Abstract
Biliary atresia (BA), the most common cause of end-stage liver disease and the leading indication for pediatric liver transplantation, is associated with intrahepatic ductular reactions within regions of rapidly expanding periportal biliary fibrosis. While the extent of such biliary fibrosis is a negative predictor of long-term transplant-free survival, the cellular phenotypes involved in the fibrosis are not well established. Using a Rhesus rotavirus (RRV)-induced mouse model of BA, we demonstrate significant expansion of a cell population expressing the putative stem/progenitor cell marker PROMININ-1 (PROM1) adjacent to ductular reactions within regions of periportal fibrosis. PROM1positive (pos) cells express Collagen-1α1. Subsets of PROM1pos cells co-express progenitor cell marker CD49f, epithelial marker E-CADHERIN, biliary marker CYTOKERATIN-19, and mesenchymal markers VIMENTIN and α-SMOOTH MUSCLE ACTIN. Expansion of the PROM1pos cell population is associated with activation of Fibroblast Growth Factor (FGF) and Transforming Growth Factor-β (TGFβ) signaling. In vitro co-treatment of PROM1-expressing Mat1a−/− hepatic progenitor cells with recombinant human FGF10 and TGFβ1 promotes morphologic transformation toward a myofibroblastic cell phenotype with increased expression of myofibroblastic genes Collagen-1α1, Fibronectin, and α–Smooth muscle actin. Infants with BA demonstrate similar expansion of periportal PROM1pos cells with activated SMAD3 signaling in association with increased hepatic expression of FGF10, FGFR1, and FGFR2 as well as mesenchymal genes SLUG and SNAIL. Infants with perinatal subtype of BA have higher tissue levels of PROM1 expression than those with embryonic subtype.
Conclusion
Expansion of collagen-producing PROM1pos cells within the regions of periportal fibrosis is associated with activated FGF and TGFβ pathways in both experimental and human BA. PROM1pos cells may, therefore, play an important role in the biliary fibrosis of BA.
Keywords: liver fibrosis, progenitor cells, Rhesus Rotavirus, Prominin-1, biliary atresia
INTRODUCTION
Biliary atresia (BA) is a disease of infants characterized by progressive, fibro-inflammatory obliteration of the extrahepatic biliary tree and rapidly progressing intrahepatic biliary fibrosis. BA is the most common cause of pediatric end-stage liver disease, and if untreated, is inevitably fatal within the first two years of life (1). Primary treatment, which involves surgical drainage of bile, is successful only half of the time (2). Even with successful surgical drainage, the majority of patients with BA still experience progression of intrahepatic biliary fibrosis towards cirrhosis (1). Importantly, transplant-free survival with one’s native liver inversely correlates with the extent of intrahepatic fibrosis at the time of surgery (1, 2). Unfortunately, the mechanism of BA-associated fibrosis remains poorly understood.
Infants with BA typically demonstrate extensive intrahepatic biliary ductular reactions within regions of bridging fibrosis, as seen with other obstructive cholangiopathies (3, 4). Within and adjacent to intrahepatic ductular reactions and extrahepatic peribiliary glands are cells that express stem/progenitor cell markers (5, 6). Cholestasis, induced pharmacologically by 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) in rodents, similarly manifests ductular reactions comprised of cells expressing stem/progenitor cell markers (7). Amongst these markers is CD133, also known as PROMININ-1 (PROM1), a penta-transmembrane glycoprotein expressed by stem and cancer stem cells in a variety of organs, including liver (8, 9). The role of these stem/progenitor marker-expressing cells in BA is unclear.
Transdifferentiation of hepatic stellate cells is considered the major source of extracellular matrix-producing myofibroblasts during liver injury and fibrosis (10). There are reports that hepatocytes and cholangiocytes may contribute to liver fibrosis via epithelial-to-mesenchymal transdifferentiation in adult animals (11, 12) although more recent fate tracing studies refute this concept (13, 14). Hepatic progenitor cells (HPC) associated with ductular reactions have been implicated in extracellular matrix deposition although their role is unclear (15). Given that biliary fibrosis associated with ductular reactions is histologically and potentially mechanistically distinct from hepatocyte-specific injury-induced fibrosis, elucidating the origin of cell populations contributing to fibrosis in BA may be critical to intervening with this highly aggressive fibrogenic disease.
In this study, we characterized the expansion of a novel population of PROM1-expressing cells within regions of evolving periportal biliary fibrosis both in a murine model of RRV-infected experimental BA as well as in infants with BA.
MATERIALS AND METHODS
Mouse model of BA
BALB/c mice (Charles River Laboratories) were bred, maintained, and cared for in a manner consistent with criteria outlined in the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees of Children’s Hospital Los Angeles and Cincinnati Children’s Hospital Center.
Experimental BA was induced by inoculating newborn BALB/c mouse pups as previously described (16). Serum and liver samples were collected up to 2 weeks post-inoculation after euthanasia. Six-week old, wild-type C57BL/6J male mice (Jackson Laboratories) underwent bile duct ligation, treatment either with carbon tetrachloride (Sigma-Aldrich), or 0.1% DDC diet (Test Diet, Richmond) as previously described (14, 17). CMVcre;Rosa26rtTA/-;tet(O)-Fgf10+/− mice and littermate control mice were given water with 1% doxycycline (Clontech) two days prior to and throughout DDC treatment in order to induce Fgf10 over-expression (17).
Fluorescence Activated Cell Sorting (FACS) Analysis
Liver cell suspensions were collected as previously described (18) one and two weeks after RRV challenge. One million live cells were Fc blocked, incubated with 2 μg of anti-PROM1-Phycoerythrin (eBiosciences, San Diego, CA), and washed with FACS buffer prior to analysis using FACS Calibur (BD Biosciences, San Jose, CA). Compensation was performed using BD™ CompBeads (BD Biosciences). Gating was determined using isotype IgG-stained controls. Flow cytometric analysis was done using Flow-Jo software (Tree Star, Ashland, OR).
Immunofluorescence staining
Livers were fixed in 4% paraformaldehyde (PFA, Poly Sciences Inc., Warrington, PA) and embedded in paraffin for sectioning. Immunofluorescence staining was performed as described previously (9) (Supplemental Table 1). Signals were detected by secondary antibodies conjugated either with anti-mouse Cy3/Cy5, anti-rat Cy3/Cy5, or anti-rabbit Cy3/Cy5 (Jackson Immuno Research Lab, West Grove, PA). Fluorescence images were acquired by an LSM700 confocal system controlled by ZEN software (Carl Zeiss Microimaging, Thornwood, NY) or by a Leica DM5500B immunofluorescence microscope using Leica Suite Advanced Fluorescence (LAS AF) 6000 software (Wetzlar, Germany). Bright field images were acquired using a Leica DM1000 (DFC290) transmitted light microscope (Leica Microsystems, Switzerland) using Leica Application Suite, Version 2.7.1R1.
Western blot analysis
Total protein lysates were prepared and Western blot analyses were performed as previously described (9) (Supplemental Table 1).
Human BA tissue analysis
Human biopsy samples and relevant clinical data were collected from BA patients undergoing Kasai portoenterostomy at CHLA under a study protocol approved by the Institutional Review Board at CHLA, with informed consent obtained from patient’s parents. Microarray analysis raw data were obtained from Biliary Atresia Research Consortium database http://genet.cchmc.org (19).
PCR
Total RNA was isolated from snap-frozen human and mouse liver tissues and FACS-sorted cells using the Qiagen RNA isolation kit (Valencia, CA). cDNA synthesis, RTPCR and quantitative real-time PCR (qPCR) were performed as previously described (9) using intron spanning and gene-specific primers (Supplemental Table 2).
Mat1a−/− cell culture
PROM1-expressing Mat1a−/− cells were cultured as previously described (9) with recombinant (r) human (h) FGF10 (25 ng/mL) and/or rhTGFβ1 (5 ng/mL).
Determination of tissue fibrosis
Sirius red staining was performed as previously described (17) and quantified densitometrically using Image J software (NIH). The extent of fibrosis and ductular proliferation in human BA liver samples were scored by two clinical pathologists blinded to clinical data and PROM1 expression levels as previously described (20, 21).
Statistical analysis
Analysis of Variance with post hoc Fisher’s Protect Least Significant Difference test was performed using Statview software (SAS Institute Inc., Cary, NC) to calculate statistical significance (p < 0.05).
RESULTS
Expansion of PROMININ-1 expressing cells in the periportal fibrotic areas of RRV-infected livers
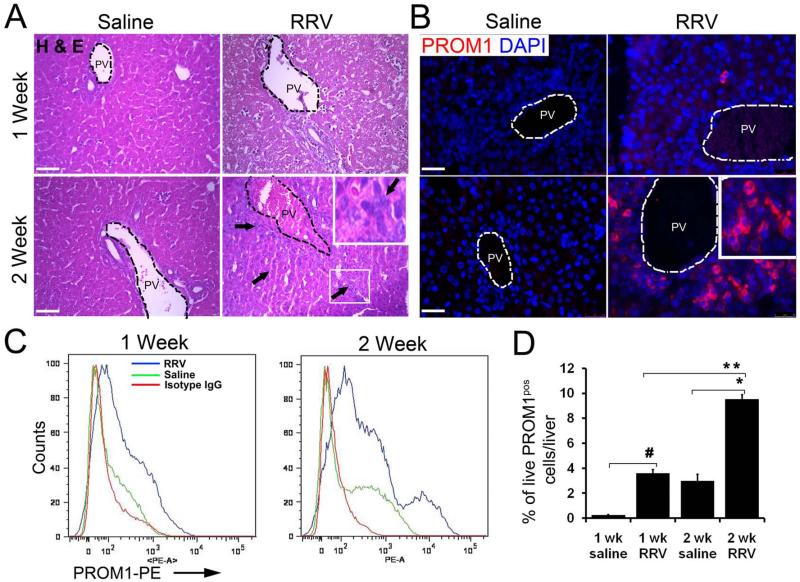
Two weeks after postnatal day zero (P0) RRV inoculation, mouse pups were jaundiced and excreted acholic stools consistent with BA as previously reported (16). RRV-challenged livers exhibited accumulation of small cells with high nuclear-to-cytoplasmic ratio near the portal vein (Figure 1A) along ductular reactions similar to human BA (Supplemental Figure 1). We observed an increase in the number of PROM1pos cells in the periportal regions of the RRV-infected livers compared to saline controls up to 2 weeks both by immunofluorescence and FACS (Figure 1B-D, Supplemental Figure 2). With P3 RRV injections, pups did not develop cholestasis and there was no increase in PROM1pos cells (Supplemental Figure 3). In P0 RRV-challenged livers, PROM1pos cells emerged predominantly adjacent to the expanding ductular cells expressing CYTOKERATIN-19 (CK19) along the proximal intrahepatic branches of the portal vein and bile ducts (Supplemental Figure 4). To lesser extents, PROM1pos cells were present in adult mouse models of ductular proliferation and fibrosis three weeks after bile duct ligation and after two weeks of 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) treatment (Supplemental Figure 5B-C) (17); PROM1pos cells were not present in livers after six weeks of carbon tetrachloride exposure (Supplemental Figure 5A) suggesting that the emergence of PROM1pos cells may be unique to biliary-specific injuries.
Figure 1.
Expansion of the PROM1-expressing cell population around the periportal regions of RRV BA livers. (A) Hematoxylin and Eosin (H&E) staining on livers from 1 and 2 week saline and RRV infected livers. Arrows indicate new small cells with high nuclear-to-cytoplasmic ratio. (B) Immunofluorescence staining for PROM1 on 1 and 2 week RRV-infected and saline control livers. PV: Portal Vein. (C, D) FACS analysis on 1 and 2 week RRV-infected and saline livers. Percentage of live PROM1pos cells compared between 1 and 2 week RRV as well as with saline controls. Nuclei were stained with DAPI. n=4-6, #p<0.0001, * p< 0.0001, ** p<0.001. Scale bar=25μm.
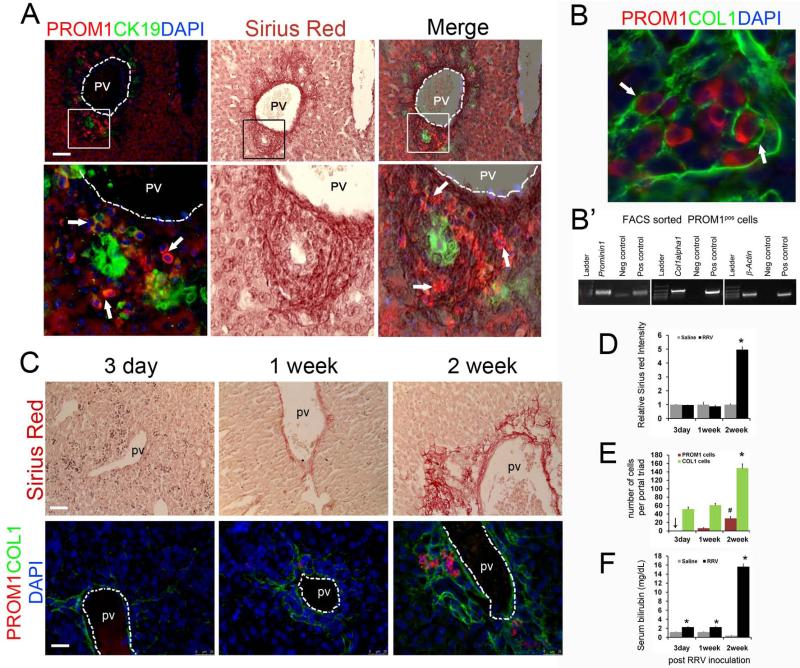
Infants with BA exhibit some degree of biliary fibrosis ranging from mild periportal fibrosis to cirrhosis marked with bridging fibrosis and hepatocyte loss (3). Overlay of immunofluorescence images with Sirius red staining demonstrated clustered PROM1pos cells adjacent to CK19pos ductular reactions predominantly within nests of new collagen deposition (Figure 2A). PROM1pos cells co-stained positively with Type 1 COLLAGEN (COL1) and FACS-sorted PROM1pos cells expressed Collagen-1α1, thus, implicating PROM1pos cells as a source of collagen synthesis in the biliary fibrosis of mice with experimental BA (Figure 2B, B’). Over the 2 weeks following RRV challenge, increases in PROM1pos and COL1pos cells paralleled Sirius red staining and serum bilirubin levels (Figure 2C-F).
Figure 2.
PROM1 cells are present within the fibrotic periportal areas in RRV BA livers: (A) Immunofluorescence staining for PROM1 and CK19. The same tissue sections were then processed for Sirius Red staining and the two images merged into one. Arrows indicate PROM1pos cells within the collagen deposited areas. (B) High-powered confocal immunofluorescence image of PROM1 and COL1 on 2-week RRV BA liver. (B’) RNA was isolated from FACS-sorted PROM1pos cells from 2-week RRV liver tissues and RTPCR was performed to show co-expression of Prominin1, Col1α1 and β actin. (C) Expansion of the PROM1pos cell population is associated with increased periportal fibrosis and biliary obstruction after RRV infection. Sirius Red staining for fibrosis and immunofluorescence staining for PROM1 and COL1 on 3-day, 1-week and 2-week post RRV liver. (D) Intensity of Sirius Red staining was quantified using ImageJ (NIH) software, normalized and compared to respective saline controls. Data represented as relative fold increase in intensity. 4-5 20x images were quantified and average value was taken for each liver sample (n=3-4, * p<0.0001 vs. 2 week saline). (E) Quantification of PROM1 and COL1 expressing cells in livers after 3-day, 1-week and 2-week RRV infection. PROM1pos cells and COL1pos cells were counted from 6-8 40× images per sample and mean value was taken for each liver tissue (n= 3-4, p #< 0.005 vs 1 week, p * < 0.001 vs. 3-day and 1-week). (F) Serum bilirubin levels in RRV infected mice (n=4-6, * p < 0.0005 compared to respective saline controls). PV=portal vein, scale bar=25μm.
PROM1pos cells exhibit dual epithelial-mesenchymal cell characteristics
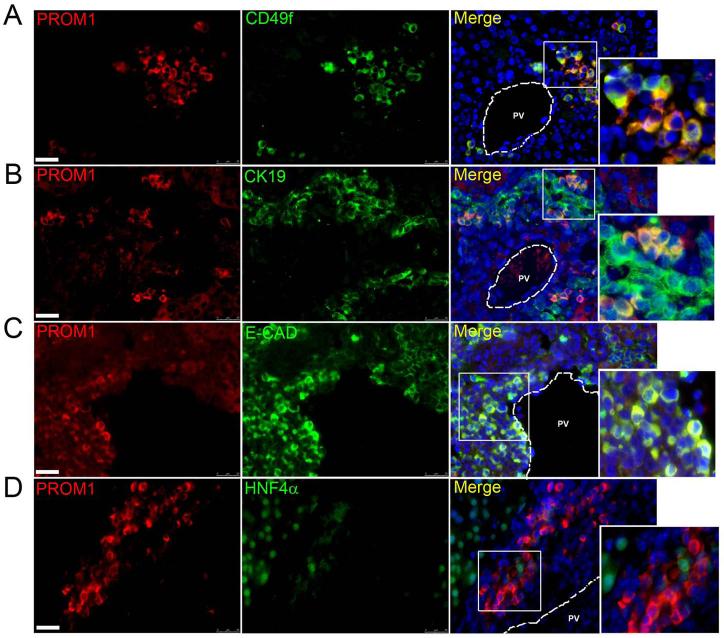
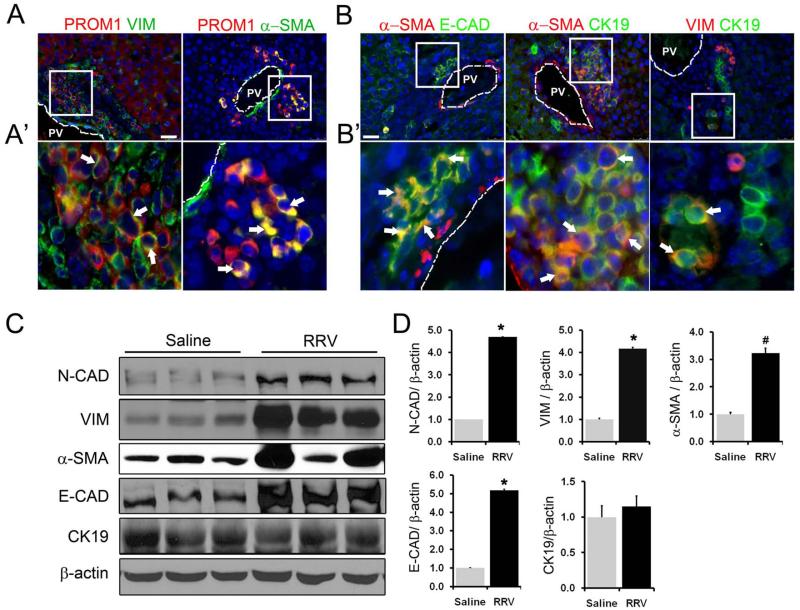
Two-week RRV-challenged livers exhibited PROM1pos cells that co-expressed CD49f (Figure 3A), a Laminin receptor highly expressed by both epithelial and mesenchymal progenitor cells (22). PROM1pos cells also co-expressed epithelial markers CK19 and E-CADHERIN (Figure 3B-C), but not HEPATOCYTE NUCLEAR FACTOR-4α, suggesting a potential absence of hepatocyte differentiation capacity at this time point (Figure 3D). ~52% of all PROM1pos cells expressed putative portal fibroblast marker, α-SMOOTH MUSCLE ACTIN (αSMA) and a subset of PROM1pos cells co-expressed mesenchymal marker VIMENTIN (Figure 4A, A’). Numerous periportal cells also co-expressed αSMA and E-CADHERIN, αSMA and CK19, or VIMENTIN and CK19 suggesting that PROM1pos cells may be both epithelial and mesenchymal in nature (Figure 4B, B’). PROM1pos cells did not express Fibroblast Specific Protein (FSP1), a marker specific for fibroblasts and activated macrophages in the injured livers (Supplemental Figure 6) (23). Western blot analysis demonstrated a significant increase in E-CADHERIN, N-CADHERIN, VIMENTIN and αSMA expression but not CK19 (Figure 4C-D). Importantly, populations of periportal PROM1posαSMAneg, PROM1posαSMApos, and PROM1negαSMApos cells all increased over two weeks post RRV inoculation (Supplemental Figure 7). In parallel, there was an observed increase in cells expressing portal fibroblast marker ELASTIN (24) as well as increased ELASTIN deposition surrounding portal veins after RRV inoculation (Supplemental Figure 8). These observations are consistent with the possibility that some collagen-producing myofibroblasts in the periportal regions of BA livers may derive from PROM1-expressing dual epithelial-mesenchymal cells.
Figure 3.
PROM1pos cells exhibit less differentiated non-hepatocyte epithelial cellular phenotype. Immunofluorescence colocalization of PROM1 in livers from 2-week RRV-infected pups with (A) progenitor cell marker, CD49f, (B) biliary epithelial marker, CK19, (C) epithelial marker, E-CADHERIN (E-CAD), and (D) hepatocyte marker HNF4α. Nuclei were stained with DAPI. Images represent four or more independent staining. Scale bar =25μm, PV= portal vein.
Figure 4.
Increased expression of mesenchymal markers in RRV BA livers (A, A’). PROM1 cells exhibit mesenchymal characteristics with the co-expression of VIMENTIN (VIM) and αSMOOTH MUSCLE ACTIN (αSMA). (B, B’) Co-expression of epithelial markers (E-CAD, CK19) with mesenchymal markers (αSMA, VIM) in RRV BA livers. Nuclei were stained with DAPI. Arrows indicate cells co-positive cells in each panel. PV=portal vein, Scale bar=25μm. (C) Western blot analysis of whole liver protein expression from 2-week saline control and RRV BA livers. (D) Densitometric quantification of Western blot data. n=3-4, *p<0.001, # p<0.05.
Expansion of the PROM1pos cell population is associated with the activation of Fibroblast Growth Factor and AKT/β-catenin pathways
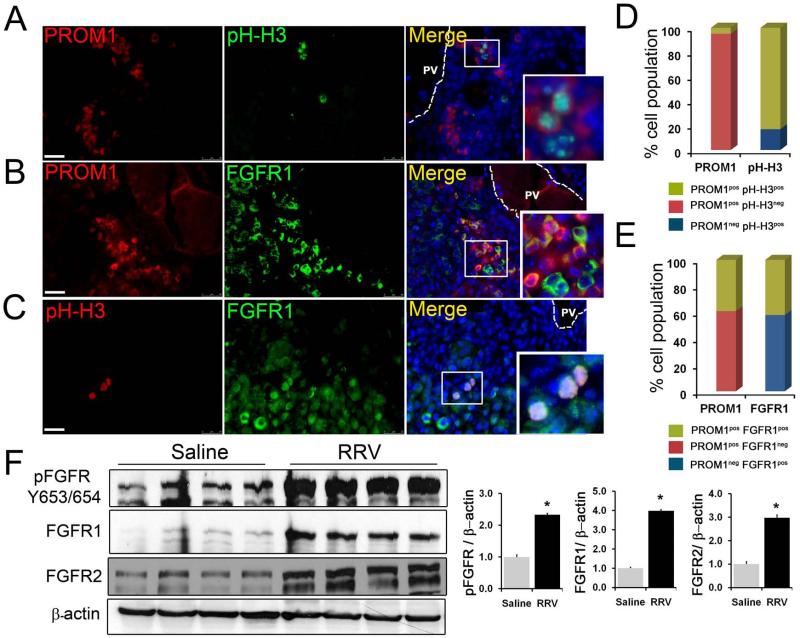
Approximately 5% of PROM1pos cells were proliferating as assessed by phospho-HISTONE-H3 (pHH3) co-positivity (Figure 5A). Notably, the vast majority of liver cell proliferation during this stage of RRV-induced liver injury was by PROM1pos cells as most pHH3pos cells also co-expressed PROM1 by immunofluorescence (Figure 5D). PROM1pos cells did not co-localize with the apoptosis marker cleaved CASPASE-3 (Supplemental Figure 9).
Figure 5.
Proliferation of PROM1pos cells is associated with activated FGF signaling. (A-C) Immunofluorescence co-staining for PROM1 with proliferation marker, phospho-Histone H3 (pHH3) and FGF Receptor1 (FGFR1). Nuclei were stained with DAPI. Images represent three or more independent staining on 2-week saline and RRV BA livers. PV= portal vein. Scale bar=25μm. (D, E) Block diagram representing the percentage cell population (n=3-4). (F) Western blot analysis of protein extracts prepared from whole livers from 2-week saline control and RRV BA livers. n=4, p<0.0.005.
Immunofluorescence analysis further demonstrated an increase in the number of periportal PROM1posFGFR1pos cells (Figure 5B). Nearly 40% of PROM1pos cells co-expressed at least one FGFR, specifically FGFR1; more than 50% FGFR1 expressing cells were co-positive for PROM1 (Figure 5E). By immunohistochemistry, FGFR2 was also present in numerous periportal cells (not shown). Co-staining with pHH3 further demonstrated that ~10% of FGFR1pos cells were proliferating (Figure 5C). Western blot analysis further demonstrated a significant 2.5-fold increase in phospho-FGFR protein levels and, hence, provided evidence for increased FGF signaling activation along with increased expression of FGFR1 and FGFR2 in RRV BA livers compared to controls (Figure 5F). Microarray data available through the Biliary Atresia Research Consortium were analyzed for relative expression of genes encoding FGFR and FGF ligands in human infants with BA compared to age-matched controls. Significant increases in expression of FGFR1 and FGFR2 but not FGFR3 or FGFR4 were observed (Supplemental Figure 10A). Of all genes encoding FGF ligands, which bind to FGFR1 and/or FGFR2, only FGF10 expression was upregulated (Supplemental Figure 10B) while FGF1, FGF3, FGF7 and FGF22 were not upregulated. Furthermore, livers from mice with BA demonstrated increased FGF10 expression localized mostly to CK8neg non-epithelial, periportal cells and a few CK8pos ductular cells (Supplemental Figure 11A). Rare FGF10pos cells were co-positive for PROM1, DESMIN or αSMA (Supplemental Figure 11B). FACS-sorted PROM1pos cells expressed Fgf10 by RTPCR (Supplemental Figure 11C). Doxycycline-induced Fgf10 overexpression during two weeks of DDC treatment of Rosa26rtTa;tet(o)-Fgf10+/− transgenic mice resulted in an increase in PROM1pos cells adjacent to evolving ductular proliferations not typically seen until later, indicating a role for FGF10 in the emergence of PROM1pos cells (Supplemental Figure 5D) (7).
Seven percent of RRV-induced PROM1pos cells co-stained positively for pSer552-β-CATENIN, the AKT-mediated activated form of β-catenin (Supplemental Figure 12A-B). More than 75% of pSer552-β-CATENINpos cells were PROM1pos. More than 90% of pSer552-β-CATENINpos cells co-expressed pHH3 and all proliferating cells were co-positive for pSer552-β-CATENIN, thus, linking this pathway in proliferating PROM1-expressing cells during RRV injury. Co-expression of FGFR1 and pSer552-β-CATENIN further demonstrated a link between these two signaling pathways in RRV BA livers in some cells (Supplemental Figure 12A). Western blot analysis further demonstrated significant activation of AKT and β-catenin pathways but not ERK pathway, another downstream mediator of FGFR signaling in RRV-challenged livers (Supplemental Figure 12C-D). Thus, we conclude that while a large percentage of PROM1pos cells co-express FGFR, only a small fraction of PROM1pos cells are proliferating via FGF-induced AKT-mediated β-catenin pathway activation.
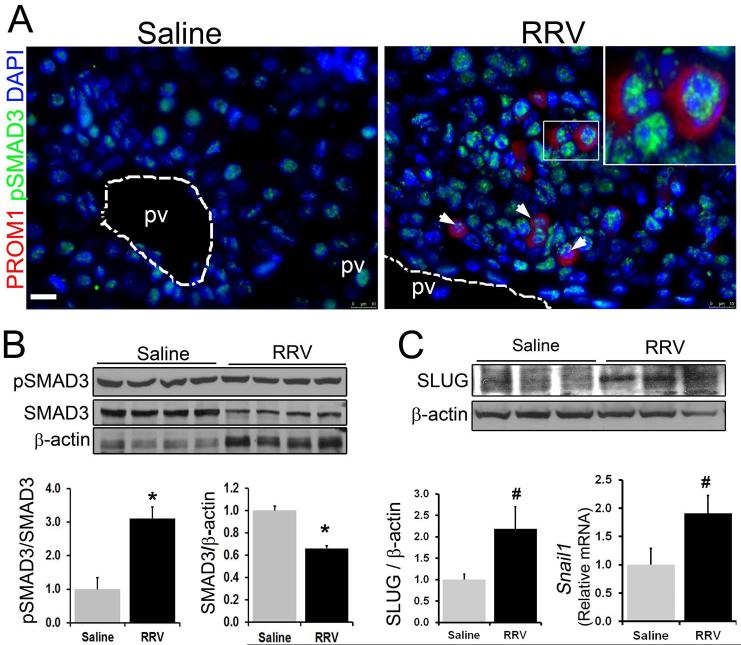
TGFβ signaling is activated in PROM1 expressing cells in BA
Several studies have demonstrated increased expression of TGFβ in the livers from patients with BA specifically in ductular reactions and surrounding inflammatory cells (25). By immunofluorescence, we identified an increased number of cells positive for pSMAD3, indicating TGFβ activation, adjacent to ductular reactions in RRV BA livers compared to saline controls. 65% of PROM1pos cells were pSMAD3pos (Figures 6A). A subset of pSMAD3pos cells also expressed VIMENTIN (not shown). Western blot analysis revealed a three-fold increase in pSMAD3 levels with a concurrent decrease in total SMAD3 levels in RRV BA liver compared to saline controls (Figure 6B). There was also increased expression of two transcriptions factors associated with TGFβ signaling activation, SLUG by Western blot analysis and Snail by qPCR (26, 27) in RRV BA livers (Figure 6C).
Figure 6.
Activation of TGFβ signaling in RRV BA livers. (A) Co-immunofluorescence staining of PROM1 with pSMAD3, downstream target of activated TGFβ signaling. Nuclei were stained with DAPI. Scale bar =25μm. Western blot analysis for (B) pSMAD3, SMAD3, and (C) SLUG in 2-week saline and RRV BA liver. (D) Quantitative PCR was performed to determine the expression level of Snail. n=4-6, *p<0.005, # 0.05.
To determine if FGF10 and TGFβ1 promote myofibroblastic differentiation of PROM1pos cells, in vitro experiments were performed using Mat1a−/− cells, an established transformed murine HPC line that expresses PROM1 along with multiple epithelial and mesenchymal genes (9). Untreated Mat1a−/− cells exhibited round, epithelioid morphology and expressed CK19 but only minimal or no VIMENTIN or pSMAD3 (Figure 7A). Treatment with recombinant (r) human (h) FGF10 had no observable effect. In contrast, treatment with rhTGFβ1 induced transformation to a spindle-shaped, fibroblastic morphology with increased VIMENTIN and pSMAD3 cell positivity as well as increases in αSma, Collagen1α1, and Fibronectin expression by qPCR analysis (Figure 7B). Co-treatment with rhFGF10 and rhTGFβ1 resulted in a three-fold augmentation in Collagen1α1 expression compared to rhTGFβ1 alone, indicating a synergism between FGF and TGFβ signaling. We, thus, surmise that the majority of FGFR signaling activation in PROM1pos cells may potentiate the effects of TGFβ pathway activation rather than promotes cell proliferation. While there was evidence of myofibroblastic transdifferentiation with TGFβ pathway activation, there was yet a clear retention of CK19 expression, implying a potential dual epithelial-mesenchymal phenotype.
Figure 7.
TGFβ signaling induces myofibroblastic differentiation of PROM1-expressing HPCs in vitro. Serum starved Mat1a−/− PROM1 expressing hepatic progenitor cells were treated with recombinant human (rh) TGFβ and/or FGF10 for 3 days. Bright field images and immunofluorescence images for pSMAD3, VIMENTIN and CK19 were shown in (A). Images represent four independent experiments for each condition. Nuclei were stained with DAPI. Scale bar 25μm. (B) Gene expression analysis of Mat1a−/− cells by quantitative PCR. Treatment of TGFβ and FGF10+ TGFβ treatment resulted in a significant increased expression of fibrotic genes Col1α1, αSma and Fibronectin compared to control and rhFGF10 treated cells. (n=4, * p< 0001 compared to control and rhFGF10 treated. # p< 0.05 compared to TGFβ treated cells.
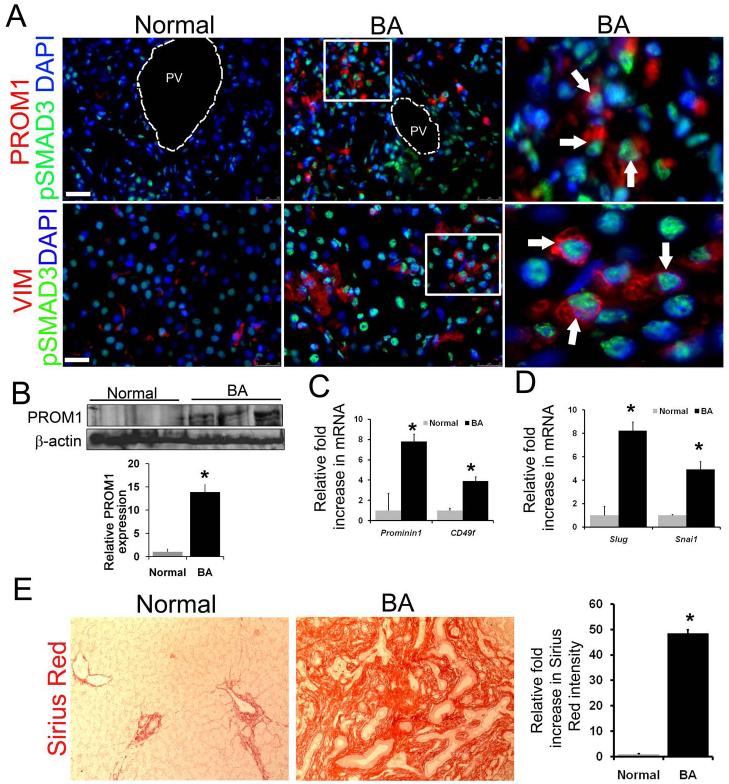
Infant BA livers demonstrated periportal pSMAD3 activation in both PROM1pos and VIMENTINpos cells (Figure 8A). Both Western blot and qPCR analyses demonstrated significant upregulation of PROM1 expression in BA livers compared to normal controls (Figure 8B,C). Microarray analysis of infant BA livers demonstrated greatest fold increases in expression of PROM1 in comparison to other putative progenitor genes including CD49f, SCA1, CD117, and CD34 (Supplemental Figure 13). SLUG and SNAIL were significantly upregulated in BA livers compared to control livers (Figure 8D). Sirius red staining demonstrated a more than 40-fold increase in collagen deposition in BA livers compared to control (Figure 8E). In summary, these data demonstrate an association between expansion of the PROM1pos cell population and TGFβ signaling activation in human BA.
Figure 8.
Expansion of PROM1pos cells with TGFβ activation in human BA livers. (A) Co-localization of PROM1pos and VIMENTINpos cells with pSMAD3 in human BA biopsy samples received at the time of Kasai operation. Nuclei were stained with DAPI. Images are representative of analyses from 4-6 individual biopsy samples. Scale bar 25μm. (B) Expression level of PROM1 in human BA biopsy samples by Western blot analysis (n=3, * p< 0.01. (C) Quantitative PCR demonstrates increased expression level of downstream TGFβ signaling targets genes, Slug and Snail in BA livers compared to normal controls. (D) Quantitative PCR demonstrates increased expression level of Prominin1 and Cd49f in BA livers compared to age matched normal controls, (n=4, BA=8 *p<0.01). (E) Sirius Red staining in normal and BA livers with densitometric quantification. All the BA tissues were received during Kasai operation. Median age of BA patients was 2 months. Age matched normal liver tissues were used as controls.(n=4, BA=7 * p<0.00001). Scale bar=25μm, PV= portal vein. Arrows indicate cells that co-express proteins on each panel.
Correlation between PROM1 expression and fibrosis in BA livers
We next sought to determine if there was a definable relationship between PROM1 expression and any clinical parameters in human BA. Data from eight patients, four of whom experienced successful biliary drainage following Kasai portoenterostomy (total bilirubin below 1.5 mg/dL within the first three months postoperatively (2)) and four of whom did not establish bile drainage, were reviewed (Supplemental Figure 14A). No trends were observed between PROM1 levels and Ishak fibrosis or ductular proliferation scores (Supplemental Figure 14B). There was, however, a trend toward higher PROM1 levels in infants with failed surgical drainage post-Kasai (7.5 ± 6.2 vs. 4.5 ± 2.5, respectively) in this underpowered patient cohort compared to those who had successful surgical drainage. The two patients with the highest total bilirubin levels in the cohort (11 and 13.4 mg/dL) also exhibited the highest relative levels of PROM1 expression (linear regression analysis R2 = 0.799). Finally, when subdivided based on age at onset of jaundice, infants with embryonic subtype of BA (at or within two weeks of birth) exhibited significantly lower PROM1 expression levels compared to infants with perinatal subtype (later than two weeks of age) at 3.4±1.2 versus 10.3±2.7, respectively (p<0.05) (Supplemental Figure 14C).
DISCUSSION
In this study, we demonstrate the expansion of a novel population of PROM1pos cells in various models of ductular proliferation-associated biliary fibrosis, and particularly RRV-induced BA. Within the regions of periportal fibrosis in experimental BA, subsets of PROM1pos cells co-express epithelial markers, mesenchymal markers, and Type 1 Collagen. There is a concurrent increase in PROM1posαSMApos and PROM1negαSMApos cells as well as in PROM1negELASTINpos portal fibroblast cells, which collectively may represent an evolving myofibroblast population. The expansion of PROM1pos cells is associated with FGF signaling activation, in part via periportal FGF10 expression. Additionally, a subset of PROM1pos cells exhibit evidence of activated profibrogenic TGFβ signaling with pSMAD3 expression in association with increased expression of SLUG and Snail, which further supports the potential role of PROM1-expressing cells in driving fibrosis in BA. Furthermore, FGF and TGFβ signaling activation promote myofibroblastic differentiation of PROM1-expressing HPCs in vitro with induced expression of Col1α1. Infants with BA exhibit similar PROM1pos cell expansion in association with FGF and TGFβ signaling activation. Finally, infants with embryonic subtype of BA had lower levels of PROM1 expression compared to those with perinatal subtype. Thus, we propose that subset of PROM1pos cells undergoes FGF/TGFβ-mediated differentiation to collagen producing cells during BA.
Diseases marked with ductular reactions, such as BA, have been associated with expansion of HPCs (6). In turn, ductular reactions and HPCs have been implicated in liver fibrogenesis (15, 28, 29) although their precise roles have remained unclear. Using a choline-deficient, ethionine-supplemented model of chronic liver injury, Van Hul et al. demonstrated that the deposition of collagen preceded the emergence of CK19pos ductular reactions and upregulation of Cd49f, E-cadherin, and α-Fetoprotein expression suggesting that matrix deposition is independent of HPC expansion (30). The authors, however, did not exclude the possibility that progenitor cells may still contribute to production and deposition of the extracellular matrix. Ding et al., demonstrated that HPC cell lines LE/6 and WB-F344 secrete Connective Tissue Growth Factor (CTGF), a profibrogenic extracellular matrix protein, in response to TGFβ, albeit in vitro (31). While our study was too underpowered for linking relative levels of PROM1 expression with degree of fibrosis, we were, nonetheless, able to establish some clear associations. Infants with perinatal subtype of BA had higher relative levels of PROM1 expression than those with embryonic subtype. The perinatal BA subtype is theorized to be more associated with postnatal exposure to fibroinflammatory infectious agents or toxins whereas the embryonic subtype is more associated with congenital malformations and, therefore, thought more likely to be due to dysregulation of biliary organogenesis (32). Prominent ductular reactions and periportal fibrosis were observed in experimental BA, similar to that of BA in human infants. The emergence of PROM1pos cells paralleled the deposition of fibrosis in experimental BA. In addition, the co-localization of PROM1 with COL1 in cells within regions of newly deposited fibrosis along with the expression of Col1α1 by FACS-sorted PROM1pos cells strongly implicates PROM1pos cells as a source of collagen deposition in BA. During the early stages of hepatogenesis, PROM1pos progenitor cells are more abundant than shortly after birth indicating either cellular attrition or loss of PROM1 expression (33). Within two weeks after RRV challenge, there is a clear re-emergence of PROM1pos cells. Half of all RRV-induced PROM1pos cells express αSMA, indicating a periportal myofibroblastic phenotype. Moreover, over time there is an increase in both PROM1posαSMApos and PROM1negαSMApos portal myofibroblast as well as an increase in ELASTIN-expressing portal fibroblast cells in RRV BA livers. Elastin fibers, which are normally present surrounding portal veins, increase during liver fibrosis (34, 35).
It is unclear if PROM1neg αSMApos cells represent a distinct and separate cell lineage from PROM1posαSMApos cells. Our data are consistent with previous lineage tracing studies, which demonstrate that neither hepatocytes nor cholangiocytes undergo epithelial-mesenchymal transdifferentiation to myofibroblasts (13, 14). PROM1-expressing cells phenotypically are neither hepatocytes nor cholangiocytes. To determine if a portal myofibroblasts derive in part from PROM1-expressing cells, lineage tracing experiments are planned but not within the scope of this report due to the necessary extensive back-crossing of existing transgenic mice into the requisite BALB/c background (36). Nevertheless, our data are consistent with the possibility that subsets of emerging PROM1pos cells during RRV-induced BA may give rise to collagen-producing portal myofibroblasts. Moreover, PROM1pos cells could also potentially give rise to the adjacent biliary ductular reactions.
We previously demonstrated that the expansion and survival of PROM1pos embryonic HPCs is partially regulated by FGF/AKT/β-catenin pathway activation (9). In this study, only a small fraction of PROM1pos cells exhibit FGF/AKT/β-catenin-mediated proliferation. In RRV BA livers, we show expression of FGF10 in periportal regions as well as evidence of FGFR/AKT/β-catenin pathway activation in some PROM1pos cells, and in human BA, FGF10 is the only FGFR1/2 ligand encoding gene which is upregulated. The enhanced expansion of PROM1pos cells with FGF10 overexpression during acute DDC injury further supports the role of this pathway in BA. Consistent with previous reports (25), our data also indicate significant TGFβ pathway activation in both experimental BA as well as in human BA. RRV is known to have high tropism for biliary epithelium, causing its initial destruction (16, 37, 38) and the subsequent release of numerous cytokines including TGFβ (39, 40). Although there are multiple potential sources of TGFβ during injury and inflammation, biliary-derived TGFβ likely drives the myofibroblastic differentiation of adjacent PROM1pos cells. Shirakihara et al. demonstrated that activation of TGFβ signaling induces myofibroblastic differentiation of epithelial cells in vitro partly due to isoform switching from epithelial Fgfr2IIIb to mesenchymal Fgfr1IIIc making the cells responsive to FGF2 (41). Harada et al. demonstrated similar observations in cultured biliary epithelial cells (42). Ding et al. showed evidence of myofibroblastic differentiation of cultured HPCs with TGFβ (31). Our in vitro gain-of-function data demonstrate a potential synergism between FGF and TGFβ signaling in the myofibroblastic differentiation of HPCs in vitro. Given evidence of FGF and TGFβ signaling activation in PROM1-expressing cells, it is likely that these pathways synergistically promote fibrogenesis in BA.
In summary, we demonstrate a potential functional linkage between the expansion of a PROM1-expressing cell population and the newly evolving fibrosis associated with BA mediated by FGF and TGFβ signaling. Defining the nature of these cells and the factors regulating their cell fate determinations could ultimately lead to novel therapeutic interventions against the fibrosis of BA.
Supplementary Material
Supplemental Figure 1: Ductular reaction in (A) 2-week RRV challenged BA liver and (B) human BA liver. Nuclei were stained with DAPI. Scale bar = 25μm.
Supplemental Figure 2: Representative scatter plots from the FACS analysis on 1- and 2-week saline and RRV BA livers. Y-axis represents side scatter and X-axis represents PROM1-PE stained cells. Total 50,000 live cell events were recorded.
Supplemental Figure 3: Minimal expansion of PROM1-expressing cells, absence of ductular reaction and periportal fibrosis at 2 weeks in mice inoculated with RRV on postnatal day 3. Images represent data from four mice. Arrows indicate PROM1 cells that were detected. Nuclei were stained with DAPI. Scale bar 25μm, PV= portal vein.
Supplemental Figure 4: Confocal image demonstrating Intralobular distribution of PROM1-expressing cells 2 weeks after RRV challenge. Note the predominance of PROM1pos cells along proximal branch points of the portal vein along CK19pos cells.
Supplemental Figure 5: The expansion of PROM1-expressing cells is associated with biliary type fibrosis: (A) Absence of PROM1 expressing cells in CCl4 treated (experimental model of hepatic fibrosis) mice livers. (B) Periportal expansion of PROM1-expressing cells 3 weeks after bile duct ligation (BDL). Expansion of PROM1-expressing cells in mice fed 0.1% DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine) diet for 2 weeks (C) without and (D) with FGF10 overexpression. Note the greater prevalence of PROM1pos cells with FGF10 overexpression. (E) IgG control staining. Data represent three independent experiments. Panel A and B were done in parallel with olive oil control (control for CCl4) and sham surgery control for BDL. PROM1pos cells were absent in both controls, hence not shown. Nuclei were stained with DAPI. PV=portal vein, scale bar 25μm
Supplemental Figure 6: Co-immunofluorescence staining for PROM1 and FSP1 (Fibroblast Specific Protein1, marker for activated macrophages and fibroblasts). Nuclei were stained with DAPI. PV=portal vein, Scale bar=25μm. Images represent three independent staining.
Supplemental Figure 7: PROM1 cells and αSMA expressing portal myofibroblast cells increase in parellel during RRV BA disease progression. (A) At 1 week post RRV infection single positive αSMA positive portal myofibroblast cells and PROM1 cells were detected whereas at 2 weeks both single positive PROM1 and SMA positive cells and PROM1/αSMA dual positive periportal myofibroblast cells were detected. pv= portal vein, Arrows indicate PROM1/αSMA dual positive portal myofibroblast cells. Nuclei are stained with DAPI. Scale bar = 25μm (B) Quantification of number PROM1 cells and αSMA positive portal myofibroblast cells at different time points after RRV challenge. Note that at 2 week a significant increase in αSMA positive portal myofibroblast cells compared to 1 week RRV parallel to PROM1cells. n =4-5, *p < 0.05, # p< 0.005 compared to 1 week RRV.
Supplemental Figure 8: ELASTIN-expressing portal fibroblast cell layers increase in RRV BA livers. (A) Immunofluorescence staining for portal fibroblast cell marker ELASTIN and PROM1 on 3 day, 1 week and 2 week RRV BA livers. Note the presence of multiple layers of ELASTIN-expressing cells and its deposition in 2 week RRV BA liver compared to other tissues around the portal vein. Images are representative of multiple stainings on from several mice. Nuclei stained with DAPI. Scale bar = 25μm (B) IgG isotype control staining. Scale bar= 50μm
Supplemental Figure 9: PROM1pos cells are not associated with cellular apoptosis in RRV-challenged livers. (A) Livers from 2-week RRV infected mice were co-stained with PROM1 and apoptosis marker, cleaved CASPASE-3. Arrow indicates detected apoptotic cells. PV= portal vein, (B) positive control staining for Cleaved CASPASE 3 (Biovision™ Apo-IHC kit). Nuclei were stained with DAPI. Scale bar = 25μm.
Supplemental Figure 10: Microarray analysis for the expression level of (A) FGF receptors, (*p< 0.001) and (B) FGF ligands (*p< 0.01) on human BA livers (n=66, median age 2 months) and age matched normal (n=7) control livers. Raw data was acquired from Biliary Atresia Research Consortium Database. Expression levels were normalized to controls and represented as fold change.
Supplemental Figure 11: Subsets of periportal mesenchymal cells, ductular cells and PROM1pos cells are the source of FGF10 in RRV BA livers. (A) Increased expression of FGF10 in the periportal CK8neg (arrowheads) as well as CK8pos ductular cells (arrows) in RRV livers compared to saline control livers. (B) Immunofluorescence staining for FGF10 and PROM1 demonstrates co-expression (arrows) whereas co-immunofluorescence staining for FGF10 with DESMIN and αSMA demonstrate minimum co-expression. PV=portal vein, scale bar 25μm. Images represents four independent experiments each (C). RTPCR analysis of RNA from FACS-isolated PROM1 cells from 2-week RRV livers. RNA from E16.5 embryonic liver was used as positive control. Data represent two independent experiments.
Supplemental Figure 12: Activation of downstream AKT-β-CATENIN and proliferation of PROM1pos cells (A) Co-immunofluorescence staining for PROM1, pSer552-β-CAT (the AKT-mediated activated form of β-CATENIN), cell proliferation marker pHH3 and FGFR1on 2week RRV BA livers. Nuclei were stained with DAPI. Scale bar=25μm. (B) Block diagram representing percentage cell population. n=3-4 each. (C) Western blot analysis of protein extracts prepared from 2-week saline and RRV-challenged livers. (D) Densitometric quantification of expression level of proteins n=4, *p<0.005, #p<0.05. PV= portal vein.
Supplemental Figure 13: Microarray analysis for the relative expression levels of putative progenitor marker genes in human BA livers (n=66, median age 2 months) and age-matched normal control livers (n=7) (p<0.0001). Raw data was acquired from Biliary Atresia Research Consortium Database. Expression levels were normalized to normal controls and represented as fold increase.
Supplemental Figure 14: (A) Correlation of PROM1 expression levels with BA subtype, serum total bilirubin level, Ishak score, ductular reaction score, and outcome post-Kasai in BA patients at the time of Kasai operation. Relative levels of PROM1 were measured in BA liver by qPCR in comparison to age-matched controls were shown. (B) Regression analysis shows a potential correlation of cholestasis with PROM1 expression (R2=0.799) whereas neither Ishak score nor post Kasai outcome correlated with the expression level. (C). Comparison of PROM1 expression between BA subtypes. P=perinatal form, E= embryonic form, *p< 0.05, n perinatal=3, embryonic=5, TB= Total Serum bilirubin.
ACKNOWLEDGEMENTS
We thank Dr. Esteban Fernandez (Imaging core), Dr. Ann George, Mr. Jonathan Kirzner (FACS core) for their help with confocal imaging and FACS analysis respectively. We also thank Dr. Joshua Friedman (University of Pennsylvania) and Dr. Linheng Li (Stower’s Institute) for their generous gifts of antibodies to perform this study. We thank Dr. Grace Aldrovandi and William Decker for their assistance with RRV culturing. We thank Cat Goodhue for manuscript review and editing. We would also like to thank Drs. Nanda Kerkar and David Warburton for their stimulating feedback on this project. We thank Dr. Henri R. Ford for his support for this project.
Financial support:
This study was supported in part by the National Institutes of Health Grants K08 AAA01690, U01 DK084538-01, and a CHLA-TSRI core utilization grant (KW) and R01 AA020753 (KA). P.S. is supported by an American Liver Foundation Liver Scholar award. K.M. was supported by California Institute for Regenerative Medicine (CIRM) internship.
Abbreviations
- RRV
Rhesus Rotavirus
- BA
Biliary Atresia
- PROM1
PROMININ-1
- TGF
Transforming Growth factor
- FGF
Fibroblast growth factor
- DMEM
Dulbecco’s Modified Eagle Medium
- ECM
Extracellular Matrix
- FACS
Fluorescence Activated Cell Sorting
Footnotes
Conflict of interest: None
REFERENCES
- 1.Sokol RJ. Corticosteroid treatment in biliary atresia: Tonic or toast? Hepatology. 2007;46:1675–1678. doi: 10.1002/hep.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Superina R, Magee JC, Brandt ML, Healey PJ, Tiao G, Ryckman F, Karrer FM, et al. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254:577–585. doi: 10.1097/SLA.0b013e3182300950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 4.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 5.Dipaola F, Shivakumar P, Pfister J, Walters S, Sabla G, Bezerra JA. Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury in mice. Hepatology. 2013;58:1486–1496. doi: 10.1002/hep.26485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamp LA, Braxton DR, Wu J, Akopian V, Hasegawa K, Chandrasoma PT, Hawes SM, et al. The GCTM-5 epitope associated with the mucin-like glycoprotein FCGBP marks progenitor cells in tissues of endodermal origin. Stem Cells. 2012;30:1999–2009. doi: 10.1002/stem.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavila N, James D, Utley S, Cu N, Coblens O, Mak K, Rountree CB, et al. Fibroblast growth factor receptor-mediated activation of AKT-beta-catenin-CBP pathway regulates survival and proliferation of murine hepatoblasts and hepatic tumor initiating stem cells. PLoS One. 2012;7:e50401. doi: 10.1371/journal.pone.0050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, et al. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–1258. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 13.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Utley S, James D, Mavila N, Nguyen MV, Vendryes C, Salisbury SM, Phan J, et al. Fibroblast Growth Factor signaling regulates the expansion of A6-expressing hepatocytes in association with AKT-dependent β-catenin activation. Journal of Hepatology. 2014;60:1002–1009. doi: 10.1016/j.jhep.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, Veltmaat JM, et al. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyer K, Kaimal V, Pacheco C, Mourya R, Xu H, Shivakumar P, Chakraborty R, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med. 2010;2:33. doi: 10.1186/gm154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 21.Lee WS, Looi LM. Usefulness of a scoring system in the interpretation of histology in neonatal cholestasis. World J Gastroenterol. 2009;15:5326–5333. doi: 10.3748/wjg.15.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 23.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- 25.Nadler EP, Patterson D, Violette S, Weinreb P, Lewis M, Magid MS, Greco MA. Integrin alphavbeta6 and mediators of extracellular matrix deposition are up-regulated in experimental biliary atresia. J Surg Res. 2009;154:21–29. doi: 10.1016/j.jss.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Dhasarathy A, Phadke D, Mav D, Shah RR, Wade PA. The transcription factors Snail and Slug activate the transforming growth factor-beta signaling pathway in breast cancer. PLoS One. 2011;6:e26514. doi: 10.1371/journal.pone.0026514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herfs M, Hubert P, Kholod N, Caberg JH, Gilles C, Berx G, Savagner P, et al. Transforming agrowth factor-beta1-mediated Slug and Snail transcription factor up-regulation reduces the density of Langerhans cells in epithelial metaplasia by affecting E-cadherin expression. Am J Pathol. 2008;172:1391–1402. doi: 10.2353/ajpath.2008.071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 29.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Van Hul NK, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–1635. doi: 10.1002/hep.22820. [DOI] [PubMed] [Google Scholar]
- 31.Ding ZY, Jin GN, Liang HF, Wang W, Chen WX, Datta PK, Zhang MZ, et al. Transforming growth factor beta induces expression of connective tissue growth factor in hepatic progenitor cells through Smad independent signaling. Cell Signal. 2013;25:1981–1992. doi: 10.1016/j.cellsig.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Schievenbusch S, Sauer E, Curth HM, Schulte S, Demir M, Toex U, Goeser T, et al. Neighbor of Punc E 11: expression pattern of the new hepatic stem/progenitor cell marker during murine liver development. Stem Cells Dev. 2012;21:2656–2666. doi: 10.1089/scd.2011.0579. [DOI] [PubMed] [Google Scholar]
- 34.Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, Hartland SN, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- 35.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen C, Kuske M, Bruns E, Biermanns D, Wussow PV, Mildenberger H. Progress in developing animal models for biliary atresia. Eur J Pediatr Surg. 1998;8:137–141. doi: 10.1055/s-2008-1071140. [DOI] [PubMed] [Google Scholar]
- 37.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest. 2009;119:2281–2290. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coots A, Donnelly B, Mohanty SK, McNeal M, Sestak K, Tiao G. Rotavirus infection of human cholangiocytes parallels the murine model of biliary atresia. J Surg Res. 2012;2:275–281. doi: 10.1016/j.jss.2012.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamireau T, Le Bail B, Boussarie L, Fabre M, Vergnes P, Bernard O, Gautier F, et al. Expression of collagens type I and IV, osteonectin and transforming growth factor beta-1 (TGFbeta1) in biliary atresia and paucity of intrahepatic bile ducts during infancy. J Hepatol. 1999;31:248–255. doi: 10.1016/s0168-8278(99)80221-9. [DOI] [PubMed] [Google Scholar]
- 40.Jafri M, Donnelly B, Bondoc A, Allen S, Tiao G. Cholangiocyte secretion of chemokines in experimental biliary atresia. J Pediatr Surg. 2009;44:500–507. doi: 10.1016/j.jpedsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, Miyazono K, et al. TGF-beta regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011;30:783–795. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harada K, Sato Y, Ikeda H, Isse K, Ozaki S, Enomae M, Ohama K, et al. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol. 2009;217:654–664. doi: 10.1002/path.2488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Ductular reaction in (A) 2-week RRV challenged BA liver and (B) human BA liver. Nuclei were stained with DAPI. Scale bar = 25μm.
Supplemental Figure 2: Representative scatter plots from the FACS analysis on 1- and 2-week saline and RRV BA livers. Y-axis represents side scatter and X-axis represents PROM1-PE stained cells. Total 50,000 live cell events were recorded.
Supplemental Figure 3: Minimal expansion of PROM1-expressing cells, absence of ductular reaction and periportal fibrosis at 2 weeks in mice inoculated with RRV on postnatal day 3. Images represent data from four mice. Arrows indicate PROM1 cells that were detected. Nuclei were stained with DAPI. Scale bar 25μm, PV= portal vein.
Supplemental Figure 4: Confocal image demonstrating Intralobular distribution of PROM1-expressing cells 2 weeks after RRV challenge. Note the predominance of PROM1pos cells along proximal branch points of the portal vein along CK19pos cells.
Supplemental Figure 5: The expansion of PROM1-expressing cells is associated with biliary type fibrosis: (A) Absence of PROM1 expressing cells in CCl4 treated (experimental model of hepatic fibrosis) mice livers. (B) Periportal expansion of PROM1-expressing cells 3 weeks after bile duct ligation (BDL). Expansion of PROM1-expressing cells in mice fed 0.1% DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine) diet for 2 weeks (C) without and (D) with FGF10 overexpression. Note the greater prevalence of PROM1pos cells with FGF10 overexpression. (E) IgG control staining. Data represent three independent experiments. Panel A and B were done in parallel with olive oil control (control for CCl4) and sham surgery control for BDL. PROM1pos cells were absent in both controls, hence not shown. Nuclei were stained with DAPI. PV=portal vein, scale bar 25μm
Supplemental Figure 6: Co-immunofluorescence staining for PROM1 and FSP1 (Fibroblast Specific Protein1, marker for activated macrophages and fibroblasts). Nuclei were stained with DAPI. PV=portal vein, Scale bar=25μm. Images represent three independent staining.
Supplemental Figure 7: PROM1 cells and αSMA expressing portal myofibroblast cells increase in parellel during RRV BA disease progression. (A) At 1 week post RRV infection single positive αSMA positive portal myofibroblast cells and PROM1 cells were detected whereas at 2 weeks both single positive PROM1 and SMA positive cells and PROM1/αSMA dual positive periportal myofibroblast cells were detected. pv= portal vein, Arrows indicate PROM1/αSMA dual positive portal myofibroblast cells. Nuclei are stained with DAPI. Scale bar = 25μm (B) Quantification of number PROM1 cells and αSMA positive portal myofibroblast cells at different time points after RRV challenge. Note that at 2 week a significant increase in αSMA positive portal myofibroblast cells compared to 1 week RRV parallel to PROM1cells. n =4-5, *p < 0.05, # p< 0.005 compared to 1 week RRV.
Supplemental Figure 8: ELASTIN-expressing portal fibroblast cell layers increase in RRV BA livers. (A) Immunofluorescence staining for portal fibroblast cell marker ELASTIN and PROM1 on 3 day, 1 week and 2 week RRV BA livers. Note the presence of multiple layers of ELASTIN-expressing cells and its deposition in 2 week RRV BA liver compared to other tissues around the portal vein. Images are representative of multiple stainings on from several mice. Nuclei stained with DAPI. Scale bar = 25μm (B) IgG isotype control staining. Scale bar= 50μm
Supplemental Figure 9: PROM1pos cells are not associated with cellular apoptosis in RRV-challenged livers. (A) Livers from 2-week RRV infected mice were co-stained with PROM1 and apoptosis marker, cleaved CASPASE-3. Arrow indicates detected apoptotic cells. PV= portal vein, (B) positive control staining for Cleaved CASPASE 3 (Biovision™ Apo-IHC kit). Nuclei were stained with DAPI. Scale bar = 25μm.
Supplemental Figure 10: Microarray analysis for the expression level of (A) FGF receptors, (*p< 0.001) and (B) FGF ligands (*p< 0.01) on human BA livers (n=66, median age 2 months) and age matched normal (n=7) control livers. Raw data was acquired from Biliary Atresia Research Consortium Database. Expression levels were normalized to controls and represented as fold change.
Supplemental Figure 11: Subsets of periportal mesenchymal cells, ductular cells and PROM1pos cells are the source of FGF10 in RRV BA livers. (A) Increased expression of FGF10 in the periportal CK8neg (arrowheads) as well as CK8pos ductular cells (arrows) in RRV livers compared to saline control livers. (B) Immunofluorescence staining for FGF10 and PROM1 demonstrates co-expression (arrows) whereas co-immunofluorescence staining for FGF10 with DESMIN and αSMA demonstrate minimum co-expression. PV=portal vein, scale bar 25μm. Images represents four independent experiments each (C). RTPCR analysis of RNA from FACS-isolated PROM1 cells from 2-week RRV livers. RNA from E16.5 embryonic liver was used as positive control. Data represent two independent experiments.
Supplemental Figure 12: Activation of downstream AKT-β-CATENIN and proliferation of PROM1pos cells (A) Co-immunofluorescence staining for PROM1, pSer552-β-CAT (the AKT-mediated activated form of β-CATENIN), cell proliferation marker pHH3 and FGFR1on 2week RRV BA livers. Nuclei were stained with DAPI. Scale bar=25μm. (B) Block diagram representing percentage cell population. n=3-4 each. (C) Western blot analysis of protein extracts prepared from 2-week saline and RRV-challenged livers. (D) Densitometric quantification of expression level of proteins n=4, *p<0.005, #p<0.05. PV= portal vein.
Supplemental Figure 13: Microarray analysis for the relative expression levels of putative progenitor marker genes in human BA livers (n=66, median age 2 months) and age-matched normal control livers (n=7) (p<0.0001). Raw data was acquired from Biliary Atresia Research Consortium Database. Expression levels were normalized to normal controls and represented as fold increase.
Supplemental Figure 14: (A) Correlation of PROM1 expression levels with BA subtype, serum total bilirubin level, Ishak score, ductular reaction score, and outcome post-Kasai in BA patients at the time of Kasai operation. Relative levels of PROM1 were measured in BA liver by qPCR in comparison to age-matched controls were shown. (B) Regression analysis shows a potential correlation of cholestasis with PROM1 expression (R2=0.799) whereas neither Ishak score nor post Kasai outcome correlated with the expression level. (C). Comparison of PROM1 expression between BA subtypes. P=perinatal form, E= embryonic form, *p< 0.05, n perinatal=3, embryonic=5, TB= Total Serum bilirubin.