Abstract
Objective
To assess whether regional lymph node dissection (LND) could improve the prognosis of patients with metastatic renal cell carcinoma (RCC).
Methods
We reviewed data on 258 patients who underwent cytoreductive nephrectomy at our institution, some of whom received a concurrent LND. Primary outcome measure was overall survival. A Cox proportional hazards regression model included, age, pathologic stage, lymphadenopathy, tumor size, modified MSKCC criteria, site of metastatic disease, and LND. We created a logistic regression model to evaluate risk factors for node-positive disease. Survival analyses were performed for lymph node template (hilar vs other) and number of nodes removed (0–3, 4–7, or ≥8).
Results
Of 258 patients, 177 (69%) underwent LND, and positive nodes were found in 59 (33%). Five-year overall survival was 21% for patients who underwent LND and 31% for patients who did not. We found no significant difference in survival among patients receiving or not receiving LND. Five-year overall survival was 27% and 9% for negative and positive nodal status, respectively (p <0.0005). For patients who underwent LND, the presence of lymphadenopathy was a significant predictor of node-positive disease (odds ratio 25.0, 95% CI 9.04–69.4, p <0.0001).
Conclusions
LND performed during cytoreductive nephrectomy was not associated with a survival benefit. Lymph node–positive disease was identified as a poor prognostic variable; therefore, LND should be considered as a staging procedure for clinical trials.
Keywords: lymph node dissection; cytoreductive nephrectomy; carcinoma, metastatic; carcinoma, renal cell
INTRODUCTION
The prognosis for patients with metastatic renal cell carcinoma (RCC) has historically been poor, with a 5-year survival rate of less than 10%.(1) In 2001, two randomized controlled trials reported improved overall survival when radical nephrectomy was performed in patients with metastatic renal cell carcinoma (RCC) treated with interferon-alpha 2b.(2, 3) Combined analysis of the 331 patients randomized in these studies showed a median overall survival of 13.6 months for cytoreductive nephrectomy plus interferon versus 7.8 months for interferon alone.(4) Both trials stipulated that radical nephrectomy should include removal of tumor outside of Gerota’s fascia, but whether lymph node dissection (LND) affected survival was not evaluated. Although the role of cytoreductive nephrectomy in the era of angiogenesis-targeted agents has been debated,(5) it still remains part of the standard of care in the multimodal approach to treating metastatic kidney cancer. Whether surgeons should extend the surgery to regional lymph nodes has not been thoroughly investigated.
While the development of angiogenesis-targeted agents has improved outcomes for patients with metastatic disease, similar advances in surgical treatment have not been developed.(6-8) Two studies have evaluated the relative benefit of LND during cytoreductive nephrectomy.(9, 10) Vasselli et al found that survival for patients with completely resected lymphadenopathy was comparable to that for patients without radiographic evidence of preoperative lymphadenopathy who did not undergo LND.(9) Pantuck et al reported that patients with node-positive disease undergoing LND (112/129) had better overall survival (approximately 5 months) than those who did not undergo LND (17/129).(10) Despite these positive results, Kates et al, using data from the Surveillance, Epidemiology and End Results registry, found that only 16% of patients with metastatic RCC underwent LND from 1998 to 2005, with no differences over time.(11) Although there is some evidence that metastasectomy at the time of nephrectomy is beneficial, whether this applies to lymphadenopathy has not been established.(12)
The aim of our study was to further evaluate the utility of performing LND during cytoreductive nephrectomy. Our primary objective was to determine whether LND imparted a survival benefit in patients undergoing cytoreductive nephrectomy, with further analyses to determine whether LND template or number of nodes removed are important variables.
MATERIALS AND METHODS
After receiving approval from our institutional review board for this retrospective study, we identified 258 patients with distant metastatic RCC who underwent partial or radical cytoreductive nephrectomy at our institution from January 1992 to January 2013. Distant metastatic disease could be a clinical or pathologic diagnosis. Chart review was performed to determine site of metastatic disease at the time of nephrectomy and if lymphadenopathy was diagnosed preoperatively on cross-sectional radiographic imaging or described in operative notes. Radiographic evidence of lymphadenopathy was defined as any retroperitoneal lymph node ≥1 cm in long axis. Operative and pathology reports were reviewed to confirm that LND was performed, as well as the number of lymph nodes removed and the lymph node template performed. Number of nodes removed was categorized as 0–3, 4–7 or ≥8. Lymph node template was categorized as either hilar or other, which included paracaval, precaval, retrocaval, and interaortocaval for right-sided tumors, and paraaortic, preaortic, and interaortocaval for left-sided tumors. Patients for whom only incidental perinephric nodal tissue was discovered in the nephrectomy specimen were counted as having no LND. Performance of LND and the extent of dissection were not standardized: some of our surgeons perform an extended LND in nearly all cases while others perform LND more selectively, either for staging or in the presence of surgically resectable lymphadenopathy.
We created a Cox proportional hazards regression model for analysis of overall survival. Patients were followed until death or time of their last follow-up. The Cox model included LND (yes or no), age (in 10-year intervals), pathologic tumor stage (pT1, pT2, or ≥pT3), lymphadenopathy (yes or no), site of metastatic disease (lung only or non-lung) and tumor size (per cm). In addition, we used a modified version of the MSKCC criteria, a risk-scoring system that has been shown to predict prognosis in patients with advanced RCC. Our version excludes prior nephrectomy and, rather than using a point system, categorizes patients as having favorable or unfavorable risk. Patients were included in the unfavorable group if any of the following was true: 1) serum lactate dehydrogenase greater than 1.5 times the upper limit of normal; 2) serum calcium more than 10 mg/dl after correction for serum albumin; 3) serum hemoglobin less than the lower limit of normal; or 4) Karnofsky Performance Status less than 80%.(13)
To evaluate risk factors for node-positive disease, we created a logistic regression model that included presenting symptoms (incidental, local, or systemic), tumor size, lymphadenopathy (yes or no), and modified MSKCC criteria (favorable vs unfavorable risk). All statistical analyses were performed using Stata 12.0 (Stata Corp., College Station, TX).
RESULTS
Of 258 patients undergoing cytoreductive nephrectomy, 177 (69%) underwent a concurrent LND, and positive lymph nodes were found in 59 (33%). Table 1 describes patient demographics and disease characteristics. Patients who underwent LND were younger than those who did not (median age 60.3 vs 63.6 years, respectively, p = 0.024) and had less favorable pathologic characteristics, including higher stage (p <0.0001), larger tumor size (median 9.2 vs 6.0 cm, p <0.0001), non-clear cell histology (p = 0.003), and the presence of lymphadenopathy (p <0.0001). Surgical variables associated with LND included left-sided tumors (p = 0.001), partial nephrectomy (p <0.0001), and no concurrent metastasectomy (p = 0.004).
Table l.
Patient characteristics. All values are median (IQR) or frequency (%)
| No LND (N=81) |
LND (N=177) |
p-value | |
|---|---|---|---|
| Age | 63.6 (56.3, 69.0) | 60.3 (52.4, 68.4) | 0.024 |
| Gender | |||
| Female | 23 (28%) | 54 (31%) | 0.7 |
| Male | 58 (72%) | 123 (69%) | |
| ASA | |||
| I/II | 36 (44%) | 89 (51%) | 0.3 |
| III/IV | 45 (56%) | 86 (49%) | |
| Pathological T Stage | |||
| pT1 | 25 (31%) | 11 (6.2%) | <0.0001 |
| pT2 | 5 (6.2%) | 19 (11%) | |
| >=pT3 | 51 (63%) | 147 (83%) | |
| Pathological N Stage | - | ||
| pN0 | 0 (0%) | 118 (67%) | |
| pN1 | 0 (0%) | 59 (33%) | |
| pNX | 81 (100%) | 0 (0%) | |
| Tumor Size (cm) | 6.0 (3.8, 8.2) | 9.2 (7.0, 11.9) | <0.0001 |
| Histology | |||
| Clear Cell | 79 (98%) | 150 (85%) | 0.003 |
| Other | 2 (2.5%) | 27 (15%) | |
| Motzer Criteria | |||
| Favorable | 29 (44%) | 57 (36%) | 0.3 |
| Unfavorable | 37 (56%) | 100 (64%) | |
| Location | |||
| Right | 52 (64%) | 74 (42%) | 0.001 |
| Left | 29 (36%) | 103 (58%) | |
| Lymphadenopathy | |||
| No | 64 (90%) | 94 (56%) | <0.0001 |
| Yes | 7 (9.9%) | 75 (44%) | |
| Nodal Template | - | ||
| Hilar Only | - | 30 (17%) | |
| Other | - | 147 (83%) | |
| Metastasectomy | |||
| No | 43 (53%) | 126 (72%) | 0.004 |
| Yes | 38 (47%) | 50 (28%) | |
| Site of Metastatic Disease | |||
| Pulmonary only | 33 (41%) | 66 (37%) | 0.6 |
| Any non-pulmonary | 48 (59%) | 111 (63%) | |
| Surgery Type | |||
| Radical | 23 (28%) | 11 (6.2%) | <0.0001 |
| Partial | 58 (72%) | 166 (94%) | |
| Minimally Invasive Surgery | |||
| No | 67 (83%) | 137 (77%) | 0.3 |
| Yes | 14 (17%) | 40 (23%) | |
| Neoadjuvant Therapy | |||
| No | 70 (86%) | 161 (91%) | 0.3 |
| Yes | 11 (14%) | 16 (9%) |
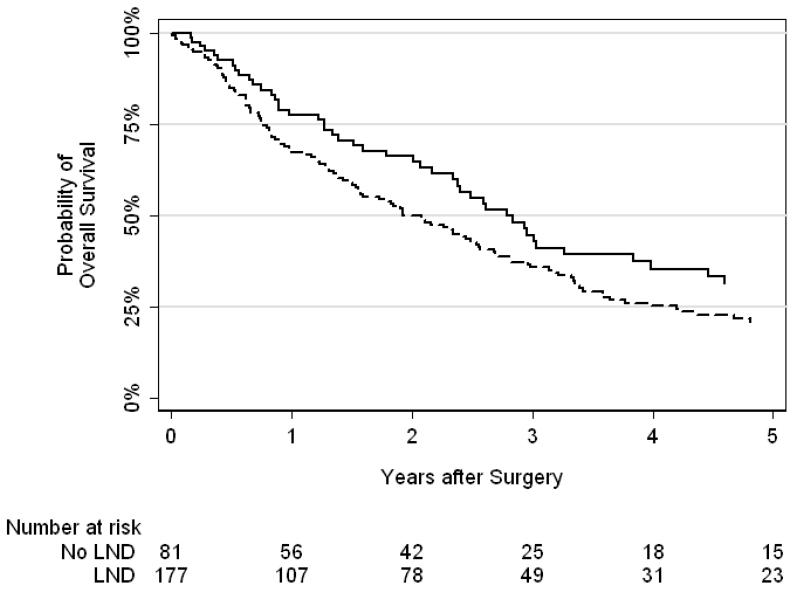
In total, 183 patients died, with a median follow-up of 2.6 years for patients who were still alive at last follow-up. A Kaplan-Meier curve for overall survival stratified by LND or no LND is shown in Figure 1. Five-year overall survival was 21% for patients who received LND and 31% for those who did not. Our Cox proportional hazards regression model for overall survival is presented in Table 2. After adjusting for age, pathologic stage, modified MSKCC criteria, lymphadenopathy, site of metastatic disease and tumor size, we found no significant difference in survival between patients who underwent LND and those who did not (HR 1.06, 95% CI 0.67–1.66; p = 0.8).
Figure 1.
Kaplan-Meier curves for overall survival after cytoreductive nephrectomy (solid line = no LND, dashed line = LND).
Table 2.
Multivariable Cox proportional hazards regression models for survival.
| HR (95% CI) | p-value | |
|---|---|---|
| Lymph Node Dissection | 1.06 (0.67, 1.66) | 0.8 |
| Patient Age (per 10 years) | 0.85 (0.72, 1.00) | 0.049 |
| Pathological Stage | 0.044 | |
| T1 | Ref. | Ref. |
| T2 | 1.17 (0.50, 2.74 | 0.7 |
| ≥T3 | 1.92 (1.01, 3.66)) | 0.048 |
|
Modified MSKCC Criteria
(Unfavorable vs. Favorable) |
2.70 (1.87, 3.88) | <0.0001 |
| Lymphadenopathy | 1.46 (1.00, 2.13) | 0.047 |
| Tumor Size (cm) | 1.02 (0.96, 1.08) | 0.6 |
| Site of Metastatic Disease (Non-lung vs. Lung only) | 0.64 (0.45, 0.89) | 0.009 |
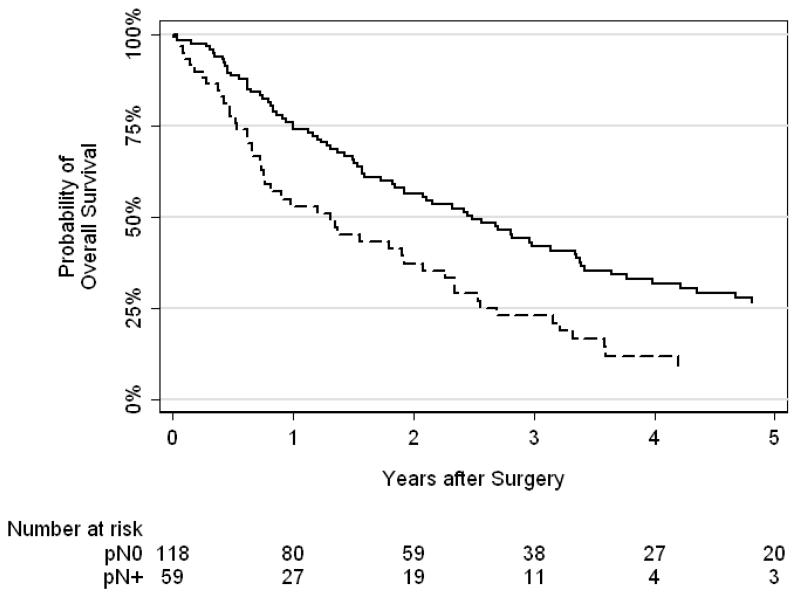
Kaplan-Meier curves showing overall survival by lymph node status are presented in Figure 2. We found a statistically significant difference between lymph node status and overall survival (p <0.0005). Five-year overall survival was 27% for patients with negative nodes and 9% for those with positive nodes. Results of our logistic regression model to predict lymph node status are presented in Table 3. For patients who underwent LND, the presence of lymphadenopathy was the only significant predictor of positive lymph node status (odds ratio 25.0, 95% CI 9.04–69.4, p <0.0001).
Figure 2.
Kaplan-Meier curves for overall survival by lymph node status (solid line = negative nodes, dashed line = positive nodes). There was a statistically significant difference between lymph node status and overall survival (p <0.0005).
Table 3.
Multivariable logistic regression model for positive lymph nodes.
| OR (95% CI) | p-value | |
|---|---|---|
| Presenting Symptoms | 0.3 | |
| Incidental | Ref. | Ref. |
| Local | 1.95 (0.69, 5.51) | - |
| Systemic | 2.07 (0.67, 6.39) | - |
| Lymphadenopathy | 25.0 (9.04, 69.4) | <0.0001 |
| Modified MSKCC Criteria (Unfavorable vs. Favorable) |
1.33 (0.50, 3.50) | 0.6 |
| Tumor Size (cm) | 0.94 (0.83, 1.05) | 0.3 |
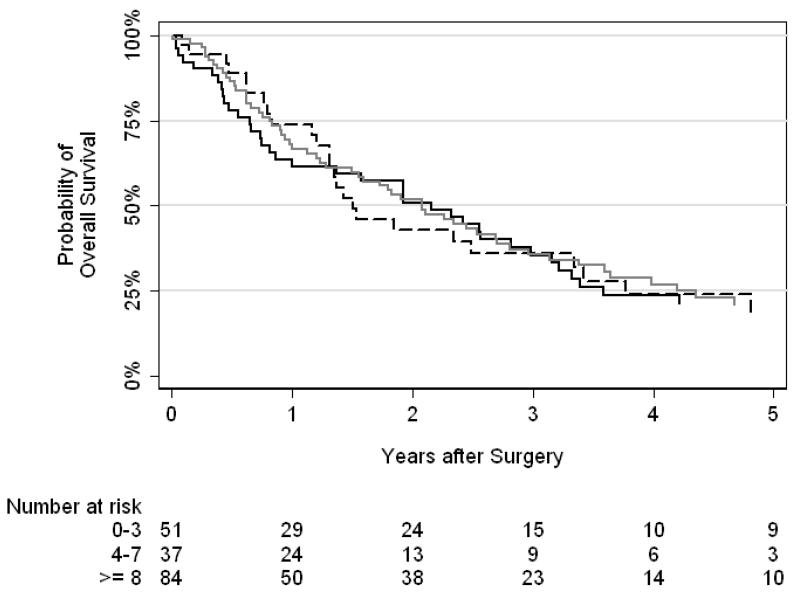
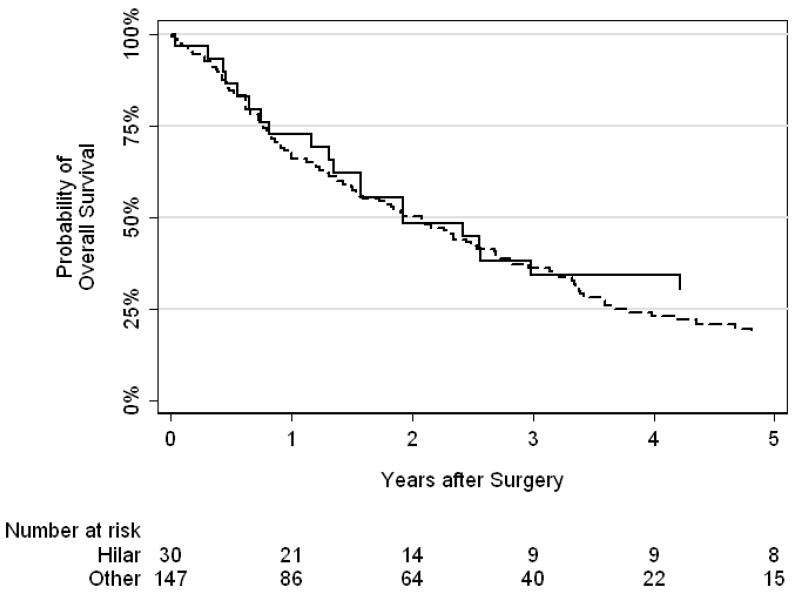
Kaplan-Meier curves for number of nodes removed (0–3, 4–7, or ≥8) and nodal template performed (hilar vs other) are presented in Figures 3 and 4, respectively. We did not find a significant difference between number of nodes removed (p = 0.9) or nodal template (p = 0.5) and overall survival.
Figure 3.
Kaplan-Meier curves for overall survival by number of nodes removed (solid black line = 0–3 nodes, dashed black line = 3–7 nodes, gray line = ≥8 nodes). There was no association between number of nodes removed and overall survival (p=0.9)
Figure 4.
Kaplan-Meier curves for overall survival by nodal template performed (solid line = hilar, dashed line = other). There was no association between nodal template and overall survival (p=0.5)
DISCUSSION
This is one of the largest series to investigate the role of LND during cytoreductive nephrectomy. Although our Kaplan-Meier curve shows that LND was associated with worse overall survival, this is likely due to the higher-risk pathologic features of most patients undergoing LND. We found no association between LND and survival when adjusting for age, pathologic stage, lymphadenopathy, tumor size, and modified MSKCC criteria. Furthermore, extending LND by number of nodes or by template of dissection did not demonstrate a survival benefit.
Our findings should be considered in context with two other large series of patients receiving LND during cytoreductive nephrectomy.(9, 10) Vasselli et al evaluated 154 patients, 72 of whom had preoperative lymphadenopathy. Patients with lymphadenopathy were categorized as having completely resected, incompletely resected, or unresectable abdominal and retroperitoneal disease. Thirteen patients with lymphadenopathy underwent complete resection of disease; although their survival time (median 8.6 months) was shorter than the 82 patients without lymphadenopathy (median 14.7 months), this difference did not reach statistical significance (p = 0.07).(9) Pantuck et al evaluated LND in 900 patients, including a subset of 129 patients undergoing cytoreductive nephrectomy prior to planned systemic immunotherapy. The 112 patients undergoing LND had better overall survival than the 17 patients without LND (p = 0.002). Notably, there was no difference in complications with or without LND.(10) Together, these 2 studies are limited by the number of patients evaluated and the lack of reporting on the extent of LND performed.
Pantuck et al also reported that patients with node-positive metastatic disease had worse survival rates than those with node-negative metastatic disease. In our subset of patients who underwent LND, those with node-positive disease had a 5-year survival of only 9%, compared to 27% for node-negative patients. This suggests that while LND has not been shown to be therapeutic, it may still be important for staging in clinical trials of multimodal therapy.
In our study, lymphadenopathy was not an independent predictor of survival. In contrast, Vasselli et al found that lymphadenopathy was associated with shorter survival. In the current cohort, node-positive disease was found in 59% of patients with lymphadenopathy vs 66% reported by Pantuck et al. These rates of nodal involvement are higher than those reported in the non-metastatic setting, where enlarged nodes are often indicative of inflammation or follicular hyperplasia rather than malignancy.(14)
Interestingly, we found that patients with lung only metastasis had worse survival than those with non-lung metastatic disease. This is in contrast to the previous randomized trial of interferon-alpha 2b that showed improved survival in patients with lung only metastatic disease.(2) This is likely due to the retrospective nature of our study design, but it could represent the changing landscape of metastatic renal cell carcinoma characterized by targeted agents and more aggressive use of metastatectomy.
The ability to predict preoperatively whether a patient had node-positive or node-negative disease would be advantageous, as avoiding unnecessary LND could reduce potential morbidity. We evaluated presenting symptoms, modified MSKCC criteria, tumor size, and lymph node staging as variables, and found that only lymphadenopathy was predictive of node-positive disease. However, as our predictive model included only patients who underwent LND, and considering that LND was preferentially performed in patients with lymphadenopathy, this finding should be considered with caution. Previous algorithms for predicting node-positive disease in the non-metastatic setting have been published, though with limited utility.(15, 16) One of these algorithms requires the use of expert frozen section analysis and the other was only validated in patients undergoing hilar lymph node dissections.
Notably, we were unable to demonstrate a benefit of performing a more extended node dissection either by number of nodes or by template. We compared an extended node dissection, which included the ipsilateral great vessel and/or the interaortocaval region, to a hilar-only dissection because there is some evidence that the extended dissection should be favored due to the unpredictability of positive node landing sites.(17) While there are no other data reporting on extent of LND during cytoreductive nephrectomy, there are conflicting data in the non-metastatic setting.(18-20) We are not aware of any standardization for extent of LND or minimal number of nodes that constitute an extended LND. In renal cell carcinoma, neither template of dissection nor number of nodes removed has been evaluated in a prospective manner.
It should be noted that patients in this cytoreductive nephrectomy cohort had very good outcomes compared to patients treated during the cytokine era.(4) Median overall survival for patients managed with cytoreductive nephrectomy at our institution was greater than 2 years. A recent study showed an improving trend in median overall survival rates for metastatic RCC, from 1.04 years (95% CI 0.97–1.09) in 1991–1995 to 1.63 years (95% CI 1.28–1.79) in 2001–2002.(21) This trend has coincided with a temporal shift from poor risk to more intermediate and favorable risk metastatic RCC. This shift may offer an opportunity for more patients to undergo cytoreductive surgery, which has been associated with improved survival in the cytokine era and is being investigated in an ongoing randomized phase III trial of sunitinib alone versus sunitinib with nephrectomy (Clinical Trial to Assess the Importance of Nephrectomy [CARMENA, NCT00930033]).
We did not investigate LND in the setting of angiogenesis-targeted therapies. These newer agents have become standard-of-care over prior cytokine therapy.(6-8) Because patients could be treated by a number of different therapies over the 20-year period in this study, it would have been impossible to test for the interaction between LND and different agents. Considering the differences between node-negative and node-positive patients, we believe that staging LND remains an important procedure particularly for patients with lymphadenopathy. These findings support the use of pathological nodal status in stratifying patients in systemic therapy trials.
This study is not without important limitations. LND was not standardized and the performance of LND was based on surgeon discretion. Preoperative imaging and pathological reports were assessed by chart review rather than radiological or pathological re-review. Re-review, with a focus on lymph nodes, may have provided a more accurate assessment of the presence of lymphadenopathy, the number of nodes removed, and the number of nodes involved. Standardization of LND templates may have improved our ability to evaluate this parameter. However, it is unlikely that any potential differences would have changed our primary outcome measurement of overall survival as stratified by LND vs no LND.
More importantly, differences in the LND vs no LND groups suggest that there may be underlying confounders that we did not account for. Patients with higher stage, larger tumor size, non-clear cell histology, and preoperative lymphadenopathy were more likely to undergo LND, which implies that surgeons selectively performed LND in the setting of more aggressive disease. However, patients who did not undergo metastasectomy were also less likely to undergo LND, and these patients may have had a greater burden of metastatic disease. The performance of metastasectomy. The decision to perform metastasectomy is based on extent and location of disease, performance status, and discussion between the patient, surgical team and medical oncologists. Although we controlled for measured confounders of survival (age, tumor size, stage, lymphadenopathy, site of metastatic disease and modified MSKCC criteria), we can assume there are likely other, unmeasured confounders that could bias our results, which show no difference in overall survival between receiving an LND or not. Considering the substantial selection bias that cannot be adjusted for in a retrospective study, survival comparisons may be held in question. Therefore, we believe there is strong justification for a randomized controlled trial to assess the survival benefit of LND during cytoreductive nephrectomy.
We were unable to demonstrate a survival difference between patients undergoing LND vs no LND during cytoreductive nephrectomy. We did confirm that lymphadenopathy is often associated with node-positive disease, and that node-positive disease is a poor prognostic variable in the metastatic setting. As long as the paradigm of treatment remains multimodal, prospective studies will be needed to further advance the surgical treatment of metastatic RCC.
Acknowledgements
This investigation was supported by the Hanson Family Renal Cancer Research Fund, the Sidney Kimmel Center for Prostate and Urologic Cancers, and the National Cancer Institute/National Institutes of Health under Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant T32 CA082088. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
REFERENCES
- [1].Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. The New England journal of medicine. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- [2].Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. The New England journal of medicine. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- [3].Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- [4].Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. The Journal of urology. 2004;171:1071–6. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- [5].Pantuck AJ, Belldegrun AS, Figlin RA. Cytoreductive nephrectomy for metastatic renal cell carcinoma: is it still imperative in the era of targeted therapy? Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:693s–6s. doi: 10.1158/1078-0432.CCR-06-1916. [DOI] [PubMed] [Google Scholar]
- [6].Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England journal of medicine. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- [7].Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- [8].Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vasselli JR, Yang JC, Linehan WM, White DE, Rosenberg SA, Walther MM. Lack of retroperitoneal lymphadenopathy predicts survival of patients with metastatic renal cell carcinoma. The Journal of urology. 2001;166:68–72. [PubMed] [Google Scholar]
- [10].Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. The Journal of urology. 2003;169:2076–83. doi: 10.1097/01.ju.0000066130.27119.1c. [DOI] [PubMed] [Google Scholar]
- [11].Kates M, Lavery HJ, Brajtbord J, Samadi D, Palese MA. Decreasing rates of lymph node dissection during radical nephrectomy for renal cell carcinoma. Annals of surgical oncology. 2012;19:2693–9. doi: 10.1245/s10434-012-2330-6. [DOI] [PubMed] [Google Scholar]
- [12].Russo P, Synder M, Vickers A, Kondagunta V, Motzer R. Cytoreductive nephrectomy and nephrectomy/complete metastasectomy for metastatic renal cancer. The Scientific World Journal. 2007;7:768–78. doi: 10.1100/tsw.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Silberstein JL, Adamy A, Maschino AC, et al. Systematic classification and prediction of complications after nephrectomy in patients with metastatic renal cell carcinoma (RCC) BJU international. 2012;110:1276–82. doi: 10.1111/j.1464-410X.2012.11103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Studer UE, Scherz S, Scheidegger J, et al. Enlargement of regional lymph nodes in renal cell carcinoma is often not due to metastases. The Journal of urology. 1990;144:243–5. doi: 10.1016/s0022-5347(17)39422-3. [DOI] [PubMed] [Google Scholar]
- [15].Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. A protocol for performing extended lymph node dissection using primary tumor pathological features for patients treated with radical nephrectomy for clear cell renal cell carcinoma. The Journal of urology. 2004;172:465–9. doi: 10.1097/01.ju.0000129815.91927.85. [DOI] [PubMed] [Google Scholar]
- [16].Hutterer GC, Patard JJ, Perrotte P, et al. Patients with renal cell carcinoma nodal metastases can be accurately identified: external validation of a new nomogram. International journal of cancer Journal international du cancer. 2007;121:2556–61. doi: 10.1002/ijc.23010. [DOI] [PubMed] [Google Scholar]
- [17].Capitanio U, Becker F, Blute ML, et al. Lymph node dissection in renal cell carcinoma. European urology. 2011;60:1212–20. doi: 10.1016/j.eururo.2011.09.003. [DOI] [PubMed] [Google Scholar]
- [18].Capitanio U, Suardi N, Matloob R, et al. Staging lymphadenectomy in renal cell carcinoma must be extended: a sensitivity curve analysis. BJU Int. 2013;111:412–8. doi: 10.1111/j.1464-410X.2012.11313.x. [DOI] [PubMed] [Google Scholar]
- [19].Joslyn SA, Sirintrapun SJ, Konety BR. Impact of lymphadenectomy and nodal burden in renal cell carcinoma: retrospective analysis of the National Surveillance, Epidemiology, and End Results database. Urology. 2005;65:675–80. doi: 10.1016/j.urology.2004.10.068. [DOI] [PubMed] [Google Scholar]
- [20].Terrone C, Guercio S, De Luca S, et al. The number of lymph nodes examined and staging accuracy in renal cell carcinoma. BJU international. 2003;91:37–40. doi: 10.1046/j.1464-410x.2003.04017.x. [DOI] [PubMed] [Google Scholar]
- [21].Patil S, Manola J, Elson P, et al. Improvement in overall survival of patients with advanced renal cell carcinoma: prognostic factor trend analysis from an international data set of clinical trials. The Journal of urology. 2012;188:2095–100. doi: 10.1016/j.juro.2012.08.026. [DOI] [PubMed] [Google Scholar]