Abstract
The recent dramatic increase of the prevalence and range of amphibian host species and populations infected by ranaviruses such as Frog Virus 3 (FV3) raises concerns about the efficacies of amphibian antiviral immunity. In this context, the potential negative effects of water contaminants such as the herbicide atrazine, at environmentally relevant levels, on host antiviral immunity remains unclear. Here we describe the use of the amphibian Xenopus laevis as an ecotoxiciology platform to elucidate the consequences of exposure to ecologically relevant doses of atrazine on amphibian antiviral immunity. X. laevis were exposed at tadpole and adult stages as well as during metamorphosis to atrazine (range from 0.1 to 10.0 ppb) prior to infection with FV3. Quantitative analysis of gene expression revealed significant changes in the pro-inflammatory cytokine, TNF-α and the antiviral type I IFN gene in response to FV3 infection. This was most marked in tadpoles that were exposed to atrazine at doses as low 0.1 ppb. Furthermore, atrazine exposure significantly compromised tadpole survival following FV3 infections. In contrast, acute atrazine exposure of mature adult frogs did not induce detectable effects on anti-FV3 immunity, but adults that were exposed to atrazine during metamorphosis exhibited pronounced defects in FV3-induced TNF-α gene expression responses and slight diminution in type I IFN gene induction. Thus, even at low doses, atrazine exposure culminates in impaired development of amphibian antiviral defenses.
Keywords: Water pollutants, ranavirus, antiviral immunity, immune toxicant
1. Introduction
As the result of increasing global prevalence of infections of poikilothermic vertebrates by pathogens belonging to the Ranavirus genus (RV, Iridoviridae) and the alarming spread of RVs such as Frog Virus 3 (FV3) to new hosts, these pathogens are now receiving considerable attention as commercially and ecologically-relevant etiological agents (Bandin and Dopazo, 2011; Chinchar, 2002; Chinchar et al., 2009; Greer et al., 2005; Jancovich et al., 2010). While this recognition has focused scientific attention on amphibian host immunity, it also highlights the growing concerns that other stressors, such as water contamination may increase host susceptibility to disease, thus providing an additional explanation for the emergence of infectious agents such as RVs (Collins and Storfer, 2003).
Atrazine (2-chloro-4-ethylamino-6-isopropyl-amino-s-triazine) is is a broad-spectrum herbicide that has been globally distributed and has a propensity to reach water sources via agricultural runoffs. The U.S. Environmental Protection Agency (EPA) maintains a maximum contaminant level (MCL) for atrazine at 3 parts-per-billion (ppb; 3 μg/L) in drinking water (Environmental Protection Agency, 2012), but atrazine levels exceed this limit in certain water sources (Environmental Protection Agency, 2013; Shipitalo and Owens, 2003). Moreover, exposure to ecologically relevant atrazine concentrations has been linked to pathological conditions across several vertebrate classes. Such exposure in rodents, for example, affects the population dynamics and function of several immune cell subsets (Filipov et al., 2005; Rooney et al., 2003; Rowe et al., 2006; Zhao et al., 2013). Given their aquatic nature, amphibians are likely to be exposed regularly to any water contaminants and therefore are more susceptible to potential adverse health effects resulting from these exposures. Indeed, atrazine has previously been described as an amphibian endocrine disruptor at concentrations below the MCL (Hayes et al., 2002; Hayes et al., 2010; Rooney et al., 2003). These observations raise the question of whether there are other possible physiological consequences of exposure including alterations to host defense mechanisms. Several studies support this idea. For instance, a 2010 meta-analysis performed in 2010 on 27 studies of amphibian and fish species revealed consistent sub-lethal deleterious effects on immune function (Rohr and McCoy, 2010). Particularly notable were associations between levels of exposure atrazine approaching, at, or below current standards and diminished peritoneal leukocyte number, reduced phagocyte activities, and increased susceptibility to pathogens (Brodkin et al., 2007; Christin et al., 2013; Forson and Storfer, 2006).
In addition to gaps in understanding the direct immunomodulatory effects of atrazine, the long-term implications of exposure to atrazine, and many other waterborne environmental contaminants, remain unclear. Much of aforementioned work was conducted using immunologically mature animals. While this approach is valuable and informative, several recent studies suggest that exposure to low levels of environmental agents during critical periods of development do not cause obvious immediate effects; rather, they set the stage for altered pathophysiology later in life. Given its strength as an FV3 infection model (Chen and Robert, 2011; De Jesus Andino et al., 2012; Morales et al., 2010), the frog Xenopus laevis further lends itself as an ecologically, economically, and physiologically relevant platform with which to study the consequences of atrazine exposure at different developmental periods on aquatic vertebrate antiviral immunity. Thus, in the work reported herein, we tested the hypothesis that tadpole exposure to current environmental levels of atrazine result in long lasting, deleterious effects on the development of amphibian antiviral immunity, thus increasing susceptibility to pathogens such as FV3.
To address the effects of low dose atrazine exposure on amphibian antiviral immune responses, three life stages (tadpoles, metamorphic, and adults) of X. laevis were exposed to this herbicide and the capacities of exposed animals to upregulate expression of immuno-relevant genes (TNF-α, Type I IFN, Mx1, IL-1β, IFN-γ, IL-10, CSF-1) following infection with FV3 was assessed.
2. Materials and Methods
2.1. Animals
All outbred Xenopus laevis tadpoles and adult frogs were acquired from the X. laevis research resource for immunology at the University of Rochester (http://www.urmc.rochester.edu/mbi/resources/Xenopus/). For tadpole survival and expression experiments, stage 50 and 56 tadpoles were used, respectively (Nieuwkoop and Faber, 1967). One-year-old frogs were used for all adult experiments. All animals were handled in accordance with stringent laboratory and University Committee on Animal Research regulations (Approval number 100577/2003-151).
2.2. Atrazine
Atrazine (Chem Service Inc.) was dissolved in DMSO to create an initial stock solution from which subsequent working solutions were prepared. A concentration of DMSO equivalent to the highest dose of atrazine was used as a control in all experiments. Fresh atrazine (or DMSO control) was reapplied with each water change for exposures lasting longer than 7 days.
2.3. Frog Virus 3 stocks and infection
Fathead minnow cells (FHM; American Type Culture Collection, ATCC No.CCL-42) used for virus production were maintained in DMEM (Invitrogen) containing 10% fetal bovine serum (Invitrogen), streptomycin (100μg/ML), and penicillin (100 U/mL) at 30°C with 5% CO2. FV3 was grown using a single passage through FHM cells and was subsequently purified by ultracentrifugation on a 30% sucrose cushion. Plaque assays on a FMH monolayer were used to quantify FV3. For tadpole infections by water bath, tadpoles were exposed to 5×106 plaque forming units (PFU)/mL of FV3 in 4 mL of clean water for 1 hr. For intraperitoneal (i.p.) infection, tadpoles were injected with 5×104 plaque forming units (PFU) of FV3 in 10μL aliquots of amphibian phosphate buffered saline (APBS). Post-metamorphic froglets were infected by i.p. injection of 1×105 PFU in 20μL, while adult frog infections were performed using 1×106 PFU FV3 in 100μL. For all i.p. infections, uninfected control animals were mock-infected (i.p.) with an equivalent volume of APBS.
2.4. Atrazine exposure and FV3 challenge
Stage 54–56 tadpoles were exposed to 0.0, 0.1, 1.0, 10.0 ppb atrazine in 1.5L containers; 12 animals per treatment (Fig. 1). Adult frogs were exposed to 0.0, 1.0, 10.0 ppb atrazine in 400mL of water for one week and either injected with APBS (mock-infection; 3 animals/treatment) or infected with FV3 (5 animals/treatment). Six days post infection (dpi), animals were euthanized by overdose of MS-222 and kidneys were extracted. For metamorphosis studies, stage 56 tadpoles were exposed to atrazine (1.0 ppb) atrazine or DMSO control in 1.5L water until the completion of metamorphosis, at which point the resulting froglets were removed and acclimatized in clean water for 3 weeks before mock or FV3 infection. Animals were euthanized using MS-222 at 6 dpi for kidney extraction (Fig. 1, bottom panel).
FIG 1.
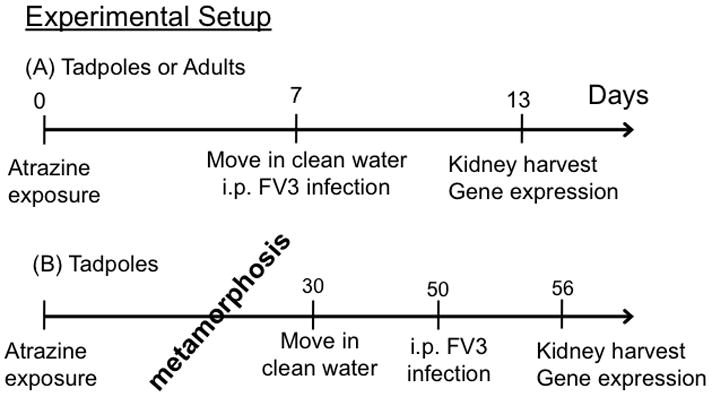
Schematic of treatment strategy. (A) Acute treatment of tadpoles or adults.(B) Treatment of animals during metamorphosis.
2.5. Tadpole survival studies
Two independent FV3 survival experiments were conducted, in which animals were infected by either water bath or i.p. injection. In both experiments, 40–44 stage 50 tadpoles were distributed equally across 4 treatments; 0.0, 0.1, 1.0, 10.0 ppb atrazine in 1.5L of water. Following one week of exposure, tadpoles were infected with FV3, either via water bath infections (in 4 mL of water containing 5×106 PFU/mL FV3 for 1h) or by i.p. inoculation and moved to 1.5L of clean water for monitoring. Tadpoles were checked daily; dead animals were immediately removed, frozen, and stored at −20°C.
2.6. Quantitative gene expression analyses
RNA and DNA were extracted from frog kidneys using Trizol reagent, as per the manufacturer’s protocol (Invitrogen). DNA was isolated from tadpole carcasses by DNAEasy Blood & Tissue kit (Qiagen). RNA was treated with DNAse (Ambion), and 500 ng of treated RNA was used to synthesize cDNA (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA).
The qRT-PCR analysis was performed using the ABI 7300 real-time PCR system with PerfeCT SYBR Green FastMix, ROX (Quanta) and ABI sequence detection system software (SDS). GAPDH endogenous control was used in conjunction with the delta^delta CT method to analyze cDNA for gene expression. FV3 viral loads were assessed by absolute qRT-PCR by analysis of isolated DNA in comparison to a serially diluted standard curve. Briefly, an FV3 DNA Pol II PCR fragment was cloned into the pGEM-T vector (Promega). This construct was amplified in bacteria, quantified and serially diluted to yield 1010–101 plasmid copies of the vDNA POl II. These dilutions were employed as a standard curve in subsequent absolute qRT-PCR experiments to derive the viral genome transcript copy numbers, relative to this standard curve. All primer sequences are listed in Table 1.
Table 1.
| Primer Name | Sequences |
|---|---|
| FV3 DNA Pol II | F: 5′-AACCCTCGCCTGGCATCTA -3′ R: 5′-TGGTGGTCCTCAGCATCCT -3′ |
| TNFα | F: 5′-TGTCAGGCAGGAAAGAAGCA -3′ R: 5′-CAGCAGAGCAAAGAGGATGGT -3′ |
| Type I IFN | F: 5′-GCTGCTCCTGCTCAGTCTCA-3′ R: 5′-GAAAGCCTTCAGGATCTGTGTGT-3′ |
| MX1 | F: 5′-AGGAGTGGTCAACAGGAGCC -3′ R: 5′-TGTTCCGCCGCTGTTCCTCT -3′ |
| IL-10 | F: 5′-TGCTGGATCTTAAGCACACCCTGA -3′ R: TGTACAGGCCTTGTTCACGCATCT -3′ |
| CSF-1 | F: 5′-ATCGAACTCTGTCCAAGCTGGATG -3′ R: 5′-GGACGAAGCAAGCATCTGCCTTAT -3′ |
| IFN-gamma | F: 5′-CTGAGGAAATACTTTAACTCCATTGACC -3′ R: 5′-TTGTAACATCTCCCACCTGTATTGTC -3′ |
| IL-1β | F: 5′-CATTCCCATGGAGGGCTACA -3′ R: 5′-TGACTGCCACTGAGCAGCAT-3′ |
| GAPDH | F: 5′-GACATCAAGGCCGCCATTAAGACT -3′ R: 5′-AGATGGAGGAGTGAGTGTCACCAT -3′ |
2.7. Statistical analysis
The Mann-Whitney U and ANOVA test were used for statistical analysis of expression and viral load data. Analyses were performed using a Vassar Stat online resource (http://vassarstats.net/utest.html). Statistical analysis of survival data was performed using a Log-Rank Test (GraphPad Prism 6). A probability value of p<0.05 was used in all analyses to indicate significance.
3. Results
3.1. Atrazine exposure enhances X. laevis tadpole susceptibility to FV3 infection
To assess whether acute atrazine exposure influences tadpole susceptibility to FV3 infection, we exposed Stage 50 tadpoles to atrazine for 1 week, then infected them with FV3 via either water bath or by i.p. injection and then monitored animal survival (Fig. 1). Both routes of infection significantly reduced survival of tadpoles exposed to 10 ppb atrazine when compared to tadpoles in the respective control groups, indicating that exposure to atrazine increased susceptibility to FV3 (Fig. 2A, B). Survival was also significantly reduced following i.p. FV3 inoculation of tadpoles that had been exposed to 1 ppb of atrazine. No discernible effects were observed following animal exposure with the lowest atrazine dose, regardless of the route of infection. Collectively, these data suggest that atrazine modulates tadpole host resistance to FV3 infection in a dose-dependent manner.
FIG 2.
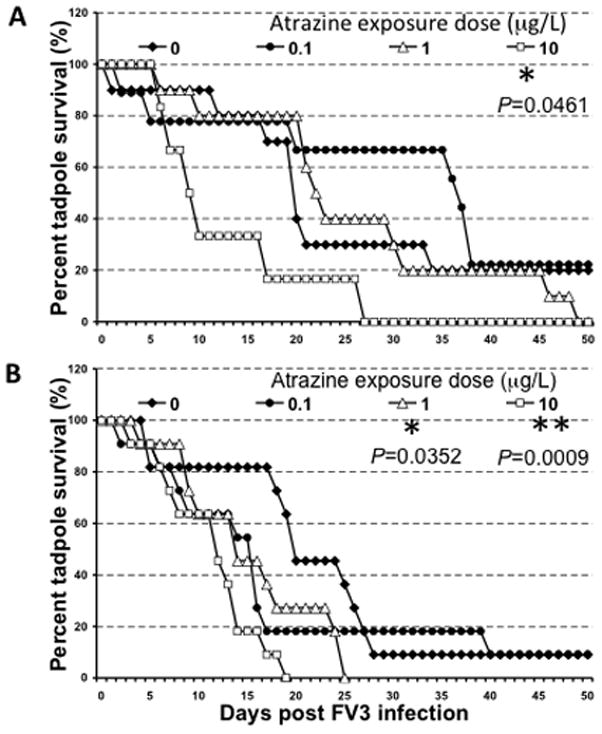
Survival curve of FV3-infected tadpoles (stage 50–52) that were exposed for 1 week to 0, 0.1, 1.0 or 10 ppb atrazine. (A) Water bath infection. (B) Infection by intra-peritoneal (i.p.) injection. Survival was monitored for 50 days following infection, during which tadpoles were checked daily. Groups of water bath-infected animals contain between 6 and 10 individuals (0.0 ppb, N=10; 0.1 ppb, N=9; 1.0 ppb, N=10; 10.0 ppb, N=6) and groups of i.p. infected animals each contain 11 (N=11). Statistical significance: (*) P<0.05 and ** P<0.005 (Log Rank Test).
We next determined whether short-term exposure to atrazine influences the expression of signature antiviral genes in pre-metamorphic tadpoles (stage 55–56) infected with FV3. Specifically, at 6 days post-infection (dpi), which correspond to the peak of anti-FV3 immune response (De Jesus Andino et al., 2012), gene expression in kidneys (main target organ of FV3 in Xenopus (De Jesus Andino et al., 2012)); was assessed by qRT-PCR. To control for the degree of FV3 infection, the virus load was determined for each tadpole by absolute qRT-PCR analysis of FV3 DNA Pol II on total DNA. Virus load did not differ significantly across treatment groups (Fig. 3A). Notably, expression of both TNF-α and Type I IFN genes was significantly and drastically decreased in infected tadpoles that had been exposed to 0.1, 1.0 and 10 ppb of atrazine, as compared to controls (Fig. 4). Although change in expression of the Type I IFN-inducible Mx1 was not statistically significant in atrazine-exposed FV3-infected groups relative to sham-atrazine exposed tadpoles, there was a trend of lower FV3-induced Mx1 gene expression along with higher dose of atrazine. A similar tendency of lower FV3-induced expression of the macrophage growth factor CSF-1 gene with higher dose of atrazine exposure was noted, albeit not statistically significant (Fig. 4). The expression of the anti-inflammatory Interleukin-10 (IL-10) gene upon FV3 infection was not affected by atrazine treatment (Fig. 4). Likewise, the gene expression of IFN-γ and IL-1β were not significantly altered by any conditions described here (data not shown). We also noted a statistically significant decrease of the basal gene expression of TNF-α in tadpoles exposed at 0.1 ppb atrazine as well as IL-10 at all doses (Fig 4, V).
FIG 3.
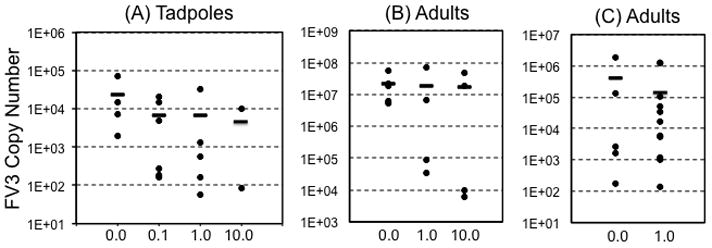
FV3 copy number 6 days after FV3 infection as determined by absolute qRT-PCR. (A) Individual carcasses of tadpoles that were exposed for 1 week to 0, 0.1, 1.0 or 10 ppb atrazine (N=4; 6; 4; 2); (B) Kidneys of adults that were exposed for 1 week to 0, 1 or 10 ppb atrazine (N=5; 4; 4); (C) Kidneys of adults that were exposed to atrazine during metamorphosis to 0 or 1.0 ppb atrazine (N=5; 11). Horizontal bars denote means. There was no statistical difference between treatment groups and controls (ANOVA and Mann-Whitney U Test).
FIG 4.
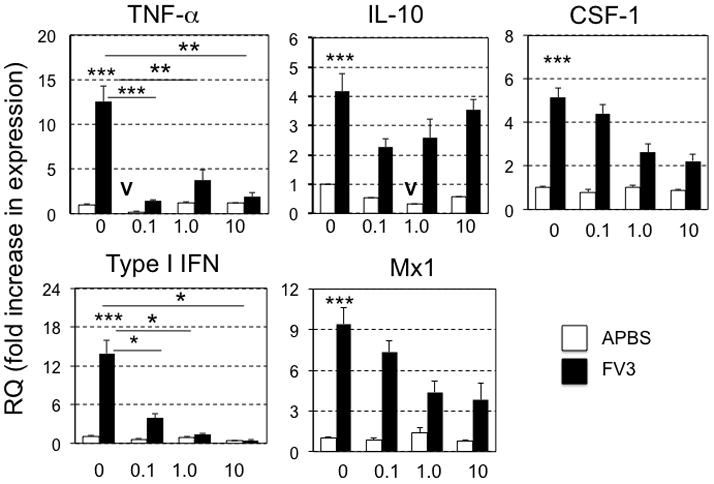
Quantitative gene expression analysis by qRT-PCR in stage 56 X. laevis tadpole kidneys for TNF-α, IL-10, CSF-1, Type I IFN, Mx1, 6 days after i.p. injection with either FV3 or mock-infection with APBS. Results are means ± SEM comprised of 5 to 6 individuals per group and are representative from two different experiments. Statistical significance (ANOVA): (*) P<0.05, ** P<0.005, *** P<0.001 (V) Denotes significant decrease (P<0.05) of basal gene expression in uninfected, atraizine-treated animals compared to non-atrazine exposed controls. Gene expression is represented as fold increase (RQ: relative quantification) relative to GAPDH endogenous control and standardized to uninfected non-atrazine exposed controls (fixed as 1).
3.2. Acute atrazine exposure results in only minimal alteration of antiviral gene expression in adult frogs
Given the effects of acute atrazine exposure on tadpole antiviral immunity, we next determined whether the same exposure program would have a similar effect on antiviral immunity of mature adult frogs. Accordingly, 1 year-old frogs were exposed to 1.0 or 10.0 ppb atrazine in their water, and then infected with FV3. No adult frogs died from FV3 infections throughout the course of these experiments (data not shown). Also, at 6 dpi, the kidney viral titers (as measured by qRT-PCR) of these FV3-inoculated frogs did not significantly differ across different treatments (Fig. 3). Moreover, at 6 dpi, there were no statistically significant differences in TNF-α gene expression as compared to adult frogs exposed to DMSO controls (Fig. 5). Similarly, FV3-induced Type I IFN and Mx1 gene expression levels were not affected by atrazine exposure. It is noteworthy that there was individual variation in the expression of these genes among infected frogs, which may explain why the apparent decrease in Mx1 expression in infected animals exposed to 10 ppb of atrazine did not reach statistical significance.
FIG 5.
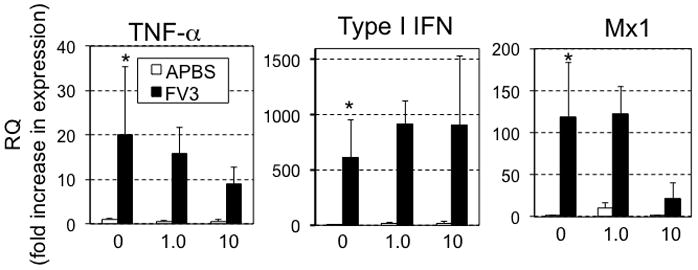
Quantitative gene expression analysis by qRT-PCR in 1-year old X. laevis adult kidneys for TNF-α, Type I IFN, Mx1 6 days after i.p. injection with either FV3 or mock-infection with APBS. Results are presented as means ± SEM from mock-infected (N=3/group) and infected frogs (N=4–5/group). Statistical significance (ANOVA): (*) P<0.05 between non-infected and infected sham-atrazine exposed controls. There was no statistical difference between atrazine exposed and sham-exposed infected groups. Gene expression is represented as fold increase (RQ: relative quantification) relative to GAPDH endogenous control and standardized to uninfected non-atrazine exposed controls (fixed as 1).
3.3 Exposure to atrazine during metamorphosis alters effective antiviral gene expression responses
Since exposure of tadpoles to atrazine resulted in changes in anti-FV3 immune gene expression, whereas the same exposure did not markedly affect adult frogs, we next examined if atrazine exposure during metamorphosis, a key developmental transition, would lead to discernible changes in immune responses to FV3 infection in post-metamorphic frogs. To this end, pre-metamorphic tadpoles (Stage 56) were exposed to atrazine (1.0 ppb) throughout metamorphosis (~4–6 weeks) until its completion (complete tail resorption and front limb development; stage 66; (Nieuwkoop and Faber, 1967)]) at which time the frogs were permitted to recover for 3 weeks in clean water (Fig. 1). Subsequently, frogs were infected (ip) with FV3 and immune gene expression in kidneys was assessed at dpi 6 (Fig. 6). The viral loads were not significantly different between the above treatment groups (Fig. 3). However, FV3-elicited TNF-α gene expression was significantly reduced in adult frogs that had been previously exposed to 1.0 ppb of atrazine during metamorphosis (Fig. 6). Indeed, TNF-α expression following 1.0 ppb exposure was only barely above the level in uninfected controls, whereas it was markedly increased in DMSO-exposed infected frogs. This suggests long-lasting effects of exposure during development on post-metamorphic ability to mount certain inflammatory responses to RV infection. Although the expression responses of the type I IFN gene were attenuated in the atrazine-exposed groups, this decrease was not statistically significant (Fig. 6). Similarly, Mx1 gene expression responses to FV3 infections exhibit a slight decrease in atrazine-exposed animals that did not reach statistical significance, whereas IL-10 expression was unaffected (Fig. 6).
FIG 6.
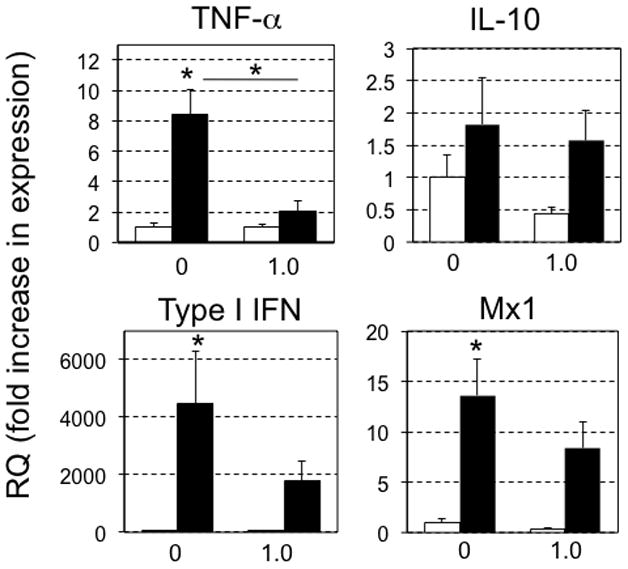
Quantitative gene expression analysis by qRT-PCR in post-metamorphic X. laevis kidneys for TNF-α, IL-10, Type I IFN, Mx1 6 days after i.p. injection with either FV3 or mock-infection with APBS. Results are means + SEM from 2–5 individuals in mock-infected groups (N=2–5), from 3 to 4 individuals per infected control group (N=3–5), and from 7 to 10 individuals per infected exposed group (N=7–10). Statistical significance (ANOVA): (*) P<0.05. Gene expression is represented as fold increase (RQ: relative quantification) relative to GAPDH endogenous control and standardized to uninfected non-atrazine exposed controls (fixed as 1).
4. Discussion
The threat to amphibian populations represented in the emerging ranavirus pathogens are compounded by ecotoxicological contaminants (Bandin and Dopazo, 2011; Chinchar, 2002; Chinchar et al., 2009; Greer et al., 2005; Jancovich et al., 2010). Considering that exposure to water pollutants may be further decreasing the capabilities of these species to thwart immune challenges. Indeed, as a common water pollutant, atrazine may well be contributing to amphibian immune susceptibility and hampering the ability of aquatic vertebrates to effectively clear infectious agents such as FV3. The deleterious effects of acute atrazine exposure on amphibian physiology are well documented (Brodkin et al., 2007; Christin et al., 2013; Forson and Storfer, 2006; Rohr and McCoy, 2010). The present work underlines that even acute exposure to atrazine concentrations below current standards has negative impacts on the abilities of developing animals to elicit pro-inflammatory, innate, and antiviral immune responses to viral pathogens. Notably, as is the case with most anuran species, the X. laevis tadpoles are naturally more susceptible to FV3 infection than adults, where the latter typically clear and survive infection (Gantress et al., 2003; Gray et al., 2009; Gray et al., 2007; Mazzoni et al., 2009). The significantly decreased mean survival of atrazine-exposed, FV3-infected tadpoles further emphasizes the adversity that the exposure to this pollutant has on the development of successful anti-RV tadpole immunity. These findings are not surprising, considering that atrazine exposure causes deficiencies in both the pro-inflammatory (TNF-α) and the antiviral (type I IFN) components of developing animals. It is alarming, however, that these adverse effects were seen even at doses as low as 0.1 and 1.0 ppb (10 to 100 time below the EPA standard). Further analysis at different time points after infection and of additional physiologically relevant organs and tissues may lend more insight into the immuno-toxicological consequences of amphibian atrazine exposure.
The TNF-α response appears to be of importance to the Xenopus antiviral immunity considering the robust upregulation of this gene in adult frogs upon FV3 infection (Morales et al., 2010), and the subsequent cellular NF-κB activation (Mawaribuchi et al., 2008). Notably, the Xenopus tadpole upregulation of the TNF-α gene expression during FV3 infections is substantially delayed and more modest compared to adult frogs (De Jesus Andino et al., 2012). This poor pro-inflammatory inefficiency is believed to contribute to the relative tadpole susceptibility to RVs (De Jesus Andino et al., 2012). Thus, the additional defect in TNF-α gene responses to FV3 likely contributes to the decreased survival rates of these animals in the face of FV3 challenge. Furthermore, the intact expression response of the IL-10 gene may further compound any proinflammatory defects resulting from atrazine exposure. This is noteworthy considering the potent anti-inflammatory nature of IL-10 (Seppola et al., 2008; Standiford et al., 1995) combined with the fact that many pathogens not only benefit from, but also actively elicit this immunosuppressive mediator as part of their immune evasion strategies (Redpath et al., 2001). Indeed, as our results indicate, FV3 infection of tadpoles (control of atrazine-exposed) results in significant increases of IL-10 expression, although at present it is unclear whether this anti-inflammatory response is actively elicited by the virus or the host. Regardless, the atrazine-induced defects in the tadpole TNF-α response, combined with the fact that the anti-inflammatory IL-10 component remains intact and is elevated upon infection likely converge in further dampening of any potential inflammatory responses to FV3.
Similarly to the atrazine-treated tadpoles, froglets exposed to atrazine during metamorphic development also exhibited severely impaired TNF-α gene responses to FV3 infections. This indicates that exposure to doses as low as 1.0 ppb (10x below EPA MCL) confers broad deleterious effects to the development of the amphibian immunity. As alluded to above, a potent, timely TNF-α response to FV3 infections is thought to be a critical determinant of the efficacies in X. laevis adult RV clearance (De Jesus Andino et al., 2012). Thus, the lasting deleterious effects to this pro-inflammatory response observed following atrazine exposure of metamorphing animals is especially alarming, considering the ramifications of such a defect to the capacity of atrazine-exposed amphibians to clear emerging pathogens such as RVs.
Antiviral properties of the type I interferon response are well characterized in mammals (Durbin et al., 2000; Muller et al., 1994). We have recently identified a Xenopus type I IFN homolog and have demonstrated that this amphibian cytokine is a potent antiranaviral agent (Grayfer et al., 2014). Notably, our findings indicate that the tadpole type I IFN response to FV3 is considerably more modest and delayed as compared to adult frogs; presumably accounting at least in part for the tadpole susceptibility and adult frog resistance to RVs (Grayfer et al., 2014). Thus, the atrazine-conferred dramatic defects to this type I IFN responses add up to the retarded pro-inflammatory tadpole susceptibility to RV infections. Although the defect of anti-FV3 type I IFN gene expression response resulting from atrazine exposure during metamorphosis did not achieve statistical significance, the trend in decreased response compared to control cohorts is suggestive of weaker antiviral defenses.
Notably our tadpole survival studies indicate that atrazine-exposed animals are much more susceptible to, and more readily succumb from FV3, than respective controls. Furthermore, we have recently demonstrated that FV3 pathology and infection-related mortality is not directly linked to viral loads, where even low viral titers are sufficient to culminate in tadpole deaths (Grayfer et al., 2014). Thus, ultimately immune control and clearance of RVs may be much more crucial to amphibian survival in the face of FV3 challenge; wherein the compromise to amphibian immune parameters responsible for this control would be detrimental, as reported here for atrazine-exposed, immuno-compromised X. laevis. It is of note that tadpole exposed to 0.1 μg/L of atrazine and then virally challenged by water bath actually faired better than animals reared in clean water prior to FV3 challenge by this route. Considering some studies suggesting that atrazine may exacerbate inflammatory outcomes (Rayner et al., 2007; Stanko et al., 2010), it is possible that at this combination of lower dose and infection route, the inflammatory response elicited by virus may actually have been further intensified by the atrazine-induced toxicity resulting in better viral clearance. Indeed, although our findings suggest that the tadpole kidney anti-FV3 inflammatory response is severely compromised by atrazine exposure, the peripheral inflammatory response may be exaggerated by this same exposure. Alternatively, atrazine toxicity may result in the selective loss of peripheral tadpole cell populations targeted by the virus, which would also manifest in enhanced tadpole survival on the account of decreased capacity of FV3 to infect these animals.
The present findings clearly indicate that even at doses well below MCL standards, atrazine exposure results in severe and chronic defects to the development and efficacies of the amphibian antiviral immune defenses. Where previous studies have demonstrated adverse effects of high doses (400 ppb) of atrazine on X. laevis tadpole immunity (Langerveld et al., 2009), it is striking to consider that, as reported here, acute exposure at doses as low as 0.1 ppb are sufficient to significantly hinder antiviral responses and immune development. Our findings underline the severity with which exposure to common water pollutants such as atrazine may be afflicting aquatic populations; warranting the reevaluation of the common policies regarding certain agricultural practices and their short and long-term ecological consequences.
Highlights.
We developed Xenopus leavis as a reliable immune-ecotoxiciology platform
We examined the effects of low doses of atrazine on Xenopus antiviral immunity
Atrazine severely impairs tadpole innate immunity and survival to FV3 infections
Atrazine exposure during metamorphosis induces chronic antiviral immune defects
Adults are more resistant to atrazine-mediated immune defects
Acknowledgments
We thank Tina Martin for animal husbandry and Dr. Eva-Stina Edholm for her critical review of this manuscript. This work was supported by R24-AI-059830 from NIH, Morris Foundation. LG was supported by NSERC-PDF, a LSRF PDF from the Howard Hughes Medical Institute, and a Pilot Project Grant from the Rochester Environmental Health Sciences Center (P30-ES01247).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandin I, Dopazo CP. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet Res. 2011;42 doi: 10.1186/1297-9716-42-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin MA, Madhoun H, Rameswaran M, Vatnick I. Atrazine is an immune disruptor in adult northern leopard frogs (Rana pipiens) Environmental toxicology and chemistry/SETAC. 2007;26:80–84. doi: 10.1897/05-469.1. [DOI] [PubMed] [Google Scholar]
- Chen G, Robert J. Antiviral immunity in amphibians. Viruses. 2011;3:2065–2086. doi: 10.3390/v3112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchar VG. Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Archives of virology. 2002;147:447–470. doi: 10.1007/s007050200000. [DOI] [PubMed] [Google Scholar]
- Chinchar VG, Hyatt A, Miyazaki T, Williams T. Family Iridoviridae: Poor Viral Relations No Longer. Curr Top Microbiol. 2009;328:123–170. doi: 10.1007/978-3-540-68618-7_4. [DOI] [PubMed] [Google Scholar]
- Christin MS, Menard L, Giroux I, Marcogliese DJ, Ruby S, Cyr D, Fournier M, Brousseau P. Effects of agricultural pesticides on the health of Rana pipiens frogs sampled from the field. Environmental science and pollution research international. 2013;20:601–611. doi: 10.1007/s11356-012-1160-1. [DOI] [PubMed] [Google Scholar]
- Collins JP, Storfer A. Global amphibian declines: sorting the hypotheses. Divers Distrib. 2003;9:89–98. [Google Scholar]
- De Jesus Andino F, Chen G, Li Z, Grayfer L, Robert J. Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology. 2012;432:435–443. doi: 10.1016/j.virol.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin JE, Fernandez-Sesma A, Lee CK, Rao TD, Frey AB, Moran TM, Vukmanovic S, Garcia-Sastre A, Levy DE. Type I IFN modulates innate and specific antiviral immunity. Journal of immunology. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. Atrazine Interim Reregistration Eligibility Decision (IRED) Q&A’s - January 2003. Environmental Protection Agency; Washington, D.C: 2012. [Google Scholar]
- Environmental Protection Agency. Basic Information about Atrazine in Drinking Water. Environmental Protection Agency; Washington, D.C: 2013. [Google Scholar]
- Filipov NM, Pinchuk LM, Boyd BL, Crittenden PL. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicological sciences: an official journal of the Society of Toxicology. 2005;86:324–332. doi: 10.1093/toxsci/kfi188. [DOI] [PubMed] [Google Scholar]
- Forson DD, Storfer A. Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecological applications: a publication of the Ecological Society of America. 2006;16:2325–2332. doi: 10.1890/1051-0761(2006)016[2325:airsit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gantress J, Maniero GD, Cohen N, Robert J. Development and characterization of a model system to study amphibian immune responses to iridoviruses. Virology. 2003;311:254–262. doi: 10.1016/s0042-6822(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Miller DL, Hoverman JT. Ecology and pathology of amphibian ranaviruses. Diseases of aquatic organisms. 2009;87:243–266. doi: 10.3354/dao02138. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Miller DL, Schmutzer AC, Baldwin CA. Frog virus 3 prevalence in tadpole populations inhabiting cattle-access and non-access wetlands in Tennessee, USA. Diseases of aquatic organisms. 2007;77:97–103. doi: 10.3354/dao01837. [DOI] [PubMed] [Google Scholar]
- Grayfer L, De Jesus Andino F, Robert J. The amphibian (Xenopus laevis) type I interferon response to Frog Virus 3: new insight into ranavirus pathogenicity. J Virol. 2014 doi: 10.1128/JVI.00223-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer AL, Berrill M, Wilson PJ. Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Diseases of aquatic organisms. 2005;67:9–14. doi: 10.3354/dao067009. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, Adame L, Chan E, Buchholz D, Stueve T, Gallipeau S. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis) Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4612–4617. doi: 10.1073/pnas.0909519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancovich JK, Bremont M, Touchman JW, Jacobs BL. Evidence for Multiple Recent Host Species Shifts among the Ranaviruses (Family Iridoviridae) J Virol. 2010;84:2636–2647. doi: 10.1128/JVI.01991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerveld AJ, Celestine R, Zaya R, Mihalko D, Ide CF. Chronic exposure to high levels of atrazine alters expression of genes that regulate immune and growth-related functions in developing Xenopus laevis tadpoles. Environmental research. 2009;109:379–389. doi: 10.1016/j.envres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Mawaribuchi S, Tamura K, Okano S, Takayama S, Yaoita Y, Shiba T, Takamatsu N, Ito M. Tumor necrosis factor-alpha attenuates thyroid hormone-induced apoptosis in vascular endothelial cell line XLgoo established from Xenopus tadpole tails. Endocrinology. 2008;149:3379–3389. doi: 10.1210/en.2007-1591. [DOI] [PubMed] [Google Scholar]
- Mazzoni R, de Mesquita AJ, Fleury LF, de Brito WM, Nunes IA, Robert J, Morales H, Coelho AS, Barthasson DL, Galli L, Catroxo MH. Mass mortality associated with a frog virus 3-like Ranavirus infection in farmed tadpoles Rana catesbeiana from Brazil. Diseases of aquatic organisms. 2009;86:181–191. doi: 10.3354/dao02096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales HD, Abramowitz L, Gertz J, Sowa J, Vogel A, Robert J. Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis. J Virol. 2010;84:4912–4922. doi: 10.1128/JVI.02486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. 2. Amsterdam: North Holland; 1967. [Google Scholar]
- Rayner JL, Enoch RR, Wolf DC, Fenton SE. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male Long-Evans rats. Toxicology and applied pharmacology. 2007;218:238–248. doi: 10.1016/j.taap.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Redpath S, Ghazal P, Gascoigne NR. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends in microbiology. 2001;9:86–92. doi: 10.1016/s0966-842x(00)01919-3. [DOI] [PubMed] [Google Scholar]
- Rohr JR, McCoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environmental health perspectives. 2010;118:20–32. doi: 10.1289/ehp.0901164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Matulka RA, Luebke RW. Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicological sciences: an official journal of the Society of Toxicology. 2003;76:366–375. doi: 10.1093/toxsci/kfg250. [DOI] [PubMed] [Google Scholar]
- Rowe AM, Brundage KM, Schafer R, Barnett JB. Immunomodulatory effects of maternal atrazine exposure on male Balb/c mice. Toxicology and applied pharmacology. 2006;214:69–77. doi: 10.1016/j.taap.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppola M, Larsen AN, Steiro K, Robertsen B, Jensen I. Characterisation and expression analysis of the interleukin genes, IL-1beta, IL-8 and IL-10, in Atlantic cod (Gadus morhua L.) Molecular immunology. 2008;45:887–897. doi: 10.1016/j.molimm.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Shipitalo MJ, Owens LB. Atrazine, deethylatrazine, and deisopropylatrazine in surface runoff from conservation tilled watersheds. Environmental science & technology. 2003;37:944–950. doi: 10.1021/es020870b. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. Journal of immunology. 1995;155:2222–2229. [PubMed] [Google Scholar]
- Stanko JP, Enoch RR, Rayner JL, Davis CC, Wolf DC, Malarkey DE, Fenton SE. Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats. Reproductive toxicology. 2010;30:540–549. doi: 10.1016/j.reprotox.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Liu J, Zhao F, Liu W, Li N, Suo Q, Zhao J, Zhao L. Sub-acute exposure to the herbicide atrazine suppresses cell immune functions in adolescent mice. Bioscience trends. 2013;7:193–201. [PubMed] [Google Scholar]


