Abstract
Several unnatural derivatives of narciclasine were prepared in which the C-7 carbon was replaced with nitrogen. The 7-aza derivative and its N-oxide were prepared by the coupling of iodopicolinic acid with a conduramine unit derived chemoenzymatically from bromobenzene. Intramolecular Heck reaction was used to construct the carbostyryl ring system. The compounds were submitted to biological screening against cancer cell lines. Full experimental and spectra data are provided for all new compounds.
Pancratistatin (1) and narciclasine (3) represent some of the most potent cytotoxic components of the Amaryllidaceae constituents. These compounds, and their 7-deoxy derivatives, 7-deoxypancratistatin (2) and lycoricidine (4), have been the focus of many synthetic ventures in the last three decades.1 Many unnatural or truncated derivatives of pancratistatin have been prepared and evaluated for biological activity with the intent to produce more bioavailable agents then the highly water-insoluble natural products.2
Most of the effort aimed at unnatural or truncated derivatives was focused on structural modifications of pancratistatin or 7-deoxypancratistatin. Modifications of narciclasine, one of the most potent of Amaryllidaceae constituents,1d are less common.2b,3
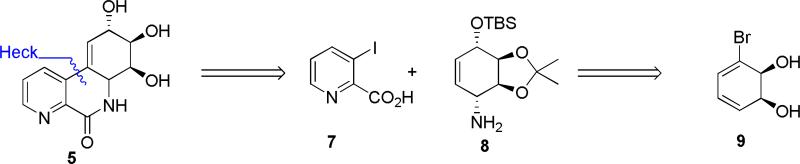
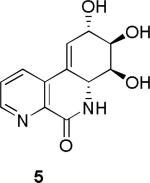
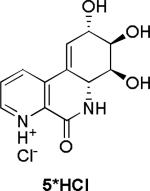
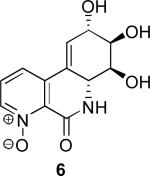
Initially, we chose to investigate a truncated derivative of narciclasine such as 5, without the methylenedioxy bridge and with nitrogen instead of carbon at C-7, Figure 2. The rationale for this design was based on the assumption that the N-oxide 6 derived from 5 could potentially mimic the donor-acceptor functionality responsible for increased activity in the alkaloids containing the 7-hydroxy-moiety.2g,l
Figure 2.
Retrosynthetic plan for aza-narciclasine analogues.
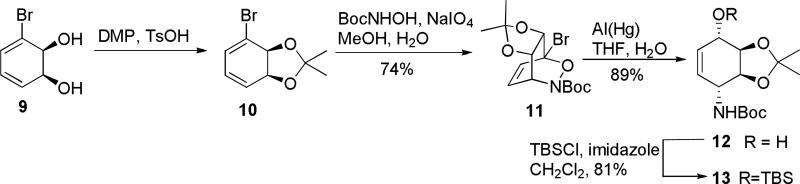
The synthesis begins with the preparation of the conduramine 8, which is generated from the diene diol 9, by a similar strategy that was utilized in the in the total synthesis of lycoricidine.4 Coupling of conduramine 8 with iodo picolinic acid 7 would be followed by the Heck reaction, in analogy with the strategy used in lycoricidine synthesis. Diol 9, Scheme 1, is produced in the yields of 18-20 g/L by the whole cell fermentation of bromobenzene with E. coli JM109 (pDTG601A), a recombinant organism developed by Gibson and over-expressing toluene dioxygenase.5
Scheme 1.
Diol 9 was protected with acetonide group and used immediately for hetero Diels-Alder reaction with fragment generated by oxidation of t-butyloxycarbohydroxamic acid , Scheme 1. Reduction of bicyclic product 11 with aluminum amalgam led to alcohol 12. This sequence was performed on a multigram scale and did not require the isolation of any intermediates.
Protection of alcohol 12 with t-butylsilyl group led to protected conduramine 13.
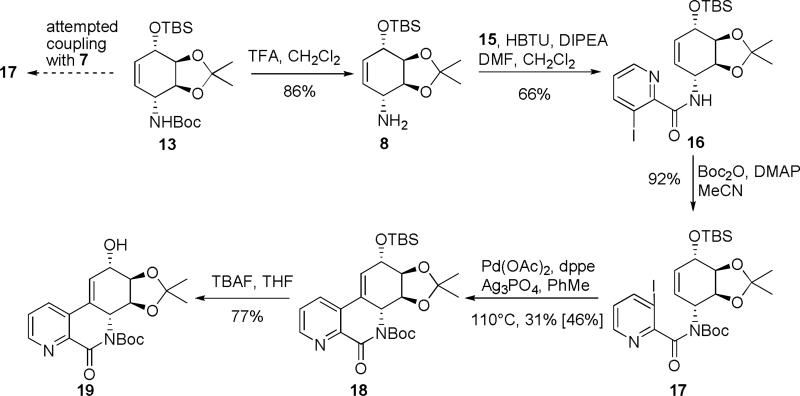
Second coupling fragment, iodo acid 7, was prepared by a simple direct ortho-metalation of picolinic acid with lithium tetramethylpiperidide (LTMP) and I2 via a known literature procedure, Scheme 2.6 The free acid 7 was not obtained in good yield and proved somewhat unstable upon prolonged storage. Therefore the lithium salt 15 was ultimately used as a coupling partner because of its increased stability.
Scheme 2.
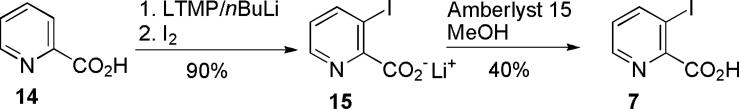
Coupling of the protected conduramine 13 with 3-iodopicolinic acid 7 was initially attempted to produce 17 directly. Unfortunately, under a variety of coupling conditions: 1,1‘-carbonyldiimidazole (CDI), N,N’-dicyclohexylcarbodiimide/hydroxybenzotriazole (DCC/HOBt), or N,N,N’,N’-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate (HBTU), no desired product was observed, Scheme 3. Therefore conduramine 13 was selectively deprotected with trifluoroacetic acid to afford the free amine 8. Coupling of amine 8 and lithium salt 15 provided amide 16, in a moderate yield that can probably be explained by steric hindrance of ortho-iodo group. Reprotection of the amide with di-tert-butyl dicarbonate in the presence of DMAP provided carbamate 17. All of the currently existing Heck approaches to narciclasine skeleton4,7 require tertiary amide or imide moiety for successful transformation; most of them also use toxic thallium (I) salts as a base. Nevertheless, both products 14 and 15 were submitted to a range of standard Heck coupling conditions. We decided to avoid the use of thallium salts and therefore silver phosphate was used as a substitute.7c The only conditions that led to the desired product 18, involved the reaction of 17 in the presence of Pd(OAc)2/1,2-bis(diphenylphosphino)ethane and silver phosphate. The summary of the various conditions attempted is shown in Table 1.
Scheme 3.
Table 1.
Conditions attempted for the Heck cyclization.
| Conditions | Outcome |
|---|---|
| Pd(dppf)2Cl2, dppf, Ag3PO4 dioxane, 60°C, 24 h | No reaction |
| Pd(OAc)2, dppe, Ag3PO4, Et3N toluene, 80°C, 18 h | Traces of 18 |
| Pd(OAc)2, PPh3, Et3N, AgNO3, MeCN, 24 h, r.t to 80°C | No reaction |
| Pd(OAc)2, dppe, AgNO3, Cs2CO3, toluene, 24 h, 110°C | 18, 35% (45 % brsm) |
| Pd(OAc)2, BINAP, Ag3PO4, Et3N toluene, 80°C, 18 h | No reaction |
No product of the Heck reaction was ever observed under similar conditions when the unprotected amide 16 was used. Deprotection of the silyl group with TBAF led to an alcohol 19.
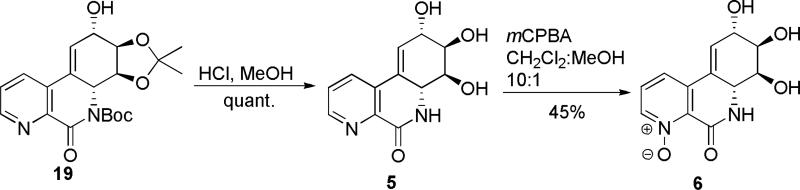
The synthesis was completed by acid-catalyzed deprotection of the Boc carbamate 19 to furnish the HCl salt of 5, which upon chromatography with basic eluent was isolated as a free base 5. Oxidation of 5 to its respective N-oxide 6 was performed, as shown in Scheme 3, and subjected to screening in two cancer cell lines to compare the cytotoxicity results to those of the recently tested pancratistatin C-1 homologues.2j
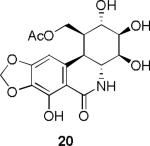
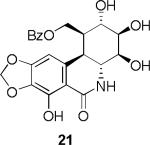
In our previous study it has been shown2r,t that C-1 analogues 20 and 21 also displayed pronounced activity against pancreatic (BxPC-3), prostate (DU-145), and lung (NCI-H460) cancer cell lines. The respective IC50 values for these three lines for C-1 acetate 20 were 0.07, 0.06 and 0.07 μM and for C-1 benzoate 21: 0.01, 0.01. 0.03 μM. For the latter compound, IC50 values exceeded those of the natural product narciclasine (3): 0.05, 0.03, 0.05 μM respectively.
The activity of pancratistatin and narciclasine against cancer cell lines is known to be ~100 times higher than that of the 7-deoxy-derivatives. It appears that the 7-hydroxy group is crucial for maintaining effective inhibition of cancer cell growth. The notion that the 7-N-oxide functionality would somehow mimic this requirement was shown not to be correct and the aza-derivatives were found to be inactive. The compounds tested also lacked the methylene dioxy group, which is a known contributor to the pharmacophore of these compounds. Our next goal will be the preparation of aza-derivatives of narciclasine that retain the 7-hydroxy moiety as well as the methylene dioxy unit. The results of these endeavors will be reported in due course.
Supplementary Material
Figure 1.
Amaryllidaceae alkaloids and aza-narciclasine.
Scheme 4.
Table 2.
Activity of aza-analogues with C-1 homologues as standards [IC50 (μM)]
| Cell line |
|
|
|
|
|
|---|---|---|---|---|---|
| HeLa (cervical cancer) | <0.4 μM | <0.4 μM | Non-inhibitory | >100 μM | >100 μM |
| MCF7 (breast cancer) | <0.4 μM | <0.4 μM | Non-inhibitory | >100 μM | >100 μM |
Acknowledgements
The authors are grateful to the following agencies for financial support of this work: Natural Sciences and Engineering Research Council of Canada (NSERC) (Idea to Innovation and Discovery Grants), Canada Research Chair Program, Canada Foundation for Innovation (CFI), TDC Research, Inc., TDC Research Foundation, the Ontario Partnership for Innovation and Commercialization (OPIC), The Advanced Biomanufacturing Centre (Brock University), and by grants from the National Institute of General Medical Sciences (P20GM103451). We also appreciate the skillful assistance of Razvan Simionescu and Tim Jones for their help with NMR and mass spectrometry analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.a Rinner U, Hudlicky T. Synlett. 2005:365. [Google Scholar]; b Chapleur Y, Chrétien F, Ahmed SI, Khaldi M. Current Organic Synthesis. 2006;3:341. [Google Scholar]; c Manpadi M, Kornienko A. Org. Prep. Proced. Int. 2008;40:109. doi: 10.1080/00304940809458083. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kornienko A, Evidente A. Chem. Rev. 2008;108:1982. doi: 10.1021/cr078198u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Chrétien F, Ahmed SI, Masion A, Chapleur Y. Tetrahedron. 1993;49:7463. [Google Scholar]; b Banwell MG, Cowden CJ, Gable RW. J. Chem. Soc., Perkin Trans. 1994;1:3515. [Google Scholar]; c McNulty J, Mo R. Chem. Commun. 1998:933. [Google Scholar]; d Akgün H, Hudlicky T. Tetrahedron Lett. 1999;40:3081. [Google Scholar]; e McNulty J, Mao J, Gibe R, Mo R, Wolf S, Pettit GR, Herald DL, Boyd MR. Bioorg. Med. Chem. Lett. 2001;11:169. doi: 10.1016/s0960-894x(00)00614-4. [DOI] [PubMed] [Google Scholar]; f Rinner U, Siengalewicz P, Hudlicky T. Org. Lett. 2001;4:115. doi: 10.1021/ol0169877. [DOI] [PubMed] [Google Scholar]; g Hudlicky T, Rinner U, Gonzalez D, Akgun H, Schilling S, Siengalewicz P, Martinot TA, Pettit GR. J. Org. Chem. 2002;67:8726. doi: 10.1021/jo020129m. [DOI] [PubMed] [Google Scholar]; h Pandey G, Murugan A, Balakrishnan M. Chem. Commun. 2002;624 doi: 10.1039/b200624c. [DOI] [PubMed] [Google Scholar]; i Phung AN, Zannetti MT, Whited G, Fessner W-D. Angew. Chem. Int. Ed. 2003;42:4821. doi: 10.1002/anie.200352023. [DOI] [PubMed] [Google Scholar]; j Rinner U, Hillebrenner HL, Adams DR, Hudlicky T, Pettit GR. Bioorg. Med. Chem. Lett. 2004;14:2911. doi: 10.1016/j.bmcl.2004.03.032. [DOI] [PubMed] [Google Scholar]; k Rinner U, Hudlicky T, Gordon H, Pettit GR. Angew. Chem. Int. Ed. 2004;43:5342. doi: 10.1002/anie.200460218. [DOI] [PubMed] [Google Scholar]; l McNulty J, Larichev V, Pandey S. Bioorg. Med. Chem. Lett. 2005;15:5315. doi: 10.1016/j.bmcl.2005.08.024. [DOI] [PubMed] [Google Scholar]; e Moser M, Banfield SC, Rinner U, Chapuis J-C, Pettit GR, Hudlicky T, Can J. Chem. 2006;84:24. [Google Scholar]; m Shukla KH, Boehmler DJ, Bogacyzk S, Duvall BR, Peterson WA, McElroy WT, DeShong P. Org. Lett. 2006;8:4183. doi: 10.1021/ol061070z. [DOI] [PubMed] [Google Scholar]; n Kireev AS, Nadein ON, Agustin VJ, Bush NE, Evidente A, Manpadi M, Ogasawara MA, Rastogi SK, Rogelj S, Shors ST, Kornienko A. J. Org. Chem. 2006;71:5694. doi: 10.1021/jo0607562. [DOI] [PubMed] [Google Scholar]; o McNulty J, Nair JJ, Griffin C, Pandey S. J. Nat. Prod. 2008;71:357. doi: 10.1021/np0705460. [DOI] [PubMed] [Google Scholar]; p Manpadi M, Kireev AS, Magedov IV, Altig J, Tongwa P, Antipin MY, Evidente A, van Otterlo WAL, Kornienko A. J. Org. Chem. 2009;74:7122. doi: 10.1021/jo901494r. [DOI] [PMC free article] [PubMed] [Google Scholar]; q Collins J, Rinner U, Moser M, Hudlicky T, Ghiviriga I, Romero AE, Kornienko A, Ma D, Griffin C, Pandey S. J. Org. Chem. 2010;75:3069. doi: 10.1021/jo1003136. [DOI] [PMC free article] [PubMed] [Google Scholar]; r Vshyvenko S, Scattolon J, Hudlicky T, Romero AE, Kornienko A. Bioorg. Med. Chem. Lett. 2011;21:4750. doi: 10.1016/j.bmcl.2011.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; s Lee S, Hwang S, Yu S, Jang W, Lee Y, Kim S. Arch. Pharmacal Res. 2011;34:1065. doi: 10.1007/s12272-011-0703-1. [DOI] [PubMed] [Google Scholar]; t Vshyvenko S, Scattolon J, Hudlicky T, Romero AE, Kornienko A, Ma D, Tuffley I, Pandey S. Can. J. Chem. 2012;90:932. doi: 10.1139/v2012-073. [DOI] [PMC free article] [PubMed] [Google Scholar]; u Nieto-Garcia O, Alonso R. Org. Biomol. Chem. 2013;11:515. doi: 10.1039/c2ob27127c. [DOI] [PubMed] [Google Scholar]
- 3.a Mondon A, Krohn K. Chem. Ber. 1975;108:445. [Google Scholar]; b Pettit GR, Eastham SA, Melody N, Orr B, Herald DL, McGregor J, Knight JC, Doubek DL, Garner LC, Bell JA. J. Nat. Prod. 2005;69:7. doi: 10.1021/np058068l. [DOI] [PubMed] [Google Scholar]; c Matveenko M, Banwell MG, Joffe M, Wan S, Fantino E. Chem. Biodiversity. 2009;6:685. doi: 10.1002/cbdv.200800319. [DOI] [PubMed] [Google Scholar]; d Ingrassia L, Lefranc F, Dewelle J, Pottier L, Mathieu V. r., Spiegl-Kreinecker S, Sauvage S. b., El Yazidi M, Dehoux M. l., Berger W, Van Quaquebeke E, Kiss R. J. Med. Chem. 2009;52:1100. doi: 10.1021/jm8013585. [DOI] [PubMed] [Google Scholar]
- 4.a Hudlicky T, Olivo HF. J. Am. Chem. Soc. 1992;114:9694. [Google Scholar]; b Hudlicky T, Olivo HF, McKibben B. J. Am. Chem. Soc. 1994;116:5108. [Google Scholar]
- 5.a Zylstra GJ, Gibson DT. J. Biol. Chem. 1989;264:14940. [PubMed] [Google Scholar]; b available from Sigma-Aldrich, cat # 489492.
- 6.Carson MW, Giese MW, Coghlan M. J. Org. Lett. 2008;10:2701. doi: 10.1021/ol800834q. [DOI] [PubMed] [Google Scholar]
- 7.a Chida N, Ohtsuka M, Ogawa S. Tetrahedron Lett. 1991;32:4525. [Google Scholar]; b Martin S, Tso H. Heterocycles. 1993;35:85. [Google Scholar]; c McIntosh MC, Weinreb SM. J. Org. Chem. 1993;58:4823. [Google Scholar]; d Yadav JS, Satheesh G, Murthy CVSR. Org. Lett. 2010;12:2544. doi: 10.1021/ol100755v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.