Abstract
Strep throat causing Streptococcus pyogenes was detected in vitro and in simulated clinical samples by performing touch spray ionization - mass spectrometry. MS analysis took only seconds to reveal characteristic bacterial and human lipids. Medical swabs were used as the substrate for ambient ionization. This work constitutes the initial step in developing a noninvasive MS-based test for clinical diagnosis of strep throat. It is limited to the single species, S. pyogenes, which is responsible for the vast majority of cases. The method is complementary to and, with further testing, a potential alternative to current methods of point-of-care detection of S. pyogenes.
Introduction
Pharyngitis is diagnosed >10 million times annually in the United States,1 with pediatric cases having an estimated economic impact of 224 - 539 million dollars.2 Bacterial infection accounts for as much as 30%3 of all pharyngitis cases with nearly all caused by Streptococci (i.e. strep throat), other minor bacterial causes include Neisseria gonorrhoeae, Corynebacterium diphtheria, and Arcanobacterium haemolyticum.4 Further, group A streptococcal (GAS), Streptococcus pyogenes, infection is responsible for nearly all Streptococci caused bacterial pharyngitis, therefore the target pathogen of current strep throat screening methods.4 The remaining fraction of cases (~70%) are caused primarily by viruses, lacking major risk of disease progression and for which antibiotic therapy is ineffective. Whereas, diagnosis of strep throat is crucial in children, elderly patients, and in regions in which rheumatic and scarlet fever are prevalent as life-threatening complications are possible and patient discomfort can be significant. The clinical symptoms of pharyngitis (e.g. sore throat) often do not allow for ready differentiation between bacterial and viral infection,1 requiring rapid screening methods.
Patients are commonly tested for the presence of GAS at the point-of-care using a rapid antigen detection test (RADT) that provides results in 15-20 minutes.5 RADTs are commonly based on the detection of group A streptococcal carbohydrate, a bacterial membrane constituent, using lateral flow immunochromatography which provides visual indication of test results. The rate of true positives of RADTs is ~70-90%; however, the rate of false negatives can be substantial (~10- 20%).6-9 Studies have shown that personnel training and interpretation are critical for reliable test results.10 The performance of RADT tests are inverted from those typically desired in screening methods in which false negatives should be mitigated at the expense of false positives. Therefore in clinical practice, a positive RADT supports treatment while a negative result commonly leads to further testing using additional RADTs or culture.2, 5 Certainly, RADT false negatives contribute to physician over-prescription in response to the possibility of development of life-threating conditions (e.g. chronic rheumatic heart disease).3, 4 By comparison, throat culture, the gold standard for diagnosis of GAS infection, possess a rate of true positives >90% with negligible false negatives.4 However, throat culture is used primarily as a confirmatory test with definitive results requiring 24-48 hours for growth and interpretation, delaying antimicrobial treatment.
The lipids that provide cellular structure and contribute to their functioning are various and ubiquitous in tissue. Their distribution in type and amount is characteristic of cell type and metabolic and disease state.11, 12 Desorption electrospray ionization (DESI)13 mass spectrometry (MS) as well as other ambient ionization MS techniques14-16 enable ready detection of the membrane lipids of cells, including phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerols (PG) and sulfatide (ST) in the negative ionization mode. Detection of bacteria too can be performed using lipid analysis by MS methods including matrix assisted laser desorption ionization (MALDI)17 and electrospray ionization (ESI).18 Using ambient ionization direct and very rapid detection of intact bacteria is possible.19 The classification of bacteria by genus and species can be achieved by MS data using multivariate statistics.20
Touch spray (TS) is an ambient ionization method that emphasises rapid analysis, minute sample amounts, and the capability of in situ analysis. TS allows the sampling device as the substrate for ionization.21, 22 Sampling probes commonly used for touch spray ionization are metallic objects with roughened surfaces and sharp tip (e.g. teasing probes). The application of solvent and a high voltage generates a strong electric field and results in the field emission of analyte-containing charged droplets. The droplets undergo evaporation and coulombic fission by mechanisms similar to those of electrospray ionization. Touch spray is most readily applicable to screening for the presence or absence of particular compounds, such as in agrochemical detection or in the recognition of different disease states.23 Semi-quantitative performance is possible, depending on sampling methodology and user experience.23 Related ambient ionization methods including probe electrospray ionization (PESI),22 paper spray (PS),11 and “toothpick” spray24 provide similar MS analysis capabilities. PESI, commonly performed using acupuncture needles, transfers minute amounts of biofluid which is electrosprayed upon application of a high voltage. When applied to cancer diagnostics,25 PESI provides the ability to detect endogenous lipids characteristic of malignant or non-malignant disease states.
While the features possessed by PESI and current TS probes are advantageous for MS analysis in which minor damage to tissue is inconsequential (e.g. during surgical removal of tumors), they are not suitable for non-invasive procedures. In particular, they do not allow sampling of a patient's pharynx. Applications in which sampling is to be non-invasive, such as strep throat, require alternative probes. Medical swabs are a commonly used medical device for sampling orifices and body surfaces with their specific construction based on application. Most medical swabs are comprised of a polymeric handle (cellulose or plastic) and a rounded adsorbent tip (e.g. cotton). Paper spray and related MS experiments21, 24 have been used to generate ions from porous materials although commercially available medical products not been used in this manner. This paper details the underlying science and initial stages of development of a non-invasive diagnostic technique based on ambient ionization mass spectrometry. It is important to note that the method is narrowly applied in this initial study and that the study is the forerunner to a clinical study in which qualitative answers will be sought to the simple question, “Does this patient have strep throat?”
Methods
All experiments were performed using a linear ion trap mass spectrometer (LTQ, Thermo Scientific, San Jose, CA). Full scan mass spectra were recorded in the negative ionization mode with automatic gain control from m/z 100-2000. This polarity was chosen because the medical swabs used showed significant interferences from polymers and other chemicals in the swabs in the positive ion mode. The following instrument parameters were used: capillary temperature 275°C, capillary voltage -50V, tube lens voltage -100V, spray voltage -5.0 kV, maximum injection time 50 ms, and 2 microscans. Swabs were affixed to a ring stand via a three finger clamp in front of the MS inlet and oriented vertically, approximately 8-10 mm away from and 5-6 mm above the MS inlet. Pure methanol (HPLC-grade, Mallinckrodt Baker Inc., Phillipsburg, NJ) spray solvent (38 – 40 microliters) was applied manually via pipette to the swab. (Other solvents tried including methanol-water (1:1), dimethylformamide-acetonitrile (1:1), ethanol, ethanol-water (1:1), and ethanol-water (4:1), gave inferior results while acetonitrile gave comparable results to methanol.) High voltage (5 kV) was applied to the metallic handle via the instrument high voltage cable and a copper clip.
Touch spray-MS experiments were performed using sterile medical swabs possessing an aluminium handle and rayon swab (Copan Diagnostics, Murrieta, CA) unless otherwise noted. Additional swabs manufactured by Puritan Medical Products (Guilford, ME) were also tested (Supplemental Fig 1), including swabs constructed of various materials in various geometries (i.e. greater or lesser curvature at apex).
Streptococcus pyogenes and Streptococcus agalactiae samples were provided by bioMérieux, Inc. (Hazelwood, MO) as frozen samples stored in cryovials at -80 °C on TSA with glycerol. Bacteria were cultured on TSA with 5% sheep blood (Remel, Lenexa, KS) at 35±1°C for approximately 24 h and subcultured for an additional 48 h prior to MS analysis. A VWR forced air incubator (Chicago, IL) was used for culturing and all materials were autoclaved prior to disposal.
Results and Discussion
Medical swabs were used for touch spray ionization - mass spectrometry with the future intention of extracting chemical information relevant to patient care, namely the detection of S. pyogenes, in a non-invasive procedure; herein we describe the initial steps in developing such a method. Medical swabs allow direct sampling (in vitro and in vivo) of many potential sources of diagnostic information including bacterial cultures and patient throat swabs, Fig 1A. Sampling is performed remote from the mass spectrometer, no preparative steps are required prior to analysis, and ionization occurs directly from the sampling device. The medical swabs were used as received.
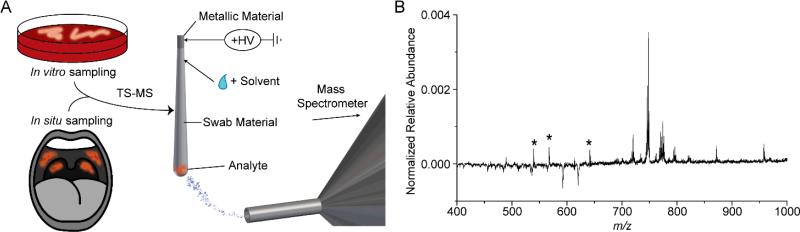
Fig 1.
(A) Schematic of sampling from culture or patient throat swab and subsequent analysis by TS-MS. (B) Negative ionization mode TS-MS spectra of a single colony of S. pyogenes sampled from culture. Peaks of negative relative abundance, after subtraction, and those annotated by asterisks are attributable to background. The other peaks with positive relative abundance are tentatively identified as bacterial phospholipids.
Initial evaluation of MS signal quality using swabs as substrates was tested on mouse brain tissue sections. Nasopharyngeal swabs with metallic handles were tested, all swab tip materials (cotton, rayon, and polyester) tested yielded mass spectra of high quality (Supplemental Fig 1). The distribution and relative abundances of lipids detected in the negative ionization mode are reminiscent of those seen in DESIMS spectra,26 particularly for the rayon and cotton tipped swabs. Glycerophospholipids (GPL) in the range from m/z 700-900, especially the ions m/z 788 (PS 36:1), 834 (PS 40:6), 885 (PI 38:4), and 888 (ST 20:4), correctly reflect the primarily grey matter composition of the samples. Polyester swabs provided the lowest signal in the GPL region of the spectra and contained substantial background ions, m/z 349.5 and 377.5. Cotton swabs provided more background in the m/z 500-600 region, as opposed to rayon which yielded the least spectral background from the swab itself. Swabs possessing metallic handles were observed to produce stable spray conditions, as opposed to paper or plastic handles (data not shown). While not commonly used in medical practice, metallic handle swabs are commercially available and their use would not represent a significant change in current throat swab protocol.
The hemispherical shape of the swabs necessitated use of higher voltages for droplet emission than required with previous touch spray probes, but the values were well within the instrumental range. The orientation of swabs relative to the mass spectrometer was found to effect signal quality and reproducibility. Swabs oriented vertically (Supplemental Fig 2) provided the best reproducibly, as the variability in emitted droplet direction was reduced. The results presented offer a proof-of-concept of the utility of swab based MS although no particular swab type has been optimized for the specific application of detecting strep throat.
In Vitro Detection of S. pyogenes
A single, isolated colony of S. pyogenes (estimated 106-8 bacteria) was sampled using a rayon tipped medical swab (Supplemental Fig 3). The colony was adsorbed to the swab tip by gently touching and subsequently transferred to the mass spectrometer for analysis. Solvent and high voltage were applied using a pipette and copper clip, respectively. Mass spectra could be recorded for ~5-10 seconds, the time varying slightly with the quantity of solvent applied. The average spectrum resulting from a single solvent addition was used to obtain the most reproducible data. It was possible to acquire data from more than one solvent addition with the lipid signal falling over the course of multiple additions, as one would expect in an extraction-like process. In the experiments covered in this manuscript, data were taken from the first solvent addition unless the first solvent additional failed to provide any MS signal (rare occurrence). Background MS signals arising from the swab material, the solvent, and agar in the case of microorganism analysis were noted, primarily in the range of m/z <600, Supplemental Fig 4. Background and S. pyogenes negative ion mode mass spectra were normalized by their respective total ion current (TIC) values and subtracted, as displayed in Fig 1B. Peaks associated with the background are reflected in negative normalized abundances and by those annotated with an asterisk. By contrast, the series of peaks with positive normalized relative abundances are attributed to S. pyogenes, tentatively identified as bacterial lipids: m/z 717.6 (PG 32:2), m/z 719.6 (PG 32:1), m/z 745.6 (PG 34:2), m/z 747.6 (PG 34:1), m/z 771.4 (PG 36:3) and m/z 773.6 (PG 36:2). The lipids detected and their relative ratios are nearly identical to those reported previously by electrospray ionization – mass spectrometry analysis of S. pyogenes.27 TS-MS analysis of S. pyogenes provided adequate reproducibility in bacterial lipid ratio as shown in Supplemental Fig 4, provided that approximately the same amount of material (estimated at 1 microgram) was sampled.
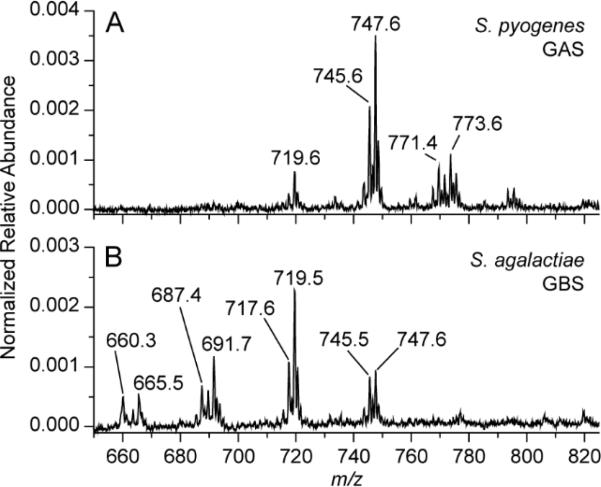
In addition to in vitro detection of S. pyogenes, the capability for TS-MS using medical swabs to provide differential diagnosis of Streptococcal infections was tested. TS-MS spectra resulting from S. pyogenes and Streptococcus agalactiae, a beta-hemolytic group B streptococcus (GBS), are displayed in Fig 2. The lipids profiles of the two Streptococcus spp. are visibly different in the m/z 600-800 region. S. agalactiae possesses a greater relative abundance of m/z 665.5, 687.4, 691.6, and 717.6, while ions at m/z 719.6 and 747.6 displayed an inverted relative abundance compared to S. pyogenes. The ability for TSMS to detect differences in the lipid profile of bacteria possessing similar culturing features (i.e. beta-hemolysis) is notable, while the ability to differentiate Streptococcus spp. is more clinically relevant. The visual differentiation of S. agalactiae serves to illustrate capability for differential diagnosis from a single testing methodology while simultaneously presenting another possible and independent application of this methodology, as rapid GBS detection is sought-after in neonatal care.28 TS-MS using medical swabs has unexplored potential in providing differential diagnosis, not readily possible with current RADT diagnostic tests, while requiring further development.
Fig 2.
Negative mode TS-MS spectra of S. pyogenes (A) and S. agalactiae (B) sampled obtained using a single colony from culture. Annotated peaks are tentatively identified as bacterial lipids.
Detection of S. pyogenes from Simulated Throat Swabs
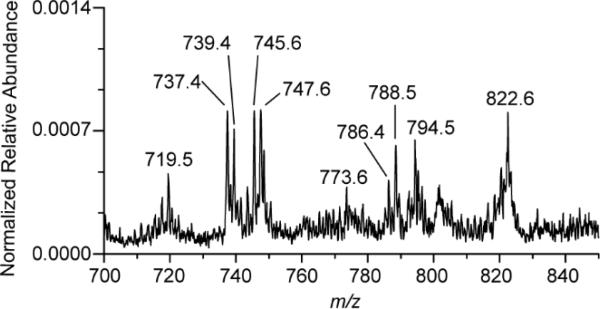
A clinical throat swab was simulated using human oral fluid, saliva containing cheek epithelial cells, and S. pyogenes. A rayon medical swab was dipped into ~1 mL of human oral fluid, absorbing an estimated 40 μL, and then subsequent used to sample a single colony of S. pyogenes from culture - simulating a clinical throat swab. The swab was then analyzed by TS-MS without pretreatment, yielding MS signal. A predominate ion at m/z 465.5 (tentatively identified as cholesterol sulfate) was detected in oral fluid (Supplemental Fig 5A) and in the simulated throat swabs (data not shown) and is presumably of human epithelial cell origin, consistent with literature reports of the occurrence of buccal cells at approximately 7.8%.29 The phospholipid region (m/z 700-900), Fig 3, contained ions corresponding to bacterial lipids while additional ions at m/z 737.4, 739.4, 786.4, 788.5, 794.5, 820.5, and 822.6 were also detected. Bacterial lipids, m/z 719.5 and 773.6 were similar in relative abundance to seen in in vitro experiments whereas m/z 745.5 and 747.6 differed. The observed differences were determined to be due to the contributions of ions of bacteria and human lipids at those m/z values. A TS-MS spectrum of human saliva containing cheek epithelial cells, Supplemental Fig 5B, included ions m/z 745.5 and 747.6. Nevertheless, the ions m/z 719.5 and 773.6 were only detected when S. pyogenes was present. Little lipid signal was observed from the oral fluid without check cells. Matrix effects are certainly present (e.g. ionization suppression) and are likely to vary between patients.
Fig 3.
Negative ionization mode TS-MS spectrum displaying phospholipid ions corresponding to bacterial and human components of a throat swab.
Future implementation of multivariate statistics upon a collection of clinical samples and formal mathematical rules will likely address issues of biological variance in patient oral fluids, bacterial load (related to infection type and progression), etc (not explored in detail here) representing a developed model for diagnosis. Further, the chemical specificity afforded by MS could allow for the deconvolution of bacterial and human lipids using post-acquisition processing, which could be further enhanced with additional stages of mass analysis (i.e. MS2).
Conclusions
This study details the initial development of a new non-invasive diagnostic technique, directed towards a specific clinical condition (strep throat) and aims to address a problem of diagnosis of bacterial infections at the point-of-care. Touch spray-MS performed using medical swabs allowed the rapid analysis of S. pyogenes requiring only seconds to obtain test results. Detection of bacterial lipids origination from S. pyogenes was performed in vitro and from simulated throat swabs using a single colony. Further, in vitro experimentation provided visual differentiation of S. pyogenes and S. agalactiae, the latter having significant neonatal care application in regards to rapid detection, a testament to the chemical specificity provided by MS-based methods and the possibility of differential diagnosis. While simulated throat swabs did not perfectly mirror clinical samples, e.g. oral fluid composition and contaminates, the ability to distinguish a limited number of human from bacteria lipids was demonstrated. It is clear that infections of the pharynx, mouth, and respiratory system can be caused by various bacteria, fungi, and viral agents amongst which this study targets only the most common bacterial pathogen causing strep throat. Future development of swab design and materials should facilitate MS analysis, improving performance including reproducibility, background reduction and improved signal levels. Additionally, extensions of the method to other microorganisms and the ability to detect bacterial and human lipids concurrently in clinical samples might provide additional data pertinent to disease. Applications in therapeutic drug monitoring, drug testing, and forensic applications are also envisioned as great benefit exists when switching from detection in blood to oral fluid.30, 31
Supplementary Material
Acknowledgements
Funding for this research was supported by grants from the National Institute of Health (1R21EB009459 01) and the Institut Mérieux (12097523). The authors thank Adam J. Pepper, MPH, PA-C for medical insights and bioMérieux collaborators, David H. Pincus, Bradford G. Clay, and Gaspard Gervasi. V.P. acknowledges the Italian L’Oreal / UNESCO Women in Science 2013 Award.
References
- 1.Gieseker KE, Roe MH, MacKenzie T, Todd JK. Pediatrics. 2003;111:e666–e670. doi: 10.1542/peds.111.6.e666. [DOI] [PubMed] [Google Scholar]
- 2.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, Martin JM, Van Beneden C. Clinical Infectious Diseases. 2012;55:e86–e102. doi: 10.1093/cid/cis629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerc O, Greub G. Clinical Microbiology and Infection. 2010;16:1054–1061. doi: 10.1111/j.1469-0691.2010.03281.x. [DOI] [PubMed] [Google Scholar]
- 4.Bisno AL. New England Journal of Medicine. 2001;344:205–211. doi: 10.1056/NEJM200101183440308. [DOI] [PubMed] [Google Scholar]
- 5.Campbell S, Landry ML. Advanced Techniques in Diagnostic Microbiology. Springer; 2013. pp. 31–51. [Google Scholar]
- 6.Gerber MA, Shulman ST. Clinical microbiology reviews. 2004;17:571–580. doi: 10.1128/CMR.17.3.571-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos O, Weckx LLM, Pignatari ACC, Pignatari SSN. Brazilian Journal of Infectious Diseases. 2003;7:297–300. doi: 10.1590/s1413-86702003000500003. [DOI] [PubMed] [Google Scholar]
- 8.Camurdan AD, Camurdan OM, Ok I, Sahin F, Ilhan MN, Beyazova U. International journal of pediatric otorhinolaryngology. 2008;72:1203–1206. doi: 10.1016/j.ijporl.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Küçük Ö, Biçer S, Giray T, Çöl D, Erdağ GÇ, Gürol Y, Kaspar ÇE, Vitrinel A. The Indian Journal of Pediatrics. 2013:1–5. doi: 10.1007/s12098-013-1067-y. [DOI] [PubMed] [Google Scholar]
- 10.Fox JW, Cohen DM, Marcon MJ, Cotton WH, Bonsu BK. Journal of clinical microbiology. 2006;44:3918–3922. doi: 10.1128/JCM.01399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooks RG, Manicke NE, Dill AL, Ifa DR, Eberlin LS, Costa AB, Wang H, Huang G, Ouyang Z. Faraday discussions. 2011;149:247–267. doi: 10.1039/c005327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberlin LS, Tibshirani RJ, Zhang J, Longacre TA, Berry GJ, Bingham DB, Norton JA, Zare RN, Poultsides GA. Proceedings of the National Academy of Sciences. 2014;111:2436–2441. doi: 10.1073/pnas.1400274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiseman JM, Ifa DR, Song Q, Cooks RG. Angewandte Chemie International Edition. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- 14.Laskin J, Heath BS, Roach PJ, Cazares L, Semmes OJ. Analytical chemistry. 2011;84:141–148. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce CY, Barr JR, Cody RB, Massung RF, Woolfitt AR, Moura H, Thompson HA, Fernandez FM. Chemical Communications. 2007:807–809. doi: 10.1039/b613200f. [DOI] [PubMed] [Google Scholar]
- 16.Nemes P, Woods AS, Vertes A. Analytical chemistry. 2010;82:982–988. doi: 10.1021/ac902245p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vékey K, Telekes A, Vertes A. Mass spectrometry of infectious pathogens. 2007 [Google Scholar]
- 18.Ho YP, Reddy PM. Mass spectrometry reviews. 2011;30:1203–1224. doi: 10.1002/mas.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meetani MA, Shin YS, Zhang S, Mayer R, Basile F. Journal of mass spectrometry. 2007;42:1186–1193. doi: 10.1002/jms.1250. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JI, Talaty N, Costa AB, Xia Y, Tao WA, Bell R, Callahan JH, Cooks RG. International Journal of Mass Spectrometry. 2011;301:37–44. [Google Scholar]
- 21.Liu J, Wang H, Manicke NE, Lin J-M, Cooks RG, Ouyang Z. Analytical chemistry. 2010;82:2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- 22.Hiraoka K, Nishidate K, Mori K, Asakawa D, Suzuki S. Rapid Communications in Mass Spectrometry. 2007;21:3139–3144. doi: 10.1002/rcm.3201. [DOI] [PubMed] [Google Scholar]
- 23.Kerian KS, Jarmusch AK, Cooks RG. Analyst. 2014 doi: 10.1039/c4an00548a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu B, So P-K, Chen H, Yao Z-P. Analytical chemistry. 2011;83:8201–8207. doi: 10.1021/ac2017713. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura K, Chen LC, Mandal MK, Nakazawa T, Yu Z, Uchiyama T, Hori H, Tanabe K, Kubota T, Fujii H, Katoh R, Hiraoka K, Takeda S. Journal of The American Society for Mass Spectrometry. 2012;23:1741–1749. doi: 10.1007/s13361-012-0447-2. [DOI] [PubMed] [Google Scholar]
- 26.Eberlin LS, Ferreira CR, Dill AL, Ifa DR, Cheng L, Cooks RG. ChemBioChem. 2011;12:2129–2132. doi: 10.1002/cbic.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosch JW, Hsu FF, Caparon MG. Journal of bacteriology. 2007;189:801–806. doi: 10.1128/JB.01549-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergeron MG, Ke D, Ménard C, François FJ, Gagnon M, Bernier M, Ouellette M, Roy PH, Marcoux S, Fraser WD. New England Journal of Medicine. 2000;343:175–179. doi: 10.1056/NEJM200007203430303. [DOI] [PubMed] [Google Scholar]
- 29.Wertz PW, Cox PS, Squier CA, Downing DT. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1986;83:529–531. doi: 10.1016/0305-0491(86)90291-9. [DOI] [PubMed] [Google Scholar]
- 30.Bosker WM, Huestis MA. Clinical chemistry. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Corcia D, Lisi S, Pirro V, Gerace E, Salomone A, Vincenti M. Journal of Chromatography B. 2013 doi: 10.1016/j.jchromb.2013.01.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.