Abstract
Background
Young children with brain tumors are often treated with high-dose chemotherapy after surgery to avoid brain tissue injury associated with irradiation. The effects of systemic chemotherapy on healthy brain tissue in this population, however, are unclear. Our objective was to compare gray and white matter integrity using MRI procedures in children with brain tumors (n=7, mean age 8.3 years), treated with surgery and high-dose chemotherapy followed by autologous hematopoietic cell rescue (AuHCR) an average of 5.4 years earlier, to age- and gender-matched healthy controls (n=9, mean age 9.3 years).
Methods
Diffusion tensor imaging data were collected to evaluate tissue integrity throughout the brain, as measured by mean diffusivity (MD), a marker of glial, neuronal, and axonal status, and fractional anisotropy (FA), an index of axonal health. Individual MD and FA maps were calculated, normalized, smoothed, and compared between groups using analysis of covariance, with age and sex as covariates.
Results
Higher mean diffusivity values, indicative of injury, emerged in patients compared with controls (p<0.05, corrected for multiple comparisons), and were especially apparent in the central thalamus, external capsule, putamen, globus pallidus and pons. Reduced FA values in some regions did not reach significance after correction for multiple comparisons.
Conclusions
Children treated with surgery and high-dose chemotherapy with AuHCR for brain tumors an average of 5.4 years earlier show alterations in white and gray matter in multiple brain areas distant from the tumor site, raising the possibility for long-term consequences of the tumor or treatment.
Keywords: mean diffusivity, diffusion tensor imaging, pediatrics, cancer, medulloblastoma
Introduction
Brain tumors are the second most common type of cancer in children, and over 20,000 children were diagnosed with brain tumors in the US between 2004 and 2008 [1]. Survival rates vary, depending upon type and location of tumor, and averaging 66% to 70% [1]. Despite successful treatment, survivors often show detrimental neurocognitive consequences years later, contributing to lower employment rates, continued dependency on parents, and social isolation [2]. Several sources of neural pathology may underlie those consequences, including the presence of the tumor and subsequent hydrocephalus, surgical intervention, or treatment, including chemotherapy and/or cranial irradiation. Most patients will undergo surgical resection of the tumor prior to further treatment with chemotherapy and/or cranial irradiation. The relationship between neurocognitive deficits and cranial irradiation has been so strongly supported [3-5] that many young children under 6 years of age are treated on protocols without irradiation [6, 7]. Less is known about the potential detrimental consequences of systemic chemotherapy on healthy neural tissue in this population.
High-dose, marrow-ablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) is a frontline treatment for brain tumors in very young children and believed to be less damaging than radiation therapy [6, 8]. There is evidence that chemotherapy leads to long-term brain tissue damage, including white [9] and gray matter loss [10], and neurocognitive deficits in children with acute lymphoblastic leukemia (ALL) [9, 11, 12]; however, in addition to systemic chemotherapy, these children received intrathecal chemotherapy with methotrexate (MTX) and/or cytosine arabinoside, drugs known to be neurotoxic, especially via instillation into cerebrospinal fluid (CSF). Certain chemotherapeutic agents are toxic to oligodendrocytes [13], hippocampal cells [14], and cerebellar granule cells. In fact, in vitro models have demonstrated that some chemotherapy agents are more toxic to neural progenitor cells than to cancer cells [13, 15, 16]. Thus, although considered less toxic to brain cells than radiation therapy, chemotherapy may also be associated with brain tissue injury.
Several quantitative MRI procedures are sensitive to brain injury or brain changes. Among those, diffusion tensor imaging techniques provide indices of diffusion of water between tissues. Measures derived from the diffusion tensor, such as mean diffusivity (MD), can assess cellular and fiber injury across gray and white matter, while fractional anisotropy (FA) is sensitive to changes in axonal integrity in white matter. For example, significantly lower FA values, suggesting white matter damage, appear in brain regions of survivors of ALL and medulloblastoma treated with chemotherapy and cranial irradiation [10, 17]. However, reports of DTI studies in the population of children with brain tumors treated with surgery and high-dose chemotherapy with AuHCR are lacking.
The purpose of this study was to examine DTI, MD and FA values in childhood survivors of brain tumors treated with surgery and high-dose chemotherapy with AuHCR, compared to age and gender matched healthy controls. We tested the hypotheses that FA would be decreased, indicating loss of tissue and diminished fiber integrity, and that MD would be increased, indicating long-term cellular and fiber injury in patients with brain tumors previously treated with surgery and systemic high-dose chemotherapy with AuHCR, when compared to age and gender matched controls.
Materials and Methods
Study Design
A two-group comparative cross-sectional design was used to compare FA and MD values throughout the brain between seven pediatric brain tumor patients and nine matched healthy controls. The UCLA Institutional Review Board and the Children's Hospital Los Angeles (CHLA) Committee for Clinical Investigations approved the study, and informed consent was obtained from parents of all subjects. Assent was obtained from subjects 7 years of age and older.
Sample & Setting
Sixteen childhood brain tumor survivors who met the following inclusion criteria were identified by the pediatric neuro-oncology team at CHLA: 1) a history of brain tumor with no current evidence of disease; 2) prior treatment on a chemotherapy-only regimen followed by AuHCR; 3) age between 5 and 13 years at time of enrollment; 4) off-therapy for at least 6 months; and 5) speaking either English or Spanish. Exclusion criteria were 1) residual disease; 2) history of cranial irradiation; 3) concurrent diagnosis of neurofibromatosis or other serious neurological anomaly; 4) history of prolonged posterior fossa syndrome postoperatively (longer than one week); and 5) presence of a pacemaker or other implanted metal device (precluding MRI).
Information about the study was mailed to families of all eligible subjects, and parents were instructed to return a postcard if interested in enrolling. Twelve families (75%) responded. Five were ultimately excluded because of: the presence of residual tumor (1 patient); a congenital brain malformation (1): a supratentorial primitive neuroectodermal tumor (PNET) with possibility of tumor location at that site interfering with DTI interpretation (1); and families unable to travel for data collection (2 patients). Six enrolled patients had posterior fossa tumors (medulloblastoma) located in the cerebellum, and one had an intra- ventricular tumor (choroid plexus carcinoma).
For the comparison group, nine healthy controls were recruited from children of staff at the institution. Inclusion criteria for this convenience sample were 1) fluency in English or Spanish, 2) current age between 5 and 13 years, and 3) the ability to complete a 30-minute MRI without sedation. Exclusion criteria were 1) any neurological abnormality including past history of head trauma, seizures, or autism, 2) learning disability, hyperactivity or attention deficit disorder, or 3) presence of dental braces or other metal appliances in the body.
Measures
Magnetic resonance imaging data were collected over 14 months using a 3.0 Tesla Philips Achieva MRI scanner. High-resolution T1- and T2-weighted and DTI images were collected on patients, while only T1-weighted and DTI images were obtained on controls to minimize time spent in the scanner for these young children without sedation. DTI images were collected using an 8-channel phased-array head coil with spin-echo echo-planar sequence [repetition time (TR)=8,000 ms, echo-time (TE)=55 ms, field of view (FOV)=260 × 260 mm, slice thickness=1.9 mm], with a 144 × 144 matrix size, 80 axial slices and no interslice gap. Diffusion-weighted images were collected in 12 directions for each slice, with a maximum b value of 1000 s/mm2. A single excitation was used. High resolution T1-weighted images were obtained with a voxel size of 1.0 × 1.0 × 1.6 mm and 136 × 136 matrix; FOV 26 cm; with the same parameters previously listed.
High-resolution T1- and T2-weighted images were used for anatomical localization and visual assessment.
Procedures
Families were asked to arrive one hour before MRI registration time to complete the informed consent and/or assent process in a private conference room. The MRI protocol lasted one hour for patients, including pre- and post-contrast images for clinical surveillance purposes in addition to DTI scans. For controls, the MRI protocol took 30 minutes to complete. Five patients received propofol anesthesia for the MRI, as was standard for their routine scans, and the remaining 2 patients and 9 controls utilized MRI-compatible movie goggles for distraction during the scan. All children were able to complete the study.
After assessment by a neuroradiologist, images were de-identified and coded for analysis.
Data Analysis
Demographic data were processed using the Statistical Package for Social Sciences, version 17 (SPSS, IBM Corp.). The MRI data were processed using Statistical Parametric Mapping, version 8 (SPM8) and custom MATLAB software. After conversion from DICOM to NIFTI format, each subject's anatomical T1 images were segmented into gray matter, white matter and CSF using the unified segmentation procedure [18]. The procedure also calculated spatial normalization parameters for warping images into standard Montreal Neurological Institute (MNI) template space.
Diffusion tensor imaging scans were analyzed with the SPM8 Diffusion Toolbox. The diffusion tensor was calculated at each voxel, from which whole-brain maps of FA and MD were derived. The b0 images from the DTI series were co-registered to T1 anatomical scans, and indices were spatially normalized using T1 parameters. Normalized FA and MD images were smoothed with a 10 mm Gaussian filter, and analyzed with a voxel-based approach in SPM8. Voxel-based morphometry [19] was used to define regional differences in MD and FA values between patients and controls, using an ANCOVA model at each voxel, and with corrections for multiple comparisons across voxels.
Because of the presence of a right hippocampal glioma and history of ventricular tumor in one patient, the analyses were run a second time excluding that patient.
Results
Sample Characteristics
Seven pediatric brain tumor patients (mean age 8.3 years ± 3.01, range 5 to13 years) and nine matched healthy controls (mean age 9.3 years ± 2.56, range 6 to 13 years) were included in these analyses. Four of the seven patients (55%) had either no hydrocephalus or mild hydrocephalus at diagnosis. Each patient underwent one surgical resection of the tumor. Initial presentation, length of time off-treatment, and health status of patients at the time of study are included in Table 1. No patient had previously received either cranial or craniospinal irradiation, or had received either intraventricular or intrathecal chemotherapy as part of their tumor management. The patient and control groups were similar in age, gender and race, but significantly different in level of parental education (Table 2).
Table 1.
Tumor Types and Treatment for Patient Group
| Subject | Age at Diagnosis | Presentation | Surgical Approach | Tumor Type, Location | Hydrocephalus at Diagnosis | Chemotherapy | Age at Study | Years off therapy | Status at Time of Study |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 54 months | Asymptomatic, incidental finding | Left posterior parietal craniotomy | Choroid plexus carcinoma, left occipital horn | None | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa Temozolomi de | 8.0 | 3.5 | No deficits, does well in school. Had stable low grade glioma of right hippocam pus |
| 02 | 27 months | 4 week history ataxia, poor appetite, emesis, lethargy | Subocci pital cranioto my | Desmoplastic medullo- blastoma, 4th ventricle | Milda | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa | 5.4 | 2.5 | Hearing loss, wears hearing aids, above average academically |
| 03 | 14 months | Poor history, probable developmental delay at diagnosis | Posterior fossa craniotomy | Medullo- blastoma, posterior fossa | Moderat ea | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa | 13.0 | 11.4 | Neurocog nitive deficits (verbalizes difficulty with memory, in specialed), vision loss, ataxia, facial nerve palsy. |
| 04 | 16 months | Ataxia | Suboccipital craniotomy | Desmoplastic medullo- blastoma, posterior fossa | Moderat ea | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa | 4.5 | No overt deficits, does well in school. Incidental stable midline AVM | |
| 05 | 55 months | Hearing loss, ataxia for 4-5 days | Left suboccipital craniotomy | Primary leptomeningeal medulloblastoma involving left cerebellum, 4th ventricle, nfundibulum,bilateralcerebellar-pontineangle | None | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa Methotrexate Temozolomide | 9.3 | 4.8 | Hearing loss, neurogenic bladder. Achieves average grades in school. |
| 06 | 19 months | Acute ataxia, extensor posturing | Posterior fossa cranioto my | Desmoplastic medulloblastoma, posterior fossa | Mild | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa Methotrexate | 5.9 | 3.3 | No problems in kindergarten per parent report. |
| 07 | 28 months | 4-5 month history headache, ataxia,decreased appetite | Posterior fossa craniotomy | Medulloblastoma, posterior fossa | Moderatea | Vincristine Cisplatin Cyclophosph amide Etoposide Carboplatin Thiotepa | 10.5 | 8.0 | Needs glasses to read, residual left-sided weakness. Does well in school. |
ventriculoperitoneal (VP) shunt placed
Table 2.
Characteristics of Patients and Controls
| Patients n = 7 | Controls n = 9 | P | |
|---|---|---|---|
| Age at study Gender | 8.33 years | 9.30 years | .54 |
| 3 male | 6 male | .52 | |
| 4 female | 3 female | ||
| Race | 6 Caucasian | 6 Caucasian | .34 |
| 1 non-Caucasian (Asian) | 3 non-Caucasian (1 Asian, 2 African American) | ||
| Highest Parental Education | 1 some high school | 5 college degree | .04 |
| 1 high school diploma | 4 graduate degree | ||
| 2 community college | |||
| 2 college degree | |||
| 1 graduate degree |
Global Brain Assessment
Anatomical scans (T1-weighted, T2-weighted, FLAIR) were evaluated by a pediatric neuroradiologist for clinical abnormalities, and in patients, for recurrent disease. Although no patient had recurrence of the primary brain tumor, one patient had a previously diagnosed second brain tumor (stable low-grade glioma of the right hippocampus), and another had a midline arterio-venous malformation (AVM) that had been noted on previous scans. Neither of those areas showed significant regional changes in our analyses, and the analysis was run again without the patient with the history of choroid plexus carcinoma with subsequent hippocampal glioma, with no significant difference in findings. No controls had abnormal findings.
Regional Analysis
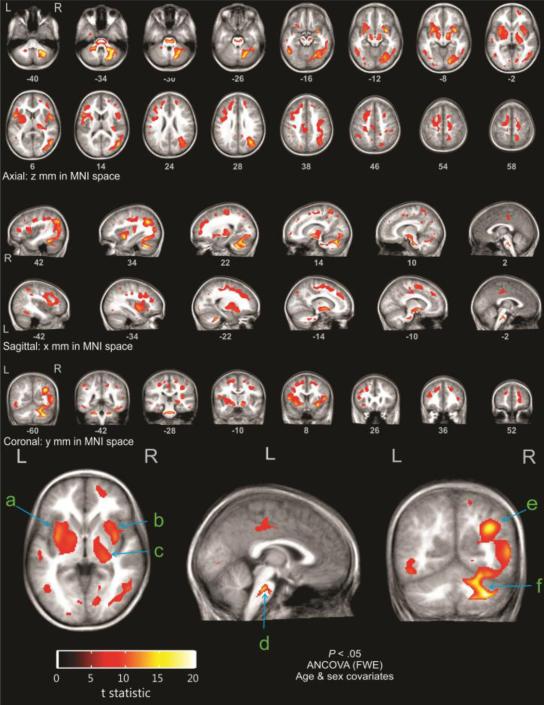
With age and sex as covariates, DTI analyses showed that while there was a trend toward lower FA in the patient group, there were no areas of significant difference in FA values between patients and controls, at the threshold of p<0.05, with family-wise error correction. However, at the same threshold (p<0.05, with stringent family-wise error correction for multiple comparisons), there were multiple areas of significantly higher MD in the patient versus the control group (Figure 1). These findings reflect a very large effect size, indicative of significant chronic injury (t statistic threshold = 8.05). Brain regions showing increased MD were widespread, and most were located far from the tumor sites. Altered values in the cerebellum were expected, due to history of tumor and surgery; thus, those values will not be discussed as significant findings. Mean diffusivity was similarly increased throughout the cerebral white matter, principally involving long and short association fibers (subcortical u fibers, arcuate fasciculus, cingulum, superior and inferior longitudinal fasciculi), external and extreme capsules, and centrum semiovale. Gray matter nuclei with increased MD included the medial and lateral nuclei of the globus pallidus, putamena, claustrum, and central thalamus, including the inter-thalamic adhesion. The insular cortex and medial pons also demonstrated significantly higher MD.
Figure 1.
Highlighted areas represent regions of significantly increased mean diffusivity in patients as compared to controls: a) globus pallidus, b) putamen, c) thalamus, d) medial pons, e) insula, f) cerebellum. The areas are concentrated in the subsulcal white matter, the insula, basal ganglia and central thalami
Discussion
This study examined DTI values (MD and FA) in survivors of childhood brain tumors treated with surgery and high-dose chemotherapy with AuHCR, without cranial irradiation, compared to age and gender matched controls. We found that MD was globally and regionally higher in the patient group, and no significant differences in FA were observed. Areas of increased MD appeared in widespread regions throughout the brain in patients, compared to healthy controls.
Parental education, an indicator of socioeconomic status (SES), significantly differed between the patient and control groups. Imaging studies have not shown a difference in overall gray and white matter volumes related to SES, but larger volumes in the hippocampi, amygdala and a portion of the left temporal gyrus were observed in higher SES subjects [20]. In addition, educational level was a significant predictor of FA in the superior longitudinal fasciculus and cingulum [21] in older adolescents. These are two white matter areas where we found elevated MD in patients, but no significant difference in FA between patients and controls. Neural structural differences related to SES are complex, so this important variable should be noted.
Mean diffusivity is a measure of molecular motion through tissue, and reflects the quality of intercellular barriers. Changes in cell size, shape and integrity will result in altered MD and increases, indicating a reduction in barriers, are interpreted as a generalized marker of structural injury [22-24]. Higher MD values occur in areas with edema, loss of axons, and demyelination, typically signifying chronic structural injury [23]. Fractional anisotropy, which may denote structural integrity and degree of alignment within fiber tracts, shows comparatively low values in areas with reduced tissue organization [22, 25], including axonal damage [26]. Thus, the observed differences in DTI indices likely reflect pathological changes, but are not specific to any one type of pathology.
The lack of significant differences in FA in children after brain tumor treatment is contrary to what is reported in the literature. Children with posterior fossa pilocytic astrocytoma treated with surgery alone demonstrated significantly lower FA in the cerebellum, callosal and frontal areas, and corona radiata, than healthy controls. Those with medulloblastoma treated with surgery followed by chemotherapy and cranial irradiation showed significantly lower FA in the cerebellum, frontal and callosal areas [17]. The authors of that study attributed their findings to two possibilities: the effect of hydrocephalus, or neuronal projection damage to the frontal areas from cerebellar surgery.
In our small sample, 45% of patients had moderate hydrocephalus at diagnosis, and 86% had undergone cerebellar surgery. It has been reported that the structures most likely to display DTI value differences related to hydrocephalus, specifically lower FA, are the genu of the corpus callosum and posterior limb of the internal capsule [27]. These low FA values in large part return to normal after hydrocephalus or brain compression resolves with shunting or other surgical intervention [28, 29] [30], and MD did not significantly differ in any areas in patients with hydrocephalus, as compared to controls [30]. In this study, there was no indication of tissue change in the corpus callosum or internal capsule. The patients were 2.5 – 11.4 years after acute presentation, and those whose hydrocephalus was not resolved with tumor debulking were shunted at that time. Therefore, it is unlikely that our findings are related to hydrocephalus, but perhaps to cerebellar surgery and/or chemotherapy. In complex diseases such as brain tumors, it is common to observe a combination of brain tissue injury such as demyelination, axonal loss, and gliosis, and DTI findings cannot distinguish between these pathologies [24].
Our findings of widespread tissue change, especially injury to key deep midline cortical structures indicated by elevated MD, merit further exploration. There appeared to be a pattern of structural alterations, most notably in deep cortical brain structures (thalamus, caudate, putamen, globus pallidus) that exert major influences on other brain regions [31], serving as essential pathways in the transmission of information between brainstem, cerebellum and thalamus to frontal and other cortical areas [31-33]. It is unclear whether this pattern is present in children with posterior fossa brain tumors treated with surgery alone, or with the addition of cranial irradiation. These alterations may have long-term neurocognitive implications, such as difficulties with processing speed, memory and executive functioning. In addition to cognitive deficits, injury to basal ganglia structures may result in movement disorders and other physical disabilities [34, 35]. Two patients in this study had chemotherapy-related hearing loss, and two had residual physical disabilities (ataxia and hemiparesis) (Table 1).
The majority of patients in this study (6 of 7, or 86%) attended regular classes at school at the time of study, and had average- to above-average grades. One child, who displayed some language and other developmental delays at diagnosis, and who had also been off-therapy the longest (11.4 years), was the most physically and cognitively disabled, attending special education classes. This child was also the youngest at diagnosis (14 months of age). It is known that cranial irradiation in very young children is detrimental to long-term cognitive outcome, and that cognitive scores continue to decline in the years following radiation treatment [36]. Less is known about the neurocognitive effects of systemic chemotherapy in the very young, which may be less toxic than irradiation [37], but still a contributing factor to deficits in executive functioning [38]. One study of outcome of children with brain tumors treated with high-dose chemotherapy found no significant association with age at diagnosis and neuropsychological outcome; however, there was a significant inverse relationship between time since diagnosis and full-scale IQ [39]. There is not enough evidence at this point to determine whether neurocognitive decline may continue in the years after chemotherapy in young children. In this study, it is not possible to know whether the patients have successfully compensated thus far for deep cortical brain tissue changes, or whether they may have mild memory or processing dysfunction that is not yet apparent, but might become more prevalent in later years of middle and high school. The specific relationship between major structural brain changes and biobehavioral impact is complex, and remains unknown.
Elevated MD in the specific structures noted may possibly be related to iron overload. This population of children treated with high-dose chemotherapy is at-risk for iron overload as a consequence of multiple blood transfusions. In the elderly, iron deposits are commonly found in the caudate, putamen, and globus pallidus, which results in significantly higher FA and MD in these regions [40]. While ferritin level was not a variable in this study, a retrospective analysis found that only 2 of the 8 patients had ferritin levels reported, and of those, one was normal and one was notably high. It is conceivable that iron in these structures contributed to the findings of high MD without significantly lower FA.
Diffusion tensor indices based on high directional data should be studied further, since those indices are sensitive to other pathologies, and the FA data here are inconsistent with the literature. Our limited DTI protocol may have resulted in a less reliable FA measure (see Limitations).
Since these data are cross-sectional, it is not possible to determine when the brain alterations first developed in these children. Changes may have occurred at the time of treatment, during the period up to 11 years subsequent to treatment, or alternatively, structural differences in patients as compared to controls may have been present prior to surgery. When compared to DTI studies in children with brain tumors who received cranial irradiation, again, the literature refers to findings of decreased FA [17, 41, 42] [43], and MD is apparently not measured. In adults, after 50-55 Gy cranial irradiation, areas of elevated MD seen initially after treatment returned to baseline 8 months later [44].
Patients in this study were young at diagnosis (14-55 months of age), and time off-therapy and age at study were more varied. Inclusion criteria for age was 5-13 years, because completing an MRI without sedation necessitated healthy controls be at least 5 years of age, and we sought to avoid pubertal brain changes by having 13 as the upper age limit (the oldest females were 9 and pre-pubertal). Patterns of cortical thickness change during childhood and adolescence, with subcortical brain development likely accelerating with puberty [45]. Other changes noted during maturation of children from 0-10 years include a decrease in water diffusion and increased anisotropy [25]. Although patients and controls were age- and gender-matched, results should be interpreted with caution due to this variability.
Limitations
Limitations include small sample size and the cross-sectional design. The limited sample size resulted from numerous exclusion criteria necessary to limit variability as much as possible, as well as difficulties in recall of earlier patients. Despite this limitation, the major findings showed a very large effect size. Another limitation of the study was the inclusion of one patient with a ventricular tumor and a second temporal glioma, and one patient with a midline AVM. However, the significant results of elevated MD reported here were found in areas distant from these abnormalities, and a separate analysis excluding the patient with the hippocampal glioma demonstrated the same results as those including that patient. Five of the seven patients, but no controls, received propofol anesthesia during their MRI scans. Although it is theoretically possible that anesthesia altered diffusion findings, there are no reports of such effects in the literature.
The 12-direction DTI protocol may have been a limitation, but was feasible, given the time limitations of keeping a child in the scanner. More directions would be preferable, but the MD index, in particular, can be reliably measured with modest numbers of directions, since that measure is an average, and does not rely on distinguishing directional differences. In contrast, FA may likely be more impacted by the low number of directions in this study [46]. However, other pediatric oncology studies using DTI protocols with 6-16 directions demonstrated significant FA findings related to cognition [21, 47], and to effects of chemotherapy and/or cranial irradiation [48, 49]. Mean diffusivity was not measured in these studies. In addition, comparison of diffusion-weighting schemes using 6-30 directions demonstrated clinically insignificant differences in FA and MD indices between protocols [50].
Conclusion
Patients treated with surgery and myelo-ablative chemotherapy with AuHCR for a brain tumor showed indications of neural injury several years later. While this study included a small number of patients, few studies are available to determine the effects of surgery and high-dose, including marrow-ablative, systemic chemotherapy on the brain. The injury and cell loss noted here may be attributable to chemotherapy, to general pathological changes associated with cerebellar lesions and to surgery, or to a combination of all factors. Larger, longitudinal studies will ultimately be needed to confirm these findings, with the inclusion of patients who had posterior fossa tumors treated with surgery alone acting as controls for those treated additionally with chemotherapy.
The number of children with brain tumors treated with chemotherapy alone, without cranial irradiation, has been relatively small, and there is little information about the effects of such treatment on the young brain. As more children are treated in this manner to avoid or delay cranial irradiation, careful monitoring of brain structure will help determine long-term consequences of this treatment approach. Clinicians should be aware that surgery followed by high-dose chemotherapy with AuHCR has potential long-term consequences to brain structure, although these are likely less than those associated with radiotherapy. Furthering our understanding of the impact of systemic chemotherapy on the brain in children will enable the provision of accurate information to parents who are consenting to treatment, and potentially assist in identifying those children in need of early neurocognitive and developmental intervention.
Acknowledgements
We thank Rajesh Kumar, PhD for his assistance in developing the DTI protocol, and Natasha Lepore, PhD for her thoughtful review of this paper.
This study was funded by the National Institute of Nursing Research [F31NR011560]; the Clinical &Translational Science Institute with funds provided by the National Center for Research Resources, National Institutes of Health [MO1 RR00043CHLA]; and Alex's Lemonade Stand Foundation.
The content is solely the responsibility of the authors and does not necessarily represent official views of the NINR or the NIH.
Ethical Standards
This study was approved by the Institutional Review Board of Children's Hospital Los Angeles and the University of California, Los Angeles, and conformed to the requirements of the United States Health Insurance Portability and Privacy Act (HIPPA).
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest, as none has a financial relationship with the NINR, Alex's Lemonade Stand Foundation, or the CTSI.
References
- 1.CBTRUS . Central Brain Tumor Registry of the United States Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2004-2008. CBTRUS; 2012. www.cbtrus.org. Accessed June 23 2012. [Google Scholar]
- 2.Anderson NE. Late complications in childhood central nervous system tumour survivors. Curr Opin Neurol. 2003;16:677–683. doi: 10.1097/01.wco.0000102623.38669.e5. doi:10.1097/01.wco.0000102623.38669.e5 00019052-200312000-00006 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 4.Moore BD, 3rd, Copeland DR, Ried H, Levy B. Neurophysiological basis of cognitive deficits in long-term survivors of childhood cancer. Arch Neurol. 1992;49:809–817. doi: 10.1001/archneur.1992.00530320033009. [DOI] [PubMed] [Google Scholar]
- 5.Waber DP, Tarbell NJ, Kahn CM, Gelber RD, Sallan SE. The relationship of sex and treatment modality to neuropsychological outcome in childhood acute lymphoblastic leukemia. Journal of Clinical Oncology. 1992;10:810–817. doi: 10.1200/JCO.1992.10.5.810. [DOI] [PubMed] [Google Scholar]
- 6.Marachelian A, Butturini A, Finlay J. Myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for childhood central nervous system tumors. Bone Marrow Transplantation. 2008;41:167–172. doi: 10.1038/sj.bmt.1705953. [DOI] [PubMed] [Google Scholar]
- 7.Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS, Termuhlen A, Abromowitch M, Sposto R, Finlay JL. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–1175. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 8.Sands SA, Oberg JA, Gardner SL, Whiteley JA, et al. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54:429–436. doi: 10.1002/pbc.22318. [DOI] [PubMed] [Google Scholar]
- 9.Carey ME, Haut MW, Reminger SL, Hutter JJ, Theilmann R, Kaemingk KL. Reduced frontal white matter volume in long-term childhood leukemia survivors: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2008;29:792–797. doi: 10.3174/ajnr.A0904. doi:ajnr.A0904 [pii] 10.3174/ajnr.A0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porto L, Preibisch C, Hattingen E, Bartels M, Lehrnbecher T, Dewitz R, Zanella F, Good C, Lanfermann H, DuMesnil R, Kieslich M. Voxel-based morphometry and diffusion-tensor MR imaging of the brain in long-term survivors of childhood leukemia. Eur Radiol. 2008;18:2691–2700. doi: 10.1007/s00330-008-1038-2. [DOI] [PubMed] [Google Scholar]
- 11.Moore IM, Miketova P, Hockenberry M, Pasvogel A, Carey ME, Kaemingk K. Methotrexate-induced alterations in beta-oxidation correlate with cognitive abilities in children with acute lymphoblastic leukemia. Biol Res Nurs. 2008;9:311–319. doi: 10.1177/1099800407313268. [DOI] [PubMed] [Google Scholar]
- 12.Stenzel SL, Krull K, Hockenberry M, Jain N, Kaemingk K. Oxidative stress and neurobehavioral problems in pediatric acute lymphoblastic leukemia patients undergoing chemotherapy. J Pediatr Hematol Oncol. 2010;32 doi: 10.1097/MPH.0b013e3181c9af84. al. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. The Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 14.James SE, Burden H, Burgess R, Xie Y, Yang T. Anti-cancer drug induced neurotoxicity and identification of Rho pathway signaling modulators as potential neuroprotectants. NeuroToxicology. 2008;29:605–612. doi: 10.1016/j.neuro.2008.04.008. al. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich J, Han R, Yang Y, Mayer-Proeschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Journal of Biology. 2006;5:22.21–22.23. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mignone RG, Weber ET. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thioTEPA. Brain Research. 2006;1111:26–29. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 17.Rueckriegel SM, Driever PH, Blankenburg F, Ludemann L, Henze G, Bruhn H. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2010;76:859–866. doi: 10.1016/j.ijrobp.2009.02.054. doi:10.1016/j.ijrobp.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston K. Multimodal image coregistration and partitioning - a unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner J, Friston KJ. Voxel-based morphometry - the methods. Neuroimage. 1999;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 20.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Science. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble KG, Korgaonkar MS, Grieve SM, Brickman A. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev Science. 2013;16:653–664. doi: 10.1111/desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. Am J Neuroradiol. 2001;22:952–958. [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging. 2006;24:1252–1258. doi: 10.1002/jmri.20759. doi:10.1002/jmri.20759. [DOI] [PubMed] [Google Scholar]
- 24.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 26.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. doi:10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan W, Mangano FT, Air EL, Holland SK, Jones BV, Altaye M. Anisotropic diffusion properties in infants with hydrocephalus: a diffusion-tensor imaging study. Am J Neuroradiol. 2009;30:1792–1798. doi: 10.3174/ajnr.A1663. al. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L. Diffusion tensor imaging in hydrocephalus: initial experience. Am J Neuroradiol. 2006;28:1717–1724. [PMC free article] [PubMed] [Google Scholar]
- 29.Osuka S, Matsushita A, Ishikawa E, Saotome K, Yamamoto T. Elevated diffusion anisotropy in gray matter and the degree of brain compression. J Neurosurg. 2012;117:363–371. doi: 10.3171/2012.4.JNS112305. al. e. [DOI] [PubMed] [Google Scholar]
- 30.Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K. Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. J Neurosurg. 2010;5:385–391. doi: 10.3171/2009.11.PEDS09343. al. e. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system. Springer; New Yourk: 2008. [Google Scholar]
- 32.Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- 33.Humphries MD, Stewart RD, Gurney KN. A physiologically plausible model of action selection and oscillatory activiy in the basal ganglia. J Neurosci. 2006;26:12921–12942. doi: 10.1523/JNEUROSCI.3486-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- 35.Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- 36.Mabbott DJ, Monsalves E, Spiegler BJ, Bartels U, Janzen L. Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 2011 doi: 10.1002/cncr.26127. al. e. doi:10.1002/cncr.26127. [DOI] [PubMed] [Google Scholar]
- 37.Gottardo NG, Gajjar A. Chemotherapy for malignant brain tumors of childhood. J Child Neurol. 2008;23:1149–1159. doi: 10.1177/0883073808321765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward C, Phipps K, de Sousa C, Butler S, Gumley D. Treatment factors associated with outcomes in children less than 3 years of age with CNS tumours. Childs Nerv Syst. 2009 doi: 10.1007/s00381-009-0832-8. [DOI] [PubMed] [Google Scholar]
- 39.Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54:429–436. doi: 10.1002/pbc.22318. [DOI] [PubMed] [Google Scholar]
- 40.Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol Aging. 2010;31:482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. doi:10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khong P, Leung LHT, Fung ASM, Fong DYT, Qiu D, Kwong DLW. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. Journal of Clinical Oncology. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 42.Leung LHT, Ooi GC, Kwong DLW, Chan GCF, Cao G, Khong PL. White-matter diffusion anisotropy after chemo-irradiation: a statistical parametric mapping study and histogram analysis. Neuroimage. 2004;21:261–268. doi: 10.1016/j.neuroimage.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Qiu D, Kwong DLW, Chan GCF, Leung LHT, Khong P. Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter sensitivity? 2007. 69 3. [DOI] [PubMed]
- 44.Haris M, Kumar S, Raj MK, Das KJ, Sapru S, Behari S, Rathore RK, Narayana PA, Gupta RK. Serial diffusion tensor imaging to characterize radiation-induced changes in normal-appearing white matter following radiotherapy in patients with adult low-grade gliomas. Radiat Med. 2008;26:140–150. doi: 10.1007/s11604-007-0209-4. [DOI] [PubMed] [Google Scholar]
- 45.Houston SM, Herting MM, Sowell ER. The neurobiology of childhood structural brain development: conception through adulthood. In: Geyer M, Ellenbrock B, Marsden C, editors. Current Topics in Behavioral Neuroscience. Springer-Verlag; Berlin: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. Am J Neuroradiol. 2008;29:843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aukema EJ, Caan MWA, Oudhuis N, Majoie C, Vos FM. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. al. e. [DOI] [PubMed] [Google Scholar]
- 48.Prabhu SP, Ng S, Vajapeyam S, Kieran MW, Pollack IF, Geyer R, Haas-Kogan D, Boyett JM, Kun LE, Poussaint TY. DTI assessment of the brainstem white matter tracts in pediatric BSG before and after therapy: a report from the Pediatric Brain Tumor Consortium. Childs Nerv Syst. 2011;27:11–18. doi: 10.1007/s00381-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uh J, Merchant TE, Li Y, Feng T, Gajjar A, Ogg RJ, Hua C. Differences in brainstem fiber tract response to radiation: a longitudinal diffusion tensor imaging study. Int J Radiat Oncol Biol Phys. 2013;86:292–297. doi: 10.1016/j.ijrobp.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage. 2007;36:1123–1138. doi: 10.1016/j.neuroimage.2007.02.056. doi:10.1016/j.neuroimage.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]