alcohol consumption, the current study found no medication effect on drinking. Subject population, length of treatment, and statistical power considerations may account for this disparity. First, subjects in the current study were heavy-drinking, non-treatment-seeking alcoholics, but in three of the studies that have reported varenicline effects on drinking, subjects were lighter-drinking smokers who did not meet DSM-IV diagnostic criteria for current alcohol dependence. Thus, the current study’s relatively heavier drinkers, coupled with their lack of motivation to reduce their alcohol consumption, may account for the absence of a varenicline effect on drinking. Second, treatment length may have been too brief in the current study. Of the previous positive varenicline studies, two observed significant medication effects on drinking beginning only after three weeks of treatment (Fucito, et al., 2011, Mitchell, et al., 2012); the third reported varenicline effects on an acute alcohol self-administration (bar lab) paradigm (McKee, et al., 2009). Subjects in the other study to report a varenicline effect on drinking (Litten, et al., 2013) were treatment-seeking alcoholics who received varenicline or placebo for 13 weeks; in that study, varenicline separated from placebo beginning in the sixth week of treatment. Finally, the current pilot study was powered to detect a large effect for the imaging paradigm, but power to detect a drinking effect of the size reported in the Litten et al. (2013) study (d = 0.4; i.e., 10 percentage points difference in %HDD between groups) was low (0.23 for a one-tailed test). Given the presence of an effect on neural cue reactivity here, a larger, longer study better powered to detect a drinking effect may be warranted.
Although it did not reduce drinking, varenicline did affect one aspect of alcohol craving—the ability to control alcohol-related thoughts. This combination of findings is consistent with the results of a recent small RCT among treatment-seeking alcoholics (Plebani, et al., 2013), which also reported no effect of varenicline on heavy drinking days, but did observe a sustained small reduction in alcohol craving. Interestingly, the varenicline group in the current study demonstrated an increase in control over alcohol-related thoughts only at week 2, after medication had reached steady state. Although this effect was relatively small, and its clinical relevance is unknown, it is possible that, had the study lasted longer or the subjects been motivated to cut down or stop drinking, this increase in control might have led to reduced drinking over time. Preclinical and clinical data indicate that varenicline blocks cue-induced reinstatement of alcohol consumption among rats that have acquired alcohol self-administration (Wouda, et al., 2011), and may enhance cognition among both healthy controls (Mocking et al., 2013) and abstinent smokers (Loughead et al., 2010). Given that subjects continued to drink heavily during the treatment period, other potential mechanisms for varenicline’s effects on drinking, including potentiation of the sedative (Kamens, et al., 2010a) and negative subjective effects of alcohol (Childs, et al., 2012), may also have contributed to an increase in perceived behavioral control.
In addition to its effect on alcohol craving, varenicline also reduced alcohol cue-elicited activation of bilateral OFC, a region believed to underlie the process by which salience is attributed to conditioned stimuli (Kringelbach and Rolls, 2004). We have previously reported that the μ-opioid receptor antagonist naltrexone reduces alcohol cue-elicited lateral OFC activation (Myrick, et al., 2008, Schacht, et al., 2013c), and an arterial spin-labeling fMRI study of varenicline’s effects on cigarette cue-elicited brain activation in heavy smokers reported that varenicline increased resting perfusion in lateral OFC, which was correlated with decreased cue-elicited activation in other regions (Franklin, et al., 2011). The OFC receives dopaminergic afferents from the VTA and VS and reciprocally projects to these regions, allowing it to associate dopamine release with temporally coincident drug cues and ultimately entrain dopamine firing to these cues (Koob and Volkow, 2010). Thus, varenicline could have disrupted alcohol cue-elicited activation in this region through attenuation of dopaminergic inputs. It is not clear why this effect did not extend to cue-elicited VS activation. However, alcoholics have been reported to display disruption of the positive correlation between OFC activity and striatal dopamine release (Volkow, et al., 2007); therefore, attenuation of the OFC cue response may not have been sufficient to disrupt the VS response (and, perhaps, to inhibit drinking behavior).
Given that varenicline reduced one aspect of alcohol craving and reduced cue-elicited OFC activation, it is somewhat puzzling that these constructs were not associated with each other. Although two small studies have reported associations between cue-elicited lateral OFC activation and in vivo craving (Filbey, et al., 2008, Myrick, et al., 2004), a large study (N = 328) of individuals with a wide range of drinking behavior recently reported associations between cue-elicited activation of left OFC and alcohol use disorder severity (Claus, et al., 2011). Given OFC’s role in salience attribution, greater cue-elicited activation of this region may thus reflect the strength of the conditioned association between alcohol cues and the effects of alcohol, and treatment-mediated reduction of this activation might be associated with reduction of states less consciously accessible than craving (e.g., implicit approach bias (Wiers, et al., 2011)). Alternatively, the reliability of subjective craving may simply be influenced by too many extraneous variables (i.e., scanner environment, demand characteristics). The notorious difficulty in measuring subjective craving (Sayette, et al., 2000) is precisely the reason that researchers have sought objective measures (i.e., cue-elicited brain activation) of this phenomenon.
This study had several important strengths and limitations. It is among the first studies to use neuroimaging to study the effects of varenicline in alcohol dependence, and to test the medication among non-treatment-seekers. The medication groups were well matched on demographic characteristics, especially smoking. However, the sample size was small, and there were relatively few heavy smokers (i.e., those who smoked ≥ 20 cigarettes per day). Given that most varenicline effects on drinking have been reported among smokers, further study of alcohol cue reactivity among individuals who both drink and smoke heavily is warranted. Further, varenicline has a relatively long titration period, but subjects were scanned after only seven days of steady-state (2 mg) dosing. This interval was chosen to maximize subject retention for the scan (which was 100%), but future studies should attempt to scan subjects after a longer treatment period, over which varenicline effects on drinking might be apparent. Finally, due to funding constraints, there was no baseline scan. Although subjects were randomly assigned to active or placebo medication, it is possible that the observed medication effects were confounded by pre-existing differences in neural cue reactivity or cerebral blood flow.
Two final limitations relate to the ROIs used in the current study. The medial OFC can suffer from gradient-echo fMRI signal dropout due to susceptibility-induced magnetic field gradients. Our ROI was anterior to the area of most significant signal loss, and all subjects had data in all voxels in the ROI. Nonetheless, since medial OFC ALC – BEV activation was not significant in the placebo group, the lack of a varenicline effect, despite effects in adjoining lateral OFC, could be due to low power as a result of low signal. More broadly, although the use of a priori ROIs in which at least two previous studies had reported a medication effect on alcohol or nicotine cue-elicited activation was a strength, four of the seven studies used to generate these ROIs were from our laboratory, suggesting that the effects reported here could be specific to the cue reactivity task used. More studies of medication effects on the neural substrates of alcohol cue reactivity are clearly needed, particularly for novel medications whose mechanism of action in alcohol dependence is unknown.
Conclusions
In conclusion, this study found that, among heavy-drinking, non-treatment-seeking alcohol-dependent individuals, varenicline improved control over alcohol-related thoughts and reduced alcohol cue-elicited activation of bilateral OFC, but did not affect cue-elicited activation of other brain areas (i.e., VS, medial OFC and mPFC) and did not reduce drinking or smoking. Thus, although these data suggest that varenicline’s neurobiological mechanism of action in alcohol dependence may involve attenuation of alcohol cue-elicited brain activation, since varenicline did not reduce drinking in this study, this question remains unresolved. Future neuroimaging studies should attempt to replicate these results after a longer period of varenicline treatment and among individuals with greater motivation to reduce their drinking.
Acknowledgments
Funding: This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), including the Charleston Alcohol Research Center (P50 AA010761). NIAAA had no involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the paper, or in the decision to submit for publication. Drs. Schacht and Anton are supported by K99 AA021419 and K05 AA017435, respectively.
Portions of this work were presented at the 34th Annual Meeting of the Research Society on Alcoholism (June 2011, Atlanta) and the 52nd Annual Meeting of the American College on Neuropsychopharmacology (December 2013, Hollywood, FL).
Footnotes
Author contributions
RFA and HM were responsible for the study concept and design. XL, SH, and HM acquired the data. JPS performed the data analysis and drafted the manuscript. PKR assisted with the data analysis and interpretation of findings. RFA provided critical revision of the manuscript. All authors critically reviewed content and approved the final version for publication.
References
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–31. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res. 2012;36:906–14. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–96. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–96. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–26. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–63. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. The nicotinic acetylcholine receptor partial agonist varenicline increases the ataxic and sedative-hypnotic effects of acute ethanol administration in C57BL/6J mice. Alcohol Clin Exp Res. 2010a;34:2053–60. doi: 10.1111/j.1530-0277.2010.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010b;208:613–26. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–81. [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, et al. A Double-Blind, Placebo-Controlled Trial Assessing the Efficacy of Varenicline Tartrate for Alcohol Dependence. J Addict Med. 2013 doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Mandelkern M, Alvarez-Estrada M, Brody AL. A single administration of low-dose varenicline saturates alpha4beta2* nicotinic acetylcholine receptors in the human brain. Neuropsychopharmacology. 2012;37:1738–48. doi: 10.1038/npp.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, et al. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–21. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, et al. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. NeuroImage. 2013;78:176–85. doi: 10.1016/j.neuroimage.2013.03.055. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking RJ, Patrick Pflanz C, Pringle A, Parsons E, McTavish SF, Cowen PJ, et al. Effects of a short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacology. 2013;38:476–84. doi: 10.1038/npp.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–72. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O’Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Lunny K, Bujarski S, Moallem N, Krull JL, Miotto K. The effects of varenicline on stress-induced and cue-induced craving for cigarettes. Drug Alcohol Depend. 2013;131:136–42. doi: 10.1016/j.drugalcdep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Anton RF, Latham PK, Moak DH. Factor structure and predictive validity of the Obsessive Compulsive Drinking Scale. Alcohol Clin Exp Res. 1999;23:1484–91. [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189-210–S189-210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: A hierarchical linear modeling approach. NeuroImage. 2011;56:61–8. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013a;18:121–33. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacology (Berl) 2013b doi: 10.1007/s00213-013-2996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Voronin KE, Randall PK, Li X, Henderson S, et al. Interacting Effects of Naltrexone and OPRM1 and DAT1 Variation on the Neural Response to Alcohol Cues. Neuropsychopharmacology. 2013c;38:414–22. doi: 10.1038/npp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol Addict Behav. 2004;18:148–59. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–9. [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–42. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011;22:490–7. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216:267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]