Abstract
Background
The combination of highly caffeinated ‘energy drinks’ with alcohol (ethanol) has become popular among young adults and intoxication via such beverages has been associated with an elevated risk for harmful behaviors. However, there are discrepancies in the human literature regarding the effect of caffeine on alcohol intoxication, perhaps due to confounding factors such as personality type, expectancy, and history of exposure. Animal models of co-exposure are resistant to such issues, however, the consequences of voluntary co-consumption have been largely ignored in the animal literature. The primary goal of this work was to characterize a mouse model of binge caffeine and ethanol co-consumption employing the limited-access ‘Drinking-in-the-Dark’ paradigm (DID).
Methods
Caffeine was added to a 20% alcohol solution via DID. Alcohol/caffeine intake, locomotor behavior, ataxia, anxiety-like behavior, and cognitive function were evaluated as a consequence of co-consumption in adult male C57BL/6J mice.
Results
Caffeine did not substantially alter binge alcohol intake or resultant BECs, nor did it alter alcohol’s anxiolytic effects on the elevated plus maze or cognitive interfering effects in a novel object recognition task. However, no evidence of alcohol-induced sedation was observed in co-consumption groups that instead demonstrated a highly stimulated state similar to that of caffeine alone. The addition of caffeine was also found to mitigate alcohol-induced ataxia.
Conclusions
Taken together, our mouse model indicates that binge co-consumption of caffeine and alcohol produces a stimulated, less ataxic and anxious, as well as cognitively altered state; a state that could be of great public health concern. These results appear to resemble the colloquially-identified ‘wide awake drunk’ state that individuals seek via consumption of such beverages. This self-administration model therefore offers the capacity for translationally-valid explorations of the neurobiological consequences of binge co-consumption in order to assess the public health risk of this drug combination.
Introduction
Mixing alcohol (ethanol) with caffeinated beverages has long been a common practice (e.g. rum and cola). The rise of ‘energy drinks’ (containing large quantities of caffeine; ~80–300 mg/container), however, has changed how people consume caffeine and alcohol combinations. These beverages are characterized by a blend of naturally-occurring stimulants (e.g. caffeine), sugars (e.g. glucose), and amino acids (e.g. taurine) (Marczinski et al., 2012). Although there are many purportedly ‘performance-enhancing’ ingredients in these beverages, caffeine is largely considered to be the primary psychoactive ingredient (Giles et al., 2012, Peacock et al., 2013b). As energy drinks contain more caffeine than standard cola (~30–50 mg) and alcohol mixers are often consumed in a binge fashion (e.g. ‘Jagerbomb’ shots), a larger quantity of caffeine is allowed to accumulate, enhancing its potential to alter alcohol intoxication. This may explain, in part, demonstrated associations between co-consumption and a more hazardous intoxicated state than alcohol intoxication alone.
There is some evidence that energy drink co-consumption is associated increased alcohol intake (O’Brien et al., 2008, O’Brien et al., 2013, Thombs et al., 2010), although this has not been consistently demonstrated (Verster et al., 2012, de Haan et al., 2012). An additional concern is that co-consumption has been associated with an increased risk for dangerous behavior such as driving under the influence (Thombs et al., 2010), unwanted sexual encounters (O’Brien et al., 2013) and aggression (Jones et al., 2012). The notion that caffeinated alcohol consumption is more hazardous has been contested, however, arguing that the likely higher incidence of co-consumption among sensation-seeking persons muddies the relationship (de Haan et al., 2012). Contradictory evidence recently showed, however, that co-consumption was associated with higher odds of hazardous behavior; even after controlling for sensation seeking tendency and the amount of alcohol consumed (O’Brien et al., 2013). It therefore appears that the consequences of co-consumption, indeed, warrant public health concern and further study.
Some human studies have found the addition of an energy drink or caffeine to alcohol to attenuate alcohol-induced decrements in reaction time (Heinz et al., 2013) and short-term memory (Drake et al., 2003), as well as increase subjective stimulation (Peacock et al., 2013a). However, others have found no such effects on certain measures of cognitive/psychomotor performance (Marczinski and Fillmore, 2006, Verster et al., 2012, Peacock et al., 2013a) or subjective intoxication (Alford et al., 2012). Such discordance is not surprising given differences in when the testing interval occurred following consumption and the wide range of caffeine and alcohol doses employed in these studies.
Animal models can mitigate the influence of potential confounds in human studies such as expectancy, personality, and degree of prior alcohol and/or caffeine experience. However, little work has been published in this regard (for review, see Lopez-Cruz et al., in press). The available literature suggests that caffeine administration may both increase (Kunin et al., 2000) and decrease (Rezvani et al., 2013) ethanol intake, as well as reduce various aspects of ethanol-induced motor (Dar, 1988, Yacoubi et al., 2000) and cognitive impairment (Spinetta et al., 2008). Caffeine has also been demonstrated to enhance the locomotor stimulant properties of ethanol in mice (Hilbert et al., 2013). One interpretational issue with the existing studies, however, is that such consequences following voluntary co-consumption have yet to be explored, hindering the translational validity of their findings.
In the current study, caffeine was added to the 20% alcohol solution used in a mouse model of binge-like alcohol consumption, Drinking-in-the-Dark (DID). As caffeine is largely considered to be the primary psychoactive ingredient in ‘energy drinks’, we chose to focus on this compound. An altered intoxication state, appearing to mirror findings from human studies, was observed; thereby bolstering enthusiasm for a translationally valid animal model with which the neurobehavioral consequences of this drug combination can be explored.
Methods
Animals
Adult male (PND 56 ± 3) C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and allowed 10–14 days to acclimate before experimentation (PND 66–70 ± 3 for Experiments 1 and 2 and PND 90–120 for Experiment 3 at the start of testing). Animals were maintained on a 12-hour reverse light/dark cycle with lights OFF at 0700 (Experiment 1), 0900 (Experiment 2), or 1300 (Experiment 3). Temperature and humidity were held constant near 20° C and 50%, respectively. Food and water were available ad libitum. Principles of laboratory animal care were followed and experiments were performed under a protocol approved by the IUPUI School of Science Institutional Animal Care and Use Committee.
Drinking Solutions
Ethanol (190 proof; Pharmco Inc., Brookfield, CT) was diluted to 20% (v/v) in tap water. Caffeine (Sigma-Aldrich) was dissolved in tap water (0.03 % w/v or 0.05% w/v). The 0.03% concentration is roughly equivalent to that of standard energy drinks (e.g. Red Bull©). The higher 0.05% concentration of caffeine was included as the rapid metabolism of the mouse may hinder the ability of caffeine to alter alcohol intoxication and/or intake. For the combined ethanol and caffeine solution, 20% ethanol was mixed with caffeine at either a 0.03% (w/v) or 0.05% (w/v) concentration.
Drinking in the Dark
Beginning 3 hours into the dark cycle, singly housed male B6 mice had their water bottles replaced by a 10 ml sipper tube containing tap water vehicle (W), ethanol (20% v/v; E), caffeine (0.03 or 0.05% w/v; C3 or C5), or combined ethanol and caffeine (EC3 or EC5) for 2 hours. During this period, the only fluid available was the designated test fluid.
Home Cage Locomotor Activity Monitors
Details concerning the exact monitors (Columbus Instruments Inc., Columbus, OH) used in the current study were previously published (Linsenbardt and Boehm, 2012). Each day, animals’ cages were moved into the activity monitors immediately prior to lights out to allow a full 3 hours for habituation prior to DID and mice were allowed to sit for at least 30 minutes following DID before being moved out of the monitors.
Balance Beam
Our balance beam apparatus and procedure were recently described (Linsenbardt et al., 2011). Ataxia was assessed by recording the number of times an animal’s hind feet slipped off of the edges of the beam as it was traversed. All tests occurred in dim red light.
Elevated Plus Maze
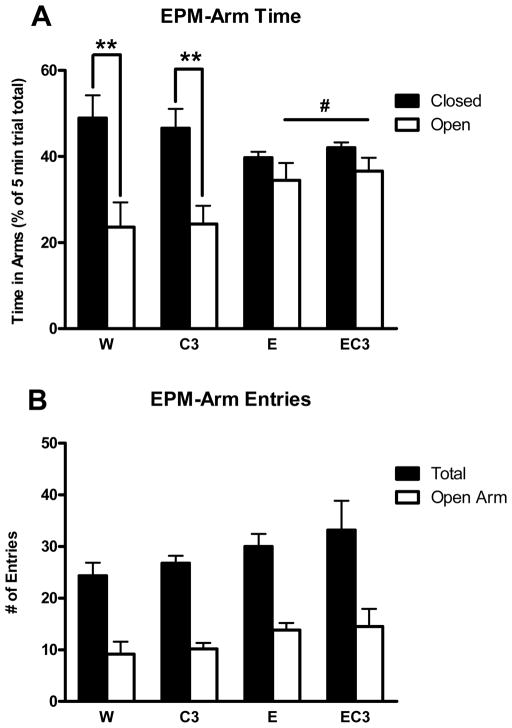
Detailed methods for the elevated plus maze (EPM) are described elsewhere (Moore et al., 2011). Mice were placed in the center of the EPM and behavior was recorded by a camcorder mounted above the apparatus for 5 minutes. The luminance at the center of the EPM was 90 lux (± 3). Videos were coded for time spent in the open and closed arms separately with anxiety-like behavior being characterized by animals spending significantly more time in the closed relative to the open arms.
Novel Object Recognition
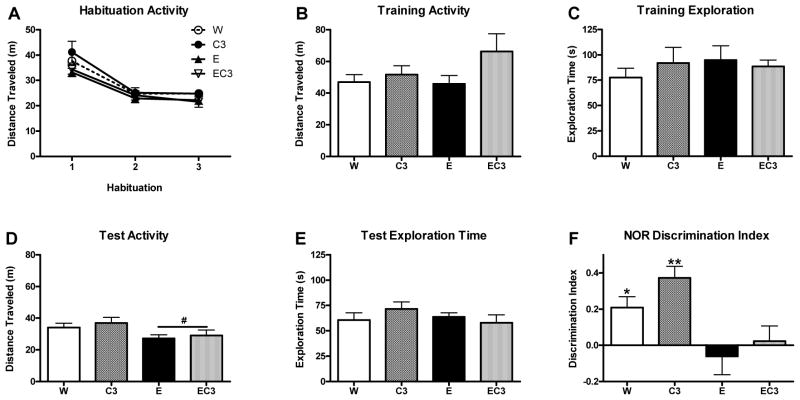
Our procedure was modeled after Ryabinin and colleagues (2002). All procedures occurred under red light (~8 lux). Animals were habituated to the testing arena (L: 40cm × W: 40 cm × H: 28cm) following lights out for 10 minutes on 3 successive days. The following day, mice were placed in the arena for a training trial immediately following the 2-hr DID session with two identical objects in opposite corners of the arena for 10 minutes. The following test day, mice were placed in the arena with a single object they had explored the previous day as well as a novel object. During all sessions, activity was recorded via video tracking using ANY-Maze software (Stoelting). Total exploration time (time spent sniffing or contacting both objects) was recorded by an experimenter blind to condition via direct video observation during both the training and test sessions. A photo of the objects can be found in the supplementary material.
Experiment 1: The effect of caffeine and ethanol co-consumption on home cage locomotor activity
Mice (n = 12–13) were given daily limited access to W, E, C3, C5, EC3, or EC5 for 14 days via DID, during which home cage locomotor activity was recorded. Mice from the E, EC3, and EC5 groups had periorbital blood samples collected following access on day 14 for BEC analysis by an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Experiment 2: The effect of caffeine and ethanol co-consumption on ethanol-induced ataxia
Mice (n = 12) were given access to W, E, C3, or EC3 15 days. Only the 0.03% concentration of caffeine was used from this point forward because the 0.05% concentration may have interfered with alcohol intake/BEC (Figure 1) in Experiment 1. Ataxia testing on the balance beam was conducted following DID on days 8 and 15. On day 16, mice were administered an acute challenge of ethanol (1.75 g/kg, i.p.; 20% v/v in saline) in place of DID and were tested for ataxia 10 minutes later to allow for full ethanol absorption. Blood samples were taken immediately following the test and again at 40 and 90 minutes following injection in order to evaluate the effect of consumption history on ethanol metabolism.
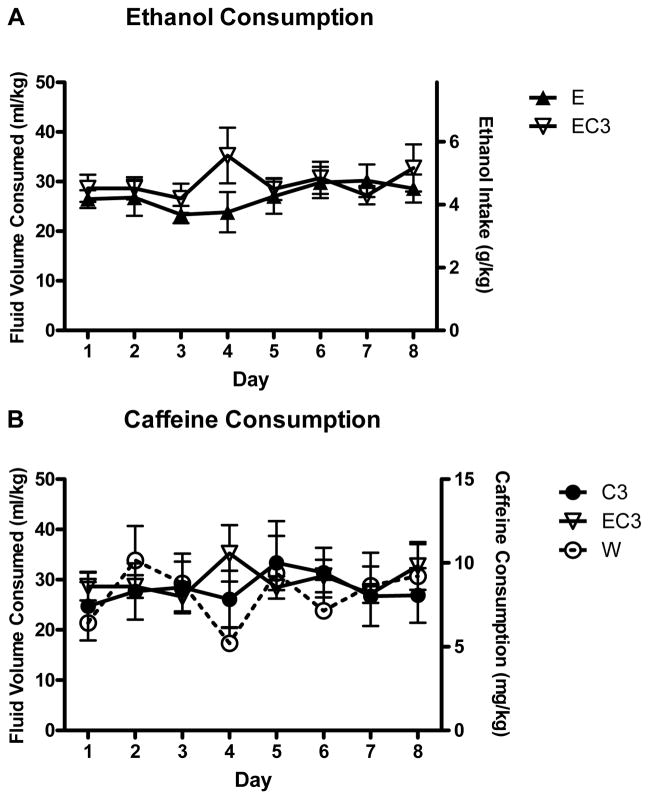
Figure 1.
Daily fluid intakes over the course of the 14 day DID procedure in Experiment 1 (n = 12–13). Where represented, the water (W) group is the same 12 animals and these animals only had access to water in DID. The symbols for W groups only indicate fluid volume consumed, not ethanol or caffeine intake. A) Average ethanol intake in animals that had access to some form of ethanol. B) Average caffeine intake for animals that had access to 0.03% caffeine. C) Average caffeine intake for animals that had access to 0.05% caffeine.
Experiment 3: The effect of caffeine and ethanol co-consumption on anxiety-like behavior and novel object recognition
Mice (n = 6) were given access to W, E, C3, or EC3 for 8 days via DID. Following DID on day 3, each mouse was transported into the EPM testing room and placed in the center of the apparatus and video was recorded for the 5 minute test. On days 5–7, mice were habituated to the NOR arena. Immediately after DID on day 8, mice were placed in the arena for training. On day 9, no DID occurred and the animals were tested for NOR at the same time they were previously trained on day 8. Behavioral testing did not occur until day 3 to limit the influence of DID novelty on behavior (Linsenbardt and Boehm, 2012). As our data from Experiment 1 also suggested that ethanol-induced locomotor sedation could interfere with task performance after ~ 8–9 days of consecutive access, we compacted the timeline of this experiment.
Statistical Analysis
Data were analyzed by Analysis of Variance (ANOVA) or t-test. As we were interested in addressing the potential interaction between caffeine and ethanol, ‘ethanol’ [yes (EC3/E) or no (C3/W)] and ‘caffeine’ [yes (C3/EC3) or no (W/E)] were factors in the relevant ANOVA analyses. This allowed us to evaluate whether or not there were main effects of caffeine/ethanol, or an interaction. Repeated measures ANOVAs also included day or time as the within-subjects factor. The descriptors ‘ethanol’ and ‘caffeine’ in the results section below refer to these aforementioned designations. The significance level was set at p < 0.05 and Newman-Keuls post-hoc statistics were run where applicable. All analyses were carried out with Statistica 7 software (Statsoft).
RESULTS
Experiment 1: The effect of caffeine and ethanol co-consumption on home cage locomotor activity
Fluid Consumption and BEC
The 0.03% analysis (W, E, C3, EC3) of total fluid intake found that ethanol-consuming groups (E/EC3) consumed significantly less fluid than the non-ethanol consuming groups (C3/W); F1,48 = 7.944; p < 0.01, Figures 1A/B. This pattern is consistent with what we typically observe in DID (Linsenbardt and Boehm, 2012, Linsenbardt and Boehm, 2013). For the 0.05% analysis (W, E, C5, EC5), no factors or interactions reached statistical significance (all p’s > 0.083; Figures 1A/C).
Ethanol intake (E, EC3, EC5) did not differ between groups across DID (Figure 1A; p > .05), although the EC5 group trended towards a lower mean BEC following DID on day 14 (E = 164.0 mg/dL ± 13.9; EC3 = 174.5 mg/dL ± 16.07; EC5 = 130.3 mg/dL ± 9.51; F2,38 = 2.96, p = 0.06). A main effect of caffeine concentration was found for caffeine intake (F1,47 = 31.08, p < 0.001) with the C5 and EC5 groups consuming significantly more caffeine overall than both the C3 and EC3 groups (Figures 1B/C). A caffeine concentration × ethanol interaction trended toward significance (F1,47 = 3.26, p = 0.07) suggesting slightly greater caffeine intake in C3 versus EC3 mice and this was confirmed by a follow up test (F1,24 = 5.649, p < 0.05).
Locomotion
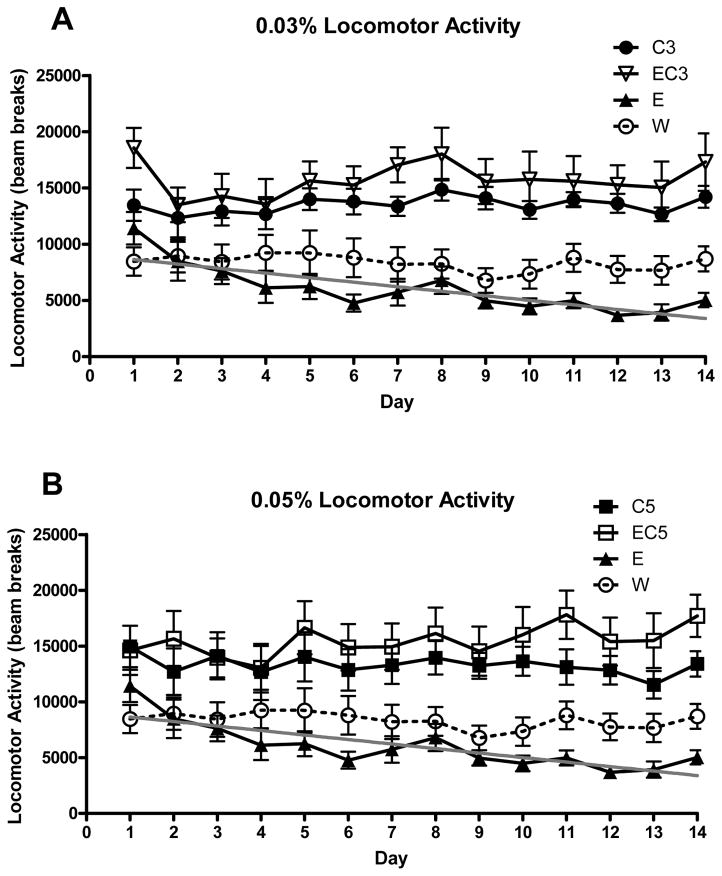
Analyses were split by caffeine concentration. For the 0.03% concentration analysis (W, E, C3, EC3), a main effect of caffeine was found (F1,48 = 40.968, p < 0.001) with the C3/EC3 groups being more active (p < 0.05) (Figure 2A). Ethanol was not a significant factor for activity due to the extreme difference between E and EC3. The overall activity of the E and W groups did not significantly differ (p > 0.05), however a planned comparison of the activity data slopes over days between these groups revealed a significant difference (F1,360 = 7.877, p < 0.01) with the E group demonstrating a negative trend, indicating locomotor sedation (Figure 2A). Both caffeine (F13,624 = 2.901, p < 0.001) and ethanol (F13,624 = 2.286, p < 0.01) significantly interacted with day. Follow-up analyses determined that the EC3 group exhibited particularly high activity on day 1 (p < .05) and W/C3 groups progressively decreased their activity over the course of the experiment (Figure 2A; p < .05). For the 0.05% caffeine analysis, again a main effect of caffeine was found (F1,47 = 26.173, p < 0.001) with the C5/EC5 groups being more active (Figure 2B). A significant caffeine × day interaction was also detected (F13,611 = 2.28, p < 0.01), with non-consuming groups (E/W) exhibiting steady decline in activity across days, although this was largely driven by the E group. A significant ethanol × caffeine × day interaction was detected (F13,611 = 3.08, p < 0.001) with the degree of difference between the C5 and W group being smaller and fluctuating to a greater extent than the difference between EC5 and W.
Figure 2.
Home cage locomotor activity during DID access periods over the course of the 14 day DID procedure in Experiment 1 (n = 12–13). The E and W groups in both panels represent the same groups of animals. A) Average locomotor activity for animals that had access to 0.03% caffeine relative to W and E animals. Gray regression line demonstrates statistically significant negative slope of the measurements for the E group over days (R2 = 0.154, p < 0.001). B) Average locomotor activity for animals that had access to 0.05% caffeine relative to the same W and E animals.
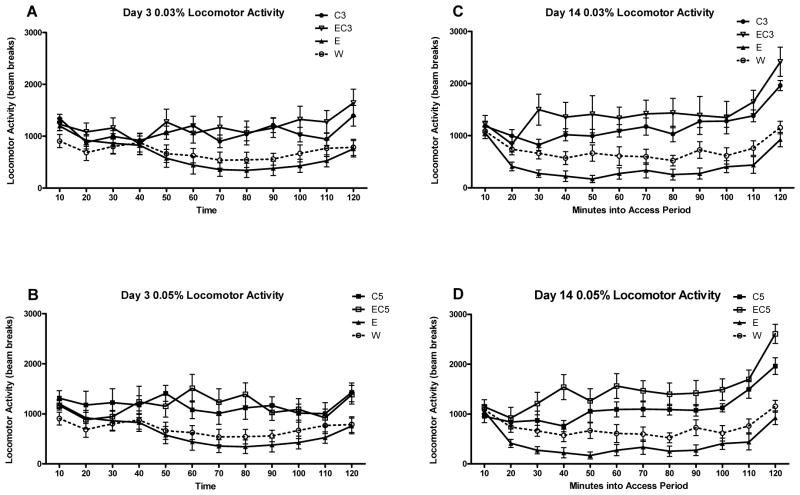
As ambulatory activity during DID sessions was also of interest, we analyzed the pattern of home cage activity early on (day 3; Figure 3, panels A&B) and late (day 14; Figure 3, panels C&D) in the 14-day DID procedure. Day 3 was chosen rather than days 1 or 2 due to previous findings in our lab that the novelty of the DID procedure can influence the locomotor effects of ethanol (Linsenbardt and Boehm, 2012). On day 3, a main effect of caffeine was found with C3/EC3 groups exhibiting greater activity (F1,48 = 13.676, p < 0.001). A significant caffeine × time interaction (F11,528 = 4.516, p < 0.001) also indicated that this difference was apparent from the 50 minute time point onward (p < .05). The analysis of the 0.05% caffeine concentration also indicated that caffeine groups (C5/EC5) exhibited greater activity (F1,47 = 13.102, p < 0.001). Caffeine also interacted with time (F11,517 = 3.348, p < 0.001) with this difference also occurring at the 50 minute time point and beyond (p < .05) with the exception of the 110 minute bin. A significant caffeine × ethanol × time interaction (F11,517 = 2.386, p < 0.01) was also found with caffeine promoting significant differences in locomotor activity between the EC5 and E groups within the 60–90 minute bins (p < .05).
Figure 3.
Home cage locomotor activity during DID access periods on days 3 and 14 of Experiment 1 (n = 12–13). The E and W groups in the 0.03% and 0.05% panels are identical. A,B) Average locomotor activity in 10 minute bins for animals that had access to 0.03% or 0.05% caffeine relative to W and E animals during DID on day 3. C,D) Average locomotor activity in 10 minute bins for animals that had access to 0.03% or 0.05% caffeine relative to W and E animals during DID on day 14.
The analysis of day 14 home cage data for the 0.03% concentration found a main effect of caffeine (F1,48 = 32.591, p < 0.001) with the C3/EC3 groups being significantly more active. An ethanol × caffeine interaction was also significant (F1,48 = 5.594, p < 0.05), indicating that caffeine produced a substantial difference between the E and EC3 groups. In addition, caffeine significantly interacted with time (F11,528 = 3.743, p < 0.001), indicating that the EC3/C3 groups were significantly more active at the 30 minute time point and beyond (p < .05). For the 0.05% analysis, a main effect of caffeine (F1,47 = 45.9, p < 0.001), as well as an interaction with ethanol (F1,47 = 9.67, p < 0.01) were found. Post-hoc testing revealed that the EC5 group was significantly more active than all other groups, overall (p < .05). In addition, caffeine significantly interacted with time (F11,517 = 6.97, p < 0.001), indicating that the C5/EC5 groups were significantly more active at the 30 minute time point and beyond (p < .05).
Experiment 2: The effect of caffeine and ethanol co-consumption on ethanol-induced ataxia
Fluid Consumption
Ethanol-consuming groups consumed more fluid, overall (F1,44 = 4.169, p < .05; Figure 4). Significant caffeine × day (F14,294 = 3.061, p < 0.001) and ethanol × day interactions were also found (F14,616 = 3.023, p < 0.001), with the C3/W groups drinking particularly low volumes of fluid on days 12–14 (p < 0.05); an effect likely representative of reactivity to the first ataxia test. Overall ethanol or caffeine intake did not differ between the EC3 and E/C3 groups, respectively, across the experiment (p > 0.05).
Figure 4.
Daily fluid intake over the course of the 15 day DID procedure in Experiment 2 (n = 12). A) Average ethanol intake in animals that had access to some form of ethanol. Ethanol intake was not different between groups prior to ataxia test 1 on day 8 (E = 3.914 g/kg ± 0.246, EC3 = 4.284 g/kg ± 0.24; p > 0.05) or ataxia test 2 on day 15 (E = 4.248 g/kg ± 0.268, EC3 = 4.703 g/kg ± 0.324; p > 0.05). B) Average caffeine intake for animals that had access to some form of caffeine. Arrows denote the ataxia tests conducted after DID access on days 8 and 15.
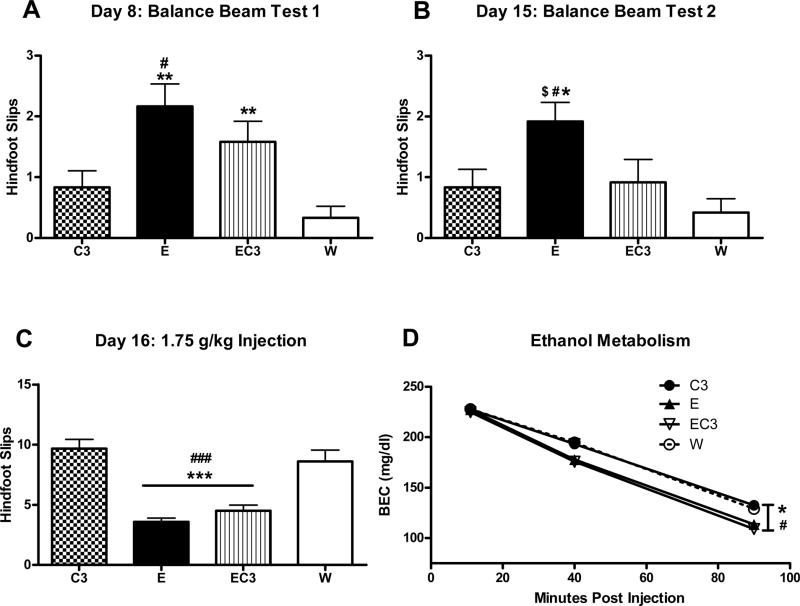
Ataxia
Following fluid access on day 8, a main effect of ethanol was found with the E/EC3 groups being significantly ataxic on the balance beam (Figure 5A; F1,44 = 18.77, p < 0.001). An ethanol × caffeine interaction approached significance (F1,44 = 3.3, p = 0.076) with ethanol trending towards producing a greater difference between E/W than EC3/C3. Ethanol-consuming groups (E/EC3) were also found to be significantly more ataxic following access on day 15 (Figure 5B; F1,44 = 6.56, p < 0.05), however, a significant caffeine × ethanol interaction (F1,44 = 5.254, p < 0.05) revealed that E group drove this main effect as the EC3 group was significantly less ataxic than the E group (p < .05) and not different from the C3 or W groups (p > .05).
Figure 5.
Balance beam assessment of ataxia immediately following DID access A) on day 8 and B) day 15 of Experiment 2. C) Functional tolerance to ethanol alone was also assessed on day 16 with a 1.75 g/kg injection of ethanol (i.p.), where no DID access was given, 10 minutes before animals were tested on the beam. D) BEC values from samples collected at 11, 40, and 90 minutes after the 1.75 g/kg ethanol challenge on day 16. *p < 0.05, **p < 0.01, ***p < 0.001 versus the W group. #p < 0.05, ###p < 0.001 versus the C3 group. $p < 0.05 versus the EC3 group; n = 12.
Ethanol-consuming (E/EC3) mice were found to be significantly less ataxic following the 1.75 g/kg ethanol challenge on day 16 (F1,44 = 62.035, p < 0.001), illustrating significant behavioral tolerance (Figure 5C). Finally, metabolic tolerance was observed in ethanol-consuming (E/EC3) mice as they cleared ethanol more rapidly than those that did not (F1,44 = 16.77, p < 0.001) (Figure 5D). Caffeine exposure had no effect on ethanol metabolism (p > 0.05).
Experiment 3: The effect of caffeine and ethanol co-consumption on anxiety-like behavior and novel object recognition
Fluid Intake
No differences between groups were detected for overall fluid, ethanol, or caffeine intake over days (p > 0.05; Figure 6). Intake was also not different between groups on day 3 prior to EPM testing or day 8 prior to NOR training (p > .05)
Figure 6.
Daily fluid intake over the course of the 8 day DID procedure in Experiment 3 (n = 6). A) Average ethanol intake in animals that had access to some form of ethanol. B) Average caffeine/fluid intake for animals that had access to some form of caffeine or water.
Elevated Plus Maze
Following DID on day 3, a main effect of ethanol was found with E/EC3 groups spending significantly more time in the open arms of the maze (F1,19 = 6.84, p < 0.05). Follow up t-tests confirmed that both the W (t10 = 3.248, p < .01) and C3 (t8 = 3.615, p < .01) groups exhibited significant anxiety-like behavior as indicated by a strong preference for the closed, relative to the open arms of the EPM whereas the E and EC3 groups showed no such preference (p’s > 0.05; Figure 7A). No differences were found between groups in total or open arm entries (p’s > 0.05) (Figure 7B).
Figure 7.
Elevated plus maze assessment of anxiety-like behavior following DID access on day 3 in Experiment 3. A) Time spent in the open and closed arms in each group expressed as a percentage of the total 5 minute test. B) Entries into the open arms or total arm entries as an index of activity. **p < 0.01 for difference between open and closed arms within the designated group; #p < 0.05 for main effect of ethanol on the open arms with the E/EC3 groups spending significantly more time in the open arms than the W/C3 groups; n = 6 (5 for C3).
Novel Object Recognition
Across the 3 habituation days (days 5–7), the activity of all animals in the NOR arena significantly decreased as indicated by a main effect of day (F2,38 = 95.22, p < 0.001; Figure 8A), suggesting habituation occurred. Following DID on day 8, no group differences were detected for total exploration time or activity during training (Figure 8B and C; p’s > 0.05). The activity of E/EC3 mice was slightly lower during the test on day 9 (F1,19 = 5.926, p < 0.05; Figure 8D); importantly, however, exploration time did not differ between groups (Figure 8E; p’s > 0.05). To quantify NOR, a discrimination index was computed ((time with novel object - time with familiar object)/total exploration time). As expected, the W (t5 = 3.501, p < 0.05) and C3 (t4 = 5.765, p < 0.01) groups exhibited NOR as evidenced by indices significantly greater than ‘0’ (Figure 8F). However, neither the E nor EC3 group exhibited significant NOR (p’s > 0.05).
Figure 8.
Behavioral measures over the course of the NOR procedure in Experiment 3. A) Activity during the 3 habituation periods on days 5–7. B,D) Total distance traveled in meters during training on day 8 and the NOR test on day 9. C,E) Total time spent exploring both objects during training on day 8 or the NOR test on day 9. F) Discrimination index on day 9 indicating the degree of novel object preference with a ‘0’ value indicating indifference. p* < 0.05, **p < 0.01 versus ‘0’; #p < 0.05 for main effect of ethanol consumption history; n = 6 (5 for C3).
Discussion
The present study demonstrated that caffeine altered some neurobehavioral consequences of binge alcohol consumption typically seen in B6 mice using the DID paradigm. Although ethanol consumption or resultant BEC was not altered, co-consumption produced no evidence of alcohol-induced sedation at equivalent alcohol dosage and even produced a highly stimulated state. Furthermore, caffeine was found to antagonize alcohol-induced ataxia. However, co-consumption had no effect on alcohol-induced anxiolysis or cognitive interference assessed by the EPM and NOR, respectively. Therefore, the capacity of caffeine to alter ethanol intake may not be the primary consequence of co-consumption, but rather how caffeine may alter the nature of intoxication.
To our knowledge, this study is the first to evaluate voluntary binge-like caffeine or caffeinated ethanol consumption in mice. Previous studies have either systemically injected caffeine (Kunin et al., 2000, Rezvani et al., 2013) or forced continuous access for long durations (Hughes, 2011). In the present study, we demonstrated that male B6 mice will not only voluntarily consume behaviorally relevant levels of caffeine, but will also consume combined ethanol/caffeine solutions via DID, reaching BECs equivalent to animals that consume ethanol alone. This is a key strength of this voluntary consumption model as the EC and E groups match their ethanol dosing, allowing for more streamlined interpretation of the capacity of caffeine to alter effects of alcohol. Although a different question, caffeine may alter alcohol intake probed at later time points (beyond 2 hours). Male B6 mice will drink alcohol to the point of sedation in the DID paradigm (Linsenbardt and Boehm, 2013; current study), but this is not observed in combination with caffeine. Therefore, extending the access period may allow more time for this activity difference to influence ethanol intake differences between groups.
Findings from Experiment 1 highlighted that mice consuming EC solutions demonstrated no evidence of sedation and were similarly stimulated to those that consumed caffeine alone. We hypothesized that caffeine would antagonize the alcohol-induced sedation we typically observe over repeated cycles of DID. Although caffeine appeared to reverse this aspect of alcohol intoxication, we cannot directly conclude that this was not due to the robust stimulant effect of caffeine. However, caffeine administration can reverse the sedative-hypnotic effects of ethanol at a much higher dose of ethanol than mice self-administered in the present study (El Yacoubi et al., 2003). Furthermore, we observed that caffeine interferes with alcohol-induced motor impairment on the balance beam (Figure 5A/B) and considering these data, we speculate that reversal of sedation is likely. Additionally, considering the equivalent degree of stimulation in the EC3 and C3 groups (Figure 2A), in light of significantly lower caffeine intake by EC3 group (Figure 1B), as well as greater activity of the EC5 group towards the end of the experiment (Figure 2B), there may be some evidence of a mild additive effect as has been previously demonstrated with systemic administrations (Hilbert et al., 2013). Finally, the observation that the EC3 group was significantly more active than all other groups at equivalent ethanol intakes and lower caffeine intake on day 1 (Figure 2A) suggests a potential interaction of co-consumption with novelty.
It was also found that after 15 days, caffeine antagonized ethanol-induced ataxia in Experiment 2 (Figure 5B). Previous studies suggest that the dose-response curve for caffeine on ethanol-induced motor impairment is U-shaped, with mid-range doses appearing to antagonize ataxia and low/high doses having no effect or even increasing impairment (Dar, 1988, Dar et al., 1987). Our data may suggest that mice consumed a mid-range dose, effectively reducing motor impairment. As the reduction in ataxia was only a trend following access on day 8 in the EC3 group, the duration of co-consumption may be important for the observed effect of caffeine on ethanol-induced ataxia. It may therefore be that caffeine facilitated the development of functional tolerance. The 1.75 g/kg ethanol challenge on day 16 detected functional tolerance development in all mice that consumed alcohol (E/EC3), consistent with previous observations of our lab (Linsenbardt et al., 2011). While this finding may appear to conflict with the observations of ataxia test 2, EC3 mice had only experienced ethanol intoxication in combination with caffeine, also likely at lower peak ethanol concentrations than was produced by the injection. Finally, it is important to note that differences between the E and EC groups outlined above cannot be due to differential ethanol pharmacokinetics (Figure 5D). Previous studies have also shown that caffeine (Kunin et al., 2000) or energy drink administration (Ferreira et al., 2004) does not alter ethanol metabolism in rodents when simultaneously administered.
Caffeine did not alter all measures alcohol intoxication, however, with mice exhibiting significant anxiolysis and affected NOR as a consequence of both E and EC consumption. An acute injection of ethanol has been previously demonstrated to produce anxiolysis on the EPM (Homanics et al., 1999) as well as impaired NOR performance in B6 mice (Ryabinin et al., 2002), however this study is the first, to our knowledge, to demonstrate such effects as a consequence of voluntary binge consumption. On their own, these new findings are important for the field as validations of binge alcohol intoxication produced by the DID model. However, they also highlight that co-consumption of caffeine selectively alters certain aspects of voluntary binge alcohol intoxication, but not others.
Considering the locomotor effects of co-consumption demonstrated in Experiment 1, there may be concern that these effects influenced behavior on the other tasks. However, our activity measures in the other tasks (EPM and NOR) indicate no activity differences produced by consumption of the various fluids (Figures 6B and 8B). Therefore, this locomotor effect does not seem apparent outside of the home cage and likely does not interfere with our other behavioral assessments. Furthermore, the NOR results may be reflective of state-dependent learning rather than actual ethanol impairment of NOR. Although state-dependent learning is a possibility, these data nevertheless suggest that ethanol-consumption (regardless of caffeine presence) influenced the exhibition of NOR in some capacity, implying that this effect of ethanol intoxication persisted.
The mechanisms by which caffeine alters ethanol intoxication may be related to actions at common effector systems. The adenosine system has been implicated in alertness and wakefulness; effects produced by caffeine via the antagonism of adenosine receptors (Fredholm et al., 1999). Ethanol also acts upon the adenosine system, preventing reuptake via the inhibition of equilibrative nucleoside transporter 1 (ENT1) (Nagy et al., 1990). In the current study, ethanol consumption produced significant markers of intoxication across all tasks, however, caffeine was only found to alter aspects of alcohol-induced motor impairment (sedation and ataxia). Therefore, caffeine may have more potently modulated alcohol-affected neurotransmission in regions involved in ambulation and coordination (e.g. motor cortex, striatum, cerebellum). The resultant increase in extracellular adenosine induced by ethanol likely contributes to its sedative-hypnotic and motor-impairing effects which are blunted in ENT1 knockout mice (Choi et al., 2004) and can be mitigated by an injection of caffeine in wildtype mice (El Yacoubi et al., 2003). In general, the A1 subtype of the adenosine receptor appears to be more important for caffeine’s ability to antagonize ethanol-induced motor incoordination whereas the A2A receptor appears to be more important in regulating the sedative-hypnotic effects of ethanol (see Lopez-Cruz et al., 2013 for review). It is not clear why caffeine modulated aspects of alcohol intoxication mediated by both of these receptor subtypes, but did not affect recognition memory or anxiety-like behavior, both of which have been demonstrated to be positively influenced by acute caffeine administration in the absence of ethanol (Prediger et al., 2005, El Yacoubi et al., 2000). One reason may be that the oral doses of caffeine consumed by mice in the current study (~10–15 mg/kg) do not reach the same peak concentrations in brain resulting from the injected doses (10–100 mg/kg; i.p.) that produced these effects. Another potential issue is that ethanol influences a host of other neurotransmitter systems (e.g. GABA, DA, GLU) which may convolute caffeine’s ability to modify ethanol’s effect on such complex cognitive-behavioral modalities as recognition memory and anxiety. Future work addressing the role of adenosine receptor subtypes in specific brain regions involved in these various behaviors will clarify the receptor/phenotype specificity of caffeine’s potential to alter alcohol intoxication.
Our results echo a number of intriguing findings in the human literature. Heightened subjective stimulation has been observed following energy drink co-consumption relative to alcohol alone (Attwood et al., 2012, Peacock et al., 2013a). Caffeine and energy drinks have been shown to antagonize fatigue (Drake et al., 2003, Marczinski et al., 2012) as well as improve decrements in reaction time (Drake et al., 2003, Azcona et al., 1995) associated with alcohol use; observations potentially associated with a stimulated state. In addition, caffeine has been found to attenuate some aspects of psychomotor impairment produced by ethanol consumption (Drake et al., 2003, Howland et al., 2011). However, ethanol-associated lightheadedness and cognitive deficits were still found to persist in individuals that consumed a combined solution (Drake et al., 2003, Heinz et al., 2013), suggesting that some aspects of intoxication remain when individuals are under the influence of both compounds.
In conclusion, the present study characterized an animal model of binge ethanol and caffeine co-consumption that has the capacity to significantly improve the translational validity of preclinical research on this topic. These findings align with human self-report data on the motivation to consume caffeinated alcoholic beverages as well as empirical data. The altered neurobehavioral state of co-consumption relative to ethanol intoxication alone is of concern as intoxicated individuals may not only be less sedated, but stimulated, less ataxic, and less anxious; a state which may influence decisions to engage in impulsive/risky behaviors (O’Brien et al., 2008, Amlung et al., 2013, O’Brien et al., 2013). This new approach can be valuable in advancing our understanding of the motivation for and consequences of binge ethanol and caffeine co-consumption as well as elucidating their neurobiological mechanisms. As this type of drinking behavior is rather new, its public health consequences are likely not yet fully realized and enhancing our understanding of its acute and protracted neurobiological effects should be paramount.
Supplementary Material
Acknowledgments
This work was supported by NIAAA grants AA016789 (S. B.) and AA07462 (B.F.). We would also like to thank Zachary Masney, Eric Herod, and Benjamin Dowden for their assistance in completing the work described above.
References
- Alford C, Hamilton-Morris J, Verster J. The effects of energy drink in combination with alcohol on performance and subjective awareness. Psychopharmacology. 2012;222:519–532. doi: 10.1007/s00213-012-2677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Few LR, Howland J, Rohsenow DJ, Metrik J, Mackillop J. Impulsivity and alcohol demand in relation to combined alcohol and caffeine use. Experimental and Clinical Psychopharmacology. 2013;21:467–474. doi: 10.1037/a0034214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood A, Rogers P, Ataya A, Adams S, Munafò M. Effects of caffeine on alcohol-related changes in behavioural control and perceived intoxication in light caffeine consumers. Psychopharmacology. 2012;221:551–560. doi: 10.1007/s00213-011-2601-0. [DOI] [PubMed] [Google Scholar]
- Azcona O, Barbanoj MJ, Torrent J, Jane F. Evaluation of the central effects of alcohol and caffeine interaction. British Journal of Clinical Pharmacology. 1995;40:393–400. doi: 10.1111/j.1365-2125.1995.tb04562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D-S, Cascini M-G, Mailliard W, Young H, Paredes P, Mcmahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nature neuroscience. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Dar MS. The biphasic effects of centrally and peripherally administered caffeine on ethanol-induced motor incoordination in mice. Journal of Pharmacy and Pharmacology. 1988;40:482–487. doi: 10.1111/j.2042-7158.1988.tb05282.x. [DOI] [PubMed] [Google Scholar]
- Dar MS, Jones M, Close G, Mustafa SJ, Wooles WR. Behavioral interactions of ethanol and methylxanthines. Psychopharmacology. 1987;91:1–4. doi: 10.1007/BF00690916. [DOI] [PubMed] [Google Scholar]
- De Haan L, De Haan HA, Van Der Palen J, Oivier B, Verster J. Effects of consuming alcohol mixed with energy drinks versus consuming alcohol only on overall alcohol consumption and negative alcohol-related consequences. Int J Gen Med. 2012;5:953–960. doi: 10.2147/IJGM.S38020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Turner L, Scofield HM, Roth T. Caffeine reversal of ethanol effects on the multiple sleep latency test, memory, and psychomotor performance. Neuropsychopharmacology. 2003;28:371–378. doi: 10.1038/sj.npp.1300026. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois J-M. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A2A adenosine receptor antagonists. Psychopharmacology. 2000;148:153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology. 2003;45:977–985. doi: 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, Hartmann Quadros IM, Trindade ÁA, Takahashi S, Koyama RG, Souza-Formigoni MLO. Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiology & Behavior. 2004;82:841–847. doi: 10.1016/j.physbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews. 1999;51:83–133. [PubMed] [Google Scholar]
- Giles GE, Mahoney CR, Brunyé TT, Gardony A, Taylor HA, Kanarek RB. Differential cognitive effects of energy drink ingredients: caffeine, taurine, and glucose. Pharmacology Biochemistry and Behavior. 2012 doi: 10.1016/j.pbb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, De Wit H, Lilje TC, Kassel JD. The combined effects of alcohol, caffeine, and expectancies on subjective experience, impulsivity, and risk-taking. Experimental and Clinical Psychopharmacology. 2013;21:222. doi: 10.1037/a0032337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert ML, May CE, Griffin WC., III Conditioned reinforcement and locomotor activating effects of caffeine and ethanol combinations in mice. Pharmacology Biochemistry and Behavior. 2013;110:168–173. doi: 10.1016/j.pbb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacology Biochemistry and Behavior. 1999;63:21–26. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Howland J, Rohsenow DJ, Arnedt JT, Bliss CA, Hunt SK, Calise TV, Heeren T, Winter M, Littlefield C, Gottlieb DJ. The acute effects of caffeinated versus non-caffeinated alcoholic beverage on driving performance and attention/reaction time. Addiction. 2011;106:335–341. doi: 10.1111/j.1360-0443.2010.03219.x. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol. 2011;45:365–372. doi: 10.1016/j.alcohol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Jones SC, Barrie L, Berry N. Why (not) alcohol energy drinks? A qualitative study with Australian university students. Drug and Alcohol Review. 2012;31:281–287. doi: 10.1111/j.1465-3362.2011.00319.x. [DOI] [PubMed] [Google Scholar]
- Kunin D, Gaskin S, Rogan F, Smith BR, Amit Z. Caffeine promotes ethanol drinking in rats: Examination using a limited-access free choice paradigm. Alcohol. 2000;21:271–277. doi: 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Role of novelty and ethanol history in locomotor stimulation induced by binge-like ethanol intake. Alcoholism: Clinical and Experimental Research. 2012;36:887–894. doi: 10.1111/j.1530-0277.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Alterations in the rate of binge ethanol consumption: implications for preclinical studies in mice. Addiction Biology. 2013 doi: 10.1111/adb.12052. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcoholism: Clinical and Experimental Research. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cruz L, Salamone JD, Correa M. The impact of caffeine on the behavioral effects of ethanol related to abuse and addiction: A review of animal studies. Journal of Caffeine Research. 2013;3:9–21. doi: 10.1089/jcr.2013.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Clubgoers and their trendy cocktails: Implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Experimental and Clinical Psychopharmacology. 2006;14:450–458. doi: 10.1037/1064-1297.14.4.450. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR. Effects of energy drinks mixed with alcohol on information processing, motor coordination and subjective reports of intoxication. Experimental and Clinical Psychopharmacology. 2012;20:129–138. doi: 10.1037/a0026136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Linsenbardt DN, Melón LC, Boehm SL. Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: Anxiety-like, locomotor, and consummatory behaviors. Developmental Psychobiology. 2011;53:141–156. doi: 10.1002/dev.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso D, Franklin C, Gordon A. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. Journal of Biological Chemistry. 1990;265:1946–1951. [PubMed] [Google Scholar]
- O’brien MC, Mccoy TP, Egan KL, Goldin S, Rhodes SD, Wolfson M. Caffeinated Alcohol, Sensation Seeking, and Injury Risk. Journal of Caffeine Research. 2013 doi: 10.1089/jcr.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’brien MC, Mccoy TP, Rhodes SD, Wagoner A, Wolfson M. Caffeinated cocktails: Energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Academic Emergency Medicine. 2008;15:453–460. doi: 10.1111/j.1553-2712.2008.00085.x. [DOI] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Martin FH, Carr A. The Impact of Alcohol and Energy Drink Consumption on Intoxication and Risk-Taking Behavior. Alcoholism: Clinical and Experimental Research. 2013a;37:1234–1242. doi: 10.1111/acer.12086. [DOI] [PubMed] [Google Scholar]
- Peacock A, Martin FH, Carr A. Energy drink ingredients. Contribution of caffeine and taurine to performance outcomes. Appetite. 2013b;64:1–4. doi: 10.1016/j.appet.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats: Involvement of adenosine A1 and A2A receptors. Neurobiology of Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Sexton HG, Johnson J, Wells C, Gordon K, Levin ED. Effects of Caffeine on Alcohol Consumption and Nicotine Self-Administration in Rats. Alcoholism: Clinical and Experimental Research. 2013;37:1609–1617. doi: 10.1111/acer.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Miller MN, Durrant S. Effects of acute alcohol administration on object recognition learning in C57BL/6J mice. Pharmacology Biochemistry and Behavior. 2002;71:307–312. doi: 10.1016/s0091-3057(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Spinetta M, Woodlee M, Feinberg L, Stroud C, Schallert K, Cormack L, Schallert T. Alcohol-induced retrograde memory impairment in rats: Prevention by caffeine. Psychopharmacology. 2008;201:361–371. doi: 10.1007/s00213-008-1294-5. [DOI] [PubMed] [Google Scholar]
- Thombs DL, O’mara RJ, Tsukamoto M, Rossheim ME, Weiler RM, Merves ML, Goldberger BA. Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addictive Behaviors. 2010;35:325–330. doi: 10.1016/j.addbeh.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Verster JC, Aufricht C, Alford C. Energy drinks mixed with alcohol: misconceptions, myths, and facts. International journal of general medicine. 2012;5:187. doi: 10.2147/IJGM.S29313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi ME, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. British journal of pharmacology. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.