Abstract
Objective
Fucosylation catalyzed by fucosyltransferases (FUTs) is an important post-translational modification involved in a variety of biological processes. The purpose of the current study is to determine the roles of fucosylation in rheumatoid arthritis (RA) and the efficacy of reestablishing the immune homeostasis by using 2-Deoxy-D-galactose (2-D-gal), a fucosylation inhibitor.
Methods
Q-PCR was performed to determine the expression of fucosyltransferases (FUTs) in RA and osteoarthritis (OA) synovial tissues and FACS sorted cells from RA synovial fluids. The in vivo inhibitory effect of 2-D-gal was evaluated in a collagen-induced arthritis (CIA) model. The in vitro effects of 2-D-gal on inflammatory macrophage differentiation, cytokine production, antigen uptake, processing, and presenting functions were analyzed.
Results
FUTs that are involved in terminal or sub-terminal, but not core or O-fucosylation, were upregulated in RA, compared to OA synovial tissues. The expression of terminal FUTs was highly positively correlated with that of TNF encoding for tumor necrosis factor α. Terminal FUTs were predominately expressed in M1 macrophages. In vivo, 2-D-gal treatment precluded CIA development with reduced inflammatory macrophages and Th17 in draining lymph nodes, decreased TNF-α, IL-6 and antibodies to type II collagen in the serum. In vitro, 2-D-gal skewed the differentiation of M1 macrophages to IL-10 producing M2 macrophages. Furthermore, 2-D-gal significantly inhibited antigen presenting function of M1 macrophages.
Conclusion
Terminal fucosylation is a novel hallmark of inflammatory macrophages. Inhibition of terminal FUTs reshapes the differentiation and functions M1 macrophages, leading to resolution of inflammation in arthritis.
Modificaiton by L-fucose (6-deoxy-L-galactose) can be found typically in N-and O-linked glycans and glycolipids. Fucosylation is the final step in the biosynthesis of oligosaccharides and considered as a terminal glycan modification. Fucose-containing glycans play an important regulatory role in a wide range of physiological and pathologic events, including cell and tissue development, angiogenesis, fertilization, cell adhesion, inflammation, tumor apoptosis and metastasis (1). It has been shown that fucosylation of IgG heavy chain and alpha 1-acid glycoprotein (AGP) is increased in RA patients compared to healthy individuals by lectin binding assay (2, 3). However, the roles of fucosylation in the pathogenesis of RA had not been investigated. Fucosyltransferases (FUTs) are enzymes that catalyze the transfer of a fucose residue from the guanosine diphosphate-fucose (GDP-fucose) donor to the acceptor molecules. Thirteen FUTs have been identified in human which catalyze α(1,2)-, α(1,3/4)-, α(1,6)-, and O-fucosylation (Figure 1A). α(1,2)- and α(1,3/4)-FUTs catalyze the fucosylation that localized at the terminal or sub-terminal of the acceptor molecules, whereas α(1,6)-FUTs catalyze the core fucosylation that occurs at the innermost moiety of N-glycan. O-fucosylation, regulated by POFUT1/2, is the direct O-linkage of fucose to serine and threonine residue in epidermal growth factor-like (EGF) repeat and thrombospondin type 1 repeat (TSR) (1, 4).
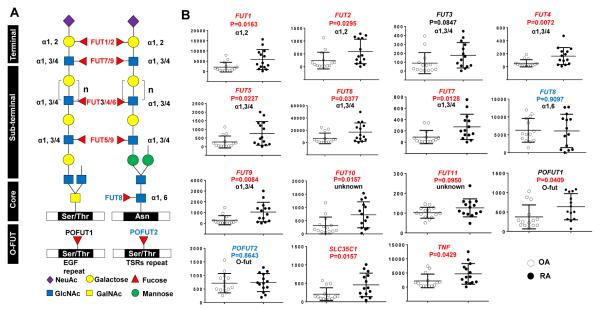
Figure 1. Expression profile of fucosyltransferase (FUT) genes in human rheumatoid arthritis (RA) and osteoarthritis (OA) synovial tissue.
A, Fucosylated sites of human FUTs including α(1,2)-, α(1,3/4)-, α(1,6)-, and OFUTs. Fucosylated sites are shown on O- and N-glycans. Ser/Thr, serine/threonine; Asn, asparagine; Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Man, mannose; NeuAc, sialic acid. B, Expression of FUTs, SLC35C1 (encodes for GDP-fucose transporter), and TNF in RA and OA synovial tissues. Copy numbers of genes of interest (GOI) were presented as (GOI/Gapdh) ×105. Values are the mean (± SEM) (n = 14). P values are indicated for each gene. Terminal and sub-terminal FUTs that exhibit a significant difference between RA and OA are indicated by red color; whereas O-FUT and core-FUT that do not exhibit a significant difference are indicated by blue color.
Macrophages are the central players in the pathogenesis of RA and are considered as important therapeutic targets in RA (5-7). They produce a number of inflammatory mediators and their professional antigen-presenting role has also been implicated in the pathogenesis of RA (5, 8). Plasticity and flexibility are key characteristics of macrophages, which have been shown to be regulated at the transcription, posttranscriptional, as well as the protein levels (9). In the present study, we have discovered a novel post-translational fucosylation mechanism that orchestrates the developmental and functional plasticity of macrophages. We identified that α(1,2)-and α(1,3/4) FUTs were predominantly expressed in M1 inflammatory macrophages and highly correlated with TNF in synovial tissues in arthritis. Our current studies are the first to describe that the posttranslational terminal fucosylation is a hallmark of inflammatory macrophages and demonstrate that fucosylation inhibitor (2-D-gal) can regulate the plasticity of inflammatory M1 macrophage differentiation and functions, leading to a M2 anti-inflammatory phenotype and the resolution of inflammation in arthritis.
MATERIALS AND METHODS
Mice
DBA/1J, C57BL6, and the MOG35-55 specific 2D2 TCR transgenic mice were obtained from the Jackson Laboratory. All animal procedures were approved by The University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee.
Induction of CIA and treatments
CIA was induced on DBA/1J mice that were 8- to 12-weeks old. Mice were immunized by intradermal administration of bovine Type II collagen (Chondrex, Inc.) emulsified in complete Freund’s adjuvant (CFA), followed by injection of bovine CII in incomplete Freund’s adjuvant (IFA) on day 21, as described previously (10). The severity of arthritis was assessed daily (10). 2-D-gal (250mg/kg BW, Sigma), fucose (250mg/kg BW, Sigma) or normal saline was administrated IP every 2 days initiated on day 0 until about day 60 when mice were sacrificed.
Subjects
Synovial tissue samples from 14 RA and 14 osteoarthritis (OA) patients were obtained from the UAB Tissue Procurement Center as described previously (11, 12). For analysis of synovial fluids, 5 RA patients were recruited from the UAB Rheumatology Clinic (mean age = 53 years, ranging from 38 to 79 years old; mean duration of disease of 14 years, ranging from 6 to 22 years). All RA patients met the American College of Rheumatology 1987 revised criteria for RA (13). All tissue and fluid was obtained for clinically indicated purposes. These studies were conducted in compliance with the Helsinki Declaration and approved by the institutional review board at UAB. All participants provided informed consent.
Isolation of human RA synovial fibroblasts and synovial fluid mononuclear cells
Synovial fibroblasts and synovial fluid mononuclear cells were isolated from RA synovial tissues or synovial fluid as described previously (12, 14).
Quantitative reverse transcription PCR (qRT-PCR)
RNA isolation, first-strand cDNA synthesis and qRT-PCR were carried out as described previously (15). All primers used in the present study are described in Supplementary Table 1.
Flow cytometric and phospho-flow analysis
Single-cell suspensions were stained using mouse-specific Abs, including FITC–anti-CD11b (BD Biosciences), Alexa 647–anti-IL-23p19 (eBioscience), PE–anti-TNF-α, PE/Cy7–anti-Ly6C for macrophages and subsets. For CD4 T cell and subset staining, cells were stained with FITC–anti-CD4, PE/Cy7–anti-Thy1.2, APC–anti-IFN-γ, and PE–anti-IL-17. Intracellular and intranuclear staining was performed as described previously (15). For macrophages treated with 2-D-gal in vitro, cells were stained with Bio-ICAM followed by streptavidin eFluor 450 (eBioscience) and APC–anti-CD11b, FITC–anti-CD80, and PE–anti-CD86. 2D2 CD4 T cells were identified by staining with APC–anti-CD4, PE/Cy7–Vβ11. DQ-Ovalbumin (DQ-OVA, Invitrogen) was detected in FITC channel. For macrophages loaded with Eα-GFP peptides, cells were stained with bio-YAe (eBioscience), followed by streptavidin eFluor 450 (eBioscience) and APC–anti-CD11b (Biolegend).
To determine the degree of ERK1/2 intracellular signaling, we performed phospho-flow studies according to the protocol from BD Biosciences. Intracellular pERK1/2 was stained with Rabbit anti-ERK1 (T202/Y204)/ERK2 (T185/Y187) (R&D systems), followed by Alexa488–goat anti-rabbit IgG (Invitrogen).
Data were acquired on a BD LSRII flow cytometer and analyzed using FlowJo software (Tree Star, Inc.).
Cell sorting
Unless specified, all reagents used for FACS analysis were purchased from Biolegend (San Diego, CA). Human synovial fluid mononuclear cells were stained with PE–anti-CD16, PE/Cy7–anti-CD14; FITC–anti-CD68, PE–anti-CD80; PE/Cy7–anti-CD4, PE–anti-CD45RA, PerCP/Cy5.5–anti-CCR2, PE/Cy7–anti-CCR4, Alexa 700–anti-CCR5, FITC–anti-CCR6, Pacific Blue–anti-CXCR3, and PE–anti-CD161 Abs and sorted into CD68+CD80+ (M1 macrophages), CD68+CD80− (M2 macrophages), CD14+,CD16− (classical monocytes), total CD4+ T cells, CD4+CD45RA+ (naïve CD4), CD4+CD45RO+ (memory CD4), CD4+CXCR3+CCR6− (Th1) (16), CD4+CXCR3−CCR4+CCR6+CD161+ (Th17) (16) with purities of > 96%. FACS sorting was performed on a FacsAria II cell sorter (BD Biosciences).
Mouse joint histology
All mouse joint tissues were processed and stained as described previously (12, 15, 17).
Generation of bone marrow-derived macrophages, 2-D-gal treatment, peptide loading and CD4 T cell proliferation assay
Mouse bone marrow-derived M1 and M2 macrophages were differentiated by using GM-CSF and M-CSF as described (18). For Eα-GFP peptide study, cells from C57BL6 mice were used; for all other in vitro experiments, cells from C57BL6 mice or DBA/1J were used. Cell counts were measured by Cell Counting Kit-8 (Dojindo Molecular Technologies, INC.) and cell viability was estimated based on the cell count ratio of experimental to control condition. For antigen presenting function studies, macrophages were loaded with 200μg/ml DQ-OVA (Invitrogen) for 30 min or 100 μg/ml Eα-GFP (19, 20) for 16 hr prior to analysis. For CD4 T cell proliferation assay, M1 macrophages were pretreated with and without 2-D-gal for 2 days, followed by loading of MOG35-55 and cultured with CFSE (Invitrogen)-labeled CD4 T cells isolated from 2D2 TCR transgenic mice for an additional 3 days.
ELISA
Cytokine levels were measured by ELISA according to the manufacturer’s manual (Biolegend). Bone marrow-derived macrophages were stimulated with 200 ng/ml LPS for 16 hr before cytokine analysis.
Statistics
Statistical analyses were performed using the two-tailed Student’s t test, one-way ANOVA, and bivariate correlation analysis. P values <0.05 were considered statistically significant.
RESULTS
Terminal α(1,2)- and sub-terminal α(1,3/4)- FUTs are upregulated in RA compared to OA synovial tissues
Thirteen FUTs have been identified in human which catalyze α(1,2)-, α(1,3/4)-, α(1,6)-, and O-fucosylation (Figure 1A). Synovial tissues were obtained as previously described (12). There was a statistically significant increase in most of the FUTs that catalyzed terminal and sub-terminal fucosylation in RA compared to OA synovial tissue, including FUT1, FUT2, FUT4, FUT5, FUT6, FUT7, and FUT9, as well as FUT10 and FUT11, whose function is less well defined (Figure 1B). Consistent with this, SLC35C1, which encodes the GDP-fucose transporter, was also upregulated in RA synovial tissues. In contrast, the expression of FUTs that catalyzed core- and O-fucosylation was not increased in RA compared to that of OA synovial tissues (Figure 1B, FUT8 and POFUT2), except that of POFUT1 (Figure 1B) which exhibited a marginally significant difference between RA and OA synovial tissues. These results suggested that terminal and sub-terminal fucosylation, but not core-and O-fucosylation might be associated with the pathogenesis of RA.
Terminal α(1,2)- and sub-terminal α(1,3/4)- FUTs are the hallmarks of M1 inflammatory MΦs which exhibit a strong positive correlation with expression of TNF
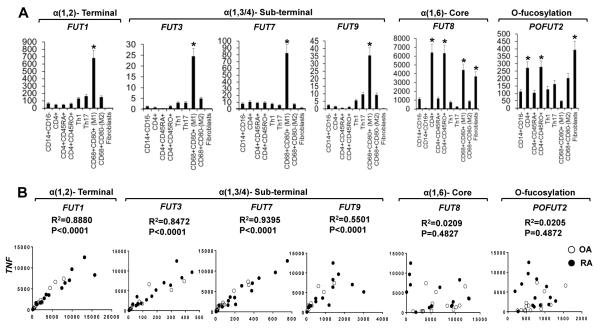
The above findings suggested that α(1,2)- and α(1,3/4)- FUTs may share some common roles as opposed to α(1,6)- Fut and O-fucosylation related POFUTs in the pathogenesis of RA. We therefore determined if this is cell-type specific. RA synovial fluid cells (N = 5) were sorted into classical monocytes, M1, and M2 macrophages, total CD4 T cells, naïve CD4 T cells, memory CD4 T cells, Th1 and Th17. Synovial fibroblasts were isolated from synovial fragments or synovial tissues by culture (<3 passages). Real-time PCR revealed that terminal α(1,2)- and α(1,3/4)- FUTs including FUT1, FUT3, FUT7, and FUT9 are mainly expressed by M1 inflammatory macrophages, whereas α(1,6)- FUT8 and O-fucosylation related POFUT2 was mainly expressed by CD4 T cells and fibroblasts (Figure 2A). Among the CD4 T cell subsets, CD45RO+ memory CD4 T cells expressed significantly higher levels of POFUT2 and α(1,6)- FUT8, compared to CD45RA+ naïve CD4 T cells (Figure 2A). We consistently observed a 5 to 10 fold upregulation of the expression of terminal and sub-terminal FUTs between M1 differentiated inflammatory macrophages and their precursors, classical monocytes (Figure 2A). This strongly indicated that the terminal α(1,2)- and sub-terminal α(1,3/4)- FUTs might play an indispensable role in the commitment, programming, and functional regulation of the M1 inflammatory macrophages and that this may contribute to the pathogenesis of RA. We therefore examined whether the expression of α(1,2)- and α(1,3/4)- FUTs can be correlated with that of TNF in RA and OA synovial tissues. We identified a strong positive correlation of the expression levels of TNF with that of FUT1/3/7/9 (Figure 2B), as well as FUT2/4/5/6/9/10/11 and POFUT1 (data not shown). In contrast, the expression of O-fucosylation related POFUT2 and α(1,6)- FUT8 did not correlate with the levels of TNF (Figure 2B).
Figure 2. Cellular expression pattern of FUTs in human RA synovial fluid cells and the correlation between FUTs and TNF in human synovial tissues.
A, Different sub-populations of RA synovial fluid cells were sorted as described in “MATERIALS AND METHODS” (n = 5). * = P < 0.05 (one-way ANOVA). B, Correlation between the expression of indicated FUTs and that of TNF in synovial tissues. (n = 14). P value for each correlation is indicated. Copy numbers of genes of interest (GOI) in each cell subset or synovial tissue were presented as (GOI/Gapdh) ×105. Values are the mean (± SEM).
Inhibition of fucosylation precludes the development of CIA
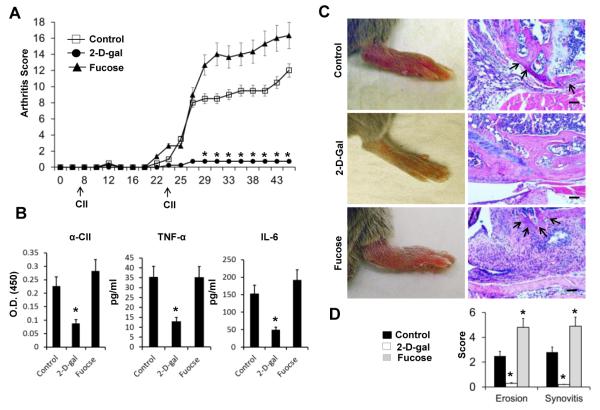
Our results indicated that expression of terminal FUTs is upregulated in M1 inflammatory macrophages in RA. Therefore, it is conceivable that inhibition of these FUTs might have an impact on the development of arthritis. To investigate this, a fucose/galactose analog, 2-Deoxy-D-galactose (2-D-gal), was used to inhibit fucosylation in the CIA disease model. It has been shown that upon cellular uptake, 2-D-gal can be converted via the Leloir pathway to the activated uridine diphosphate (UDP)-2-deoxy-galactose. This compound can then complete with UDP-galactose for incorporation into glycan chains and prevent the formation of the Fucα(1,2)-Gal linkage, making it an α(1,2)- fucosylation inhibitor (21-23). It has been demonstrated that 2-D-gal can inhibit the fucosylation of synapsin Ia/Ib (23). CIA was induced in DBA/1J mice by immunization with bovine collagen II. Normal saline (NS), 2-D-gal (250 mg/kg BW), and fucose (250 mg/kg BW) were administrated I.P. starting from day 0, every 2 days, until the mice were sacrificed. There was a highly significant inhibition of the development of CIA in mice receiving 2-D-gal compared to NS-treated control mice (Figure 3A). Interestingly, administration of equivalent amounts of fucose facilitated the development and increased severity of arthritis compared to control group (Figure 3A), which suggested that in mice under the inflammatory conditions with upregulated FUTs, relative fucose deficiency might occur. Serum levels of anti-CII antibody, TNF-α, and IL-6 were determined 2 weeks after priming with CII; all of these exhibited a significant decrease in the 2-D-gal treated group compared to the control and fucose treated groups (Figure 3B). Histologic evaluation revealed a significant decrease in synovial hyperplasia, infiltration, and bone erosion indicated by H&E staining, whereas there was an increase in fucose-treated mice compared to the control mice (Figure 3C and 3D).
Figure 3. Effects of 2-D-gal and fucose on Collagen II-induced Arthritis (CIA).
A, CIA was induced on DBA/1J mice and the administration of 2-D-gal and fucose were performed as described in “MATERIALS AND METHODS”. Clinical scores (range 0-4 for each limb) were assessed daily as indicated. B, Serum levels of anti-CII antibody, TNF-α, and IL-6 or were determined by an ELISA assay (day14). C, Gross images of hind limbs and images of hematoxylin and eosin (H & E) for representative knee joints in each group. Bone erosion is indicated by arrows. D, Quantification of bone erosion and synovitis in knee joints of the indicated groups of mice. Values are the mean (± SEM), (n = 8 each group). * = P < 0.01, compared to the control group. Bars = 100μm.
Inhibition of fucosylation decreases M1 inflammatory macrophages and Th17 T cells in CIA mice
Selectin-dependent leukocyte adhesion regulated by Fut4/7 is one important function of fucosylation. Gene knockout studies have shown that α(1,3)-Futs 4 and 7 are essential for recruitment of neutrophils and T cells to inflammatory sites and lymphocyte trafficking to secondary lymphoid organs; and leukocytosis is the major phenotype of the Fut4/7 knockout mice (24-27). In mice treated with 2-D-gal, which inhibits the formation of Fucα(1,2)-gal, no significant leukocytosis was observed (data not shown). This suggested that selectin fucosylation might not be the mechanism contributing to the effects of 2-D-gal in CIA mice.
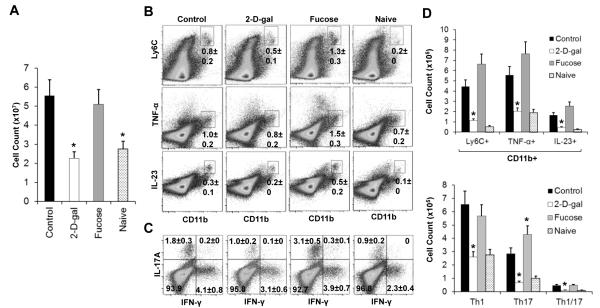
Whereas there was no difference of total cell counts in the bone marrow and spleen of 2-D-gal treated mice, there was a significant reduction of cellularity in the lymph nodes (LNs) in 2-D-gal treated CIA mice compared to the control mice (Figure 4A). Because the expression of α(1,2)- FUTs is specific to M1 macrophages and exhibits much lower expression in monocytes and other cells including different CD4 T cell subsets, which suggests that terminal fucosylation might play a critical role in M1 inflammatory macrophage development. Consistent with this, we found that there was a decrease in the percentages of CD11b+ Ly6C+, CD11b+ IL-23+, CD11b+ TNFα+ M1 inflammatory macrophages. Furthermore, the absolute cell counts of the inflammatory macrophages were significantly reduced (Figure 4B and 4D, upper panels). In contrast, administration of fucose resulted in significant increase in the M1 inflammatory macrophages (Figure 4B and 4D, upper panels). Consistent with the inhibitory effects on M1 macrophages, 2-D-gal treatment also resulted in a significant decrease in the percent and total number of CD4+IL-17A+Th17, as well as CD4+IFNγ+ Th1 cells (Figure 4C and 4D, lower panels). In contrast to 2-D-gal, fucose treatment resulted in a significant increase in Th17 cells (Figure 4C and 4D, lower panels).
Figure 4. Effects of 2-D-gal and fucose on the cellularity, inflammatory macrophages, and pathogenic CD4 T cells in the draining lymph nodes (LNs).
A, Total cell count of the draining LNs from the representative mouse of the indicated groups. B, FACS analysis of CD11b+ and Ly6C+, TNF-α+, or IL-23+ inflammatory macrophages in the draining LNs of the indicated group. C, FACS analysis of CD4 T cell subsets including Th1, Th17, and Th1/17 from the draining LNs of the indicated group. D, Total number of each inflammatory macrophage and CD4 T cell subsets for each group determined by multiplying the total cell count by the percentage of each cell subpopulation. Values are the mean (± SEM), (n = 8 each group). * = P < 0.05, compared to the control group.
Impacts of fucosylation inhibition on macrophage differentiation and plasticity
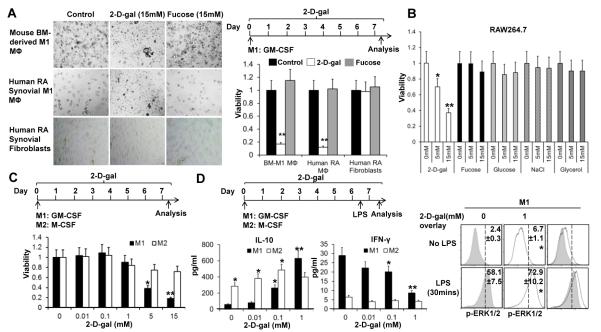
To explore whether fucosylation regulates inflammatory M1 macrophage differentiation, mouse bone-marrow derived M1 macrophages were polarized by incubation with GMCSF with and without the presence of 15mM 2-D-gal or fucose from day 0. On day 6, 2-D-gal treated bone marrow cells failed to survive and differentiate as indicated by the light microscopy images and cell count assay (Figure 5A, upper panel). Similar results of 2-D-gal were observed in RA synovial fluid macrophages (Figure 5A, middle panels). 2-D-gal did not have an impact on the survival of synovial fibroblasts (Figure 5A, lower panels). Interestingly, additional fucose did not exhibit a significant effect (Figure 5A) possibly due to the redundancy of endogenous fucose generated by the cells. 2-D-gal at the concentration of 15 mM can potentially alter the extracellular osmolarity significantly and thus the phenotypes of macrophages. This possibility was excluded by the fact that equal molar concentrations of fucose, Glucose, NaCl, and glycerol did not have the capability of reducing viability of RAW264.7 macrophages compared to 2-D-gal (Figure 5B). Our data suggested that the observed effect was 2-D-gal and M1 inflammatory macrophage specific.
Figure 5. 2-D-Gal regulates the plasticity of inflammatory macrophages.
A, A high dose of 2-D-gal selectively reduces the viability of inflammatory macrophages. Bone-marrow derived M1 macrophages and human RA synovial M1 macrophages were cultured with GM-CSF with and without 15mM of 2-D-gal or fucose for 7 days. Human RA synovial fibroblasts were used as controls. Representative images (left panels) and viability of cells (right panels) under the indicated culture conditions is shown. Viability was determined as described in the “MATERIALS AND METHODS”. B, Bar graph showing the viability of RAW264.7 cells under 2-D-gal or osmolarity control treatment. C, M1 and M2 macrophages were prepared by using GM-CSF and M-CSF with and without various concentration of 2-D-gal for 7 day (upper). Bar graphs show the viability of cells on day 7 (lower). D, Effects of 2-D-gal on cytokine production of M1 and M2 macrophages. Cells were stimulated with 200 ng/ml of LPS 16 hr before analysis (upper left). Bar graphs show the levels of IL-10 and IFN-γ in culture supernatant (lower left). Flow cytometry histograms show the effects of 2-D-gal on pERK1/2 of M1 macrophages (right panels). Values are the mean (± SEM), * = P < 0.05, ** = P < 0.01 compared to control group.
Further analysis of the impact of various concentrations of 2-D-gal on M1 inflammatory macrophage and M2 anti-inflammatory macrophage differentiation showed that lower concentrations of 2-D-gal (0.01, 0.1, and 1mM) did not exhibit an effect on the viability of both M1 and M2 macrophages (Figure 5C). However, at higher concentrations (5 and 15mM), 2-D-gal selectively reduced the cell number of M1 macrophages without inhibiting the growth of M2 macrophages (Figure 5C). This data suggested that higher concentration of 2-D-gal can specifically preclude the differentiation of macrophages under the inflammatory M1 polarization condition.
To determine whether 2-D-gal is capable of regulating cytokine expression in macrophages without altering the cell viability, we focused on the lower concentrations of 2-D-gal (0, 0.01, 0.1 and 1mM) that did not reduce cell density. Striking, 2-D-gal induced an augmentation of anti-inflammatory IL-10 production in macrophages under both M1 and M2 polarization conditions in a dose-dependent manner (Figure 5D). IL-10 has been shown to be a critical cytokine that suppresses arthritis development (28, 29). There was a gradual decline in the generation of pro-inflammatory IFN-γ in 2-D-gal treated cells within the same dosage range (Figure 5D). Due to the polarizing effects of these two cytokines on macrophages (30), reduction of IFN-γ and augmentation of IL-10 production from macrophages by 2-D-gal can further drive them towards the M2 phenotypes by an autocrine fashion. Our results strongly indicated that fucosylation inhibition can regulate macrophage plasticity and skews the differentiation of macrophages towards an anti-inflammatory phenotype. It has been shown that activation of the extracellular signal-regulated kinases (ERKs) is one critical factor modulating IL-10 expression in macrophages (31). Consistent with this, phospho-flow cytometry analysis indicated that 2-D-gal treatment upregulated the p-ERK1/2 in M1 inflammatory macrophages (Figure 5D).
Fucosylation inhibition suppresses antigen processing and presentation of inflammatory macrophages
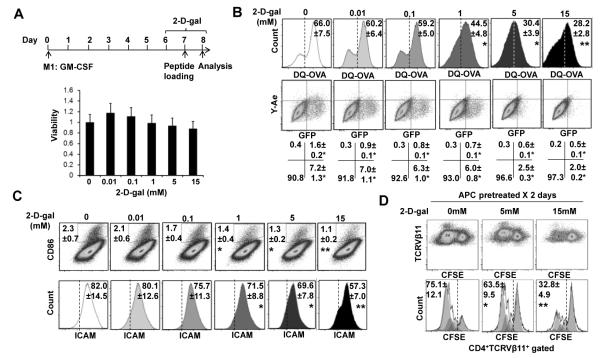
Antigen presentation function is one hallmark of M1 macrophages and there are dynamic interactions of macrophages with T cells during antigen presentation (32). Glycosylation has previously been implicated in MHC processing and antigen presentation. Specifically, the loss of N-glycan on class II MHC significantly impairs the ability of antigen-presenting cells (APCs) to present bacterial polysaccharides to T cells (33). To determine whether and how fucosylation inhibition affects the antigen-presenting functions of macrophages, antigen uptake, processing and presentation in the context of MHCII was investigated. Bone marrow derived M1 macrophages were generated in vitro by GM-CSF. Fully differentiated macrophages were then treated with varied concentrations of 2-D-gal on Day 6, and lasted for 2 days, which did not alter the viability of cells (Figure 6A). For analysis of antigen uptake and processing, M1 macrophages were pulsed with DQ-OVA which exhibit fluorescence upon proteolytic degradation. Uptake and/or proteolysis of DQ-OVA by M1 MΦ were diminished at 0.01and 0.1mM, and reached significant levels at 1, 5, and 15 mM of 2-D-Gal (Figure 6B, upper panels). To further investigate the effect of 2-D-Gal on antigen uptake, processing, MHCII loading, and surface presentation, a recombinant protein (Eα-GFP) consisting of amino acids, 46-74 of the I-Edα MHC II subunit and a GFP tag was utilized (19, 34). The inherent GFP was used to detect its presence after being uptaken. The processed I-Eα peptide can be detected using the Y-Ae monoclonal antibody, which is specific for the complex composed of the I-Eα peptide 52-68 (pEα) bound to the I-Ab MHCII. There was a significant decrease in processing and presentation of the I-Eα peptide indicated by decreased percentage of the surface Y-Ae+ population which occurred at low doses (0.01, 0.1, and 1 mM) of 2-D-gal (Figure 6B, lower panels). Higher doses of 2-D-gal (5 and 15 mM) resulted in decreased Ag uptake indicated by GFP+ cells (Figure 6B, lower panels).
Figure 6. 2-D-gal effects on antigen uptake processing, and presenting function of inflammatory macrophages.
A, Bone marrow-derived M1 macrophages using GMCSF for 6 days followed by various doses of 2-D-gal treatment for 2 days (upper). Bar graphs show that at this condition, 2-D-gal (up to 15 mM) does not reduce macrophage viability (lower). B, FACS analysis of the DQ-OVA antigen uptake (upper) and the Eα-GFP antigen uptake and/or processing (lower) in 2-D-gal treated M1 macrophages. C, FACS analysis of the expression of CD86 and ICAM in GM-CSF polarized BM macrophages 2 days post 2-D-gal treatment. D, FACS analysis of the 2D2 TCR transgenic CD4 T cell proliferative response, indicated by the attenuation of the CFSE intensity, to MOG35-55 peptide presented by various doses of 2-D-gal treated M1 macrophages stimulation. The coculture was carried out in vitro for 3 days. Values are the mean (± SEM), * = P < 0.05, ** = P < 0.01 compared to control group.
Initial contact between APC and T cells is established between adhesion molecules including lymphocyte function-associated antigen 1(LFA-1) on T cells and intercellular adhesion molecule 1 (ICAM-1) on the APCs (35). Interaction between B7 costimulatory molecules, CD80 and CD86, on APC and CD28 on T cells has been shown to provide a critical signal for T cell activation, while the lacking of this signal results in T cell anergy (36). Interestingly, the expression of CD86 and ICAM was reduced by 2-D-gal in a dose dependent manner and was significantly reduced at the concentrations of 1, 5 and 15 mM (Figure 6C), whereas that of CD80 was not changed (data not shown). Consistently, the capability of APCs loaded with MOG35-55 peptide to stimulate proliferation of CD4 T cells from 2D2 mice was reduced or abrogated by pretreating APCs with 2-D-gal (Figure 6D). Our data suggested that 2-D-gal not only suppressed the antigen uptake and presentation, but also disrupted the interaction between APCs and T cells by downregulating adhesion and costimulatory molecules on APCs.
DISCUSSION
Fucosylated carbohydrate structure plays an important role in host-microbe interactions, selectin-dependent leukocyte adhesion, tissue development, and tumor metastasis (1, 4). However, how fucosylation is involved in the pathogenesis of rheumatoid arthritis is not known. Our results indicate that in RA synovial tissue there is a significant increase of FUTs 1/2/4/5/6/7/9/10/11 compared to OA. In contrast, FUT8 and FUT12/13 (POFUT1 / 2), are not upregulated in synovial tissue of RA subjects. The expression of terminal and sub-terminal FUTs is highly correlated with that of TNF in synovial tissues. Fucosylation inhibition skewed the M1 inflammatory macrophages to a M2 anti-inflammatory phenotype. These results strongly suggest that subsets of FUTs are required for commitment to and maintenance of the pro-inflammatory phenotypes of inflammatory M1 macrophages.
Specific fucosyltransferase can have overlapping enzymatic activity. FUT1 and FUT2 both catalyze the addition of fucose to the galactose moiety of Galβ(1,4)-GlcNAc or Galβ(1,3)-GlcNAc structures, which are usually localized at the termini of the glycan. To investigate the roles of FUT1/2 in RA, Fut1/2 double knockout mouse model is one potential tool for carrying out the studies because Fut1 or Fut2 single knockout does not completely eliminate the activity of α(1,2)-fucosyltransferases. However, this approach is not feasible due to the embryonic lethality of the double knockout mice. Therefore, in the current study, we took advantage of utilizing the fucose analogue, 2-Deoxy-D-galactose (2-D-gal), which can inhibit the formation of the Fucα(1,2)Gal linkage (23), and fulfilled the goal of eliminating the activity of both FUT1 and FUT2.
2-D-Gal treatment led to a dramatic inhibition of CIA whereas fucose facilitated it. Serum levels of TNF-α, IL-6 and anti-CII antibody were significantly reduced by 2-D-gal. Immunization with CII plus CFA promotes an immune response in the draining LNs including induction of inflammatory macrophages, antigen processing and presentation to T cells, and development of adaptive Th1, Th17, and anti-CII responses (37-39). Our data suggested that inhibition of terminal fucosylation with 2-D-Gal significantly suppressed the immune responses that followed priming with CII plus CFA. One key scenario that was altered by 2-D-gal might be the antigen presentation of M1 macrophages.
In the CIA mice treated with 2-D-gal, in addition to the reduction of inflammatory macrophages, we consistently found a remarkable reduction of Th17 in the draining LNs. It was unlikely that 2-D-gal had a direct impact on Th17 cells due to the low levels of FUT1/2 in Th17 cells. We have previously reported that there is a bidirectional interaction between M1 inflammatory macrophages and Th17 in RA and other autoimmune diseases (12, 40). Therefore, it is possible that the decreased Th17 was indirectly caused by the elimination of M1 inflammatory macrophages. It is also possible that other unidentified mechanisms, such as microbiota, might be involved because fucose has been shown to be an important signal that regulates bacterial intestinal colonization (41).
Macrophages exhibit remarkable diversity and plasticity in response to environment cues, undergoing a “spectrum” of activation including M1 (classical or pro-inflammatory) and M2 (alternative or anti-inflammatory) as two extremes (42). It has been shown that the phenotypes of polarized M1 and M2 macrophages can be reversed in vitro and in vivo, which is associated with the sustaining and resolution of inflammation dynamically (43, 44). A network of transcription factors, signaling molecules, epigenetic mechanisms, and posttranscriptional regulators contribute to macrophage activation and plasticity, including IRFs/STATs, NF-κB, SOCS family, PPARs, KLF2/4, c-Myc, HIF-1α/2α, histone demethylase, JMJD3, and miR-155 (9). The current studies identified fucosylation as a novel and key mechanism regulating macrophage plasticity. Our preliminary data generated using Ulex Europaeus Agglutinin-I (UEA-1) pull down followed by Liquid Chromatography Mass (LCMS) Spectrometry further suggested that histone (s) fucosylation might be involved in this process.
Thirteen fucosyltransferases have been identified in mammals. They are responsible for α(1,2)-, α(1,3)-, α(1,4)-, α(1,6)-, and O-fucosylation on various surface localized and secreted molecules (4, 45). Lowe et al has demonstrated that O-fucosylation of Notch catalyzed by Pofut1 regulates lymphoid and myeloid homeostasis (46). Our current study is focused on the effect of 2-D-gal which inhibits the formation of Fucα(1,2)Gal linkage (23). In mice received long term 2-D-gal treatment for 4 months, we did not observe a significant change of bone marrow myeloid progenitors, including common myeloid progenitors (CMP) and granulocyte-macrophage progenitors (GMPs) compared to control mice (data not shown). The size of thymus, counts of different subsets of thymocytes including CD4 SP, CD8 SP, DN1, DN2, DN3, and DN4 were not altered by long term 2-D-gal treatment (data not shown). Therefore, it is unlikely that 2-D-gal interfered with the O-fucosylation of Notch molecules. One of the most important functions of fucosylation is cell adhesion and migration. Interaction between selectins and the fucosylated structures on their ligands is essential in this process. Two fucosyltransferases, Fut 4 and Fut7, are responsible for the α(1,3)-fucosylated reaction on the selectin ligand (24, 27). The expression of both FUT4 and FUT7 is upregulated in RA synovial tissues to those of OA, which suggests that FUT4 and FUT7 might play a role in the pathogenesis of RA. However, it is unlikely that 2-D-gal is also having an effect on these two FUTs due to the following reasons: (1) 2-D-gal prevents the α(1,2)-fucosylated reaction to the galactose moiety of Galβ(1,4)-GlcNAc or Galβ(1,3)-GlcNAc structures; (2) Blood leukocytosis, a major phenotype of Fut4−/− and Fut7−/− mice, was not observed in mice treated with long term 2-D-gal.
Our current studies are the first to demonstrate that terminal fucosylation is one hallmark of M1 inflammatory macrophages and plays a determining role in their lineage commitment and differentiation. Fucosylation inhibition can functionally skew the M1 inflammatory macrophages towards an anti-inflammatory phenotype leading to the resolution of inflammation by reshaping their cytokine profiles, suppressing the expression of costimulatory molecules, inhibiting antigen uptake and presenting function. The current studies not only extended our understanding of macrophage plasticity to a posttranslational level but also suggested that fucosylation is a novel therapeutic target in RA.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Marc K. Jenkins (University of Minnesota) for kindly providing the Eα-GFP peptide. Portions of the study were performed at the flow cytometry facility at the Rheumatic Diseases Core Center, supported by NIH grant P30-AR-048311, and the flow cytometry facility at Center for AIDS research, supported by NIH grant P30-AI-027767; as well as the Center for Metabolic Bone Disease–Histomorphometry and Molecular Analysis Core Laboratory, supported by NIH grant P30-AR-46031. All the above cores are at University of Alabama at Birmingham (UAB).
This work was supported by a grant from Arthritis Foundation (to J.L.), Daiichi Sankyo Co., Ltd (to J.D.M.), and Lupus Research Institute (to H-C. H.). Additional support was granted from The Rheumatology Research Foundation, the Department of Veterans Affairs Merit Review Grant 1I01BX000600, the National Institutes of Health Grants 1RO1 AI 071110, and P30 AR048311 (to J.D.M.), and 1RO1 AI 083705 (to H-C.H.).
Footnotes
All authors claim to have no financial interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
REFERENCES
- 1.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–84R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 2.Gornik I, Maravic G, Dumic J, Flogel M, Lauc G. Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clinical biochemistry. 1999;32(8):605–8. doi: 10.1016/s0009-9120(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 3.Ryden I, Pahlsson P, Lundblad A, Skogh T. Fucosylation of alpha1-acid glycoprotein (orosomucoid) compared with traditional biochemical markers of inflammation in recent onset rheumatoid arthritis. Clinica chimica acta; international journal of clinical chemistry. 2002;317(1-2):221–9. doi: 10.1016/s0009-8981(01)00803-8. [DOI] [PubMed] [Google Scholar]
- 4.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13(7):41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 5.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Current opinion in rheumatology. 2007;19(3):289–95. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis and rheumatism. 2009;60(5):1210–21. doi: 10.1002/art.24505. [DOI] [PubMed] [Google Scholar]
- 7.Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheumatic diseases clinics of North America. 2010;36(2):271–96. doi: 10.1016/j.rdc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinne RW, Stuhlmuller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis research & therapy. 2007;9(6):224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122(3):787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosloniec EF, Cremer M, Kang A, Myers LK. Collagen-induced arthritis. Current protocols in immunology / edited by John E Coligan [et al] 2001 doi: 10.1002/0471142735.im1505s20. Chapter 15:Unit 15 5. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Hsu HC, Chen J, Grizzle WE, Chatham WW, Stockard CR, et al. Increased expression of activation-induced cytidine deaminase is associated with anti-CCP and rheumatoid factor in rheumatoid arthritis. Scand J Immunol. 2009;70(3):309–16. doi: 10.1111/j.1365-3083.2009.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Yang P, Wu Q, Li H, Ding Y, Hsu HC, et al. Death Receptor 5-Targeted Depletion of Interleukin-23-Producing Macrophages, Th17, and Th1/17 Associated With Defective Tyrosine Phosphatase in Mice and Patients With Rheumatoid Arthritis. Arthritis and rheumatism. 2013;65(10):2594–605. doi: 10.1002/art.38057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa K, Liu W, Fleck M, Zhang H, Zhao L, Ohtsuka T, et al. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. Journal of immunology. 2003;171(2):1061–9. doi: 10.4049/jimmunol.171.2.1061. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Hsu HC, Yang P, Wu Q, Li H, Edgington LE, et al. Treatment of arthritis by macrophage depletion and immunomodulation: testing an apoptosis-mediated therapy in a humanized death receptor mouse model. Arthritis and rheumatism. 2012;64(4):1098–109. doi: 10.1002/art.33423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, Levings MK. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. Journal of immunology. 187(11):5615–26. doi: 10.4049/jimmunol.1101058. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Liu J, Feng X, Yang P, Xu X, Hsu HC, et al. Synovial fibroblasts promote osteoclast formation by RANKL in a novel model of spontaneous erosive arthritis. Arthritis and rheumatism. 2005;52(10):3257–68. doi: 10.1002/art.21354. [DOI] [PubMed] [Google Scholar]
- 18.Lari R, Fleetwood AJ, Kitchener PD, Cook AD, Pavasovic D, Hertzog PJ, et al. Macrophage lineage phenotypes and osteoclastogenesis--complexity in the control by GM-CSF and TGF-beta. Bone. 2007;40(2):323–36. doi: 10.1016/j.bone.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19(1):47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 20.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26(4):491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Bullock S, Potter J, Rose SP. Effects of the amnesic agent 2-deoxygalactose on incorporation of fucose into chick brain glycoproteins. Journal of neurochemistry. 1990;54(1):135–42. doi: 10.1111/j.1471-4159.1990.tb13293.x. [DOI] [PubMed] [Google Scholar]
- 22.Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. The Journal of biological chemistry. 2003;278(45):43885–8. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 23.Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, et al. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(1):21–6. doi: 10.1073/pnas.0503381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86(4):643–53. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 25.Wagers AJ, Lowe JB, Kansas GS. An important role for the alpha 1,3 fucosyltransferase, FucT-VII, in leukocyte adhesion to E-selectin. Blood. 1996;88(6):2125–32. [PubMed] [Google Scholar]
- 26.Smithson G, Rogers CE, Smith PL, Scheidegger EP, Petryniak B, Myers JT, et al. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. The Journal of experimental medicine. 2001;194(5):601–14. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15(1):115–26. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 28.Huang QQ, Koessler RE, Birkett R, Perlman H, Xing L, Pope RM. TLR2 deletion promotes arthritis through reduction of IL-10. Journal of leukocyte biology. 2013;93(5):751–9. doi: 10.1189/jlb.0912473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubberts E, Joosten LA, Helsen MM, van den Berg WB. Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine. 1998;10(5):361–9. doi: 10.1006/cyto.1997.0298. [DOI] [PubMed] [Google Scholar]
- 30.Zizzo G, Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. Journal of immunology. 2013;190(10):5237–46. doi: 10.4049/jimmunol.1203017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature reviews Immunology. 2010;10(3):170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 32.Underhill DM, Bassetti M, Rudensky A, Aderem A. Dynamic interactions of macrophages with T cells during antigen presentation. The Journal of experimental medicine. 1999;190(12):1909–14. doi: 10.1084/jem.190.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan SO, Bonomo JA, Zhao F, Cobb BA. MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. The Journal of experimental medicine. 2011;208(5):1041–53. doi: 10.1084/jem.20100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nature biotechnology. 1999;17(10):969–73. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- 35.Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6(4):361–9. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 36.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356(6370):607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 37.Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. European journal of immunology. 1996;26(7):1511–8. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 38.Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. Journal of immunology. 2000;164(12):6495–502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- 39.Kai H, Shibuya K, Wang Y, Kameta H, Kameyama T, Tahara-Hanaoka S, et al. Critical role of M. tuberculosis for dendritic cell maturation to induce collagen-induced arthritis in H-2b background of C57BL/6 mice. Immunology. 2006;118(2):233–9. doi: 10.1111/j.1365-2567.2006.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Hsu HC, Mountz JD. The Dynamic Duo-Inflammatory M1 macrophages and Th17 cells in Rheumatic Diseases. J orthopedics & Rheumatol. 2013;1(1):01–4. doi: 10.13188/2334-2846.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, et al. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492(7427):113–7. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 44.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer research. 2005;65(8):3437–46. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 45.Moloney DJ, Haltiwanger RS. The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-beta1,3-glucosyltransferase activity from Chinese hamster ovary cells. Glycobiology. 1999;9(7):679–87. doi: 10.1093/glycob/9.7.679. [DOI] [PubMed] [Google Scholar]
- 46.Yao D, Huang Y, Huang X, Wang W, Yan Q, Wei L, et al. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood. 2011;117(21):5652–62. doi: 10.1182/blood-2010-12-326074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.