Abstract
Background
Clinically, early life stress and anxiety disorders are associated with increased vulnerability for alcohol use disorders. In male rats, early life stress, imparted by adolescent social isolation, results in long-lasting increases in a number of behavioral risk factors for alcoholism, including greater anxiety-like behaviors and ethanol intake. Several recent studies have begun to use this model to gain insight into the relationships among anxiety measures, stress, ethanol intake and neurobiological correlates driving these behaviors. As prior research has noted significant sex differences in the impact of adolescent stress on anxiety measures and ethanol drinking, the current study was conducted to determine if this same model produces an “addiction vulnerable” phenotype in female rodents.
Methods
Female Long Evans rats were socially isolated (SI; 1/cage) or group housed (GH; 4/cage) for six weeks during adolescence. After this housing manipulation, behavioral assessment was conducted using the elevated plus maze, response to novelty in an open field environment, and the light/dark box. After behavioral testing, home cage ethanol drinking was assessed across an eight week period.
Results
No group differences were detected in any of the behavioral measures of unconditioned anxiety-like behavior. Greater ethanol intake and preference were observed in SI females but these differences did not persist.
Conclusions
The SI/GH model, which results in robust and enduring increases in anxiety measures and ethanol self-administration in male Long Evans rats, did not result in similar behavioral changes in female rats. These data, and that of others, suggest that adolescent social isolation is not a useful model with which to study neurobiological substrates linking antecedent anxiety and addiction vulnerability in female rats. Given the compelling epidemiological evidence that the relationship between chronic adolescent stress and alcohol addiction is particularly strong in women, there is clearly an urgent need to identify a more effective model with which to study these clinically important relationships in female rodents.
Keywords: stress, addiction vulnerability, adolescence, sex differences
INTRODUCTION
Early life stress is associated with increased risk for alcohol use disorders later in life, particularly among women (Enoch 2011). Alcohol dependent females are more likely to have a comorbid anxiety disorder (Kessler et al. 1997; Kessler et al. 2005); report greater anxiety following exposure to an alcohol related cue (Fox et al. 2009); and PTSD/trauma more often precedes comorbid alcohol dependence in females than males (Sonne et al. 2003). Additionally, early life stress or trauma is more consistently associated with alcohol abuse/dependence in women than men (Widom et al. 2007).
Despite the clinical evidence of a prominent role for negative affect and stress contributing to alcohol use disorders in females, the vast majority of preclinical animal studies of the relationship between antecedent anxiety-like behavior and alcohol use vulnerability have focused exclusively on male subjects. For example, in male rodents, adolescent social isolation engenders enduring increases in anxiety-like behaviors and measures of ethanol self-administration (McCool and Chappell 2009; Sanna et al. 2011; Chappell et al. 2013), as well as increased preference for the ethanol-paired chamber in a conditioned place preference paradigm (Whitaker et al. 2013). Further, male rats genetically bred for high alcohol intake (alcohol-preferring (P) rats; Marchigian Sardinian alcohol-preferring (msP) rats) also demonstrate greater anxiety-like and depressive-like behavior along with greater ethanol intake than non-alcohol preferring counterparts (Ciccocioppo et al. 1999; Ehlers et al. 2007). Repeated stress also increases ethanol intake and potentiates ethanol withdrawal-related anxiety in male rats (Breese et al. 2004). These powerful models are beginning to provide important insight into some of the neurobiological substrates linking early life stress, anxiety, and vulnerability to alcohol addiction, but need to also be explored in females.
Surprisingly, the relationship between antecedent anxiety and increased propensity to self-administer ethanol has proven more difficult to model in females. For instance, repeated restraint stress in female rats does not alter ethanol intake during access or following ethanol withdrawal (Bertholomey et al. 2011), but adolescent foot shock stress increases ethanol intake in female HAP1 mice (Chester et al. 2008). Additionally, Lopez et al. (2011) showed that female mice isolated immediately post-weaning did not consistently drink more than their GH counterparts (only drank more on days 1, 8, 10); however, female mice isolated in adulthood drank significantly less than mice group housed in adulthood. Thus, there are several models using male rodents that can be used to study neurobiological substrates linking antecedent anxiety and vulnerability to alcohol addiction, but a reliable model using females has yet to be identified.
Previous studies from our laboratory and others have shown that adolescent early life stress, imparted by social isolation (SI) during adolescence, models important aspects of addiction vulnerability in male rats. That is, compared to animals group housed (GH) during adolescence, SI engenders robust increases in anxiety-like behaviors and alcohol intake in male rodents (McCool and Chappell 2009; Chappell et al. 2013). This behavioral phenotype is not only robust, but enduring in nature, and has marked effects on measures of neural plasticity (Miyazaki et al. 2012; Whitaker et al. 2013; Yorgason et al. 2013). This model is very simple to use and is effective in at least two rodent strains using male rodents. Thus, we sought to determine if adolescent social isolation could also be used to model the relationship between antecedent anxiety-like behavior and alcohol addiction vulnerability in female rats.
MATERIALS AND METHODS
Subjects
Twenty-five adolescent female Long Evans rats aged 24 days and weighing approximately 40 grams upon arrival at the laboratory were used in these studies (Harlan Laboratories, Indianapolis, IN). Upon arrival, animals were group housed (4/cage) and given one week to acclimate to the laboratory and handling procedures before experiments were initiated. Handling and behavioral procedures were consistent with those used in previous studies in our lab with male rats (McCool and Chappell 2009; Chappell et al. 2013). Female rats were housed in a separate colony room without male rats. Rats were maintained on a 12h light/dark cycle (lights on 7:00-19:00), and food and water were available ad libitum for the duration of the studies. All animal care procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Housing Procedure
Following the one week acclimation period in which all animals were group housed, animals either remained group housed (GH; n = 12) or were socially isolated (SI; n = 13) (Method after Chappell et al. 2013). GH rats were housed in groups of four in large plexiglass cages (33.0 cm × 59.7 cm; Nalgene, Rochester, NY), whereas SI rats were singly housed in standard cages (20.3 cm × 26.7 cm; Allentown Inc., Allentown, NJ) and deprived of any physical contact and/or social interaction with their peers. These housing conditions were maintained for six weeks with rats being subject only to a once/week cage change. Rats remained in their GH/SI living condition until after the completion of behavioral tests for anxiety-like behavior (detailed below).
Measures of Unconditioned Anxiety-Like Behavior
Elevated Plus Maze, Response to Novelty, and Light/Dark Box
At the end of the six week GH/SI housing period, anxiety-like behavior was assessed using a standard elevated plus maze (EPM; Med Associates, St. Albans, VT) as described previously (Chappell et al. 2013; McCool and Chappell 2009). Measures of anxiety-like behavior included time spent on the open arms and the number of open arm entries during the 5 minute trial (300 seconds). The number of closed arm entries was used as a measure of general locomotor activity (Holmes and Rodgers 1999). This test was conducted on PND 85 for all subjects. The following week, on PND 91-92, response to novelty was measured using standard locomotor activity chambers (model-RXYZCM, Digiscan animal activity monitors, Omnitech, Columbus, OH). Subjects were placed in the center of acrylic plastic chambers (42 × 42 × 30 cm) equipped with infrared photodetectors located in arrays of eight photobeams along each wall, arranged at regular intervals along the length of the chamber, 2.5 cm above the floor. Exploratory activity in this novel environment was first measured for 60 minutes in five minute time bins. Following this period, an immovable object (500g weight) was placed in the center of the chamber and locomotor activity in response to the placement of this novel object was assessed for an additional 60 minutes. GH and SI rats were tested on separate, but consecutive days due to the number of animals and long session duration (two hours). In a final assessment of anxiety-like behavior on PND 97 before initiation of the ethanol drinking procedure, rats were exposed to the light/dark box (27 × 58 × 30 cm). An area of 24 × 27 cm was painted black and illuminated by a 40 W red light bulb (dark side). The remaining area was painted white and was illuminated by a 40 W incandescent light (light side). The two chambers were separated by a partition that contained a 10 × 10 cm doorway in the center to allow free access between the two sides. To initiate the test, subjects were placed on the light side of the chamber, facing away from the doorway. Each test session lasted five minutes. Anxiety-like measures included the latency for the rats to re-enter the light side for the first time, the total number of crosses to the light side, and the total time spent on the light side (Lack et al. 2008).
Home Cage Ethanol Drinking Procedure
On PND 100, following the completion of tests of anxiety-like behavior, all animals were singly housed in standard sized cages for the duration of the ethanol self-administration procedure. To initiate familiarity with ethanol before the two-bottle choice procedure, subjects were given three day access to a 10% ethanol solution (v/v) as their only liquid. The following week, to assess ethanol preference, rats were given concurrent access to a 10% ethanol solution and water for 23 hours for five consecutive days. For the remainder of the drinking study, ethanol consumption was assessed using an intermittent access, two-bottle choice home cage drinking procedure that has previously been shown to engender relatively high levels of ethanol intake in male Long-Evans rats (Wise 1973; Simms et al. 2008; Chappell et al. 2013; Butler et al. 2014). Rats were given access to 20% ethanol (v/v) and water on Monday, Wednesday, and Friday for 24 hours with only water available on the remaining days. Total fluid intake was measured following 30 minutes and 24 hours of ethanol access during this procedure. The placement of the ethanol and water bottles was alternated on each drinking day to control for side preferences, and ethanol and water intake were measured each day. Preference ratios were calculated at the 30 minutes and 24 hour time points, respectively, as the amount of ethanol consumed divided by the total fluid (water + ethanol) consumed. This procedure was conducted for seven consecutive weeks (21 ethanol drinking sessions), with the exception of one Friday (week 5) due to a holiday for laboratory personnel.
Statistics
For measures of anxiety-like behavior on the EPM and the Light/Dark box, one-tailed unpaired t-tests were conducted to compare GH and SI female rats. Data gathered from the response to novelty assay and drinking procedures were conducted using a two-way repeated measures (RM) ANOVA (group × time), with Bonferroni post-hoc tests interpreted when appropriate. Additional correlational analyses (Pearon’s r) were conducted to explore the relationship between anxiety-like behavior and ethanol intake. The significance level for all analyses was set at p < 0.05. Data were analyzed and graphs were generated using SigmaPlot 11.0 and GraphPad Prism4 software.
RESULTS
Elevated Plus Maze
At the end of the six week GH/SI housing period, anxiety-like behavior was assessed using three behavioral assays. On the EPM, GH and SI female rats did not differ on any parameter, including: time spent in the open arms, number of open arm entries, time spent on the closed arms, number of closed arm entries, or time spent at the junction of the four arms (Table 1).
Table 1. Elevated Plus Maze.
Group housed (GH) and socially isolated (SI) female rats did not differ on any parameter measured on the elevated plus maze, including: time spent in the open arms, number of open arm entries, time spent on the closed arms, number of closed arm entries, or time spent at the junction of the four arms.
| Group Housed | Socially Isolated | t-test, p | |
|---|---|---|---|
| Open Arm Time (sec) | 15.0 ± 5.3 | 23.02 ± 9.5 | n.s., p = 0.24 |
| Number of Open Arm Entries | 0.67 ± 0.2 | 1.5 ± 0.5 | n.s., p = 0.10 |
| Closed Arm Time (sec) | 195.5 ± 8.4 | 180.7 ± 17.7 | n.s., p = 0.23 |
| Number of Closed Arm Entries | 5.3 ± 0.7 | 5.6 ± 0.51 | n.s., p = 0.37 |
| Junction Time (sec) | 90.3 ± 9.1 | 96.3 ± 12.8 | n.s., p = 0.36 |
Response to Novelty
The response to novelty test was two hours in duration, with the novel object placed in the open field immediately following the 60 minute time point. A RM two-way ANOVA (group × time) was conducted to determine if overall locomotion (distance travelled) differed between GH and SI rats. The data revealed that the groups did not differ in total distance travelled, and there was not a significant interaction between group and time. There was, however, a main effect of time that showed differences in total distance travelled at multiple time points (F(23, 599) = 34.113, p <0.001). Of note, distance travelled at the 5 minute time point was greater than all other time points (t values ranged from 4.6 - 17.1, p values ≤ 0.001), and distance travelled was significantly increased by placement of the novel object, as compared to the previous 45 minutes of the test (t values ranged from 5.4 – 12.0, p values < 0.001) and as compared to distance travelled from the 70 minute time point until the end of the 120 minute test (t values ranged from 5.7 – 11.69, p values < 0.001). A RM two-way ANOVA (group × time) was conducted for time spent in the center of the open field. There was no main effect of group, or a significant interaction. There was a main effect of time (F(23, 599) = 10.261, p < 0.001), indicating that time in the center of the field was increased by placement of the novel object (t values ranged from 4.1 – 9.1, p values < 0.05) (Fig. 1). Together, these data suggest that GH and SI female rats do not differ in open-field locomotor activity, either before, or in response to, the introduction of a novel object.
Figure 1.
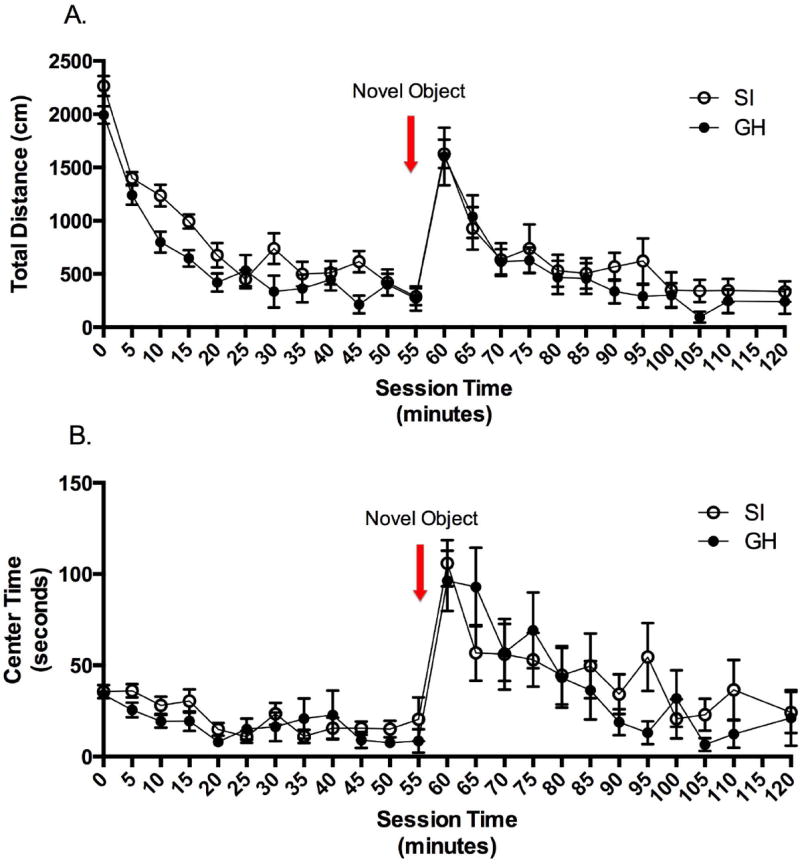
In the Response to Novelty test, no group difference was detected in distance travelled or time in the center of the field. A. Regarding distance travelled, there was a main effect of time such that the distance travelled at the 5 minute time point was greater than all other time points, and distance travelled was significantly increased by placement of the novel object. B. Regarding time in the center of the field, a main effect of time revealed that time in center of the field was increased by placement of the novel object.
Light/Dark Box
Lastly, anxiety-like behavior was assessed using the Light/Dark box. GH and SI rats did not differ in general locomotion, as there was no group difference detected in the amount of time they spent moving in the dark side of the box or the light side. There was also no group difference for time spent on the light side of the box or number of entries into the light side of the box (Table 2). It is notable that both groups spent more time in the dark side rather than the light side of the chamber, suggesting that this assay is indeed anxiogenic (SI rats: t = 3.01, p < 0.01; GH rats: t = 1.73, p < 0.05; Fig. 2). The number of entries into each side of the chamber was equivalent within SI rats (t = 0.10, p = 0.46) and within GH rats (t = 0.10, p = 0.46) (not shown).
Table 2. Light/Dark box.
In the light/dark box, GH and SI rats did not differ in general locomotion or measures of anxiety-like behavior. Time was measured in seconds.
| Group Housed | Socially Isolated | t-test, p | |
|---|---|---|---|
| Time spent moving (dark) | 116.8 ± 9.1 | 122.1 ± 6.0 | n.s., p = 0.31 |
| Time spent moving (light) | 87.5 ± 9.81 | 95.35 ± 6.9 | n.s., p = 0.26 |
| Total time (light) | 132.5 ± 14.7 | 131.0 ± 9.2 | n.s., p = 0.46 |
| # Entries (light) | 5.9 ± 0.58 | 6.4 ± 0.50 | n.s., p = 0.27 |
Figure 2.
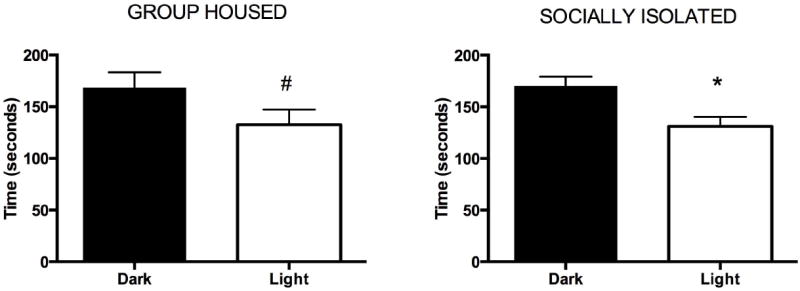
In the light/dark box, GH and SI rats spent significantly less time on the light side compared to the dark side, though the number of entries into each side of the chamber was equivalent within groups. (*p < 0.01 vs. Dark side of chamber in SI rats; #p < 0.05 vs. Dark side of chamber in GH rats).
Home Cage Ethanol Drinking
Home cage ethanol drinking was initiated with three day forced access to 10% ethanol. A RM two-way ANOVA (group × day) showed a significant main effect of day, such that more ethanol was consumed on the first day of access (Friday) as compared to each of the following two days (Saturday and Sunday) (F(2, 74) = 22.583, p <0.001) (Fig. 3). Rats drank an average of 6.4 (± 0.29) g/kg ethanol in the first day of access and decreased their daily intake to 4.7 (± 0.23) and 5.0 (± 0.11) g/kg, respectively, on the second and third days of forced access. There was no significant interaction or group difference in drinking over the three day period. After three day forced access, a two-bottle choice procedure was initiated in which each rat was given access to both water and 10% ethanol for five consecutive days. A RM two-way ANOVA (group × day) showed a main effect of group (F(1, 123) = 5.912, p < 0.05) and a main effect of day (F(4, 123) = 4.534, p < 0.01), but no significant interaction. Across the five day period, SI female rats drank more than GH female rats (F(1, 123) = 5.912, p < 0.05) (Fig. 4). Ethanol intake increased steadily across days (F(4, 123) = 4.534, p < 0.01), such that ethanol intake was greatest on Friday, with significantly greater intake compared to all other days (p values < 0.05), except Thursday. In fact, daily intake doubled across the five day period, with an average intake of 0.646 (± 0.14) g/kg on Monday and 1.317 (± 0.14) g/kg on Friday. Ethanol preference also significantly increased across the five day period (F(4, 120) = 2.707, p < 0.05), with a strong trend toward significance for SI rats to prefer ethanol more than GH rats (p = 0.053). A statistically significant increase in daily ethanol preference for SI rats was observed when comparing Monday and Friday ratios (t = -2.43, p < 0.05; Monday: 0.091 (± 0.02), Friday: 0.237 (± 0.06). The ethanol preference ratio also increased in GH rats, though the increase was not statistically significant (t = -1.465, p = 0.158; Monday: 0.057 (± 0.01), Friday: 0.190 (± 0.09) for GH rats (Fig. 4).
Figure 3.
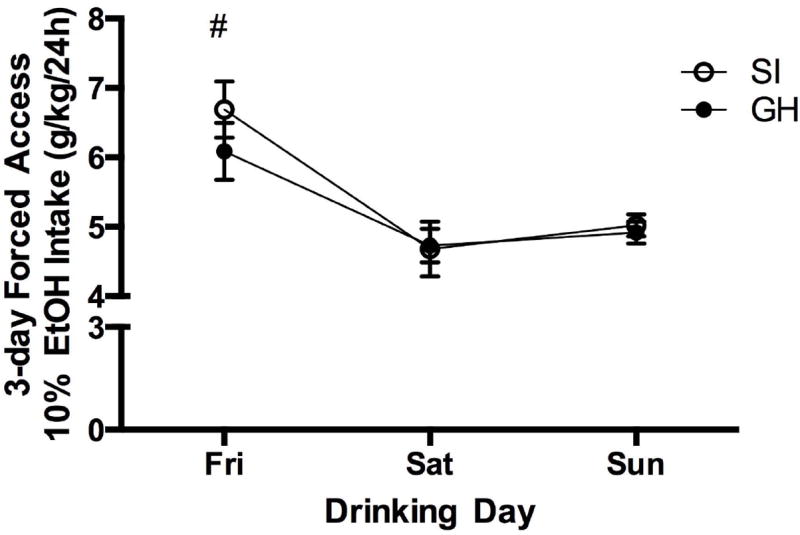
Three day forced drinking (10% ethanol). There was no effect of group, but ethanol intake (g/kg) was greater on Friday than both Saturday and Sunday (#p < 0.05).
Figure 4.
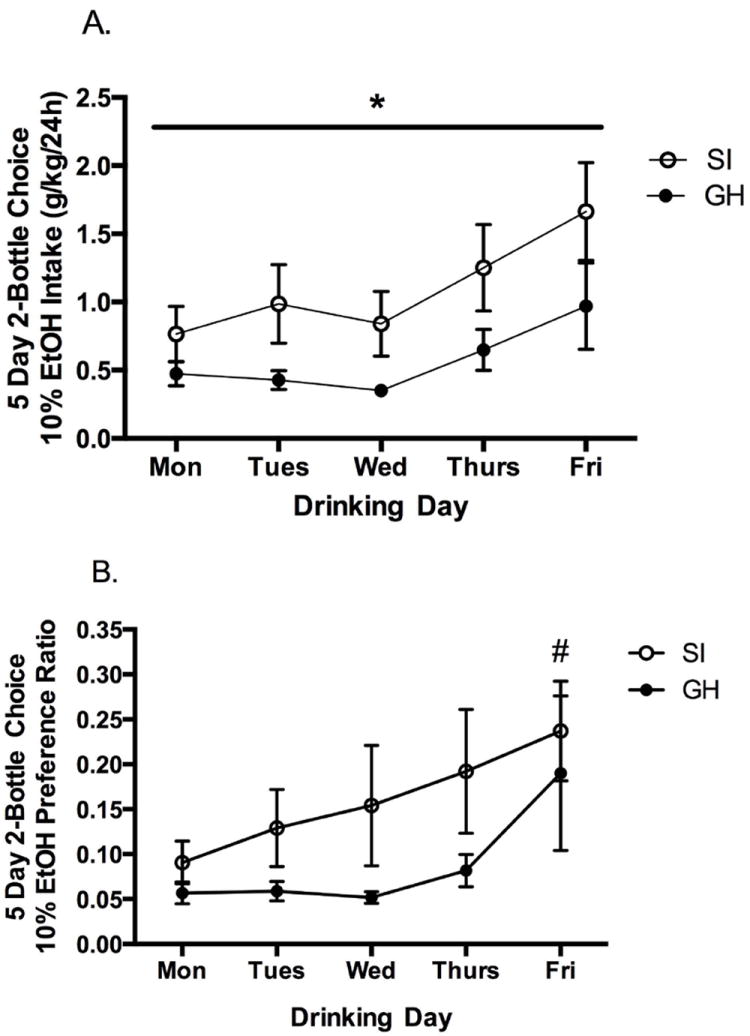
A. Five day, two-bottle choice drinking intake (water and 10% ethanol) showed a main effect of group and a main effect of day. Across the five day period, SI female rats drank more than GH female rats (*p < 0.05). Ethanol intake was greatest on Friday, with significantly greater intake compared to all other days (p values < 0.05), except Thursday. B. During the five day two-bottle choice procedure, ethanol preference was significantly increased across the five day period, with a strong trend toward significance for SI rats to prefer ethanol more than GH rats (p = 0.053) (#p < 0.05 Friday vs. Monday preference in SI rats).
The forced access and five day choice procedure for 10% ethanol were implemented to, 1) reduce novelty/aversion to ethanol, and 2) assess ethanol preference over consecutive days with a familiar ethanol concentration (10%). However, previous work has demonstrated that intermittent access to ethanol (Mon, Wed, Fri) engenders higher levels of ethanol intake compared to intake when access is uninterrupted (Wise 1973). As such, the intermittent access, two-bottle choice ethanol (20%) drinking paradigm was initiated following the forced access and five day two-bottle choice access periods (Chappell et al. 2013). Ethanol intake (g/kg) was analyzed using RM two-way ANOVAs (group × drinking session) for the 30 minute time point and 24h intake. At 30 minutes, there was a significant group × drinking session interaction (F(19, 497) = 1.883, p < 0.05), such that SI rats drank more than GH rats on sessions three and four (Bonferroni p values < 0.05), but intake in SI rats was not statistically greater than intake in GH rats at other time points (Fig. 5a). Similarly, following 24h access to ethanol, there was a significant group × drinking session interaction (F(19,497) = 3.453, p < 0.001). SI rats consumed more ethanol than GH rats on sessions two, three, and eight (Bonferroni ps < 0.05), but intake in SI rats was not statistically greater than intake in GH rats at other time points. Ethanol preference ratios were also calculated for SI and GH rats, respectively, across the seven week intermittent access drinking period. A RM two-way ANOVA (group × session) indicated a main effect of session on 30 minute preference (F(19, 494) = 2.471, p < 0.001), but no main effect of group or interaction. Analysis of data for ethanol preference ratios (24h time point) revealed a significant main effect of session (F(19, 494) = 2.721, p < 0.001) and a group × session interaction (F(19, 494) = 2.316, p < 0.01). During week one through week three, SI rats showed greater preference for ethanol on five out of the nine sessions (Bonferroni, ps < 0.05). Significant group differences were never noted in ethanol drinking or ethanol preference after week three of home cage, two-bottle choice intermittent ethanol access. Overall, the drinking data show that female SI rats drank more ethanol than female GH rats at early time points, but over sessions, SI rats decreased their ethanol intake while GH rats increased their ethanol intake; thus resulting in no significant differences in ethanol intake or preference in the last few weeks of the intermittent drinking regimen.
Figure 5.
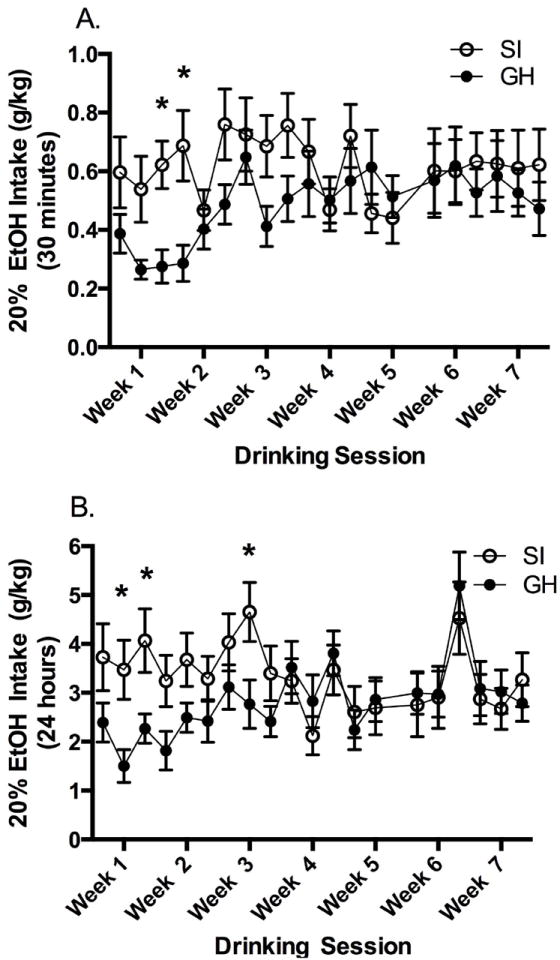
Home cage ethanol drinking in the intermittent access, two bottle choice paradigm. A. At the 30 minute time point, SI rats drank more ethanol (g/kg) than GH rats on sessions three and four (*p < 0.05). B. Following 24h access to ethanol, SI rats consumed more ethanol (g/kg) than GH rats on sessions two, three, and eight (*p < 0.05).
Correlational Analyses: Ethanol Intake and Anxiety-Like Behavior
Previous data from male Long Evans rats using this experimental protocol have shown a significant correlation between 24h ethanol intake in week 1 of the intermittent procedure and anxiety-like behavior on the elevated plus maze, such that greater ethanol intake was observed in rats that showed greater anxiety-like behavior when GH and SI groups were combined (but not when GH and SI groups were considered separately) (Chappell et al., 2013). In female rats, there was also no significant correlation between 24h ethanol intake in week 1 and time spent on the open arms of the elevated plus maze when GH and SI groups were considered separately (GH rats: r = 0.1960, p = 0.54; SI rats: r = -0.1125, p = 0.71). And in contrast to the male data, the relationship between ethanol intake and anxiety-like behavior on the elevated plus maze was also not significant when GH and SI groups were combined for analysis (r = 0.023, p = 0.914). Correlations with ethanol intake and behavior in the Light/Dark box showed a different result. For GH rats, there was no significant relationship between 24h ethanol intake in week 1 and time spent on either the Dark or Light side of the box (Dark: r = 0.004, p = 0.99; Light: r = -0.004, p = 0.99). However, for SI rats, there was a significant negative correlation between ethanol intake and time spent on the Dark side of the box (r = -0.602, p < 0.05; Fig. 7a) and, correspondingly, a significant positive correlation between ethanol intake and time spent on the Light side of the box (r = 0.602, p < 0.05; Fig. 7b). These data are consistent with the conclusion that female SI rats that drink more ethanol show less anxiety-like behavior in the Light/Dark box paradigm.
Figure 7.
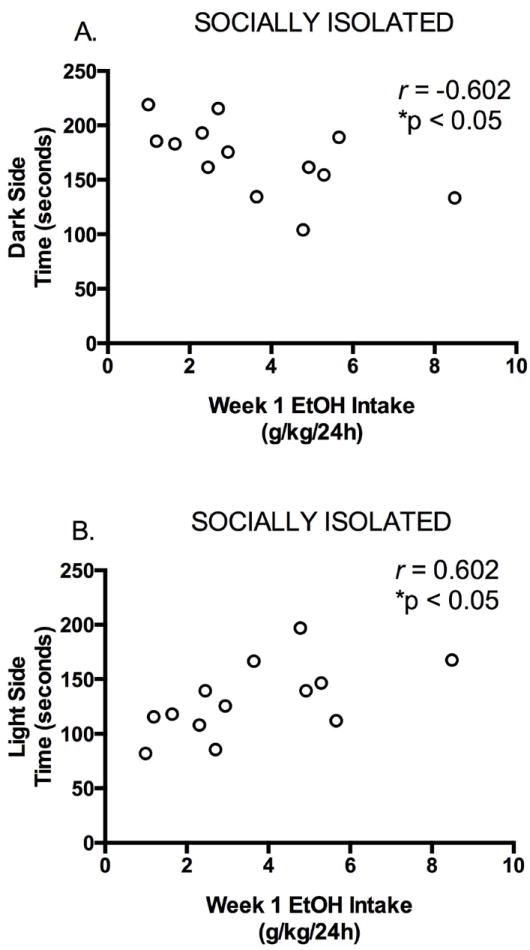
Correlations between Light/Dark box behavior and ethanol intake in SI rats. A. There was a significant negative correlation between ethanol intake and time spent on the Dark side of the box. B. Correspondingly, there was a significant positive correlation between ethanol intake and time spent on the Light side of the box.
DISCUSSION
The adolescent GH/SI model implemented in this study has been previously shown by our lab and others to result in an addiction vulnerable phenotype hallmarked by enduring increases in anxiety-like behaviors and ethanol intake in male Long Evans rats. However, the current studies do not show a similar phenotype in female Long Evans rats following the same experimental protocol. Female GH and SI rats show only modest and transient differences in behavioral assays of unconditioned anxiety-like behavior and ethanol drinking. Moreover, increased anxiety measures were not associated with greater ethanol intake in female rats. These data provide additional evidence that behavioral and neurobiological measures in models of psychological and addictive disorders may engender profoundly different patterns in males and females. Despite the clinical evidence that early life adversity is strongly associated with increased alcohol addiction vulnerability in women, it appears that adolescent social isolation does not serve as a reliable model in female rodents for anxiety-addiction correlates, but such a model is extremely desirable.
Previous reports of the effects of adolescent SI on anxiety-like behaviors in female rats have shown equivocal results, and the current EPM data contrast with previously published data gathered from male Long Evans rats that were housed in the same manner (McCool and Chappell 2009; Chappell et al. 2013). On a measure of unconditioned anxiety-like behavior (i.e., open field activity), SI female rats have shown significantly greater anxiety-like behavior, indicated by less time spent in the center of the field (Hermes et al. 2011) and greater immobility time (Lukkes et al. 2012) relative to GH conspecifics. Conversely, on the elevated plus maze, female SI rats have shown increased anxiety-like behavior (Da Silva et al. 1996), or no difference relative to GH females (Weiss et al. 2004; Weintraub et al. 2010; Bourke and Neigh 2011). Results are also equivocal in social interaction paradigms using SI female rats, such that no difference (Lukkes et al. 2012), or decreased social interaction has been reported (Hermes et al. 2011). Additionally, hyperactivity in a novel environment in males is consistently observed across rat strains and GH/SI paradigm, but female SI rats do not reliably show this difference in locomotion relative to GH counterparts (Weiss et al. 2004; Ferdman et al. 2007). It has also been suggested that SI engenders a depressive-like, but not anxiety-like, phenotype in female SI rats compared to their GH counterparts (Bourke and Neigh 2011). Perhaps most important in regard to the current study design, the elevated plus maze has been a validated test for measuring anxiety-like behavior in male rodents in large part because of the ability of diazepam to increase open arm exploration and time (Pellow et al. 1985). However, female Sprague Dawley rats fail to show an anxiolytic effect after diazepam administration in the elevated plus maze, perhaps suggesting that this test is not optimized for use in female rats (Simpson et al. 2012). On the light/dark box paradigm, however, the lack of group differences in time spent in the dark compartment and general locomotion is consistent with observations made in this assay using male Long Evans rats that were similarly housed (McCool and Chappell 2009). When data were analyzed within groups, an anxiogenic effect of the assay was detected, with mean values showing that both GH and SI rats spent slightly more time in the dark side and slightly less time in the light side of the chamber. Most interestingly, however, was the observation that ethanol intake in SI rats was actually positively correlated with lower anxiety-like behavior in the light/dark box, in that rats that spent more time in the light (anxiogenic) side of the box drank the most ethanol. These data are in the opposite direction of the correlation between ethanol intake and anxiety-like behavior in male rats. Together, these data highlight that in female rats, antecedent anxiety-like behavior is likely not driving ethanol intake as in male rats. This further underscores our need for an appropriate model in female rats to study neurobiological substrates linking antecedent anxiety and alcohol dependence. It is noteworthy that anxiety-like behavior was measured in this study using three separate approaches (EPM, Light/Dark, Open field center time), but group differences were not apparent. This is in marked contrast to the enduring increases in anxiety-like behavior observed in male rats subjected to this same adolescent isolation procedure (McCool and Chappell 2009; Chappell et al. 2013).
Adolescence is a critical period of brain development. In male Long Evans rats, there are likely myriad reasons why early life stress during adolescence increases the expression of anxiety-like behaviors and ethanol intake/preference, or group housing significantly reduces these behaviors. Greater ethanol intake in SI female rats was observed on a few of the drinking days, though this greater intake was transient, with SI and GH females drinking the same amount of ethanol in the last four weeks. Even more striking, SI rats showed greater ethanol preference under intermittent access conditions in the first three weeks, but this effect failed to persist as preference in SI rats dropped and preference in GH rats increased over the seven week period. This pattern contrasts with ethanol intake in male Long Evans rats having undergone the same housing procedure, in which SI rats persist in drinking more ethanol than GH rats across a four week intermittent drinking period (Chappell et al. 2013). Additionally, male rats’ anxiety-like behavior on the EPM is positively correlated with their initial ethanol intake, whereas no such relationship was detected in female rats. The inability to detect this phenotype in female rats may be because they are less adversely affected by social isolation; or, female rats may be more sensitive to hierarchies that develop in social groups, making group housing more adverse. For instance, in a non-human primate model of ethanol self-administration in which animals were initially group housed, ethanol intake during group housing was inversely correlated with dominance rank, with no change in alcohol preference in females following isolation (McKenzie-Quirk and Miczek 2008). Additionally, Advani et al. (2007) showed increased ethanol preference in socially isolated male mice, whereas increased ethanol preference in female mice was only observed in those females that were subjected to social isolation and maternal separation in early life. Indeed, female rats exposed to prenatal restraint stress have been shown to have greater preference for, and intake of, ethanol during adolescence compared to unstressed counterparts (Van Waes et al. 2011), which may suggest that the nature of the stressor may be of critical importance in engendering persistently greater ethanol intake in females after stress, or that females may need a longer, more severe history of stress during critical developmental periods to engender greater levels of alcohol intake/preference. Alternatively, other behavioral deficits may be more pronounced in females. For instance, McCormick et al. (2013) showed that social instability stress in female rats during adolescence led to increased fear-like behavior.
It is pertinent to note that we did not evaluate the stage of the estrous cycle in this study. However, the range of variability observed within groups in this study is not greater than would be observed in studies with male rats, suggesting that stage of the cycle likely did not introduce significant variability in the ethanol drinking observed, and brief postnatal social isolation has been shown to not alter the estrous cycle (Kosten et al. 2005). Most relevant to the current dataset that was collected over an eight week period, previous data have shown that ethanol drinking patterns can vary across the estrous cycle without changing total ethanol (g/kg) intake (Ford et al. 2002b). In accordance, intake in an operant self-administration paradigm has been shown to be similar in freely cycling female rats across phases of the estrous cycle (Roberts et al., 1998). However, it cannot be completely ruled out that hormones can affect ethanol intake, as exogenous introduction of hormones in intact female rats has been shown to affect ethanol intake (Ford et al. 2002a). Some studies have suggested a relationship between stage of the estrous cycle and anxiety-like behavior (Bitran & Dowd 1996; Mora et al. 1996), though several studies have reported no effect of estrous cycle on the elevated plus maze in adult female rats (Bitran et al., 1991; Nomikos & Spyraki, 1988). Taken together, what can be concluded is that estrous cycle stage does not uniformly affect anxiety-like behavior in females across paradigms. Though it is an important consideration, there is not compelling evidence to date that hormone fluctuations throughout the estrous cycle are a primary determinant of anxiety-like behavior or ethanol intake in female rats. As we and others seek to identify more effective models to study the interaction between early life stress, anxiety, and ethanol drinking, it will be important to determine how estrous cycle-associated hormonal fluctuations contribute to these relationships.
The goal of the current studies was to assess whether an adolescent social isolation protocol that increases a number of behavioral risk factors for alcoholism in male Long Evans rats engenders similar phenotypic changes in females. The data indicated that early life stress fails to impart the same addiction vulnerable phenotype in female rats. Given the robust and enduring behavioral and neurobiological alterations noted in SI vs. GH male rats, it is compelling that this same protocol produces only small, transient differences in SI/GH female rats and that increased anxiety-like behaviors were not positively correlated with ethanol intake in females. Current efforts are underway to develop an appropriate model to study anxiety-like behaviors in female rats and subsequent addiction vulnerability and/or resiliency.
Figure 6.
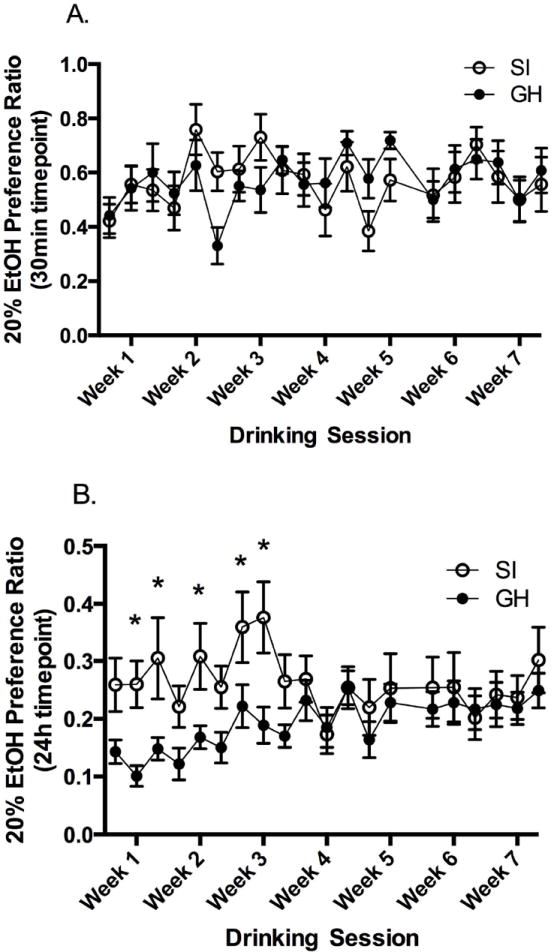
Ethanol preference ratios for home cage, two-bottle choice intermittent ethanol access. A. A RM two-way ANOVA (group × session) indicated a main effect of session on 30 min preference, but no main effect of group or interaction. B. Analysis of 24h ethanol preference ratios revealed a significant main effect of session and a group × session interaction. During week one through week three, SI rats showed greater preference than GH rats for ethanol in five out of the nine sessions (Bonferroni post-hoc tests, *p values < 0.05).
Acknowledgments
This work was supported by National Institutes of Health Grants AA17531, AA, AA21099, AA10422, and F32 AA022270-01A1.
References
- Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol. 2007;10:595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- Bertholomey ML, Henderson AN, Badia-Elder NE, Stewart RB. Neuropeptide Y (NPY)-induced reductions in alcohol intake during continuous access and following alcohol deprivation are not altered by restraint stress in alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2011;97:453–461. doi: 10.1016/j.pbb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Dowd JA. Ovarian steroids modify the behavioral and neurochemical responses of the central benzodiazepine receptor. Psychopharmacology. 1996;125(1):65–73. doi: 10.1007/BF02247394. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Ovarian endocrine states modulates the anxiolytic potency of diazepam and the efficacy of gamma-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991;105(5):653–62. doi: 10.1037//0735-7044.105.5.653. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Ariowodola OJ, Weiner JL. The impact of social isolation on HPA axis function, and anxiety-like behaviors, and ethanol drinking. Front Integr Neurosci. 2014;7:102. doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 1999;144:151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- Da Silva NL, Ferreira VM, Carobrez Ade P, Morato GS. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol Behav. 1996;60:1391–1396. doi: 10.1016/s0031-9384(96)00254-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002a;26:103–113. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002b;26:635–643. [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, Bergquist K, Anderson G, Kreek MJ, Sinha R. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44:575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ. Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev. 1999;23:971–980. doi: 10.1016/s0149-7634(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Jatlow PI, Kehoe P. Neonatal isolation alters the estrous cycle interactions on the acute and behavioral effects of cocaine. Psychoneuroendocrinology. 2005;30(8):753–761. doi: 10.1016/j.psyneuen.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res. 2012;1443:1–17. doi: 10.1016/j.brainres.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mongillo DL, Simone JJ. Age and adolescent social stress effects on fear extinction in female rats. Stress. 2013;16:678–688. doi: 10.3109/10253890.2013.840283. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201:137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Takase K, Nakajima W, Tada H, Ohya D, Sano A, Goto T, Hirase H, Malinow R, Takahashi T. Disrupted cortical function underlies behavior dysfunction due to social isolation. J Clin Invest. 2012;122:2690–2701. doi: 10.1172/JCI63060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21(7):609–20. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27(7):691–6. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22(7):1564–1569. [PubMed] [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia MP, Mostallino MC, Secci PP, Pisu MG, Biggio F, Utzeri C, Olla P, Biggio G, Follesa P. Voluntary Ethanol Consumption Induced by Social Isolation Reverses the Increase of alpha(4)/delta GABA(A) Receptor Gene Expression and Function in the Hippocampus of C57BL/6J Mice. Front Neurosci. 2011;5:15. doi: 10.3389/fnins.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Ryan C, Curley A, Mulcaire J, Kelly JP. Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:227–236. doi: 10.1016/j.pnpbp.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Sonne SC, Back SE, Diaz Zuniga C, Randall CL, Brady KT. Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. Am J Addict. 2003;12:412–423. [PubMed] [Google Scholar]
- Van Waes V, Darnaudery M, Marrocco J, Gruber SH, Talavera E, Mairesse J, Van Camp G, Casolla B, Nicoletti F, Mathe AA, Maccari S, Morley-Fletcher S. Impact of early life stress on alcohol consumption and on the short- and long-term responses to alcohol in adolescent female rats. Behav Brain Res. 2011;221:43–49. doi: 10.1016/j.bbr.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Whitaker LR, Degoulet M, Morikawa H. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron. 2013;77:335–345. doi: 10.1016/j.neuron.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, White HR, Czaja SJ, Marmorstein NR. Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. J Stud Alcohol Drugs. 2007;68:317–326. doi: 10.15288/jsad.2007.68.317. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–1031. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]


