Abstract
Cisplatin, cisplatinum, or cis-diamminedichloroplatinum (II), is a well-known chemotherapeutic drug. It has been used for treatment of numerous human cancers including bladder, head and neck, lung, ovarian, and testicular cancers. It is effective against various types of cancers, including carcinomas, germ cell tumors, lymphomas, and sarcomas. Its mode of action has been linked to its ability to crosslink with the purine bases on the DNA; interfering with DNA repair mechanisms, causing DNA damage, and subsequently inducing apoptosis in cancer cells. However, because of drug resistance and numerous undesirable side effects such as severe kidney problems, allergic reactions, decrease immunity to infections, gastrointestinal disorders, hemorrhage, and hearing loss especially in younger patients, other platinum-containing anti-cancer drugs such as carboplatin, oxaliplatin and others, have also been used. Furthermore, combination therapies of cisplatin with other drugs have been highly considered to overcome drug-resistance and reduce toxicity. This comprehensive review highlights the physicochemical properties of cisplatin and related platinum-based drugs, and discusses its uses (either alone or in combination with other drugs) for the treatment of various human cancers. A special attention is given to its molecular mechanisms of action, and its undesirable side effects.
Keywords: Cisplatin, Platinum-based drugs, Mechanisms of action, Cancer treatment
1. Introduction
Cisplatin (CAS No. 15663-27-1, MF-Cl2H6N2Pt; NCF-119875), cisplatinum, also called cis-diamminedichloroplatinum(II), is a metallic (platinum) coordination compound with a square planar geometry. It is a white or deep yellow to yellow-orange crystalline powder at room temperature. It is slightly soluble in water and soluble in dimethylprimanide and N,N-dimethylformamide. Cisplatin is stable under normal temperatures and pressures, but may transform slowly over time to the trans-isomer (IARC 1981, Akron 2009). Cisplatin has a molecular weight of 301.1 gm/mol, a density of 3.74 g/cm3, a melting point of 270° C, a log Kow of -2.19 and a water solubility of 2.53 g/L at 25° C (HSDB 2009).
Cisplatin was first synthesized by M. Peyrone in 1844 and its chemical structure was first elucidated by Alfred Werner in 1893. However, the compound did not gain scientific investigations until the 1960's when the initial observations of Rosenberg (Rosenberg, Vancamp et al., 1965) at Michigan State University pointed out that certain electrolysis products of platinum mesh electrodes were capable of inhibiting cell division in Escherichia coli created much interest in the possible use of these products in cancer chemotherapy. Since the identification of cis-dichlorodiammineplatinum (II) (cisplatin, r) as the agent responsible for this activity, much interest has been generated in the use of coordination complexes of platinum, palladium, and other noble metals in the treatment of cancer.
Cisplatin has been especially interesting since it has shown anticancer activity in a variety of tumors including cancers of the ovaries,' testes,' and solid tumors of the head and neck. It was discovered to have cytotoxic properties in the 1960s, and by the end of the 1970s it had earned a place as the key ingredient in the systemic treatment of germ cell cancers. Among many chemotherapy drugs that are widely used for cancer, Cisplatin is one of the most compelling ones. It was the first FDA-approved platinum compound for cancer treatment in 1978 (Kelland, 2007). This has led to interest in platinum (II) - and other metal-containing compounds as potential anticancer drugs (Frezza, Hindo et al., 2010).
Cisplatin is clinically proven to combat different types of cancers including sarcomas, cancers of soft tissue, bones, muscles, and blood vessels. Although such cancers have recently received better prognosis and therefore have become less life threatening (Desoize and Madoulet, 2002), significant challenges remain with regard to their cure. Also, because of drug resistance and considerable side effects, combination therapy of cisplatin with other cancer drugs have been applied as novel therapeutic strategies for many human cancers. In this research, we aim to provide a comprehensive review of the physicochemical properties of cisplatin and related platinum-based drugs, to discuss its uses (either alone or in combination with other drugs) for the treatment of various human cancers, to examine its molecular mechanisms of action, and to discuss it potential side effects.
2. Cisplatin and Other Platinum-Containing Drugs
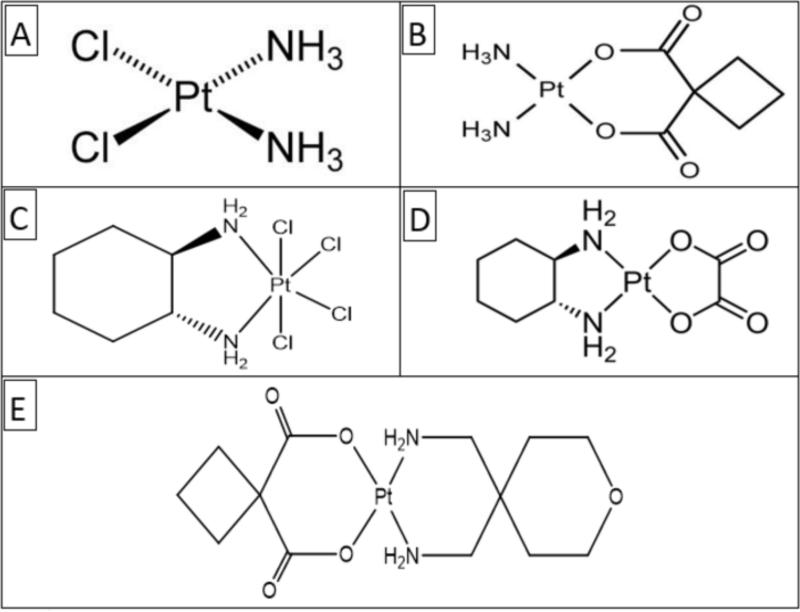
Since the early seminal work in the preclinical and clinical development of cisplatin, several thousand analogues have been synthesized and tested for properties that would enhance its therapeutic index. About 13 of these analogues have been evaluated in clinical trials, but only one (carboplatin) has provided definite advantage over cisplatin and achieved worldwide approval. Nine platinum analogues are currently in clinical trials around the world ormaplatin (tetraplatin), oxaliplatin, DWA2114R, enloplatin, lobaplatin, CI-973 (NK-121), 254-S, JM-216, and liposome-entrapped cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum (II) (LNDDP)] (Weiss and Christian, 1993). Figure 1 presents the chemical structures of cisplatin and four of its analogs including carboplatin, oxaliplatin, ormaplatin and enloplatin.
Fig. 1.
Chemical structures of selected platinum drugs: A-cisplatin; B-carboplatin; C-oxaliplatin; D-ormaplatin; E-enloplatin (Weiss and Christian, 1993).
From the molecular perspective, cisplatin represents a perfect example of how a small alteration in chemical structure can significantly affect biological activity in target cell (Goodsell, 2006). As illustrated in Figure 2, cisplatin, carboplatin and oxaliplatin are composed of doubly charged platinum ion surrounded by four ligands; with the amine ligands on the left forming stronger interactions with the platinum ion, and the chloride ligands or carboxylate compounds on the right forming leaving groups allowing the platinum ion to form bonds with DNA bases (Goodsell, 2006).
Fig. 2.
Computational molecular structures of cisplatin, carboplatin and oxaliplation. These platinum compounds are composed of doubly charged platinum ion surrounded by four ligands; with the amine ligands on the left forming stronger interactions with the platinum ion, and the chloride ligands or carboxylate compounds on the right forming leaving groups allowing the platinum ion to form bonds with DNA bases (Goodsell, 2006).
Carboplatin or Cis diammine (1,1-cyclobutanecarboxylato) platinum (II) is a chemotherapeutic drug used for cancers of ovaries, lung, head and neck. In terms of its structure, carboplatin differs from cisplatin in that it has a bidentate dicarboxylate (CBDCA) ligand in place of the two chloride ligands, which are the leaving groups in cisplatin (Figures 1 and 2). It exhibits lower reactivity and slower DNA binding kinetics, although it forms the same reaction products in vitro at equivalent doses with cisplatin. Unlike cisplatin, carboplatin may be susceptible to alternative mechanisms. Some studies show that cisplatin and carboplatin cause different morphological changes in MCF-7 cell lines while exerting their cytotoxic behavior (Natarajan, Malathi et al., 1999). The diminished reactivity limits protein-carboplatin complexes, which are excreted. The lower excretion rate of carboplatin means that more is retained in the body, and hence its effects are longer lasting (a retention half-life of 30 hours for carboplatin, compared to 1.5-3.6 hours in the case of cisplatin).
Relative to cisplatin, the greatest benefit of carboplatin is its reduced side effects, particularly the elimination of nephrotoxic effects. The main drawback of carboplatin is its myelo suppressive effect which causes the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels (Canetta, Rozencweig et al., 1985).
Carboplatin is less potent than cisplatin; depending on the type of cancer, carboplatin may only be 1/8 to 1/45 as effective. The clinical standard of dosage of carboplatin is usually a 4:1 ratio compared to cisplatin; that is, for a dose that usually requires a particular dose of cisplatin, four times more carboplatin is needed to achieve the same effectiveness. The stable property of carboplatin is a mixed blessing: once uptake of the drug occurs, its retention half-life is considerably longer than cisplatin, but it is also this inertness that causes carboplatin to go right through the human body, and up to 90% of the carboplatin given can be recovered in urine (Go and Adjei, 1999).
In order to overcome cisplatin resistance, other platinum (Pt) compounds have been developed and, in the last ten years, Pt-derivatives with reporting activity have also been synthesized. The first generation of reporting Pt-compounds was based on linking a fluorescent molecule (e.g. cyanine) to cisplatin, but more recent studies have focused on strategies to synthesize intrinsically fluorescent derivatives. Accordingly, bile acid platinum compounds have shown fluorescence intensity that is stable at room temperature for a long time; this fluorescence is maintained after binding to oligonucleotides or DNA. Because of this, the binding mode of these compounds to DNA can be easily analyzed both by flow injection and fluorescence techniques, showing that although these compounds target the nuclei, they form adduct with the DNA that are different from those due to cisplatin. In line with this, these bile acid derivatives have shown increased cytotoxicity and ability to overcome resistance as compared to cisplatin in several cell lines. Moreover, in contrast to cisplatin, the activity of these compounds does not seem to be restricted to cycling cells but they also seem to kill resting cells (Rodriguez-Fernandez, Manzano et al., 2009)
3. Uses of Cisplatin for Cancer Treatment
3.1. Cisplatin and Lung Cancer
Lung cancer remains one of the most common types of fatal malignancies (Youlden, Cramb et al., 2008). Small cell lung cancers (SCLCs) represent 15% of all lung cancers (Chen, Huynh et al., 2009). At present, platinum based treatments are key drugs for SCLC (Abrams, Lee et al., 2003). Cisplatin and carboplatin are two of the most common types of platinum based treatments used in SCLC chemotherapy (Go and Adjei, 1999). In clinical trials, cisplatin is often selected due to its strong antitumor activity, but its adverse effects include renal toxicity (Iwasaki, Nagata et al., 2005), nausea and vomiting (Kosmas, Tsavaris et al., 2001). Therefore, to avoid renal toxicity, urine volumes should be monitored and large-dose infusion is mandatory in cisplatin based chemotherapy. In clinical practice, carboplatin has been considered to be a substitute for cisplatin without any apparent loss of therapeutic efficacy since aggressive hydration is often problematic.
The standard of care for localized non-small-cell lung cancer (NSCLC) is surgery followed by, in case of stage II and III disease, adjuvant cisplatin-based chemotherapy. The Lung Adjuvant Cisplatin Evaluation program, a pooled analysis of the five largest trials, recently showed an absolute 5-year survival benefit of 5.3%with adjuvant chemotherapy as well as the NSCLC meta-analysis (Pignon, Tribodet et al., 2008).
CD133, a surface glycoprotein linked to organ-specific stem cells, has been described as a marker of cancer-initiating cells in different tumor types. It has also been reported that a CD133+, epithelial-specific antigen-positive (CD133+ESA+) population is increased in primary non-small cell lung cancer (NSCLC) compared with normal lung tissue (Bertolini, Roz et al., 2009).
3.2. Cisplatin and Ovarian Cancer
Ovarian cancer has the highest mortality among gynecologic cancers. Most patients with ovarian cancer are diagnosed at late stages due to lack of effective screening strategies and specific symptoms associated with early-stage disease. Conventional treatment for late stages of ovarian cancers is surgical excision followed by platinum/ taxane combination chemotherapy. Although this treatment regime is effective as the first-line treatment, recurrence occurs in up to 75% of ovarian cancer patients. Patients with recurrent ovarian cancer ultimately develop resistance to chemotherapy and eventually succumb to the disease (Agarwal and Kaye, 2003). About 90% of ovarian cancers arise originally from ovaries with an unknown reason, while the remainder has hereditary background, or are associated with breast and colon cancers (Lynch, Casey et al., 2009). Cisplatin derivatives are used as the mainline treatment of ovarian cancer, despite their severe side effects and development of resistance. Cisplatin is used in combination with other chemical agents or compounds to treat ovarian cancer in both the resistant and sensitive cell lines. For example Cisplatin is used along with honey venom (Alizadehnohi, Nabiuni et al., 2012), withaferin (Kakar, Jala et al., 2012), trichostatin A or 5-aza-2′-deoxycytidine (Meng, Sun et al., 2013), overcoming chemotherapy resistance of ovarian cancer cells by liposomal Cisplatin (Koch, Krieger et al., 2013)
3.3. Cisplatin and Carcinoma
Head and neck squamous cell carcinoma (HNSCC) is a common malignant disease with more than 600,000 new cases registered worldwide every year (Parkin, Bray et al., 2005). Despite improved treatment options, including surgery, radiation and chemotherapy, HNSCC is associated with a high mortality rate. The overall 5-year survival rate of approximately 50% has not changed over the last decades. Cisplatin alone is not an effective drug in treating the disease. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, doxorubicin, and or gemcitabine in patients with metastatic urothelial carcinoma has been reported (Scher, 1992;Matsuki, Takahashi et al., 2013).
3.4. Cisplatin and Breast Cancer
Breast cancer is one of the leading causes of women mortality worldwide. Chemotherapy is the only option for treating the malignant breast cancer and condition for increases the lifespan of the patient (Decatris, Sundar et al., 2004). Chemotherapeutic agents have been developed to counter the continuing breast cancer problem. However, most chemotherapeutic drugs effectively target rapidly dividing cells causing damage and are thus referred to as “cytotoxic drugs.” Cisplatin is an important chemotherapeutic agent used widely or the treatment of a variety of malignancies, including breast, testicular, ovarian, cervical, prostate, head and neck, bladder, lung and refractory non-Hodgkin's lymphomas (Tsimberidou, Braiteh et al., 2009;Dhar, Kolishetti et al., 2011). The cytotoxic effect is likely a result of inhibition of replication by cisplatin-DNA adducts and induction of apoptosis (Siddik, 2003).
3.5. Cisplatin and Brain Cancer
Glioblastomamultiforme (GBM) is the most common primary malignant brain tumor, and with rare exception, is invariably fatal (Stupp, Mason et al., 2005). The current standard of care for patients with GBMs consists of surgery and radiotherapy in combination with temozolomide, followed by repetitive cycles of temozolomide (Stupp, Hegi et al., 2009). Although the survival advantage of this combined treatment regimen was still evident at 5 years, the increase in overall median survival was only for 2.5 months. Cisplatin therapy is also used for recurrent childhood brain tumors(Khan, D'Souza et al., 1982),as well as in other cancers such as gastric cancer (Koizumi, Narahara et al., 2008), anal cancer (Ajani, Winter et al., 2008), and leukemia (Previati, Lanzoni et al., 2006).
4. Combination Therapy of Cisplatin with Other Cancer Drugs
Cisplatin combination chemotherapy is the basis of treatment of many cancers. Platinum responsiveness is high primarily but many cancer patients will ultimately relapse with cisplatin-resistant disease. Hence, drug resistance has been observed in many patients who have relapsed from cisplatin treatment. The proposed mechanisms of cisplatin resistance include changes in cellular uptake and efflux of cisplatin, increased biotransformation and detoxification in the liver, and increase in DNA repair and anti-apoptotic mechanisms (Gottesman, Fojo et al., 2002) . To overcome resistance, cisplatin is commonly used in combination with some other drugs in treating ovarian cancer, biliary tract cancer, lung cancer (diffuse malignant pleural mesothelioma), gastric cancer, carcinoma of salivary gland origin, breast, colon, lung, prostate, melanoma and pancreatic cancer cell lines, squamous cell carcinoma of male genitial tract, urothelial bladder cancer, and cervical cancer. Table 1 presents a synopsis of Cisplatin combination therapy with other cancer drugs and targeted cancers.
Table 1.
Combination therapy of cisplatin and other cancer drugs
| Combination Drug(s) | Cancer Type | Reference(s) |
|---|---|---|
| Paclitaxel | Ovarian carcinoma Breast carcinoma Lung carcinoma Melanoma Head and neck carcinoma |
Sarosy, Kohn et al., (1992)
McGuire, Rowinsky et al., (1989) Einzig, Wiernik et al., (1992) Holmes, Walters et al., (1991) Murphy, Fossella et al., (1993) Legha, Ring et al., (1990) Einzig, Hochster et al., (1991) Forastiere, (1994) |
| Paclitaxel and 5-FU | Gastric and Esophagogastric adenocarcinoma | Kim, Shin et al., (1999) |
| UFT | Non small lung carcinoma | Ichinose, Yosimori et al., (2000) |
| Doxorubicin | Diffuse malignant pleural mesothelioma | Ardizzoni, Rosso et al., (1991) |
| Cyclophosphamide and doxorubicin | Salivary gland advanced carcinoma | Dreyfuss, Clark et al., (1987) |
| Gemcitabine | Biliary cancer | Valle, Wasan et al., (2010) |
| Osthole | Lung cancer | Xu, Zhang et al., (2013) |
| Honeybee venom | Ovarian cancer | Alizadehnohi, Nabiuni, Nazari, Safaeinejad, and Irian, (2012) |
| Anvirzel | Breast, Colon, Lung, Prostate, Melanoma and Pancreatic cancer | Apostolou, Toloudi et al., (2013) |
| Bevacizumab | Non small lung carcinoma | Du, Li et al., (2013) |
| Vinbalstine and bleomycin | Metastatic granulosa cell tumors in ovary | Colombo, Sessa et al., (1986) |
| Methotrexate and bleomycin | Advanced squamous cell carcinoma of the male genital tract | Dexeus, Logothetis et al., (1991) |
| Everolimus | Urothelial bladder cancer | Pinto-Leite, Arantes-Rodrigues et al., (2013) |
| Fluorouracil, doxorubicin and cyclophosphamide | Salivary gland carcinoma | Dimery, Legha et al., (1990) |
| Metformin | Lung adenocarcinoma | Lin, Yeh et al., (2013) |
| Oxaliplatin, quercetin and thymoquinone | Ovarian cancer | Nessa, Beale et al., (2011) |
| Olaparib | PTEN deficient Lung cancer | Minami, Takigawa et al., (2013) |
| Tetra arsenic oxide | Cervical cancer | Byun, Jeong et al., (2013) |
| Vindesine | Non small lung carcinoma | Le, Brisgand et al., (1994) |
| β-galactosyl-pyrrolidinyldiazeniumdiolate | Glial cells of brain Human cervical cancer Gliosarcoma cell lines |
Deng, Zhang et al., (2013) |
4.1.Cisplatin and Paclitaxel
Paclitaxel is a mitotic agent that binds preferentially to microtubules(Parness and Horwitz, 1981) and the resulting stabilization of microtubules inhibits the reorganization of the microtubule network. Paclitaxel has been demonstrated to be active against previously treated ovarian carcinoma (Sarosy, Kohn et al., 1992; McGuire, Rowinsky et al., 1989; Einzig, Wiernik et al., 1992),breast carcinoma (Holmes, Walters et al., 1991), lung carcinoma (Murphy, Fossella et al., 1993), and melanoma (Legha, Ring et al., 1990;Einzig, Hochster et al., 1991) as well as for head and neck carcinoma (Forastiere, 1994). The combination chemotherapy with paclitaxel, cisplatin and fluorouracil is an active and tolerable as first-line and second line therapy in Chinese patients with advanced gastric and esophagogastric junction adenocarcinoma which showed a better tolerance has also been reported (Kim, Shin et al., 1999)
4.2. Cisplatin and Tegafur-uracil (UFT)
UFT is an oral anticancer agent comprised of tegafur and uracil in a 1:4 fixed molar ratio and is absorbed very well from the small intestine (Fujii, Kitano et al., 1979).Combination chemotherapy comprised of oral UFT (a combination of tegafur and uracil) and cisplatin was shown to be an effective regimen for the treatment of advanced non-small cell lung carcinoma (Ichinose, Yosimori et al., 2000).
4.3. Cisplatin and Doxorubicin
The combination of doxorubicin and cisplatin is effective and well tolerated. It might be considered for palliation of symptomatic patients with diffuse malignant pleural mesothelioma DMPM (Ardizzoni, Rosso et al., 1991). Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin showed encouraging results (Dreyfuss, Clark et al., 1987).
4.4. Cisplatin and Gemcitabine
As compared with gemcitabine alone, cisplatin plus gemcitabine was associated with a significant survival advantage without the addition of substantial toxicity. Cisplatin plus gemcitabine is an appropriate option for the treatment of patients with advanced biliary cancer (Valle, Wasan et al., 2010).
4.5. Cisplatin and Vitamin D
Vitamin D and its analogs regulate gene expression by binding to specific vitamin D receptors (VDR). Upon ligand activation and dimerization with retinoid X receptor (RXR), VDR – RXR heterodimers bind specific nucleotide sequences, vitamin D response elements (VDREs), in target genes to activate or repress their expression (Baniahmad and Tsai, 1993). A number of vitamin D target genes have been identified in several tumor cell types: p21, E-cadherin, c-Jun N-terminal kinase (JNK), c-Myc oncogene, insulin-like transforming growth factor family and their receptors (Saramaki, Banwell et al., 2006;Nagpal, Na et al., 2005).
Cisplatin is used along with vitamin D in treating squamous cell carcinoma (Light, Yu et al., 1997) and colon cancer (Milczarek, Rosinska et al., 2013). Published research has shown that pre-treatment for 72 hours of human promyelocytic leukemia (HL-60)cells with calcitriol or its new analogues significantly potentiated their sensitivity to the antiproliferative effect in vitro of cisplatin, doxorubicin or genistein. Moreover, for all cytotoxic agents tested a synergistic antiproliferative effect was observed. This effect was expressed as a significant decrease of the ID50 (inhibitory dose 50%) values for each cytotoxic agent applied after pretreatment of HL-60 cells with calcitriol or its analogues in comparison with the effect of cytotoxic agent applied alone (Siwinska, Opolski et al., 2001).
4.6. Other Possible Drug Combinations
Cisplatin is also used in combination with natural compounds like osthole in lung cancer cell lines (Xu, Zhang et al., 2013), honey bee venom in ovarian cancer cells (Alizadehnohi, Nabiuni, Nazari, Safaeinejad, and Irian, 2012), anvirzel in breast, colon, lung, prostate, melanoma and pancreatic cancer cell lines (Apostolou, Toloudi et al., 2013), bevacizumab in non-small cell lung cancer-mediated malignant pleural effusion (Du, Li et al., 2013), vinblastine and bleomycin in metastatic granulosa cell tumor of the ovary (Colombo, Sessa et al., 1986), methotrexate, bleomycin and cisplatin for advanced squamous cell carcinoma of the male genital tract (Dexeus, Logothetis et al., 1991).
Everolimus combined with cisplatin has a potential role in treatment of urothelial bladder cancer (Pinto-Leite, Arantes-Rodrigues et al., 2013), Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy has been used in advanced or recurrent salivary gland carcinoma (Dimery, Legha et al., 1990). Metformin has been shown to enhance cisplatin cytotoxicity by suppressing Stat3 activity independently of the LKB1-AMPK pathway (Lin, Yeh et al., 2013).
Synergistic interactions have been reported for -galactosyl-pyrrolidinyldiazeniumdiolate and cisplatin combination chemotherapy against glial cells of brain, human cervical and gliosarcoma cell lines (Deng, Zhang et al., 2013). Cisplatin and oxaliplatin in combination with quercetin and thymoquinone in human ovarian tumour models is the best combination to treat ovarian cancer (Nessa, Beale et al., 2011). Olaparib in combination with Cisplatin has a synergistic effect on PTEN-Deficient lung cancer Cells (Minami, Takigawa et al., 2013).
Tetraarsenic oxide and cisplatin induce apoptotic synergism in cervical cancer (Byun, Jeong et al., 2013). Vindesine and cisplatin combination chemotherapy compared with vindesine as a single agent in the management of non-small cell lung cancer seemed to be more effective (Le, Brisgand et al., 1994).
In ovarian carcinoma treatment, the combination of cisplatin and paclitaxel produced a more encouraging response rate (73%) (McGuire, Hoskins et al., 1996). A single institutional Phase II trial using cisplatin and oral UFT administration in place of protracted intravenous injection of 5-FU yielded a 35% response rate and a median survival time of 11 months in 31 patients with advanced NSCLC. (Ichinose, Takanashi et al., 1995). The addition of cisplatin to cyclophosphamide and doxorubicin (CAP) has been recently associated with overall response rates of 57% to 100% and complete response rates of 26% to 75% in small series of patients (Creagan, Woods et al., 1983;Alberts, Manning et al., 1981;Eisenberger, 1985). Cisplatin is known to have an additive or synergistic effect in combination with gemcitabine in a number of different tumor types. (Crino, Scagliotti et al., 1997; von der, Sengelov et al., 2005; Hitt, Castellano et al., 1998)
Photodynamic therapy (PDT), which is the administration of a photosensitiser followed by visible light activation, is a promising route to avoid damage to healthy cells and the surrounding tissue. Transition metal complexes as photochemotherapeutic agents are an attractive option for further development in the field of photoactivated chemotherapy (PACT). These complexes exhibit different numbers and types of excited states which are easily accessible upon light irradiation, subsequently giving rise to the formation of various photoproducts that can enable a distinct mode of action. Platinum-diazido complexes are promising candidates for PACT due to the low cytotoxicity when irradiated with visible light. (Shaili, 2014)
Cisplatin resistance is correlated with autophagy induction in a panel of ovarian cancer cells (Wang and Wu, 2014). Previous studies demonstrated that A549/CDDP cells acquired an epithelial-mesenchymal transition (EMT) phenotype, with morphological changes including acquisition of a spindle-like fibroblastic phenotype, downregulation of E-cadherin, upregulation of mesenchymal markers (vimentin, Snail and Slug), and increased capability of invasion and migration in case of human lung cancer cell lines. (demonstrated that A549/CDDP cells acquired an epithelial-mesenchymal transition (EMT) phenotype, with morphological changes including acquisition of a spindle-like fibroblastic phenotype, downregulation of E-cadherin, upregulation of mesenchymal markers (vimentin, Snail and Slug), and increased capability of invasion and migration in case of human lung cancer cell lines. (Wang, Zhang et al., 2014)
It has been hypothesized that Rac1, a Rho GTPase, is implicated in HNSCC insensitivity to chemo-radiotherapy resulting in tumour recurrence development in head and neck squamous cell carcinoma. (Skvortsov, Dudas et al., 2014). Cisplatin resistance in colorectal cancer is due to adaptive activation of Nrf2 may contribute to the development of acquired drug-resistance. (Chian, Li et al., 2014). It is also indicated that AQP5 is associated with drug resistance of colon cancer (Shi, Wu et al., 2014). In chondrosarcoma cells overexpression of EGFR contributed to cisplatin resistance (Song, Zhang et al., 2014). Due to loss of homeodomain-interacting protein kinase-2 (HIPK2) contributes to cell proliferation and tumorigenesis in bladder cancer. (Lin, Zhang et al., 2014)
5. Molecular Mechanisms of Cisplatin Pharmacology
Several membrane transporters of platinum compounds analogous to MDR1 including the efflux ATPases (MRPs, ATP7A/B), and the solute carrier importers (CTR1, the SLCs, AQP2, and AQP9), have been reported. MDR1 is an ATP-binding cassette transporter known for years as P-glycoprotein (Shen, Pouliot, Hall, and Gottesman, 2012; Johnson, Shen et al., 1996). The uptake of cisplatin is mediated by the copper transporter Ctr1 in yeast and mammals (Ishida, Lee et al., 2002). It has been further confirmed in human cells that cisplatin triggers rapid degradation of the copper membrane transporter CTR1, with diminished influx of cisplatin, resulting in resistance to the drug (Lin, Okuda et al., 2002; Holzer, Manorek et al., 2006). Genetic knockout of CTR1 results in cellular resistance to cisplatin in vivo. Cells with increased CTR1 expression exhibit increased platinum accumulation and, in most instances, increased sensitivity to cisplatin. TMEM205, a membrane protein has been associated with cellular resistance to cisplatin was identified. Analysis of TMEM205 expression profiles in normal human tissues indicates a differential expression pattern with higher expression levels in the liver, pancreas, and adrenal glands. So, overexpression of TMEM205 in CP-r cells may play a role in cellular resistance to platinum and would also be valuable as a biomarker or target in cancer chemotherapy (Shen, Pouliot, Hall, and Gottesman, 2012). Glucose Transporter 1 (Glut1) is not proposed to directly transport cisplatin, the mislocalization of the transporter exacerbates the cisplatin resistance phenotype(Shen, Pouliot, Hall, and Gottesman, 2012).
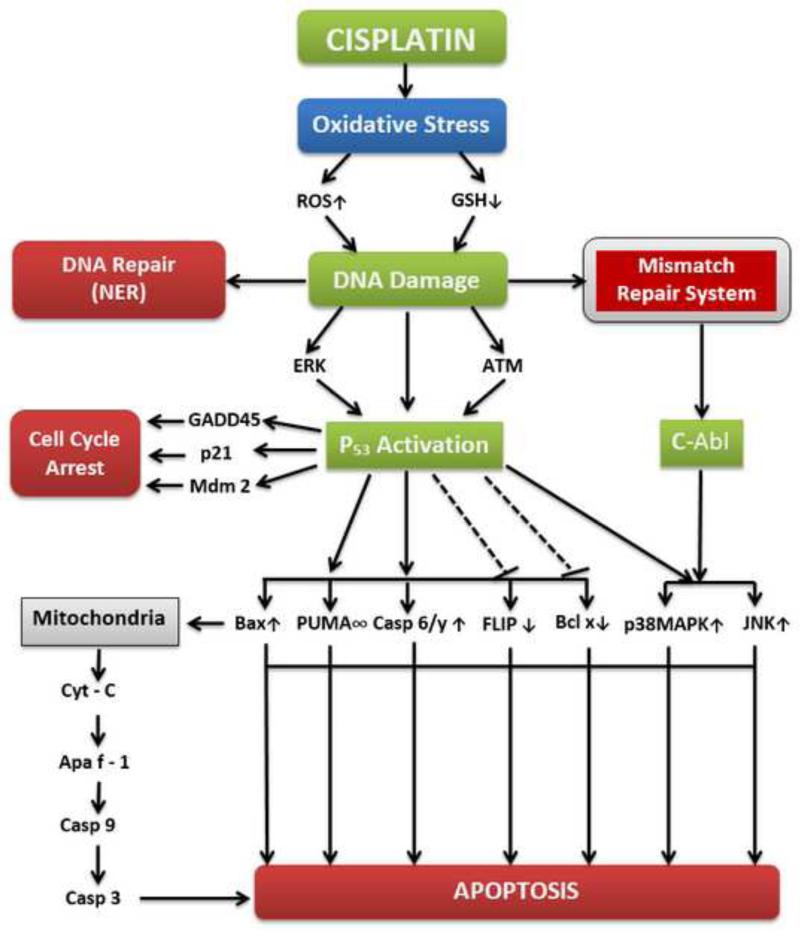
Cisplatin becomes activated once it enters the cell. In the cytoplasm the chloride atoms on cisplatin are displaced by water molecules. This hydrolyzed product is a potent electrophile that can react with any nucleophile, including the sulfhydryl groups on proteins and nitrogen donor atoms on nucleic acids. Cisplatin binds to the N7 reactive center on purine residues and as such can cause deoxyribonucleic acid (DNA) damage in cancer cells, blocking cell division and resulting in apoptotic cell death. The 1,2-intrastrand cross-links of purine bases with cisplatin are the most notable among the changes in DNA. These include the 1,2-intrastrand d(GpG) adducts 1,2-intrastrand d(ApG) adducts representing about 90% and 10% of adducts, respectively. 1,3-intrastrand d(GpXpG) adducts and other adducts such as inter-strand crosslinks and nonfunctional adducts have been reported to contribute to cisplatin's toxicity. Hence, published research from many laboratories has implicated DNA as a critical target for cisplatin cytotoxicity, the most revealing evidence being the hypersensitivity to cisplatin by both prokaryotic and eukaryotic cells deficient in DNA repair (Beck and Brubaker, 1973;Fraval, Rawlings et al., 1978). As illustrated in Figure 3, several molecular mechanisms leading to apoptosis have been implicated in cisplatin treatment of human cancers.
Fig. 3.
Overview of molecular mechanisms of cisplatin in cancer treatment.
5.1.Cisplatin-Induced Oxidative Stress
Under normal physiological conditions, cells control reactive oxygen species levels by balancing the generation of reactive oxygen species with their elimination by scavenging system (reduced glutathione-GSH, superoxide dismutase-SOD, and catalase-CAT). But under oxidative stress conditions, excessive reactive oxygen species can damage cellular proteins, lipids and DNA, leading to fatal lesions in cells that contribute to carcinogenesis. Cancer cells exhibit greater reactive oxygen species stress than normal cells do, partly due to oncogenic stimulation, increased metabolic activity and mitochondrial malfunction. Oxidative stress is the one of most important mechanisms involved in cisplatin toxicity. The mitochondrion is the primary target for cisplatin induced oxidative stress, resulting in loss of mitochondrial protein sulfhydrl group, calcium uptake inhibition and reduction of mitochondrial membrane potential (Saad, Najjar et al., 2004)
Exposure to oxidative stress can upset regular biological functions. Cisplatin also induces reactive oxygen species that trigger cell death besides DNA damage. Cell death occurs upon immediate activation of numerous signaling pathways, whereas the definite pathways depend on the (cancer) cell. The formation of reactive oxygen species depends on the concentration of cis-diamminedichloro platinum(II) and the length of exposure (Brozovic, Ambriovic-Ristov et al., 2010). The intracellular redox homeostasis is maintained by the thiol group (-SH) containing molecules. Under certain conditions a thiol group may lead to formation of thiyl radicals that in turn can interact with molecular oxygen, therefore generating reactive oxygen species (Desoize, 2002).
Excessive reactive oxygen species can induce apoptosis through both the extrinsic and intrinsic pathways (Ozben, 2007). In the extrinsic pathway of apoptosis, reactive oxygen species are generated by Fas ligand as an upstream event for Fas activation via phosphorylation, which is necessary for subsequent recruitment of Fas-associated protein with death domain and caspase 8 as well as apoptosis induction(Gupta, Hevia et al., 2012). In the intrinsic pathway, reactive oxygen species function to facilitate cytochrome c release by activating pore-stabilizing proteins (Bcl-2 and Bcl-xL) as well as inhibiting pore-destabilizing proteins (Bcl-2-associated X protein, Bcl-2 homologous antagonist/killer) (Martindale and Holbrook, 2002). An even higher reactive oxygen species level can result in both apoptosis and necrosis in cancer cells(Hampton and Orrenius, 1997). Reactive oxygen species can also induce cell death through autophagy, which is a self-catabolic process involving sequestration of cytoplasmic contents (exhausted organelles and protein aggregates), for degradation in lyposomes (Shrivastava, Kuzontkoski et al., 2011)
5.2. Cisplatin Modulation of Calcium Signaling
Cisplatin, under low intracellular chloride ion concentrations, has been shown to hydrolyze into variously charged reactive species including monoaqua [cis-(NH) PtCI(HO)]+ and diaquated [cis-(NH)Pt(HO)]2+ forms (Jennerwein and Andrews, 1995; Aggarwal, Broomhead et al., 1980). These hydrolyzed forms of cisplatin have been shown to be 1,000 times more reactive than normal cisplatin, and act through the inhibition of mitochondrial respiration by uncoupling oxidative phosphorylation (Aggarwal, 1993). This result in an efflux of calcium from the mitochondria and a temporary increase in the cellular calcium levels, which is thought to play a significant role in the disruption of normal calcium homeostasis, and hence cell function.
Mitochondrial glutathione (GSH) seems to be essential in the regulation of inner mitochondrial permeability and enzyme function by keeping SH in the reduced state. When the SH-groups of enzymes are not maintained in a reduced form, they become inactivated. Cisplatin induced toxicities, especially nephrotoxicity, seem to be related to a decrease in the intracellular concentrations of GSH and protein bound SH-groups. Nicotinamide adenine dinucleotide (NADH), which helps to maintain SH groups, declines with cisplatin treatment. Consequently, this depletion of GSH and NADH appears to result in the inhibition of some dehydrogenases, resulting in the uncoupling of oxidative phosphorylation leading to hydroxyl radical formation and oxidative stress. These free radicals attack polyunsaturated lipids and proteins and initiate lipid per oxidation. This process becomes autolytic and causes severe damage to membrane integrity (Aggarwal, 1998).
In summary, cisplatin's disruption of calcium homeostasis initiates primary events such as lipid per oxidation and enzyme inhibition. These events damage the cells through mitochondrial damage, inhibition of mitochondrial function, depletion of adenosine triphosphate (ATP) and other cofactors. This probably leads to apoptosis and tissue necrosis. Thus, it seems that elevated calcium levels, via calcium supplementation, may act as another means of cytoprotection, by competing for binding sites with cisplatin and prevent various toxicities associated with it.
5.3. Cisplatin-Induced Cell Apoptosis
Apoptosis is a controlled type of cell death which is energy-dependent leading to cell shrinkage, chromatin condensation, membrane budding, phosphatidylserine externalization, and activation of a family of cysteine proteases called caspases (Salvesen and Dixit, 1997;Cummings, Lasker et al., 2000). Caspase activation is the key step in the beginning of apoptosis, and several stimuli activate caspases, including those that activate plasma membrane death receptors (caspase 8) and cause mitochondrial dysfunction (caspase 9). Caspases are either initiators or executioners of apoptosis. Initiator caspases include caspases 8 and 9, and activation of these caspases results in activation of downstream or executioner caspases such as caspases 3 and 7 (Salvesen and Abrams, 2004). Executioner caspases are accountable for many of the biochemical characteristics of apoptosis, including cleavage and activation of poly (ADP-ribose) polymerase and of the inhibitor of caspase activator domain protein, which leads to DNA fragmentation.
Cisplatin primarily induces cell death by apoptosis and a defect in apoptotic signaling could also confer cisplatin resistance. There are two major pathways of apoptotic cell death (Nunez, Benedict et al., 1998;Kischkel, Hellbardt et al., 1995). The extrinsic pathway is initiated when ligands bind to the tumor necrosis factor-α (TNFα) receptor super family followed by oligomerization and recruitment of procaspase-8 via adaptor molecules to form the death-inducing signaling complex (DISC). The intrinsic pathway is initiated by cellular stress, such as DNA damage, resulting in release of cytochrome-c from the mitochondria causing activation of procaspase-9 through the interaction with apoptosis promoting activating factor-1 (APAF-1) and formation of an active apoptosome complex. Bcl-2 family proteins regulate DNA damage-induced apoptosis by regulating the release of mitochondrial cytochrome c in response to DNA damage. Cisplatin-induced genotoxic stress activates multiple signal transduction pathways, which can contribute to apoptosis or chemo resistance.
5.4. Cisplatin and Protein Kinase C
Protein kinase C [PKC] is a family closely related phospholipid dependent enzymes that play critical roles in signal transduction and cell regulation (Basu and Sivaprasad, 2007;Newton, 2003;Basu, 1993;Nishizuka, 1992). There have been contrasting on reports whether activation or down regulation of PKC is necessary for cisplatin sensitization (Isonishi, Andrews et al., 1990;Hirata, Kikuchi et al., 1993). The effects of PKC on cellular sensitivity/resistance to cisplatin depend on the pattern of the PKC isozymes as well as on the cellular context (Basu and Krishnamurthy, 2010).
5.5. Cisplatin and mitogen-activated protein kinase (MAPK)
Mitogen activated protein kinases are a family of structurally related serine/threonine protein kinases that coordinate various extra cellular signals to regulate cell growth and survival (Chang and Karin, 2001;Johnson and Lapadat, 2002;Marshall, 1995). Cisplatin has been shown to cause activation of ERK in several cell types although there are controversies whether activation of ERK prevents or contributes to cisplatin induced cell death (Tang, Zhou et al., 2010;Wang, Martindale et al., 2000;Yeh, Chuang et al., 2002;Nowak, 2002;Hayakawa, Ohmichi et al., 1999;Persons, Yazlovitskaya et al., 1999;Basu and Tu, 2005). Cisplatin-induced extracellular-signal-regulated kinase (ERK) activation precedes p53-mediated DNA damage response since ERK directly phosphorylates p53 causing up regulation of p21, 45kd-growth arrest and DNA damage (GADD45), and mouse double minute 2 homolog (Mdm2) (DeHaan, Yazlovitskaya et al., 2001).Thus, activation of ERK may cause cell cycle arrest allowing time for the repair of cisplatin-induced DNA damage via p53.
5.6. Cisplatin and Jun amino-terminal kinase (JNK)
c-Jun N-terminal kinase or stress activated protein kinase is activated by various stress stimuli, including DNA damage. Both cis and trans forms of cisplatin activate the JNK pathway. p73, a proapoptotic member of p53 forms a complex with JNK leads to cisplatin induced apoptosis (Jones, Dickman et al., 2007).
5.7. Cisplatin and p38 mitogen-activated protein kinase (MAPK)
Environmental stress is an important moderator of cisplatin-induced apoptosis, by activating p38 MAPK family. It has already been identified that EGFR as a substrate for p38 MAPK and cisplatin-induced receptor internalization was triggered by p38-mediated phosphorylation of the receptor (Winograd-Katz and Levitzki, 2006). p38 MAPK has been shown to mediate its effect via p18(Hamlet), a p38 MAPK-regulated protein, which interacts with p53 and stimulates the transcription of proapoptotic genes PUMA and NOXA to induce apoptosis (Cuadrado, Lafarga et al., 2007). Therefore, the p38 MAPK pathway plays a critical role in regulating cisplatin-induced apoptosis.
5.8. Cisplatin and AKT
Akt belongs to a family of serine/threonine kinases which act downstream of phosphoinositide 3-kinase (P13K) and plays a critical role in the cell survival(Brazil and Hemmings, 2001). It has been demonstrated that cisplatin-induced DNA damage caused phosphorylation of BAD at ser136 via Akt (Hayakawa, Ohmichi et al., 2000). Basically, BAD is phosphorylated in cells with cisplatin- induced DNA damage and this BAD phosphorylation is required for cell viability after cisplatin treatment in both cisplatin resistant and -sensitive cells. Previous study showed that cisplatin-induced DNA damage triggers the phosphorylation of both BAD Ser-112 via an ERK cascade and BAD Ser-136 via a PI-3K-PKB/Akt cascade. Iinhibition of either of these cascades sensitizes ovarian cancer cells to cisplatin (Hayakawa, Ohmichi et al., 2000).
It has been demonstrated that cisplatin-induced phosphorylation of BAD Ser-112 is MEK-dependent; while cisplatin-induced phosphorylation of BAD Ser- 136 is PI-3K-Akt-dependent. In addition, cisplatin induced phosphorylation of both BAD Ser-112 and Ser-136 is involved in maintaining cell viability after cisplatin treatment. All these results suggest that the ERK and PI-3K-Akt signaling cascades converge at BAD to suppress the apoptotic effect of BAD (Hayakawa, Ohmichi et al., 2000).
5.9. Cisplatin and Signaling for DNA Damage
Mutations are caused by several signals associated with stress. DNA once is damaged is followed by activation of cell cycle check points, there by delaying the cell cycle progression either to repair or to permanently eliminate the cells by inducing cell death. The response of cells to cisplatin induced DNA damage will decide the fate of a cell to live or die (Basu and Krishnamurthy, 2010).
5.10. p53 and DNA Damage Response to Cisplatin Treatment
p53 is a short lived protein, which is activated (phosphorylation) by Ataxia telangiectasia mutated (ATM) on DNA damage signal. The activated p53 now in turn activates Mdm2, which is E3 ubiquitin ligase. p53 can transactivate genes involved in cell cycle progression, DNA repair and apoptosis.p53 can regulate cisplatin induced cell death by several mechanisms like: Degradation of flice-like inhibitory protein (FLIP), direct binding and counteracting the antiapoptotic function of B-cell lymphoma-extra-large (Bcl –xL), over expression of phosphatase and tensin homolog (PTEN) and inhibition of AMPK (Basu and Krishnamurthy, 2010). Although p53 plays an important role in cisplatin induced DNA damage response, p53 negative cells also respond to Cisplatin induced DNA damage. This suggests the existence of alternate pathway for this event to occur upon stress.
5.11.Cyclobutanedicarboxylate (c-Abl) and DNA Damage Response to Cisplatin Treatment
c-Abl is tyrosine kinase receptor and contains nuclear localization motifs and nuclear export signals. Upon DNA damage, c-Abl is recruited from cytoplasm to nucleus, later associate with and phosphorylates MEK kinase 1. This complex activates jun amino-terminal kinase /stress-activated protein kinases (JNK/SAPK) (Kharbanda, Pandey et al., 2000). It is also demonstrated that activation of c-Abl and JNK is conditional upon the recognition of cisplatin induced DNA damage by the mismatch repair (MMR) system since c-Abl response is absent in MMR-deficient cells (Nehme, Baskaran et al., 1999).
The MMR/c-Abl binds to p73, a member of p53 family to start apoptosis (Gong, Costanzo et al., 1999). Phosphorylation and stabilization of p73 can increase its proapoptotic function by disassociating from p63, another p53 family member (Basu and Krishnamurthy, 2010). C-Jun will enhance the p73 stability by binding to transcription co activator Yap1, preventing proteasomal degradation of p73 and results in the recruitment of p300 to trigger transcription of proapoptotic genes (Levy, Adamovich et al., 2008).Cisplatin can trigger cleavage of c-Abl which is a substrate for caspase and proteolytic cleavage of c-Abl was shown to be important for cisplatin-induced apoptosis (Machuy, Rajalingam et al., 2004).
5.12. Cisplatin Modulation of Gene expression
In vitro studies suggest that cisplatin resistance can result from epigenetic changes at the molecular and cellular levels, including reduced accumulation of the platinum compounds by either active efflux/sequestration/secretion or impaired influx, detoxification by GSH conjugates, metallothioneins and other antioxidants, increased levels of DNA damage repair (nucleotide excision repair and mismatch repair), changes in DNA methylation status, alterations of membrane protein trafficking as a result of defective organization and distribution of the cytoskeleton, overexpression of chaperones, up- or down-regulated expression of microRNA (miRNA1), transcription factors and small GTPases, inactivation (Shen, Pouliot et al., 2012).
As a consequence of reduced uptake or retention, the formation of platinum-DNA adducts is correspondingly decreased, reducing cytotoxicity, resulting in more resistance to the platinum compound. Significant reductions in platinum-DNA adduct formation (9-fold) and ribosomal RNA gene-specific interstrand cross-link formation (12-fold) have been reported in studies with human hepatoma cisplatin-resistant 7404-CP20 cells. However the removal rates of the total platinum-DNA adducts and gene-specific interstrand cross-links were similar to those in their parental 7404 cells (Johnson et al., 1996).
Cisplatin also induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling (Mandic, Hansson et al., 2003). Because cisplatin is a DNA-damaging agent and an inducer of apoptosis, it is reasonable to expect changes in HSPs in the development of cellular resistance to the drug. HSP60 was significantly expressed in both human cervical and liver carcinoma (Shen, Pouliot, Hall, and Gottesman, 2012). Up-regulation of HSP27, HSP70, HSP72, GRP78, and HSP90 have been reported to be involved in CP-r ovarian cancer (Arts, Hollema et al., 1999;Liu, Opipari et al., 2002), breast cancer (Vargas-Roig, Gago et al., 1998), colon cancer (Belfi, Chatterjee et al., 1999), cervical HeLa cells (Huang, Ip et al., 2000) and laryngeal carcinoma cells (Brozovic, Simaga et al., 2001).
The low molecular weight GTP-binding proteins of the Rho family, including RhoA, RhoB, RhoC, and Rac, all belong to the Ras tumor suppressor gene family. Reduced expression of small GTPases such as Rab5, Rac1, and RhoA was detected in human cisplatin-resistant cells in association with decreased accumulation of radiolabeled compounds (Shen, Liang et al., 2004). The ribosomal protein L36 was found to present cisplatin resistance in human carcinoma cells (Shen, Liang et al., 2006). In human breast cancer MCF-7 cells, reduced expression of the ribosomal protein P0 was found by proteomic analysis (Smith, Welham et al., 2007). Moreover, ectopic L37 over expression can attenuate the DNA damage response mediated by p53 (Llanos and Serrano, 2010).
It has been reported that transcription factors such as Y-box binding protein-1, CCAAT-binding transcription factor 2, activating transcription factor 4, zinc finger factor 143, the nuclear transcription factor- B, the microphthalmia-associated transcription factor, the forkhead transcription factor O, and mitochondrial transcription factor A, play a major role in cisplatin resistance (Shen, Pouliot, Hall, and Gottesman, 2012). It was first found that the levels of nuclear factor (Erythroid-Derived 2)-like 2 (Nrf2) expression in Nrf2-deficient murine embryonic cells and human ovarian cancer SK-OV cisplatin-resistant cells correlate with the extent of resistance to cisplatin. Loss of Nrf2 or inhibition by siRNA resulted in increased cell death, cytotoxicity, and apoptosis in response to cisplatin treatment compared with control cells (Cho, Manandhar et al., 2008). Also, increased expression in GCF2-transfected KB-3-1 cells results in reduced RhoA expression, disruption of actin-filamin dynamics, mislocalization of the membrane protein MRP1, and reduction in cisplatin accumulation, leading to 3-fold resistance to cisplatin (Shen, Pouliot et al., 2012).
5.13. Computational Studies of Cisplatin
Cisplatin is one of the most effective anticancer drugs currently in use. Following the finding of its antitumor activity over three decades ago, strong research has been carried out to reveal the details of its cytotoxic activity and to design analogs with reduced side effects (Mantri and Baik, 2006). Recently, computational studies have been conducted to complement experimental works. The hydrolysis process of cisplatin which activates the drug was the goal of past research. Cisplatin–DNA interactions are the next theoretical studies, since DNA is the primary target of the drug. At present, to study the thermodynamics and kinetics of not only cisplatin–DNA complexes, but also of other complexes such as Pt(II)-based cisplatin analogs, other transition metal complexes, and DNA binding organic molecules, both quantum mechanical and molecular mechanical methods are being used. Future research is aiming at elucidating the role of repair enzymes in modulating the cytotoxic activity of DNA binding agents (Mantri and Baik, 2006).
The combination of dramatically improved computer hardware and robust, sophisticated, and numerically ef cient modeling software has allowed for employing high levels of theory to examine various aspects of cisplatin chemistry using computational chemistry techniques. The very rst computational studies on cisplatin aimed at better understanding the hydrolysis of cisplatin, because the activation by hydrolysis had long been established as the rate-limiting step (Basch, Krauss et al., 1986). An intriguing question had been the preferential antitumor activity of the cis isomer over the trans isomer, a fact then attributed to the steric effects of binding DNA bases. It was eventually discovered that the cis orientation of the leaving groups allowed binding of two adjacent guanine bases on the same strand, which resulted in a large kink in the DNA helix, causing polymerases to be stalled at the kink. However, it was demonstrated that the trans isomer is favored over the cis isomer in all cases due to reduction in ligand–ligand repulsion, especially in the case of anionic ligands. These differences become negligibly small when favorable hydrogen bonding interactions are possible between the ammine ligands and the other labile ligands (Basch, Krauss et al., 1986).
There is increasing awareness now that one important reason for cisplatin's performance is associated to the high mobility group (HMG) proteins that bind to DNA and protect the drug–DNA adducts against excision repair (Wang and Lippard, 2005). To enable comparison of the binding of Pt(II) to various positions in the four nucleobases in DNA and to keep computational costs to a minimum, Pt(NH3)32+ was used as the fragment that interacted with the bases. The ranking of the bases followed the order: G(N7) > C(N3) > C(O2) > G(O6) > A(N3) ≈ A(N1) > A(N7) > G(N3) > T(O4) > T(O2), based on differential Pt(II) binding energies. On the basis of the ranking above and the fact that most of the potential binding sites are in reality unavailable for binding due to Watson–Crick base pairing, the N7 position of guanine—in particular intrastrand binding to two adjacent guanines—was con rmed as the preferred binding site, in full agreement with experimental evidence (Mantri and Baik, 2006)
Density functional theory was applied to provide new insights into the structure and reactivity of cis- and transplatin and related hydrolysis product (Carloni, Sprik et al., 2000) and also to compare the electronic structures of cisplatin and its second- generation analog carboplatin (Carloni and Andreoni, 1996). Results suggested that the replacement of the chloride ligands of cisplatin by carboxylate ligands in carboplatin resulted in a greater stability of the metal-ligand bonds in the latter, leading to potentially higher activation energy for substitution reactions. Later, a more comprehensive study comparing the geometric, electronic, and vibrational properties of cisplatin using different basis sets, including pure ECP and hybrid ECP with various electron correlation methods up to the MP4 level, and DFT, was performed (Pavankumar, Seetharamulu et al., 1999).
Thermodynamics of hydrolysis of cisplatin and bis(ethylenediamine) dichloroplatinum(II) using a combination of molecular mechanics for obtaining optimized geometries of the reactants and products, and the extended Huckel method for deriving charge distributions and electronic energies were studied . This hydrolysis was studied by several others using various levels of theory, where a common approach adopted has been to use DFT to optimize the geometries of the key intermediates and reevaluate their energies using higher level ab initio methods, and/or adding solvation corrections based on continuum dielectric models. describes the interaction of transplatin and the fragments trans-PtCl2(NH3+ and Pt(NH3)32+ with G:C and A:T base pairs using DFT, followed by an MP2-based energy analysis (Nikolov, Trendafilova et al., 1994; Mantri and Baik, 2006; Zhang, Guo et al., 2001; Lau and Deubel, 2005).
Cisplatin has been known to bind to guanine with much greater preference over adenine, which is somewhat surprising because it could be argued that the inductive effects of the electron-withdrawing oxo group at the C6 position of guanine should reduce the electron density at N7 compared to adenine that has an electron-donating amino group at the C6 position, thus making the N7 of guanine less nucleophilic compared to adenine (Mantri and Baik, 2006). The major difference between guanine and adenine is that N1 of guanine is protonated, while adenine exposes an N1 lone pair. The presence of the N1 proton diminishes the delocalization of electron density over the nitrogen lone pairs of the purine skeleton resulting in greater localization of electron density on the N3 and N7 atoms of guanine, compared to adenine where the electron density is delocalized over the N1, N3, and N7 atoms (Mantri and Baik, 2006). In an attempt to identify yet another subtle electronic feature that could help in distinguishing between the reactivity of adenine and guanine, it was discovered that the [Pt] fragment is a poor π-donor; thus π-back donation does not appear to play a major role (Mantri and Baik, 2006).
Cisplatin binds to other cellular components, resulting in either deactivation of the drug and/or disruption of normal biochemical pathways. Thus, signi cant efforts have focused on understanding the binding of cisplatin with other entities found in the cells; in particular S- and N-containing ligands that are expected have the greatest af nity for platinum based on good hardness- softness matching. It was reported a thorough DFT study which compared the Pt–L bond strengths of a series of tri- ammine platinum(II) complexes with oxygen-, nitrogen- and sulfur-donor ligands as models of competing ligands encountered in a biological system (Deubel, 2002). In another study, the kinetics of the substitution reaction of various nitrogen- and sulfur ligands with the activated cisplatin complex was modeled using DFT. This study revealed that kinetically N-donor ligands were preferred over S-donors, with the selectivity originating from electrostatic rather than orbital-based interaction (Deubel, 2004).
Other areas of active study on cisplatin are degree of local bending / unwinding upon cisplatin binding, the disruption of base stacking and other local distortions, the thermodynamics of the various possible adducts, the directional preference in the formation of the bifunctional adducts, the ineffectiveness of transplatin, the effect of cisplatin binding on the dynamics of DNA, and the binding and recognition of HMG domain and/or DNA repair proteins to these adducts (Mantri and Baik, 2006). Computational models have made signi cant contributions to understanding the nature and reactivity of cisplatin and allowed for delineating many features of how it binds to DNA.
6. Toxicological Effects of Cisplatin
Cisplatin interacts with DNA, and forms covalent adduct with purine DNA bases and this platinum compound, interaction is the root cause for cytotoxic effect of cisplatin (Yousef, Saad et al., 2009). Cisplatin treatment has been associated with several toxic side effects including nephrotoxicity (de Jongh, van Veen et al., 2003), hepatotoxicity and Cardiotoxicity (Al-Majed, 2007). Many cardiac events have been reported in many case reports including electro-cardiographicchanges, arrhythmias, myocarditis, cardiomyopathy and congestive heart failure (Yousef, Saad, and El-Shennawy, 2009). Decrease in antioxidant defense system is reported due to oxidative stress through the generation of reactive oxygen species, including antioxidant enzymes and non enzymatic molecules, reduced glutathione, are major alterations in the cisplatin toxicity (Kart, Cigremis et al., 2010).
6.1. Hepatotoxicity
High dosage of Cisplatin may lead to hepatotoxicity (dos Santos, Martins et al., 2007).Oxidative stress is the main reason for cisplatin-induced toxicity possibly due to depletion of reduced glutathione GSH (Yilmaz, Iraz et al., 2004), also many studies reported that there were a significant elevation in the hepatic malonaldehyde (MDA) and reduction in the level of antioxidant enzymes in rats treated with cisplatin (Yilmaz, Sogut et al., 2005; Mansour, Hafez et al., 2006).The most sensitive biomarkers directly concerned in causing the cellular damage and toxicity are transaminases, because they are cytoplasmic in location and are released into the circulation after cellular damage . Elevation of the hepatic enzymes level in serum and bilirubin are the indicators for impaired liver functions (Iseri, Ercan et al., 2007). Cisplatin hepatotoxicity was shown to be exacerbated by increased expression of cytochrome P450-2E1 enzyme (Caro and Cederbaum, 2004). Observed histopathological changes will be necrosis and degeneration of hepatocytes with inflammatory cells infiltration around portal area with sinusoidal dilatation (Kart, Cigremis, Karaman, and Ozen, 2010; Cetin, Devrim et al., 2006). Recent studies have focused on methods for protection of cisplatin-induced Hepatotoxicity using various agents, such as selenium (Liao, Lu et al., 2008) and vitamin E (Naziroglu, Karaoglu et al., 2004; Iraz, Kalcioglu et al., 2005).
6.2.Cardiotoxicity
Leakage of lactate dehydrogenase (LDH) and creatine kinase (CK) from cardiac myocytes is due to cardiotoxicity could be a secondary event following cisplatin-induced lipid peroxidation of cardiac membranes. Degeneration and necrosis of cardiac muscle fiber cells with fibrous tissue reaction and vacuolated cytoplasm of many muscle cells and blood vessels are inflated with blood are the histological changes of cisplatin induced toxicology (Al-Majed, Sayed-Ahmed et al., 2006)
6.3.Nephrotoxicity
The kidney accumulates cisplatin to a greater degree than other organs and is the major route for its excretion. The cisplatin concentration in proximal tubular epithelial cells is about 5 times the serum concentration (Kuhlmann, Burkhardt et al., 1997). The disproportionate accumulation of cisplatin in kidney tissue contributes to cisplatin-induced nephrotoxicity (Arany and Safirstein, 2003).
Biosynthesis of amino acid lysine and methionine yields a quaternary ammonium compound called Carnitine, which is required for the transport of fatty acids from the cytosol into the mitochondria during the breakdown of lipids to generate metabolic energy. Kidney damage is caused by the inhibition of Carnitine synthesis and also by the Carnitine reabsorption by the proximal tubule of nephron, which is due to declined production of Carnitine.
Cisplatin is cleared by the kidney by both glomerular filtration and tubular secretion (Yao, Panichpisal et al., 2007; Ishida, Lee et al., 2002) Cisplatin concentrations within the kidney exceed those in blood suggesting an active accumulation of drug by renal parenchymal cells. Studies in recent years have identified two different membrane transporters capable of transporting cisplatin into cells: Ctr1 and OCT2 (Ishida, Lee et al., 2002). Later, cisplatin is biotransformed in the kidney into cysteinyl glycine conjugates and other high thiols by some localized enzymes.
6.4. Other Organ Toxicity
Other cisplatin-induced organ toxicities such as ototoxicity, gastrotoxicity, myelosuppression, allergic reactions and some reproductive toxic effects have also been reported (Hartmann, Fels et al., 2000; Hartmann and Lipp, 2003)
7. Conclusions
Cisplatin is one of the most effective anticancer agents widely used in the treatment of solid tumors. It has been extensively used for the cure of different types of neoplasms including head and neck, lung, ovarian, leukemia, breast, brain, kidney and testicular cancers. In general, cisplatin and other platinum-based compounds are considered as cytotoxic drugs which kill cancer cells by damaging DNA, inhibiting DNA synthesis and mitosis, and inducing apoptotic cell death. Several molecular mechanisms of action including induction of oxidative stress as characterized by reactive oxygen species production and lipid peroxidation, induction of p53 signaling and cell cycle arrest, down-regulation of proto-oncogenes and anti-apoptotic proteins, and activation of both intrinsic and extrinsic pathways of apoptosis. However, cisplatin chemotherapy is also associated with substantial side effects that include hepatotoxic, nephrotoxic, cardiotoxic, neurotoxic and/or hematotoxic damage. Also, some patients may relapse from cisplatin treatment with their cancers being refractory to cisplatin regimen. Hence, combination therapies of cisplatin with other drugs are common practice in the treatment of human cancers. Findings of several studies have suggested that other compounds combined with cisplatin constitute the best therapeutic approach to overcome drug resistance and reduce the undesirable side effects. Moving forward, combinatorial strategies which target multiple mechanisms, such as reducing cisplatin uptake and reducing inflammation, may offer the best chance for clinically meaningful prevention.
Acknowledgments
The research described in this publication was made possible by a grant from the National Institutes of Health (Grant No. G12MD007581) through the RCMI Center for Environmental Health at Jackson State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol.Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat.Rev.Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- Aggarwal SK. A histochemical approach to the mechanism of action of cisplatin and its analogues. J.Histochem.Cytochem. 1993;41:1053–1073. doi: 10.1177/41.7.8515048. [DOI] [PubMed] [Google Scholar]
- Aggarwal SK. Calcium modulation of toxicities due to Cisplatin. Met.Based.Drugs. 1998;5:77–81. doi: 10.1155/MBD.1998.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal SK, Broomhead JA, Fairlie DP, Whitehouse MW. Platinum drugs: combined anti-lymphoproliferative and nephrotoxicity assay in rats. Cancer Chemother.Pharmacol. 1980;4:249–258. doi: 10.1007/BF00255269. [DOI] [PubMed] [Google Scholar]
- Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, III, Thomas CR, Jr., Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- Akron The Chemical Database. [3/25/14];The Department of Chemistry at the University of Akron. 2009 http://ull.chemistry.uakron.edu/erd and search on CAS number.
- Alberts DS, Manning MR, Coulthard SW, Koopmann CF, Jr., Herman TS. Adriamycin/cis-platinum/cyclophosphamide combination chemotherapy for advanced carcinoma of the parotid gland. Cancer. 1981;47:645–648. doi: 10.1002/1097-0142(19810215)47:4<645::aid-cncr2820470404>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA. Carnitine deficiency provokes cisplatin-induced hepatotoxicity in rats. Basic Clin.Pharmacol.Toxicol. 2007;100:145–150. doi: 10.1111/j.1742-7843.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Shabanah OA. Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol.Res. 2006;53:278–286. doi: 10.1016/j.phrs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Alizadehnohi M, Nabiuni M, Nazari Z, Safaeinejad Z, Irian S. The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J.Venom.Res. 2012;3:22–27. [PMC free article] [PubMed] [Google Scholar]
- Apostolou P, Toloudi M, Chatziioannou M, Ioannou E, Knocke DR, Nester J, Komiotis D, Papasotiriou I. AnvirzelTM in combination with cisplatin in breast, colon, lung, prostate, melanoma and pancreatic cancer cell lines. BMC.Pharmacol.Toxicol. 2013;14:18. doi: 10.1186/2050-6511-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin.Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Ardizzoni A, Rosso R, Salvati F, Fusco V, Cinquegrana A, De PM, Serrano J, Pennucci MC, Soresi E, Crippa M. Activity of doxorubicin and cisplatin combination chemotherapy in patients with diffuse malignant pleural mesothelioma. An Italian Lung Cancer Task Force (FONICAP) Phase II study. Cancer. 1991;67:2984–2987. doi: 10.1002/1097-0142(19910615)67:12<2984::aid-cncr2820671208>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Arts HJ, Hollema H, Lemstra W, Willemse PH, De Vries EG, Kampinga HH, Van der Zee AG. Heat-shock-protein-27 (hsp27) expression in ovarian carcinoma: relation in response to chemotherapy and prognosis. Int.J.Cancer. 1999;84:234–238. doi: 10.1002/(sici)1097-0215(19990621)84:3<234::aid-ijc6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Baniahmad A, Tsai MJ. Mechanisms of transcriptional activation by steroid hormone receptors. J.Cell Biochem. 1993;51:151–156. doi: 10.1002/jcb.240510206. [DOI] [PubMed] [Google Scholar]
- Basch H, Krauss M, Stevens WJ, Cohen D. Binding of Pt(NH3)32+ to nucleic acid bases. Inorg.Chem. 1986;25:684–688. [Google Scholar]
- Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol.Ther. 1993;59:257–280. doi: 10.1016/0163-7258(93)90070-t. [DOI] [PubMed] [Google Scholar]
- Basu A, Krishnamurthy S. Cellular responses to Cisplatin-induced DNA damage. J.Nucleic Acids 2010. 2010 doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Tu H. Activation of ERK during DNA damage-induced apoptosis involves protein kinase Cdelta. Biochem.Biophys.Res.Commun. 2005;334:1068–1073. doi: 10.1016/j.bbrc.2005.06.199. [DOI] [PubMed] [Google Scholar]
- Beck DJ, Brubaker RR. Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J.Bacteriol. 1973;116:1247–1252. doi: 10.1128/jb.116.3.1247-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfi CA, Chatterjee S, Gosky DM, Berger SJ, Berger NA. Increased sensitivity of human colon cancer cells to DNA cross-linking agents after GRP78 up-regulation. Biochem.Biophys.Res.Commun. 1999;257:361–368. doi: 10.1006/bbrc.1999.0472. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo VS, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc.Natl.Acad.Sci.U.S.A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem.Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brozovic A, Ambriovic-Ristov A, Osmak M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev.Toxicol. 2010;40:347–359. doi: 10.3109/10408441003601836. [DOI] [PubMed] [Google Scholar]
- Brozovic A, Simaga S, Osmak M. Induction of heat shock protein 70 in drug-resistant cells by anticancer drugs and hyperthermia. Neoplasma. 2001;48:99–103. [PubMed] [Google Scholar]
- Byun JM, Jeong DH, Lee DS, Kim JR, Park SG, Kang MS, Kim YN, Lee KB, Sung MS, Kim KT. Tetraarsenic oxide and cisplatin induce apoptotic synergism in cervical cancer. Oncol.Rep. 2013;29:1540–1546. doi: 10.3892/or.2013.2243. [DOI] [PubMed] [Google Scholar]
- Canetta R, Rozencweig M, Carter SK. Carboplatin: the clinical spectrum to date. Cancer Treat.Rev. 1985;12(Suppl A):125–136. doi: 10.1016/0305-7372(85)90027-1. [DOI] [PubMed] [Google Scholar]
- Carloni P, Andreoni W. Platinum-Modified Nucleobase Pairs in the Solid State:GÇë A Theoretical Study. J.Phys.Chem. 1996;100:17797–17800. [Google Scholar]
- Carloni P, Sprik M, Andreoni W. Key Steps of the cis-Platin-DNA Interaction:GÇë Density Functional Theory-Based Molecular Dynamics Simulations. J.Phys.Chem.B. 2000;104:823–835. [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu.Rev.Pharmacol.Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Cetin R, Devrim E, Kilicoglu B, Avci A, Candir O, Durak I. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: possible protective roles of natural antioxidant foods. J.Appl.Toxicol. 2006;26:42–46. doi: 10.1002/jat.1103. [DOI] [PubMed] [Google Scholar]
- Chian S, Li YY, Wang XJ, Tang XW. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the nrf2 pathway. Asian Pac.J.Cancer Prev. 2014;15:2911–2916. doi: 10.7314/apjcp.2014.15.6.2911. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen G, Huynh M, Fehrenbacher L, West H, Lara PN, Jr., Yavorkovsky LL, Russin M, Goldstein D, Gandara D, Lau D. Phase II trial of irinotecan and carboplatin for extensive or relapsed small-cell lung cancer. J.Clin.Oncol. 2009;27:1401–1404. doi: 10.1200/JCO.2008.20.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett. 2008;260:96–108. doi: 10.1016/j.canlet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Colombo N, Sessa C, Landoni F, Sartori E, Pecorelli S, Mangioni C. Cisplatin, vinblastine, and bleomycin combination chemotherapy in metastatic granulosa cell tumor of the ovary. Obstet.Gynecol. 1986;67:265–268. doi: 10.1097/00006250-198602000-00020. [DOI] [PubMed] [Google Scholar]
- Creagan ET, Woods JE, Schutt AJ, O'Fallon JR. Cyclophosphamide, adriamycin, and cis-diamminedichloroplatinum (II) in the treatment of advanced nonsquamous cell head and neck cancer. Cancer. 1983;52:2007–2010. doi: 10.1002/1097-0142(19831201)52:11<2007::aid-cncr2820521106>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Crino L, Scagliotti G, Marangolo M, Figoli F, Clerici M, De MF, Salvati F, Cruciani G, Dogliotti L, Pucci F, Paccagnella A, Adamo V, Altavilla G, Incoronato P, Trippetti M, Mosconi AM, Santucci A, Sorbolini S, Oliva C, Tonato M. Cisplatin-gemcitabine combination in advanced non-small-cell lung cancer: a phase II study. J.Clin.Oncol. 1997;15:297–303. doi: 10.1200/JCO.1997.15.1.297. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Lafarga V, Cheung PC, Dolado I, Llanos S, Cohen P, Nebreda AR. A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO J. 2007;26:2115–2126. doi: 10.1038/sj.emboj.7601657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BS, Lasker JM, Lash LH. Expression of glutathione-dependent enzymes and cytochrome P450s in freshly isolated and primary cultures of proximal tubular cells from human kidney. J.Pharmacol.Exp.Ther. 2000;293:677–685. [PubMed] [Google Scholar]
- de Jongh FE, van Veen RN, Veltman SJ, de WR, van der Burg ME, van den Bent MJ, Planting AS, Graveland WJ, Stoter G, Verweij J. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br.J.Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decatris MP, Sundar S, O'Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat.Rev. 2004;30:53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- DeHaan RD, Yazlovitskaya EM, Persons DL. Regulation of p53 target gene expression by cisplatin-induced extracellular signal-regulated kinase. Cancer Chemother.Pharmacol. 2001;48:383–388. doi: 10.1007/s002800100318. [DOI] [PubMed] [Google Scholar]
- Deng L, Zhang E, Chen C. Synergistic interaction of beta-galactosyl-pyrrolidinyl diazeniumdiolate with cisplatin against three tumor cells. Arch.Pharm.Res. 2013;36:619–625. doi: 10.1007/s12272-013-0047-0. [DOI] [PubMed] [Google Scholar]
- Desoize B. Cancer and metals and metal compounds: part I--carcinogenesis. Crit Rev.Oncol.Hematol. 2002;42:1–3. doi: 10.1016/s1040-8428(02)00017-3. [DOI] [PubMed] [Google Scholar]
- Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev.Oncol.Hematol. 2002;42:317–325. doi: 10.1016/s1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- Deubel DV. On the Competition of the Purine Bases, Functionalities of Peptide Side Chains, and Protecting Agents for the Coordination Sites of Dicationic Cisplatin DerivativesGÇá. J.Am.Chem.Soc. 2002;124:5834–5842. doi: 10.1021/ja012221q. [DOI] [PubMed] [Google Scholar]
- Deubel DV. Factors Governing the Kinetic Competition of Nitrogen and Sulfur Ligands in Cisplatin Binding to Biological TargetsGÇá. J.Am.Chem.Soc. 2004;126:5999–6004. doi: 10.1021/ja0499602. [DOI] [PubMed] [Google Scholar]
- Dexeus FH, Logothetis CJ, Sella A, Amato R, Kilbourn R, Fitz K, Striegel A. Combination chemotherapy with methotrexate, bleomycin and cisplatin for advanced squamous cell carcinoma of the male genital tract. J.Urol. 1991;146:1284–1287. doi: 10.1016/s0022-5347(17)38069-2. [DOI] [PubMed] [Google Scholar]
- Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc.Natl.Acad.Sci.U.S.A. 2011;108:1850–1855. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimery IW, Legha SS, Shirinian M, Hong WK. Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J.Clin.Oncol. 1990;8:1056–1062. doi: 10.1200/JCO.1990.8.6.1056. [DOI] [PubMed] [Google Scholar]
- dos Santos NA, Martins NM, Curti C, Pires Bianchi ML, dos Santos AC. Dimethylthiourea protects against mitochondrial oxidative damage induced by cisplatin in liver of rats. Chem.Biol.Interact. 2007;170:177–186. doi: 10.1016/j.cbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Dreyfuss AI, Clark JR, Fallon BG, Posner MR, Norris CM, Jr., Miller D. Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin. Cancer. 1987;60:2869–2872. doi: 10.1002/1097-0142(19871215)60:12<2869::aid-cncr2820601203>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Du N, Li X, Li F, Zhao H, Fan Z, Ma J, Fu Y, Kang H. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancermediated malignant pleural effusion. Oncol.Rep. 2013;29:2332–2340. doi: 10.3892/or.2013.2349. [DOI] [PubMed] [Google Scholar]
- Einzig AI, Hochster H, Wiernik PH, Trump DL, Dutcher JP, Garowski E, Sasloff J, Smith TJ. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs. 1991;9:59–64. doi: 10.1007/BF00194546. [DOI] [PubMed] [Google Scholar]
- Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J.Clin.Oncol. 1992;10:1748–1753. doi: 10.1200/JCO.1992.10.11.1748. [DOI] [PubMed] [Google Scholar]
- Eisenberger MA. Supporting evidence for an active treatment program for advanced salivary gland carcinomas. Cancer Treat.Rep. 1985;69:319–321. [PubMed] [Google Scholar]
- Forastiere AA. Paclitaxel (Taxol) for the treatment of head and neck cancer. Semin.Oncol. 1994;21:49–52. [PubMed] [Google Scholar]
- Fraval HN, Rawlings CJ, Roberts JJ. Increased sensitivity of UV-repair-deficient human cells to DNA bound platinum products which unlike thymine dimers are not recognized by an endonuclease extracted from Micrococcus luteus. Mutat.Res. 1978;51:121–132. doi: 10.1016/0027-5107(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, Tomco D, Dou QP. Novel metals and metal complexes as platforms for cancer therapy. Curr.Pharm.Des. 2010;16:1813–1825. doi: 10.2174/138161210791209009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Kitano S, Ikenaka K, Shirasaka T. Effect of coadministration of uracil or cytosine on the anti-tumor activity of clinical doses of 1-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in rodents. Gann. 1979;70:209–214. [PubMed] [Google Scholar]
- Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J.Clin.Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr., Levrero M, Wang JY. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat.Rev.Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Goodsell DS. The molecular perspective: Cisplatin. Stem Cells. 2006;24:514–515. doi: 10.1634/stemcells.2006-CSC2. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid.Redox.Signal. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Fels LM, Knop S, Stolt H, Kanz L, Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. Invest New Drugs. 2000;18:281–289. doi: 10.1023/a:1006490226104. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert.Opin.Pharmacother. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Mittal S, Wang Y, Korkmaz KS, Adamson E, English C, Ohmichi M, McClelland M, Mercola D. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol.Cell. 2004;16:521–535. doi: 10.1016/j.molcel.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Kurachi H, Ikegami H, Kimura A, Matsuoka T, Jikihara H, Mercola D, Murata Y. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J.Biol.Chem. 1999;274:31648–31654. doi: 10.1074/jbc.274.44.31648. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, Adachi K, Tasaka K, Kanzaki T, Murata Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- Hazardous Substances Data Bank. National Library of Medicine [3/19/14]; http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB and search on CAS number.
- Hirata J, Kikuchi Y, Kita T, Imaizumi E, Tode T, Ishii K, Kudoh K, Nagata I. Modulation of sensitivity of human ovarian cancer cells to cis-diamminedichloroplatinum(II) by 12-O-tetradecanoylphorbol-13-acetate and D,L-buthionine-S,R-sulphoximine. Int.J.Cancer. 1993;55:521–527. doi: 10.1002/ijc.2910550332. [DOI] [PubMed] [Google Scholar]
- Hitt R, Castellano D, Hidalgo M, Garcia-Carbonero R, Pena M, Brandariz A, Millan JM, Alvarez Vincent JJ, Cortes-Funes H. Phase II trial of cisplatin and gemcitabine in advanced squamous-cell carcinoma of the head and neck. Ann.Oncol. 1998;9:1347–1349. doi: 10.1023/a:1008413818569. [DOI] [PubMed] [Google Scholar]
- Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, Buzdar AU, Frye DK, Hortobagyi GN. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J.Natl.Cancer Inst. 1991;83:1797–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol.Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- Huang TG, Ip SM, Yeung WS, Ngan HY. Changes in p21WAF1, pRb, Mdm-2, Bax and Bcl-2 expression in cervical cancer cell lines transfected with a p53 expressing adenovirus. Eur.J.Cancer. 2000;36:249–256. doi: 10.1016/s0959-8049(99)00247-6. [DOI] [PubMed] [Google Scholar]
- IARC . IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Vol. 26. International Agency for Research on Cancer; Lyon, France: 1981. Cisplatin. In Some Antineoplastic and Immunosuppressive Agents. pp. 151–164. [PubMed] [Google Scholar]
- Ichinose Y, Yosimori K, Yoneda S, Kuba M, Kudoh S, Niitani H. UFT plus cisplatin combination chemotherapy in the treatment of patients with advanced nonsmall cell lung carcinoma: a multiinstitutional phase II trial. For the Japan UFT Lung Cancer Study Group. Cancer. 2000;88:318–323. doi: 10.1002/(sici)1097-0142(20000115)88:2<318::aid-cncr11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Iraz M, Kalcioglu MT, Kizilay A, Karatas E. Aminoguanidine prevents ototoxicity induced by cisplatin in rats. Ann.Clin.Lab Sci. 2005;35:329–335. [PubMed] [Google Scholar]
- Iseri S, Ercan F, Gedik N, Yuksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–264. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc.Natl.Acad.Sci.U.S.A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isonishi S, Andrews PA, Howell SB. Increased sensitivity to cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells in response to treatment with 12-O-tetradecanoylphorbol 13-acetate. J.Biol.Chem. 1990;265:3623–3627. [PubMed] [Google Scholar]
- Iwasaki Y, Nagata K, Nakanishi M, Natuhara A, Kubota Y, Ueda M, Arimoto T, Hara H. Double-cycle, high-dose ifosfamide, carboplatin, and etoposide followed by peripheral blood stem-cell transplantation for small cell lung cancer. Chest. 2005;128:2268–2273. doi: 10.1378/chest.128.4.2268. [DOI] [PubMed] [Google Scholar]
- Jennerwein M, Andrews PA. Effect of intracellular chloride on the cellular pharmacodynamics of cis-diamminedichloroplatinum(II). Drug Metab Dispos. 1995;23:178–184. [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Shen D, Pastan I, Gottesman MM, Hamilton TC. Cross-resistance, cisplatin accumulation, and platinum-DNA adduct formation and removal in cisplatin-sensitive and -resistant human hepatoma cell lines. Exp.Cell Res. 1996;226:133–139. doi: 10.1006/excr.1996.0211. [DOI] [PubMed] [Google Scholar]
- Jones EV, Dickman MJ, Whitmarsh AJ. Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase. Biochem.J. 2007;405:617–623. doi: 10.1042/BJ20061778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar SS, Jala VR, Fong MY. Synergistic cytotoxic action of cisplatin and withaferin A on ovarian cancer cell lines. Biochem.Biophys.Res.Commun. 2012;423:819–825. doi: 10.1016/j.bbrc.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp.Toxicol.Pathol. 2010;62:45–52. doi: 10.1016/j.etp.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat.Rev.Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Khan AB, D'Souza BJ, Wharam MD, Champion LA, Sinks LF, Woo SY, McCullough DC, Leventhal BG. Cisplatin therapy in recurrent childhood brain tumors. Cancer Treat.Rep. 1982;66:2013–2020. [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Yamauchi T, Kumar S, Kaneki M, Kumar V, Bharti A, Yuan ZM, Ghanem L, Rana A, Weichselbaum R, Johnson G, Kufe D. Activation of MEK kinase 1 by the c-Abl protein tyrosine kinase in response to DNA damage. Mol.Cell Biol. 2000;20:4979–4989. doi: 10.1128/mcb.20.14.4979-4989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295–301. [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Krieger ML, Stolting D, Brenner N, Beier M, Jaehde U, Wiese M, Royer HD, Bendas G. Overcoming chemotherapy resistance of ovarian cancer cells by liposomal cisplatin: molecular mechanisms unveiled by gene expression profiling. Biochem.Pharmacol. 2013;85:1077–1090. doi: 10.1016/j.bcp.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- Kosmas C, Tsavaris NB, Malamos NA, Vadiaka M, Koufos C. Phase II study of paclitaxel, ifosfamide, and cisplatin as second-line treatment in relapsed small-cell lung cancer. J.Clin.Oncol. 2001;19:119–126. doi: 10.1200/JCO.2001.19.1.119. [DOI] [PubMed] [Google Scholar]
- Kuhlmann MK, Burkhardt G, Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol.Dial.Transplant. 1997;12:2478–2480. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- Lau JK-C, Deubel DV. Hydrolysis of the Anticancer Drug Cisplatin:GÇë Pitfalls in the Interpretation of Quantum Chemical Calculations. J.Chem.Theory Comput. 2005;2:103–106. doi: 10.1021/ct050229a. [DOI] [PubMed] [Google Scholar]
- Le CT, Brisgand D, Douillard JY, Pujol JL, Alberola V, Monnier A, Riviere A, Lianes P, Chomy P, Cigolari S. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patients. J.Clin.Oncol. 1994;12:360–367. doi: 10.1200/JCO.1994.12.2.360. [DOI] [PubMed] [Google Scholar]
- Legha SS, Ring S, Papadopoulos N, Raber M, Benjamin RS. A phase II trial of taxol in metastatic melanoma. Cancer. 1990;65:2478–2481. doi: 10.1002/1097-0142(19900601)65:11<2478::aid-cncr2820651114>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol.Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol.Res. 2008;57:125–131. doi: 10.1016/j.phrs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res. 1997;57:3759–3764. [PubMed] [Google Scholar]
- Lin CC, Yeh HH, Huang WL, Yan JJ, Lai WW, Su WP, Chen HH, Su WC. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am.J.Respir.Cell Mol.Biol. 2013;49:241–250. doi: 10.1165/rcmb.2012-0244OC. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang Q, Lu Y, Xue W, Xu Y, Zhu Y, Hu X. Downregulation of HIPK2 Increases Resistance of Bladder Cancer Cell to Cisplatin by Regulating Wip1. PLoS.One. 2014;9:e98418. doi: 10.1371/journal.pone.0098418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, Howell SB. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol.Pharmacol. 2002;62:1154–1159. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- Liu JR, Opipari AW, Tan L, Jiang Y, Zhang Y, Tang H, Nunez G. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res. 2002;62:924–931. [PubMed] [Google Scholar]
- Llanos S, Serrano M. Depletion of ribosomal protein L37 occurs in response to DNA damage and activates p53 through the L11/MDM2 pathway. Cell Cycle. 2010;9:4005–4012. doi: 10.4161/cc.9.19.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HT, Casey MJ, Snyder CL, Bewtra C, Lynch JF, Butts M, Godwin AK. Hereditary ovarian carcinoma: heterogeneity, molecular genetics, pathology, and management. Mol.Oncol. 2009;3:97–137. doi: 10.1016/j.molonc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuy N, Rajalingam K, Rudel T. Requirement of caspase-mediated cleavage of c-Abl during stress-induced apoptosis. Cell Death.Differ. 2004;11:290–300. doi: 10.1038/sj.cdd.4401336. [DOI] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J.Biol.Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates Cisplatin-induced oxidative stress and hepatotoxicity in rats. J.Biochem.Mol.Biol. 2006;39:656–661. doi: 10.5483/bmbrep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- Mantri Y, Baik MH. Encyclopedia of Inorganic Chemistry. John Wiley & Sons, Ltd.; 2006. Computational Studies: Cisplatin. [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J.Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Matsuki M, Takahashi A, Katou S, Takayanagi A, Takagi Y, Kamata K. [Pathological complete response to gemcitabine and cisplatin chemotherapy for advanced upper tract urothelial carcinoma: a case report]. Nihon Hinyokika Gakkai Zasshi. 2013;104:33–37. doi: 10.5980/jpnjurol.104.33. [DOI] [PubMed] [Google Scholar]
- McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, Donehower RC. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann.Intern.Med. 1989;111:273–279. doi: 10.7326/0003-4819-111-4-273. [DOI] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N.Engl.J.Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- Meng F, Sun G, Zhong M, Yu Y, Brewer MA. Anticancer efficacy of cisplatin and trichostatin A or 5-aza-2'-deoxycytidine on ovarian cancer. Br.J.Cancer. 2013;108:579–586. doi: 10.1038/bjc.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milczarek M, Rosinska S, Psurski M, Maciejewska M, Kutner A, Wietrzyk J. Combined colonic cancer treatment with vitamin D analogs and irinotecan or oxaliplatin. Anticancer Res. 2013;33:433–444. [PubMed] [Google Scholar]
- Minami D, Takigawa N, Takeda H, Takata M, Ochi N, Ichihara E, Hisamoto A, Hotta K, Tanimoto M, Kiura K. Synergistic effect of olaparib with combination of cisplatin on PTEN-deficient lung cancer cells. Mol.Cancer Res. 2013;11:140–148. doi: 10.1158/1541-7786.MCR-12-0401. [DOI] [PubMed] [Google Scholar]
- Murphy WK, Fossella FV, Winn RJ, Shin DM, Hynes HE, Gross HM, Davilla E, Leimert J, Dhingra H, Raber MN. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J.Natl.Cancer Inst. 1993;85:384–388. doi: 10.1093/jnci/85.5.384. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr.Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Natarajan G, Malathi R, Holler E. Increased DNA-binding activity of cis-1,1-cyclobutanedicarboxylatodiammineplatinum(II) (carboplatin) in the presence of nucleophiles and human breast cancer MCF-7 cell cytoplasmic extracts: activation theory revisited. Biochem.Pharmacol. 1999;58:1625–1629. doi: 10.1016/s0006-2952(99)00250-6. [DOI] [PubMed] [Google Scholar]
- Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–230. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Nehme A, Baskaran R, Nebel S, Fink D, Howell SB, Wang JY, Christen RD. Induction of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells. Br.J.Cancer. 1999;79:1104–1110. doi: 10.1038/sj.bjc.6690176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res. 2011;31:3789–3797. [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem.J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov GS, Trendafilova N, Sch+¦nenberger H, Gust R, Kritzenberger J, Yersin H. Molecular mechanical and quantum chemical study on the species involved in the hydrolysis of cis-diamminedichloroplatinum(II) and substituted bis(ethylenediamine)dichloroplatinum(II) complexes Part I. Reactants and products. Inorganica Chimica Acta. 1994;217:159–170. [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nowak G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J.Biol.Chem. 2002;277:43377–43388. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- Ohta T, Ohmichi M, Hayasaka T, Mabuchi S, Saitoh M, Kawagoe J, Takahashi K, Igarashi H, Du B, Doshida M, Mirei IG, Motoyama T, Tasaka K, Kurachi H. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in in vivo ovarian cancer models. Endocrinology. 2006;147:1761–1769. doi: 10.1210/en.2005-1450. [DOI] [PubMed] [Google Scholar]
- Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J.Pharm.Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- Pavankumar PNV, Seetharamulu P, Yao S, Saxe JD, Reddy DG, Hausheer FH. Comprehensive ab initio quantum mechanical and molecular orbital (MO) analysis of cisplatin: Structure, bonding, charge density, and vibrational frequencies. J.Comput.Chem. 1999;20:365–382. [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J.Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J.Cell Biol. 1981;91:479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons DL, Yazlovitskaya EM, Cui W, Pelling JC. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin.Cancer Res. 1999;5:1007–1014. [PubMed] [Google Scholar]
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le CT. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J.Clin.Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- Pinto-Leite R, Arantes-Rodrigues R, Palmeira C, Colaco B, Lopes C, Colaco A, Costa C, da Silva VM, Oliveira P, Santos L. Everolimus combined with cisplatin has a potential role in treatment of urothelial bladder cancer. Biomed.Pharmacother. 2013;67:116–121. doi: 10.1016/j.biopha.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Previati M, Lanzoni I, Corbacella E, Magosso S, Guaran V, Martini A, Capitani S. Cisplatin-induced apoptosis in human promyelocytic leukemia cells. Int.J.Mol.Med. 2006;18:511–516. [PubMed] [Google Scholar]
- Rodriguez-Fernandez E, Manzano JL, Alonso A, Almendral MJ, Perez-Andres M, Orfao A, Criado JJ. Fluorescent cisplatin analogues and cytotoxic activity. Curr.Med.Chem. 2009;16:4314–4327. doi: 10.2174/092986709789578169. [DOI] [PubMed] [Google Scholar]
- Rosenberg b., vancamp l., krigas t. Inhibition of cell division in escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Saad SY, Najjar TA, Alashari M. Role of non-selective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin.Exp.Pharmacol.Physiol. 2004;31:862–867. doi: 10.1111/j.1440-1681.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Abrams JM. Caspase activation - stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene. 2004;23:2774–2784. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Saramaki A, Banwell CM, Campbell MJ, Carlberg C. Regulation of the human p21(waf1/cip1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34:543–554. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosy G, Kohn E, Stone DA, Rothenberg M, Jacob J, Adamo DO, Ognibene FP, Cunnion RE, Reed E. Phase I study of taxol and granulocyte colony-stimulating factor in patients with refractory ovarian cancer. J.Clin.Oncol. 1992;10:1165–1170. doi: 10.1200/JCO.1992.10.7.1165. [DOI] [PubMed] [Google Scholar]
- Scher HI. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J.Urol. 1992;148:1625–1626. [PubMed] [Google Scholar]
- Shaili E. Platinum anticancer drugs and photochemotherapeutic agents: recent advances and future developments. Sci.Prog. 2014;97:20–40. doi: 10.3184/003685014X13904811808460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Liang XJ, Gawinowicz MA, Gottesman MM. Identification of cytoskeletal [14C]carboplatin-binding proteins reveals reduced expression and disorganization of actin and filamin in cisplatin-resistant cell lines. Mol.Pharmacol. 2004;66:789–793. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- Shen DW, Liang XJ, Suzuki T, Gottesman MM. Identification by functional cloning from a retroviral cDNA library of cDNAs for ribosomal protein L36 and the 10-kDa heat shock protein that confer cisplatin resistance. Mol.Pharmacol. 2006;69:1383–1388. doi: 10.1124/mol.105.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol.Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong J, Lu B, Jiang G, Zhao W. AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumour.Biol. 2014 doi: 10.1007/s13277-014-1956-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol.Cancer Ther. 2011;10:1161–1172. doi: 10.1158/1535-7163.MCT-10-1100. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Siwinska A, Opolski A, Chrobak A, Wietrzyk J, Wojdat E, Kutner A, Szelejewski W, Radzikowski C. Potentiation of the antiproliferative effect in vitro of doxorubicin, cisplatin and genistein by new analogues of vitamin D. Anticancer Res. 2001;21:1925–1929. [PubMed] [Google Scholar]
- Skvortsov S, Dudas J, Eichberger P, Witsch-Baumgartner M, Loeffler-Ragg J, Pritz C, Schartinger VH, Maier H, Hall J, Debbage P, Riechelmann H, Lukas P, Skvortsova I. Rac1 as a potential therapeutic target for chemo-radioresistant head and neck squamous cell carcinomas (HNSCC). Br.J.Cancer. 2014;110(11):2677–2687. doi: 10.1038/bjc.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Welham KJ, Watson MB, Drew PJ, Lind MJ, Cawkwell L. The proteomic analysis of cisplatin resistance in breast cancer cells. Oncol.Res. 2007;16:497–506. doi: 10.3727/096504007783438358. [DOI] [PubMed] [Google Scholar]
- Song YD, Zhang KF, Liu D, Guo YQ, Wang DY, Cui MY, Li G, Sun YX, Shen JH, Li XG, Zhang L, Shi FJ. Inhibition of EGFR-induced glucose metabolism sensitizes chondrosarcoma cells to cisplatin. Tumour.Biol. 2014 doi: 10.1007/s13277-014-1902-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N.Engl.J.Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Tang MK, Zhou HY, Yam JW, Wong AS. c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2. Neoplasia. 2010;12:128–138. doi: 10.1593/neo.91438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3/21/14]; The chemical book data base http://www.chemicalbook.com/ProductIndex_EN.aspxand search on product details.
- Tsimberidou AM, Braiteh F, Stewart DJ, Kurzrock R. Ultimate fate of oncology drugs approved by the us food and drug administration without a randomized Trial. J.Clin.Oncol. 2009;27:6243–6250. doi: 10.1200/JCO.2009.23.6018. [DOI] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N.Engl.J.Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int.J.Cancer. 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- von der MH, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J.Clin.Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat.Rev.Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang G, Zhang H, Zhang F, Zhou B, Ning F, Wang HS, Cai SH, Du J. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/beta-catenin/Snail signaling pathway. Eur.J.Pharmacol. 2014;723:156–166. doi: 10.1016/j.ejphar.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J.Biol.Chem. 2014 doi: 10.1074/jbc.M114.558288. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J.Biol.Chem. 2000;275:39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Christian MC. New cisplatin analogues in development. A review. Drugs. 1993;46:360–377. doi: 10.2165/00003495-199346030-00003. [DOI] [PubMed] [Google Scholar]
- Winograd-Katz SE, Levitzki A. Cisplatin induces PKB/Akt activation and p38(MAPK) phosphorylation of the EGF receptor. Oncogene. 2006;25:7381–7390. doi: 10.1038/sj.onc.1209737. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhang Y, Qu D, Liu HB, Gu X, Jiao GY, Zhao L. Combined anticancer activity of osthole and cisplatin in NCI-H460 lung cancer cells in vitro. Exp.Ther.Med. 2013;5:707–710. doi: 10.3892/etm.2013.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am.J.Med.Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- Yeh PY, Chuang SE, Yeh KH, Song YC, Ea CK, Cheng AL. Increase of the resistance of human cervical carcinoma cells to cisplatin by inhibition of the MEK to ERK signaling pathway partly via enhancement of anticancer drug-induced NF kappa B activation. Biochem.Pharmacol. 2002;63:1423–1430. doi: 10.1016/s0006-2952(02)00908-5. [DOI] [PubMed] [Google Scholar]
- Yilmaz HR, Iraz M, Sogut S, Ozyurt H, Yildirim Z, Akyol O, Gergerlioglu S. The effects of erdosteine on the activities of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacol.Res. 2004;50:287–290. doi: 10.1016/j.phrs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Yilmaz HR, Sogut S, Ozyurt B, Ozugurlu F, Sahin S, Isik B, Uz E, Ozyurt H. The activities of liver adenosine deaminase, xanthine oxidase, catalase, superoxide dismutase enzymes and the levels of malondialdehyde and nitric oxide after cisplatin toxicity in rats: protective effect of caffeic acid phenethyl ester. Toxicol.Ind.Health. 2005;21:67–73. doi: 10.1191/0748233705th216oa. [DOI] [PubMed] [Google Scholar]
- Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J.Thorac.Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem.Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo Z, You XZ. Hydrolysis Theory for Cisplatin and Its Analogues Based on Density Functional Studies. J.Am.Chem.Soc. 2001;123:9378–9387. doi: 10.1021/ja0023938. [DOI] [PubMed] [Google Scholar]