Abstract
The beta-carboline, methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM), is a potent chemoconvulsant. While it has been utilized in adult rodents, it has not been previously examined for effects across postnatal development. DMCM is a negative allosteric modulator of benzodiazepine-sensitive GABAA receptors, receptor subtypes that are particularly enriched in limbic brain regions. This raises the possibility that DMCM may be particularly effective at evoking forebrain seizures, which is a challenge in neonatal animals due to the relative immaturity of the forebrain seizure network. The ability to selectively evoke forebrain seizures is desirable when screening for drugs to use in temporal lobe epilepsy, which is characterized by seizures within the forebrain (limbic) network. To determine the profile of DMCM action across development, we examined the dose-dependent ability of DMCM to induce seizures in rats at P7, P10, P13, P14, P21 and in adulthood. We found that the highest sensitivity to DMCM occurred in P10, P13, and P14 rats. The lowest sensitivity occurred in P21 rats. Neonatal (P7) and adult (P60+) rats displayed moderate sensitivity. With moderate (0.2–0.4mg/kg) doses of DMCM, we were able to reliably evoke limbic motor seizures without tonic-clonic components in animals as young as P7. These data support the utility of DMCM in assessing seizure threshold during development and raise the possibility for future exploration of DMCM as an agent to screen anticonvulsant drugs during the postnatal period.
Keywords: development, chemoconvulsant, epilepsy, rat, neonatal, GABA
1. Introduction
The beta-carboline, DMCM (methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate), is a potent and efficacious negative allosteric modulator of benzodiazepine-sensitive GABAA receptors (Braestrup et al., 1983, 1982; Honoré et al., 1983). DMCM reduces chloride flux through the GABAA receptor by decreasing the frequency of channel openings (Jensen and Lambert, 1983; Puia et al., 1991; Vicini et al., 1987, 1986); this decrease in inhibitory efficacy results in hyperexcitability. In adult rodents DMCM exerts chemoconvulsant action (e.g., Braestrup et al., 1982; Contó et al., 2005; Petersen, 1983), however, its efficacy in neonatal rodents is under-explored. Only one prior study has examined DMCM-evoked seizures in neonatal animals (postnatal day (P) 3 rats) (Nutt and Little, 1986), a time point more consistent with in utero than postnatal exposure in humans (Dobbing and Sands, 1979, 1973). These authors failed to find a dose-dependent effect of DMCM on locomotor activity, loss of righting reflex, twitches, or body shakes within a dose-range of 1 to 50mg/kg (Nutt and Little, 1986). Ages more relevant to term neonates (e.g., P7) remain unexplored.
DMCM and other beta-carbolines have high affinity binding to benzodiazepine-sensitive GABAA receptors (e.g., those containing α1,2,3, or 5 and γ2 and 3 subunits) (Derry et al., 2004). In the adult rat brain, as revealed by autoradiography, benzodiazepine sensitive GABA receptors show particularly high density in telencephalic and diencephalic structures including the cerebral cortex, hippocampus, and amygdala (Young and Kuhar, 1980). In contrast, relatively lower levels of binding are seen in hindbrain structures (Young and Kuhar, 1980); consistent with this, there is significant beta carboline binding in cerebral cortex, hippocampus, and substantia nigra (Miyoshi et al., 1985). Moreover, DMCM preferentially increases local cerebral glucose utilization in limbic and basal ganglia structures of the rat brain after a dose (1mg/kg) that evokes limbic motor seizures (Ableitner and Herz, 1987).
Forebrain (complex partial seizure) and hindbrain (generalized tonic-clonic and running/bouncing clonic) seizure networks are separable and can function independently (see: Browning et al., 1993; Browning and Nelson, 1986; Gale et al., 2007). However, a separation between forebrain and hindbrain seizure components is difficult to reliably achieve in neonatal rodents using systemically administered chemoconvulsants, such as pentylenetetrazole (PTZ) (de Casrilevitz et al., 1971; Mares and Schickerová, 1980; Velisek et al., 1992). PTZ is channel blocker that acts on the chloride pore of the GABAA receptor (Squires et al., 1984), and thus acts on both benzodiazepine-sensitive and insensitive GABAA receptors, a larger population than that affected by DMCM.
Identification of models that can selectively evoke forebrain seizures may be advantageous for screening drugs for use in temporal lobe epilepsy, which is characterized by seizures within the forebrain (limbic) network, and for evaluating the effects of forebrain seizures in neonatal animals. The high degree of forebrain localization of beta-carboline binding (Miyoshi et al., 1985) led us to hypothesize that DMCM may be particularly efficacious at triggering complex partial seizures with a wider separation between the doses needed to evoke complex partial and tonic seizures. In the present study we examined this possibility using rats (P7, P10, P13, P14, P21 and adults) as a model. The ability of DMCM to evoke myoclonic, complex partial, and tonic/tonic-clonic seizures was examined at doses ranging from 50 to 1200μg/kg.
2. Materials & Methods
2.1 Animals
Adult Sprague-Dawley rats and female Sprague-Dawley rats with pups (Harlan Laboratories, Frederick, Maryland) were housed in the Georgetown University Division of Comparative Medicine. Animals were maintained in a temperature-controlled (21°C) room with humidity between 30 and 70%; a 12-h light cycle (0600–1800 lights on) was used and all manipulations occurred during the light phase. Six age groups were used: P7 (n = 60), P10 (n = 32), P13 (n = 47), P14 (n = 47), P21 (n = 84), and adults (>P60; n = 50). Pups were weaned at P21. Each experimental group consisted of an approximately equal number of male and female animals. Ages were selected as follows: P7 models term infants, P13–14 is the average age of eye opening, P21 is the age of weaning, and >P60 is post-puberty. Moreover, P7, P14, and P21 are ages we and others have commonly examined using other chemoconvulsant models and serve as a point of reference. All experiments were performed in compliance with the American Association for Accreditation of Laboratory Animal Care standards and were approved by the Georgetown University Animal Care and Use Committee.
2.2 Drugs
DMCM (methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate) (Tocris) was dissolved in a small volume of 1N HCl and diluted with saline. Drug doses of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/kg were administered to all age groups. An additional dose of 0.05 mg/kg was administered to P10, 0.1 mg/kg was administered to the P7, P10, P13, and adult groups, 1.2 mg/kg was administered to P7, P21, and adults, and 1.4 mg/kg was administered to P21 based on preliminary data in order to acquire the entire dose-response curve for each age group. DMCM was injected at a volume of 10 ml/kg for pups and 1 ml/kg for adults. The doses of DMCM were selected on the basis of previous reports using this compound to evoked seizures in rats (Contó et al., 2005; Nutt and Little, 1986).
2.3 Seizure Testing
Seizure testing was performed in the afternoon. Rats were weighed, labeled, and treated with DMCM via intraperitoneal (i.p.) injection. Animals were placed in clear boxes and seizure activity was observed for 20 min. During this period, no passive heating was applied to the animals.
2.4 Seizure Scoring
Because behavioral seizure manifestations differ between neonatal and adult rats, we used different scoring systems for these age groups, as described below.
Pup seizures were rated using a 5-point scoring system modified from Kubová and Mares (Kubova and Mares, 1991) as previously described (Forcelli et al., 2013, 2012): 0 = no change in behavior; 0.5 = scratching, chewing, tremors, wet dog shakes; 1 = 0.5+myoclonic jerks; 2 = unilateral clonus (a score of 2 was considered to be the lower threshold for a limbic motor/complex partial seizure), chewing/shuffling, Straub tail; 3 = bilateral facial and forelimb clonus (FFC); 4 = clonic seizure with loss of righting; 4.5 = clonic seizure with running/bouncing (swimming-like behavior; a score of 4.5 is the upper threshold for a limbic motor/complex partial seizure); 5 = tonic-clonic and tonic extensor (forelimb or hindlimb) seizure.
Adult seizures were rated using a 6-point scoring system modified from Racine (Racine, 1972) as in Forcelli et al. (Forcelli et al., 2011): 0 = no change in behavior; 0.5 = chewing, wet dog shakes; 1 = single myoclonic jerk; 2 = multiple myoclonic jerks; 2.5 = unilateral clonus (a score of 2.5 was considered to be the lower threshold for a limbic motor limbic motor/complex partial seizure); 3 = bilateral facial and forelimb clonus; 3.5 = FFC with a body twist; 3.75 = FFC with a full body roll; 4 = FFC with rearing; 4.5 = FFC with rearing and a body twist; 5 = FFC with rearing and loss of balance (a score of 5 was considered to be the upper threshold for a limbic motor limbic motor/complex partial seizure); 6 = running/bouncing seizure with tonic forelimb extension (a score of 6 indicates a “tonic-clonic” seizure).
2.5 Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad Software, LaJolla, CA). Seizure score is a non-parametric measure, and was thus analyzed using Kruskal-Wallis test with Dunn’s post-test. Latency to seizure onset is a parametric variable and was analyzed by analysis of variance with Bonferroni-correct post-hoc tests. P-values less than 0.05 were considered to be statistically significant. Median convulsant dose (CD)50 values were calculated via a log-probit analysis using SPSS (IBM).
3. Results
Analysis of sex collapsed across age and dose revealed no difference in the median seizure response (Mann Whitney U = 5970, P=0.56). Likewise, two-way analyses of variance (dose by sex) within each age revealed no main effect of sex at any age (all Ps<0.20). For these reasons, all subsequent analyses (presented below) are collapsed across sex.
3.1 Effects of DMCM in P7 rats
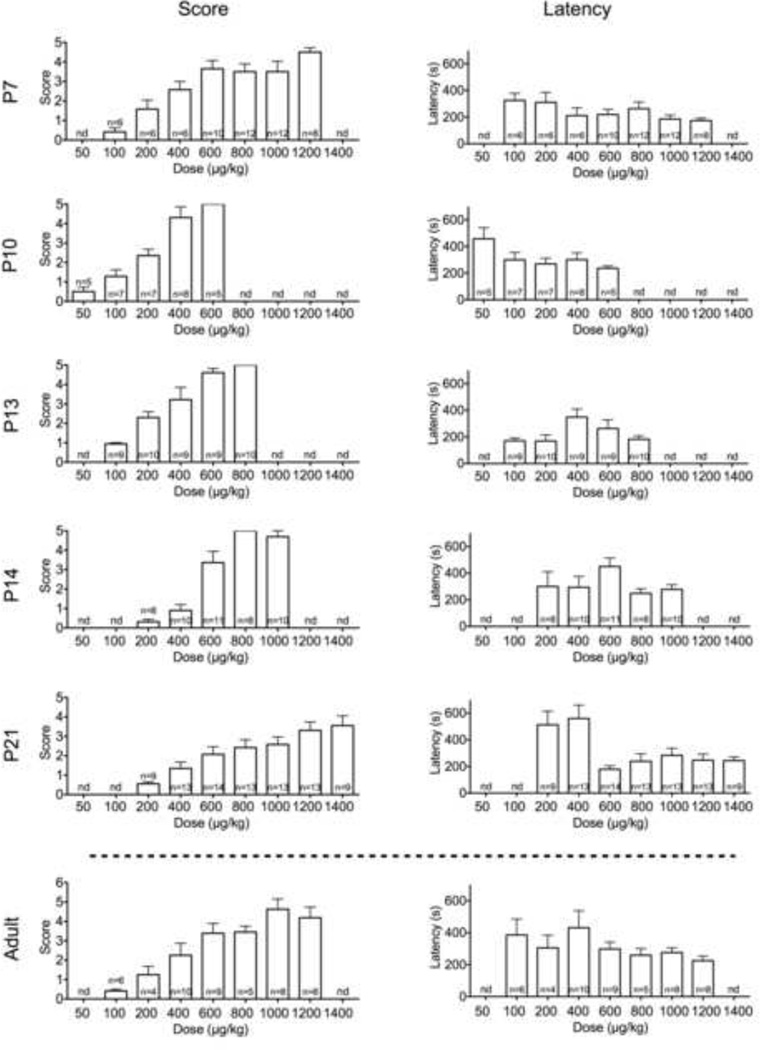
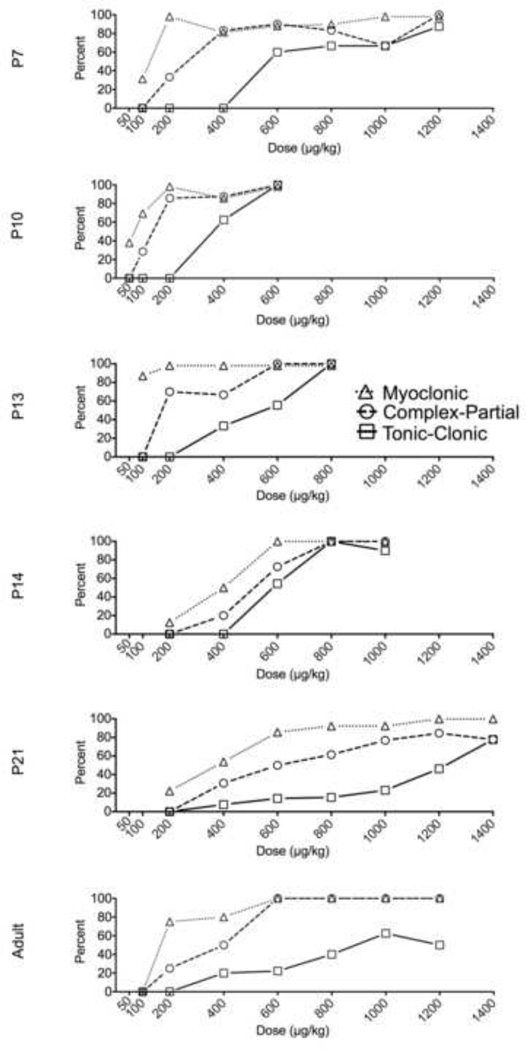
In a dose-dependent manner (H=27.5, P<0.001), DMCM induced myoclonic, complex partial (limbic-motor) and tonic-clonic seizures. As shown in Fig 1, seizure responses were detected with doses as low as 100μg/kg (myoclonic). Latency to seizure onset did not differ as a function of dose (F6,50=1.2, P=0.30). Fig 2 shows dose-response curves for each seizure of the three seizure types we analyzed (myoclonic, complex partial, and tonic-clonic). The CD50s for these seizure types are shown in Table 1. Limbic motor seizures were detected with doses as low as 200μg/kg, while tonic-clonic seizures were detected with doses as low as 600μg/kg. The relative median convulsant dose (ratio) for evoking limbic motor and tonic-clonic seizures at this age was 2.7, indicating a robust separation between the two seizure types at this age.
Fig. 1.
Severity (left) and latency (right) of DMCM-induced seizures in P7, P10, P13, P14, and P21 pups (from top). N = the number of pups tested. nd = the associated dose was not tested for the given age group.
Fig. 2.
| Limbic Motor CD50 (95% CI) |
Tonic-Clonic CD50 (95% CI) |
CD50 ratio (tonic-clonic/ limbic motor) |
|
|---|---|---|---|
| P7 | 320 (130–685) | 845 (431–1713) | 2.7 |
| P10 | 131 (44–371) | 305 (111–873) | 2.3 |
Table 1.
Median Convulsant Dose (CD50) values for each seizure endpoint, as a function of age. Values were determined by probit analysis. Values in parentheses indicate the 95% confidence intervals surrounding the CD50.
| P13 | 190 (75–443) | 357 (156–803) | 1.9 |
| P14 | 253 (105–576) | 356 (156–796) | 1.4 |
| P21 | 630 (347–1096) | 1488 (826–3128) | 2.4 |
| Adult | 265 (98–623) | 950(439–2147) | 3.6 |
3.2 Effects of DMCM in P10 rats
P10 rats showed a left-shifted dose-response to DMCM, as compared to P7 rats (Fig 1 & 2). We detected limbic motor seizure responses with doses as low as 100μg/kg. Tonic seizures were detected with doses of 400μg/kg and higher; 100% of animals displayed tonic seizures at a dose of 600μg/kg. Kruskal-Wallis test confirmed the dose-dependence of DMCM effects in P10 animals (H=22.4, P=0.002). Latency to seizure onset did not differ as a function of dose (F4,25=1.6, P=0.2). The CD50s for each seizure type is shown in Table 1. The ratio of CD50s for evoking limbic motor and tonic-clonic seizures at this age was 2.3.
3.3 Effects of DMCM in P13 rats
P13 rats showed a left-shifted dose response as compared to P7 rats, but a right-shifted curve as compared to P10 rats (Fig 1 & 2). Limbic motor seizure responses were detected with 200μg/kg doses. Tonic responses were seen with doses as low as 400μg/kg; 100% of animals displayed tonic responses when challenged with a dose of 800μg/kg. The dose-dependence was confirmed by Kruskal-Wallis test (H=32.3, P<0.001). While ANOVA revealed a marginally significant effect of dose on latency to seizure onset (F4,42=2.8, P=0.04), no post-hoc comparisons revealed significant group differences. The CD50s for each seizure type is shown in Table 1. The ratio of CD50s revealed a 1.9-fold separation between the CD50s for evoking limbic motor and tonic-clonic seizures at this age.
3.4 Effects of DMCM in P14 rats
Despite only a one day difference between P13 and P14 rats, the dose response for P14 rats was right-shifted as compared to P13 animals (Fig 1 & 2). Limbic seizure responses were seen with doses as low as 400μg/kg with tonic responses seen starting at 600μg/kg. Dose-dependence was confirmed by Kruskal-Wallis test (H=35.6, P<0.0001). Latency to seizure onset did not vary as a function of dose (F4,35=2.1, P=0.11). P14 animals displayed the smallest degree of separation (1.4-fold) between the CD50s for evoking limbic motor and tonic-clonic seizures (Table 1), a separation similar to that previously reported for PTZ (Velisek et al., 1992).
3.5 Effects of DMCM in P21 rats
P21 rats showed a dose-response function that was right shifted with respect to all other perinatal time points (Fig 1 & 2). The minimal dose needed to detect limbic motor seizures was 400μg/kg. Interestingly, even at doses of 1400μg/kg, only 78% of rats showed tonic-clonic seizures, suggesting a relative resistance to the tonic-clonic manifestations at this age. The dose-dependency of effects were confirmed by Kruskal-Wallis test (H=31.9, P<0.0001). Latency to seizure onset differed as a function of dose (F6,76=5.4, P=0.001); animals treated with a dose of 600μg/kg showed significantly longer latencies than all other groups (P<0.05). The ratio of CD50s for limbic motor and tonic-clonic seizures was 2.4 at this age (Table 1).
3.6 Effects of DMCM in adult rats
Adult rats showed myoclonic seizure manifestations in response to 100μg/kg DMCM (Fig 1). The dose-dependence of DMCM effects in adults was confirmed by Kruskal-Wallis test (H=27.9, P<0.0001). Latency to seizure onset did not differ as a function of dose (F6,41=1.2, P=0.31). There was a 3.6-fold separation between the CD50 for limbic motor seizures and the CD50 for tonic-clonic seizures at this age (Table 1).
4. Discussion
Here we have described the dose-response for DMCM-evoked seizures across postnatal development. Our findings demonstrate a mean 2.4-fold separation between the CD50 values for complex partial and tonic-clonic seizures. This separation was largest in adult animals. In immature animals, this separation was greatest at P7 and smallest at P14. The dose-response we obtained in adult animals is consistent with prior reports (Ableitner and Herz, 1987; Contó et al., 2005). Our present data support the utility of DMCM as a method of evoking complex partial seizures in postnatal animals. While the relatively small numbers of subjects of each sex preclude us from definitively ruling out a sex difference, it is worth noting that the analyses we performed suggest that the profile of DMCM seizures does not differ as a function of sex.
4.1 Potential factors contributing to developmental shifts in dose-response curves
Several possible factors may contribute to the shifts in dose-response seen across development. These factors include maturational state of limbic and hindbrain seizure networks, regional changes in GABAA receptor expression patterns, changes in chloride homeostasis, and changes in drug metabolism.
Seizure networks undergo substantial maturation during the first month of postnatal life in rodents (see discussions in (Velíšek, 2006; Velíšková, 2006)). The relative maturational states of the forebrain and hindbrain seizure networks [for discussion see (Forcelli and Gale, 2014)] are critical mediators of the behavioral seizure responses that can be detected at various postnatal ages. Early in postnatal life, tonic-clonic seizures (mediated by hindbrain/brainstem circuits) (Browning and Nelson, 1986) are more prevalent than forebrain seizure manifestations (i.e., clonic, limbic motor seizures). This is evident in responses to electrical (Kim et al., 2010) as well as chemoconvulsant (Velisek et al., 1992) evoked seizures. As the forebrain circuitry matures, complex partial manifestations are progressively easier to evoke. This is consistent with the pattern we saw with DMCM across postnatal development: the greatest separation between doses needed to evoke clonic as compared to tonic seizures was seen in adulthood. The maturation of these circuits is likely a function of synaptogenesis and pruning, and may also involve changes in neurotransmitter receptor expression.
The expression of GABAA receptor subunits changes dramatically over the course of postnatal development (Araki et al., 1992; Fritschy et al., 1994; Gambarana et al., 1991; Laurie et al., 1992). Of particular interest may be the benzodiazepine-sensitive GABAA receptors which are the site of DMCM action. With respect to the impact of the γ subunit, high-affinity binding of DMCM to recombinant α1β1γ2 receptors has been reported (Puia et al., 1991), while no significant binding is seen at recombinant α1β1γ1 receptors (Ymer et al., 1990). The α subunit is also a significant determinant of DMCM binding, with high affinity seen at α5β3γ2 GABAA receptors, and lower affinity at α4 or α6 containing receptors. Moreover, when benzodiazepine-sensitivity is removed by a point mutation in the α5 subunit (H105R), beta carboline binding and modulation of the GABA response is significantly attenuated (Kelly et al., 2002).
α5 subunit expression is high during postnatal development, and drops significantly by adulthood (Laurie et al., 1992). Likewise, γ2 subunit expression decreases over postnatal maturation (albeit to a much lower extent than α5) (Laurie et al., 1992). Thus, during early postnatal brain development, benzodiazepine-sensitive GABAA receptors predominate; perhaps this difference from adult receptor expression can explain the left-shifted dose responses during early development as compared adulthood. On the other hand, DMCM-insensitive α4-containing receptors peak around the period of greatest sensitivity to DMCM (P12), indicating that subunit expression is not sufficient to account for the developmental shift. Perhaps the precise balance between subunits within key seizure trigger zones may better explain the change over ontogeny. It is also worth noting that in situ data (while informative) do not necessarily reflect assembled and functional receptors on the cell membrane.
Neuronal chloride homeostasis, which regulates the reversal potential of GABAA currents, also has a distinct developmental profile. In adulthood, intracellular chloride concentrations are low, resulting in inhibitory effects of GABA signaling. In contrast, during early postnatal development, intracellular chloride concentrations are high; this leads to a reversal potential greater than the resting membrane potential, and thus excitatory effects of GABA signaling. For a review see: (Ben-Ari, 2002). A pro-convulsant effect of DMCM would only be expected under conditions of hyperpolarizing GABA, indeed DMCM might be expected to display anticonvulsant activity against a background of depolarizing GABA signaling.
The neuronal chloride gradient is determined by the relative expression of NKCC1, which actively transports chloride into the cell, and KCC2, which extrudes chloride. NKCC1 and KCC2 have opposite developmental expression patterns. KCC2 is expressed highly in the mature brain, and at low levels during development, whereas NKCC1 is expressed highly in the developing brain with expression decreasing across postnatal life. Levels of NKCC1 in cortex peak between P5 and 7; while levels decrease significantly between P7 and P21, they still exceed those in the adult. In contrast, there is very little expression of KCC2 until P11, at which time it is less than 20% of adult levels. By P21, this approaches 80% (Dzhala et al., 2005). The profile of maturation is not homogenous throughout the brain (Wang et al., 2002) – for example, KCC2 mRNA levels are greater in brainstem than in telencephalon during early postnatal development; similarly levels of NKCC1 in brainstem are substantially lower than in telencephalon (Balakrishnan et al., 2003). This is particularly interesting in light of the predominately brainstem seizure responses seen early in postnatal development.
Finally, while the precise metabolic fate of DMCM is unknown in rats or humans, bioassay data suggest that it may be a substrate for human CYP1A2, CYP2C19 and CYP3A4 (National Center for Biotechnology Information, n.d.); it has not been assessed as a direct substrate for conjugation. CYP1A levels are constant between P3 and P28 in the rat, but are significantly less than adult levels during that time period (Elbarbry et al., 2007; Rich and Boobis, 1997); in contrast, CYP3A4 levels in the perinatal period do not differ from the perinatal period to adulthood (Rich and Boobis, 1997). Phase II enzyme (e.g., UDP-glucuronyltransferase) activity changes considerably across postnatal development (Rachmel and Hazelton, 1986). The degree to which ontogenic changes in hepatic and renal drug clearance may contribute to differences in DMCM responses between early development and later life remains an open question.
4.2 Comparison to other seizure models
The most commonly utilized chemoconvulsant in neonatal rats is pentylenetetrazole (PTZ). We (Forcelli et al., 2013, 2012) and others (Kubová and Mares, 1993; Kubova and Mares, 1991; Mares et al., 1989) have evaluated the anticonvulsant efficacy of a wide range of compounds against PTZ seizures during ontogenesis. Mares and Schickerova reported the effects of PTZ in a variety of ages using a dose of 80mg/kg (Mares and Schickerová, 1980). This dose consistently produced tonic seizures across all ages. When a lower dose was tested in P9 rats (the youngest age tested with multiple doses) only myoclonic and not complex partial (referred to as minor seizures) were obtained. A similar pattern was seen in P12 rats. Importantly, decreasing the dose produced isolated myoclonic jerks, but not minimal seizures. A separation between clonic and tonic-clonic seizures was obtained in P18 rats in their study: with a dose of 60mg/kg, 55% of the rats displayed minimal seizures without tonic components. Velisek and colleagues found the CD50 for tonic seizures evoked by PTZ to be 70mg/kg in P7 and P12 animals; only beginning at P18 could they reliably determine a CD50 for limbic motor seizure responses (Velisek et al., 1992). A similar pattern was found by de Casrilevitz and colleagues in P18 rats (albeit with a different route of administration than that used by Mares and Schickerova and Velisek and colleagues); tonic seizures were evoked with a median effective dose of 103.4mg/kg (de Casrilevitz et al., 1971). In the earliest ages these authors reported no clonic seizures, only myoclonic and tonic-clonic responses. At 21 days, these authors reported the first reliable appearance of clonic seizures (with a ratio between the median doses for clonic and tonic-clonic of 1.05).
Other chemoconvulsants, such as kainate, have also been examined in neonatal rats. Limbic motor seizures have been reported by several groups in pups P12 and older (Albala et al., 1984; Tremblay et al., 1984; Velísková et al., 1988). Electrographically, limbic seizures were reported in this same age group, but not in younger animals (Cherubini et al., 1983). Pilocarpine has also been examined, with limbic motor seizures seen in a quarter of animals between P7 and P12, but not in younger animals (Cavalheiro et al., 1987). Interestingly, the young animals displayed a much lower rate of status epilepticus (14%) than adult (3mo old) animals (40%) (Cavalheiro et al., 1987). For detailed reviews of chemoconvulsant models in neonatal rats see: (Mareš, 2012; Velíšek, 2006).
Several non-chemical models have also been employed in immature rats, including amygdala kindling and electroconvulsive shock (ECS)-evoked seizures. Experiments with amygdala kindling in immature animals have focused on pups between 2 and 4 weeks of age. In P15 rats, afterdischarges were evoked in a majority (75%) of animals by Moshe et al, with a threshold significantly higher than all other time points (Moshé et al., 1981). Similarly, Mares et al found that while afterdischarges could be evoked reliably in rats as young as 15d, the electrographic pattern did not generalize to the same degree as in older animals (Mares et al., 1980). Interestingly, minimal ECS was capable of producing forelimb clonus and running-like movements as well as head bobbing in animals throughout the first two postnatal weeks (Kim et al., 2010).
These previous findings described above document the difficulty in reliably evoking limbic seizures in the absence of tonic seizures in developmental animals using the most common chemical and electrical methods. The separation we have found between the doses needed to generate limbic motor and tonic-clonic seizures in response to DMCM during early postnatal development puts this compound on par with (or potentially better suited than) minimal ECS and pilocarpine for cleanly evoking complex partial seizures in neonatal rats.
4.3 Potential Utility of DMCM as a screening tool during ontogenesis
DMCM seizures are attenuated by a variety of classical anticonvulsant compounds, including benzodiazepines, barbiturates, voltage gated sodium channel blockers, valproate and ethosuximide (Petersen, 1983). Importantly, DMCM has also served as a useful model for detecting anticonvulsant effects of newer AEDs, including tiagabine and levetiracetam. The efficacy of levetiracetam against DMCM seizures is particularly intriguing, because levetiracetam fails to attenuate seizures in the most common screening models for AEDs (i.e., s.c. pentylenetetrazol and the maximal electroshock test), as well as many other chemoconvulsant models (including bicuculline and picrotoxin) (Klitgaard et al., 1998). In association with our current data, this suggests that the DMCM model may be particularly well-suited for evaluating nontraditional anticonvulsants during postnatal development.
5. Conclusions
DMCM is a potent chemoconvulsant in neonatal rats. It offers a 1.4 to 3.1-fold separation between the median convulsant doses for clonic and tonic-clonic seizures. This may offer an advantage when screening antiepileptic drugs for use against complex partial seizures in early development.
Acknowledgement
PAF received support from HD046388.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds used in this article: methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM, PubChem CID: 45261899)
References
- Ableitner A, Herz A. Changes in local cerebral glucose utilization induced by the beta-carbolines FG 7142 and DMCM reveal brain structures involved in the control of anxiety and seizure activity. J. Neurosci. Off. J. Soc. Neurosci. 1987;7:1047–1055. doi: 10.1523/JNEUROSCI.07-04-01047.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albala BJ, Moshé SL, Okada R. Kainic-acid-induced seizures: a developmental study. Brain Res. 1984;315:139–148. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- Araki T, Kiyama H, Tohyama M. GABAA receptor subunit messenger RNAs show differential expression during cortical development in the rat brain. Neuroscience. 1992;51:583–591. doi: 10.1016/0306-4522(92)90298-g. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Becker M, Löhrke S, Nothwang HG, Güresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Nielsen M, Honoré T. Binding of [3H]DMCM, a convulsive benzodiazepine ligand, to rat brain membranes: preliminary studies. J. Neurochem. 1983;41:454–465. doi: 10.1111/j.1471-4159.1983.tb04763.x. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Schmiechen R, Neef G, Nielsen M, Petersen EN. Interaction of convulsive ligands with benzodiazepine receptors. Science. 1982;216:1241–1243. doi: 10.1126/science.6281892. [DOI] [PubMed] [Google Scholar]
- Browning R, Maggio R, Sahibzada N, Gale K. Role of brainstem structures in seizures initiated from the deep prepiriform cortex of rats. Epilepsia. 1993;34:393–407. doi: 10.1111/j.1528-1157.1993.tb02579.x. [DOI] [PubMed] [Google Scholar]
- Browning RA, Nelson DK. Modification of electroshock and pentylenetetrazol seizure patterns in rats after precollicular transections. Exp. Neurol. 1986;93:546–556. doi: 10.1016/0014-4886(86)90174-3. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987;465:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- Cherubini E, De Feo MR, Mecarelli O, Ricci GF. Behavioral and electrographic patterns induced by systemic administration of kainic acid in developing rats. Brain Res. 1983;285:69–77. doi: 10.1016/0165-3806(83)90110-4. [DOI] [PubMed] [Google Scholar]
- Contó MB, de Carvalho JGB, Benedito MAC. Behavioral differences between subgroups of rats with high and low threshold to clonic convulsions induced by DMCM, a benzodiazepine inverse agonist. Pharmacol. Biochem. Behav. 2005;82:417–426. doi: 10.1016/j.pbb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- De Casrilevitz M, Engelhardt E, Esberard CA. Maturation of convulsogenic activity induced by leptazol in the albino rat. Br. J. Pharmacol. 1971;42:31–42. doi: 10.1111/j.1476-5381.1971.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry JMC, Dunn SMJ, Davies M. Identification of a residue in the gamma-aminobutyric acid type A receptor alpha subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding. J. Neurochem. 2004;88:1431–1438. doi: 10.1046/j.1471-4159.2003.02264.x. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Child. 1973;48:757–67. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Elbarbry FA, McNamara PJ, Alcorn J. Ontogeny of hepatic CYP1A2 and CYP2E1 expression in rat. J. Biochem. Mol. Toxicol. 2007;21:41–50. doi: 10.1002/jbt.20156. [DOI] [PubMed] [Google Scholar]
- Forcelli P, Gale K. In: Brain circuits responsible for seizure generation, propagation, and control: Insights from preclinical research. Holmes M, editor. Epilepsy: Intech.; 2014. [Google Scholar]
- Forcelli PA, Gale K, Kondratyev A. Early postnatal exposure of rats to lamotrigine, but not phenytoin, reduces seizure threshold in adulthood. Epilepsia. 2011;52:e20–e22. doi: 10.1111/j.1528-1167.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Soper C, Duckles A, Gale K, Kondratyev A. Melatonin potentiates the anticonvulsant action of phenobarbital in neonatal rats. Epilepsy Res. 2013;107:217–223. doi: 10.1016/j.eplepsyres.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Soper C, Lakhkar A, Gale K, Kondratyev A. Anticonvulsant effect of retigabine during postnatal development in rats. Epilepsy Res. 2012 doi: 10.1016/j.eplepsyres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J. Neurosci. Off. J. Soc. Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K, Proctor M, Veliskova J, Nehlig A. Ganglia and Brainstem Anatomy and Physiology. In: Engel JJ, Pedley T, editors. Epilepsy: A Comprehensive Textbook. New York, NY: Lippincott Williams & Wilkins; 2007. pp. 367–384. [Google Scholar]
- Gambarana C, Beattie CE, Rodríguez ZR, Siegel RE. Region-specific expression of messenger RNAs encoding GABAA receptor subunits in the developing rat brain. Neuroscience. 1991;45:423–432. doi: 10.1016/0306-4522(91)90238-j. [DOI] [PubMed] [Google Scholar]
- Honoré T, Nielsen M, Braestrup C. Binding of 3H-DMCM to benzodiazepine receptors; chloride dependent allosteric regulation mechanisms. J. Neural Transm. 1983;58:83–98. doi: 10.1007/BF01249127. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Lambert JD. The interaction of the beta-carboline derivative DMCM with inhibitory amino acid responses on cultured mouse neurones. Neurosci. Lett. 1983;40:175–179. doi: 10.1016/0304-3940(83)90298-7. [DOI] [PubMed] [Google Scholar]
- Kelly MD, Smith A, Banks G, Wingrove P, Whiting PW, Atack J, Seabrook GR, Maubach KA. Role of the histidine residue at position 105 in the human alpha 5 containing GABA(A) receptor on the affinity and efficacy of benzodiazepine site ligands. Br. J. Pharmacol. 2002;135:248–256. doi: 10.1038/sj.bjp.0704459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gale K, Kondratyev A. Effects of repeated minimal electroshock seizures on NGF, BDNF and FGF-2 protein in the rat brain during postnatal development. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2010;28:227–232. doi: 10.1016/j.ijdevneu.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Kubova H, Mares P. Anticonvulsant effects of phenobarbital and primidone during ontogenesis in rats. Epilepsy Res. 1991;10:148–155. doi: 10.1016/0920-1211(91)90007-3. [DOI] [PubMed] [Google Scholar]
- Kubová H, Mares P. Anticonvulsant action of oxcarbazepine, hydroxycarbamazepine, and carbamazepine against metrazol-induced motor seizures in developing rats. Epilepsia. 1993;34:188–192. doi: 10.1111/j.1528-1157.1993.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. Off. J. Soc. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares J, Mares P, Trojan S. The ontogenesis of cortical self-sustained after-discharges in rats. Epilepsia. 1980;21:111–121. doi: 10.1111/j.1528-1157.1980.tb04051.x. [DOI] [PubMed] [Google Scholar]
- Mareš P. Models of epileptic seizures in immature rats. Physiol. Res. Acad. Sci. Bohemoslov. 2012;61(Suppl 1):S103–S108. doi: 10.33549/physiolres.932370. [DOI] [PubMed] [Google Scholar]
- Mares P, Maresová S, Maresová D. Antimetrazol action and plasma levels of valproate in developing rats. Physiol. Bohemoslov. 1989;38:97–107. [PubMed] [Google Scholar]
- Mares P, Schickerová R. Seizures elicited by subcutaneous injection of metrazol during ontogenesis in rats. Act. Nerv. Super. (Praha) 1980;22:264–268. [PubMed] [Google Scholar]
- Miyoshi R, Kito S, Mizuno K. Autoradiographic studies on benzodiazepine receptor subtypes in the rat brain. Jpn. J. Pharmacol. 1985;38:281–285. doi: 10.1254/jjp.38.281. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Sharpless NS, Kaplan J. Kindling in developing rats: variability of afterdischarge thresholds with age. Brain Res. 1981;211:190–195. doi: 10.1016/0006-8993(81)90082-2. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information, P.B.D. [accessed 5.12.14];methyl 6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate - PubChem [WWW Document] n.d. URL http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=104999&loc=ec_rcs.
- Nutt DJ, Little H. Benzodiazepine-receptor mediated convulsions in infant rats: effects of beta-carbolines. Pharmacol. Biochem. Behav. 1986;24:841–844. doi: 10.1016/0091-3057(86)90422-3. [DOI] [PubMed] [Google Scholar]
- Petersen EN. DMCM: A potent convulsive benzodiazepine receptor ligand. Eur. J. Pharmacol. 1983;94:117–124. doi: 10.1016/0014-2999(83)90448-x. [DOI] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol. Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Rachmel A, Hazelton GA. The inducibility and ontogeny of rat liver UDP-glucuronyltransferase toward furosemide. Biochem. Pharmacol. 1986;35:3777–3782. doi: 10.1016/0006-2952(86)90664-7. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroen Clin Neuro. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rich KJ, Boobis AR. Expression and inducibility of P450 enzymes during liver ontogeny. Microsc. Res. Tech. 1997;39:424–435. doi: 10.1002/(SICI)1097-0029(19971201)39:5<424::AID-JEMT5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Squires RF, Saederup E, Crawley JN, Skolnick P, Paul SM. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984;35:1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the ratIClinical, electrographic and metabolic observations. Neuroscience. 1984;13:1051–1072. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- Velišek L. Models of Seizures and Epilepsy. Elsevier; 2006. Models of Chemically-Induced Acute Seizures; pp. 127–152. [Google Scholar]
- Velisek L, Kubova H, Pohl M, Stankova L, Mares P, Schickerova R. Pentylenetetrazol-induced seizures in rats: an ontogenetic study. Naunyn. Schmiedebergs Arch. Pharmacol. 1992;346:588–591. doi: 10.1007/BF00169017. [DOI] [PubMed] [Google Scholar]
- Veliškov00E1; J. Models of Seizures and Epilepsy. Elsevier; 2006. Behavioral Characterization of Seizures in Rats; pp. 601–611. [Google Scholar]
- Velísková J, Velísek L, Mares P. Epileptic phenomena produced by kainic acid in laboratory rats during ontogenesis. Physiol. Bohemoslov. 1988;37:395–405. [PubMed] [Google Scholar]
- Vicini S, Mienville JM, Costa E. Actions of benzodiazepine and beta-carboline derivatives on gamma-aminobutyric acid-activated Cl- channels recorded from membrane patches of neonatal rat cortical neurons in culture. J. Pharmacol. Exp. Ther. 1987;243:1195–1201. [PubMed] [Google Scholar]
- Vicini S, Wroblewski J, Costa E. Pharmacological modulation of gabaergic transmission in cultured cerebellar neurons. Neuropharmacology. 1986;25:207–211. doi: 10.1016/0028-3908(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Dev. Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Ymer S, Draguhn A, Wisden W, Werner P, Keinänen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO. J. 1990;9:3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Kuhar MJ. Radiohistochemical localization of benzodiazepine receptors in rat brain. J. Pharmacol. Exp. Ther. 1980;212:337–346. [PubMed] [Google Scholar]