Abstract
Background
Sleep disturbances are both common and well-characterized in adults with alcohol use disorders (AUDs), but have received little study in adolescents with AUDs. Furthermore, a handful of studies suggest that sleep complaints are a risk factor for AUDs. However, no published studies have yet examined the longitudinal course of sleep complaints in adolescents with AUDs; in particular, it remains unclear how persistent AUD-associated sleep complaints are in this age group, and what types of sleep complaints are most relevant to alcohol use symptoms. We investigated these questions in a 5-year longitudinal study of adolescents with and without AUDs at baseline.
Methods
Participants were 696 adolescents (age 12–19) from a longitudinal study at the Pittsburgh Adolescent Alcohol Research Center. At baseline, 347 participants had a current AUD (AUD+), while 349 had no current or past AUD (AUD−). We examined sleep and alcohol involvement at baseline as well as 1, 3, and 5-year follow-up visits. Sleep variables included self-reported insomnia and hypersomnia, as well as variability in weekday-weekend sleep duration, all at baseline. Covariates included sex, age, current alcohol symptoms, and depression severity.
Results
The AUD+ group reported more overall sleep disturbance at baseline, including greater insomnia and hypersomnia complaints, and greater variability in weekday-weekend sleep duration. Group differences in insomnia and hypersomnia complaints persisted to the 5-year and 3-year follow-ups, respectively. In the AUD− group, greater insomnia complaints at baseline predicted an increase in alcohol symptoms at the 1-year follow-up, while greater variability in sleep duration at baseline predicated an increase in alcohol symptoms at the 3- and 5-year follow-ups.
Conclusions
These results complement previous findings in other samples, indicating that insomnia and other sleep problems are a chronic predicament for adolescents with AUDs. The findings also suggest that sleep disturbances may place adolescents without AUDs at an elevated risk of developing alcohol problems.
Keywords: sleep, alcohol, adolescents, alcohol use disorders
INTRODUCTION
Sleep disturbances are common among adults with alcohol use disorders (AUDs; (Brower, 2001, Roehrs and Roth, 2001)). This relationship is well-characterized in adults, but has received little study in adolescents—a striking neglect given the propensity for sleep and alcohol problems in this age group ((Johnson et al., 2006, Young et al., 2002)). Sleep in the context of adult AUDs is highly fragmented, and deep (slow-wave) sleep is generally reduced or even absent (Pressman et al., 2007). Insomnia diagnoses remain highly prevalent during recovery, present in 36–72% of individuals in early recovery (Ford and Kamerow, 1989) and up to 91% of AUD inpatients (Cohn et al., 2003). Indeed, longitudinal data in adult male AUD inpatients suggest that sleep disturbances last months or even years into abstinence (Drummond et al., 1998). However, little extant data speaks to whether sleep problems are present early on in AUDs, potentially acting as a perpetuating factor. To our knowledge, the sole published exception is a prior paper from our group that reported more sleep problems in general (based on a sum of 5 dichotomous items) among 71 adolescents with alcohol dependence relative to a reference group of 131 adolescents without AUDs (Clark et al., 2001). This prior paper focused on cross-sectional data, however, and does not speak to the chronicity or predictive power of sleep symptoms among adolescents with and without AUDs.
Sleep complaints appear to precipitate alcohol problems throughout the lifespan. Longitudinal data from Wong and colleagues (2004, 2010) indicate that childhood sleep problems, measured during ages 3 to 8 years, predict alcohol problems during adolescence and young adulthood. However, the evidence during adolescence per se is mixed, as another prospective study found that adolescent insomnia predicted increased risk of depression and suicide attempts, but not alcohol and drug use, during young adulthood (a 6–7 year follow-up; (Roane and Taylor, 2008)). In a longitudinal study of 21–30 year-old young adults, both insomnia (OR = 7.18) and hypersomnia (OR = 13.41) were risk factors for new substance use disorders. Only hypersomnia was a statistically significant risk factor for new alcohol use disorders (OR = 3.92) (Breslau et al., 1996). Finally, multiple reports have shown that sleep disturbance predicts relapse in adults with AUDs (Brower et al., 1998, Brower et al., 2001, Drummond et al., 1998). As a whole, these findings parallel evidence that insomnia is a well-documented risk factor for mood disorders (Baglioni et al., 2011), raising the possibility of overlapping pathogenic mechanisms. Given the high prevalence of sleep complaints during adolescence (Johnson et al, 2006), it is important to understand how sleep complaints may precipitate alcohol problems and/or serve to perpetuate an AUD already present.
Moreover, sleep problems are particularly concerning in the context of AUDs because they contribute to a myriad of physical (e.g., increased risk for cardiovascular disease; (Vgontzas et al., 2009) and mental health problems (e.g., two-fold increased risk of depression; (Baglioni et al., 2011)) that might further complicate or exacerbate alcohol problems. Thus, it is critical to understand the nature and course of sleep problems in AUDs as a means to identifying potential prevention or intervention targets. Adolescence is a key developmental window for understanding sleep-AUD relationships, given that both sleep disturbance and alcohol problems accelerate during this time. Co-occurring sleep disturbance and alcohol problems may have compounding effects; recent data from college students suggests that heavy drinkers with poor sleep quality suffered more severe alcohol-related consequences ((Kenney et al., 2012). However, as noted, few published studies have examined sleep problems in adolescents with AUDs.
The present report documents the largest study of sleep complaints among adolescents with AUDs to date, using data from a longitudinal case-control design (baseline, 1-, 3-, 5-year follow-ups). Notably, our design allows for relatively fine-grained temporal analyses, given that no previous studies have conducted follow-up assessments sooner than 3.5 years after baseline. We had several aims. First, we aimed to determine the nature and persistence of AUD-associated sleep complaints in this age group, compared with a control group without AUDs. We predicted that adolescents with AUDs at baseline would report greater sleep disturbance at baseline and throughout the three follow-up visits. Second, we aimed to determine which types of sleep complaints most distinguish those with and without AUD: insomnia, hypersomnia, and/or variability in weekday-weekend sleep duration. Insomnia complaints were based on self-reported problems with falling or staying asleep, while hypersomnia complaints were based on self-reports of sleeping too much; neither were based on clinical diagnoses. Variability in weekday-weekend sleep duration is of interest because it typically manifests in adolescents as a result of mismatch between adolescents’ preferred later sleep schedules and early school start times (Lazar et al., 2013, O’Brien and Mindell, 2005). Specifically, this mismatch results in shorter sleep durations on school days (sleep loss) and longer sleep durations on weekends (“catch-up” sleep). Based on prior findings, we predicted that adolescents with AUDs at baseline would report worse sleep across all three constructs. Finally, using our control sample alone, we aimed to replicate other longitudinal data showing that sleep disturbance predicts future alcohol problems. We predicted that both baseline insomnia and baseline hypersomnia complaints would predict more alcohol problems at the 1-year follow-up.
METHODS
Participants
Participants included a total of 696 adolescents (ages 12- to 19-years old) from a longitudinal study at the Pittsburgh Adolescent Alcohol Research Center. Participants were recruited from clinical and community sources. Clinical sources included hospital-based outpatient and inpatient addictions and psychiatric programs, free-standing addictions programs, and residential programs for youth with family difficulties. Community subjects were identified from the local area using survey methods and advertisements. More details on recruitment have been presented in prior publications (Clark et al., 2001).
Participants were divided into two groups: 347 adolescents with an AUD (AUD+; DSM-IV Abuse or Dependence), and 349 adolescents with no AUD or other substance use disorder (AUD−) at baseline. Participants were excluded from analysis if they were missing data on alcohol symptoms or insomnia. See Table 1 for demographic and clinical data on these groups.
Table 1.
Demographic and clinical data at baseline
| AUD− (n=349) | AUD+ (n = 347) | Statistic | p | |
|---|---|---|---|---|
| Age | 15.77 ± 1.70 | 16.69 ± 1.26 | t = −8.11 | <0.001 |
| Sex (m/f) | 151/198 | 218/129 | χ2 = 26.72 | <0.001 |
| Race/ethnicity – n (%) | χ2 = 17.62 | <0.001 | ||
| European American | 260 (74.5) | 303 (87.3) | ||
| African American | 86 (24.6) | 43 (12.4) | ||
| Native American | 2 (0.6) | 0 (0) | ||
| Asian American | 1 (0.3) | 1 (0.3) | ||
| Drug/alcohol-related diagnosis | ||||
| Alcohol use disorder | 0 | 347 (100) | ||
| Cannabis use disorder | 0 | 271 (78.1) | ||
| Other substance use disorder | 0 | 127 (36.6) | ||
| Alcohol use, past montha | 0.60 ± 0.84 | 1.58 ± 1.53 | t = 10.42 | <0.001 |
| Daily tobacco users – n (%)b | 82 (26.6) | 243 (86.2) | χ2 = 210.98 | <0.001 |
| BDIc | 6.14 ± 6.47 | 13.83 ± 10.57 | t = −11.55 | <0.001 |
| BDI (no sleep) c | 5.80 ± 6.19 | 13.18 ± 10.20 | t = −11.54 | <0.001 |
NOTE:
Six participants (two AUD− and four AUD+) were missing past month’s alcohol use data, resulting in n=347 for AUD− and n=343 for AUD+. Scale for past month’s alcohol use: 0=0 times, 1=1–2 times, 2=3–9 times, 3= 10–20 times, 4=20+ times
106 participants (84 AUD− and 22 AUD+) were missing tobacco use data, resulting in n=265 for AUD− and n=325 for AUD+
Three participants (one AUD− and two AUD+) were missing BDI data, resulting in n=348 for AUD− and n=345 for AUD+
The study was approved by the University of Pittsburgh Institutional Review Board. Active participant and parental consent were required.
Measures
Alcohol and Substance Use Disorders
Information about AUD and other substance use disorders was gathered by revised sections of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; (Martin et al., 2000)). Diagnoses were considered to be present if the subject met the diagnostic criteria in the 6 months prior to the interview. An alcohol symptoms count variable was created by summing the number of DSM-IV alcohol-related symptoms.
Recent alcohol and tobacco use was assessed during each visit. The Drug Use Screening Inventory (DUSI; (Tarter and Hegedus, 1991)) provided data on past month’s alcohol use (0=0 times, 1=1–2 times, 2=3–9 times, 3=10–20 times, 4=20+ times). We also assessed whether participants were daily tobacco users (yes/no).
Sleep and Mood Symptoms
Several sleep scales were based on items drawn from the Health Problems Checklist (HPC), a larger checklist assessing self-reported symptoms from range of health areas (Arria et al., 1995). The HPC was administered at baseline and all three follow-up assessments. A HPC-Insomnia scale was based on the sum of four dichotomous items (‘trouble staying asleep’, ‘early morning waking’,’ trouble falling asleep’, and ‘restless sleep’). These items reflect diagnostic criteria for Insomnia Disorder, and also emerged as a single factor in factor analyses (data available upon request). A HPC-Hypersomnia scale was based on a single dichotomous item (‘sleeping too much’). A HPC-Sleep Variability scale was calculated from the absolute value of the difference between two items—weekday and weekend sleep duration—which we also analyzed as independent measures.
Given evidence that AUDs are also associated with disrupted circadian rhythms (Hasler and Clark, 2013), we examined three scales reflecting circadian rhythmicity from the Dimensions of Temperament Survey-Revised (DOTS-R; (Windle and Lerner, 1986)): Rhythmicity-Sleep, Rhythmicity-Eating, and Rhythmicity-Daily Habit. Each of these scales was based on five or six 4-point response scales ranging from 0 (‘usually false’) to 3 (‘usually true’). Typical items included “I wake up at different times” (reversed scored) and “I seem to get sleepy just about the same time every night.”
The Beck Depression Inventory (BDI; (Beck et al., 1996)) was used as a continuous measure of depressive symptomatology. The BDI’s sleep item (#16) was subtracted from the total score for all analyses.
Procedure
Follow-up assessments occurred at approximately 1-, 3-, and 5-year intervals. Some follow-up visits were missed, and not all participants completed all items at every time point. Subjects who missed an assessment were still recruited to participate in subsequent follow-ups. The SCID-IV, HPC, and BDI were administered at all time points. The DOTS-R was completed only at baseline. The AUD+ group included 347 individuals at baseline, and 244, 223, and 199 individuals at the 1-year, 3-year, and 5-year visits, respectively. The AUD- group included 349 individuals at baseline, and 287, 248, and 260 individuals at the 1-year, 3-year, and 5-year visits, respectively.
Data analysis
We used SAS version 9.3 for all analyses. Baseline differences in the demographic and clinical characteristics of the AUD+ and AUD− groups were examined using two-tailed t-tests. We used MANOVAs to examine group differences in the sleep and rhythmicity measures at baseline. Because the sleep duration measures were completed by a subsample, including 253 AUD− participants and 288 AUD+ participants, we ran two separate MANOVAs to examine group differences in Insomnia and Hypersomnia, and in Weekday Sleep Duration, Weekend Sleep Duration, and Sleep Variability. We ran a third MANOVA to examine group differences in the DOTS-R subscales, which were also completed by a subsample of participants: 217 AUD− participants and 153 AUD+ participants.
We examined longitudinal associations between current alcohol symptoms and the sleep variables (HPC-Insomnia, HPC-Hypersomnia, and HPC-Sleep Variability) using random-intercept linear mixed-effect models, which are robust against missing data and thus allowed us to include all participants with baseline data and at least one follow-up assessment. Covariates included baseline age, sex, baseline AUD status, and current depression (BDI score minus the sleep items). For ease of interpretation, baseline age was centered at the grand mean of the sample (16.23 years). Given that current alcohol symptoms and depression were moderately skewed, we re-ran all models after using a square root-transformation of these variables. The models were unchanged by using the transformed variables, and thus we present the models using the original variables for ease of interpretation.
Using the Proc Glimmix statement in SAS, we fit two versions of a mixed-effect model in which the intercept (Insomnia, Hypersomnia, Sleep Variability at the baseline visit) was modeled as a random effect, and baseline age, sex, baseline AUD status, and current alcohol symptoms were modeled as fixed effects. In the first model, we included baseline AUD status x visit year and current alcohol symptoms x visit year interaction terms, in order to model any change in group and alcohol symptom effects due to time. In second model, we added current depression as a covariate. The least significant difference method was used for post-hoc comparisons with the Bonferroni significance level adjustment. Adjusted p-values for post-hoc comparisons are presented. All statistical analyses were two-sided, and the significance value was p<0.05.
To complement and extend prior reports that sleep disturbance predicts the development of new alcohol problems, we ran a series of hierarchical linear regression analyses in the AUD− group alone and focusing on the baseline to 1-, 3-, and 5-year follow-ups. Specifically, we used these regression models to predict alcohol symptoms at 1-, 3-, and 5-years from baseline sleep (insomnia, hypersomnia, and sleep variability), while accounting for baseline alcohol symptoms and relevant covariates including sex, age, and baseline depression. In Model 1, we included baseline alcohol symptoms, age and sex as predictors. In Model 2 we added baseline depression (BDI Total minus the sleep items). In Models 3, we added the three sleep variables (Insomnia, Hypersomnia, and Sleep Variability).
RESULTS
Baseline demographic and clinical characteristics (Table 1)
The AUD+ group was older, included relatively more males, a larger proportion of participants of European American descent and a smaller proportion of participants of African American descent. The AUD+ group used more alcohol and were more likely to be daily tobacco users. The AUD+ group also had a higher incidence of major depression and, on average, more severe symptoms of depression (based on the BDI).
Baseline differences in sleep and rhythmicity measures (Table 2)
Table 2.
Baseline group differences in sleep and rhythmicity measures
| Measure | AUD− M (SD) | AUD+ M (SD) | F | p |
|---|---|---|---|---|
| HPC Insomnia (0–4) | 0.27 (0.58) | 0.68 (0.92) | 49.81 | <0.001 |
| HPC Hypersomnia (0 or 1) | 0.04 (0.19) | 0.24 (0.43) | 59.35 | <0.001 |
| HPC Sleep Variability-ABS (hours) | 1.18 (1.43) | 2.14 (1.88) | 43.54 | <0.001 |
| HPC Sleep Duration (hours) | ||||
| Weekday | 7.89 (1.39) | 7.49 (1.75) | 8.51 | 0.004 |
| Weekend | 8.79 (1.72) | 9.24 (2.25) | 6.54 | 0.011 |
| DOTS-R Rhythmicity Scales | ||||
| Sleep (0–18) | 14.48 (3.81) | 12.88 (3.93) | 15.36 | <0.001 |
| Eating (0–15) | 13.61 (3.51) | 12.05 (3.83) | 16.35 | <0.001 |
| Daily Habits (0–15) | 11.94 (2.97) | 10.65 (2.82) | 17.90 | <0.001 |
The AUD+ group reported greater overall sleep disturbance (multivariate tests: F=49.01, p<0.001), as well as greater Insomnia and Hypersomnia. We also observed significant overall group differences in the measures of sleep duration and variability (F=14.68, p<0.001), with the AUD+ group reporting shorter weekday sleep duration, longer weekend sleep duration, and greater variability in sleep duration.
The AUD+ group reported less rhythmicity overall (multivariate tests: F=8.48, p<0.001)) and on each of the individual DOTS-R measures of rhythmicity, including Sleep, Eating, and Daily Habits.
Baseline differences and drop-outs (Supplemental Table 1)
There was a significantly larger attrition rate in the AUD+ group, as based on participation in the 5-year visit (Χ2=23.09, p<0.001). We examined baseline differences between participants with 5-year data and drop-outs separately for AUD+ and AUD− groups. Within each group, males were more likely to drop out by the 5-year visit and race was unrelated to drop-out rate. In the AUD− group, drop-outs were younger and had less variable weekday-weekend sleep duration. In the AUD+ group, drop-outs were older, had fewer hypersomnia complaints and had lower depression ratings.
Alcohol use disorder symptoms over time
In the AUD− group, the mean (±SD) numbers of current alcohol symptoms were 0.05±0.26, 0.14±0.60, 0.29±0.88, and 0.59±1.35 for the baseline, 1-year, 3-year, and 5-year visits, respectively. In the AUD+ group, the mean (±SD) numbers of current alcohol symptoms were 4.12±2.82, 1.49±1.98, 1.66±2.24, and 1.38±1.93 for the baseline, 1-year, 3-year, and 5-year visits, respectively.
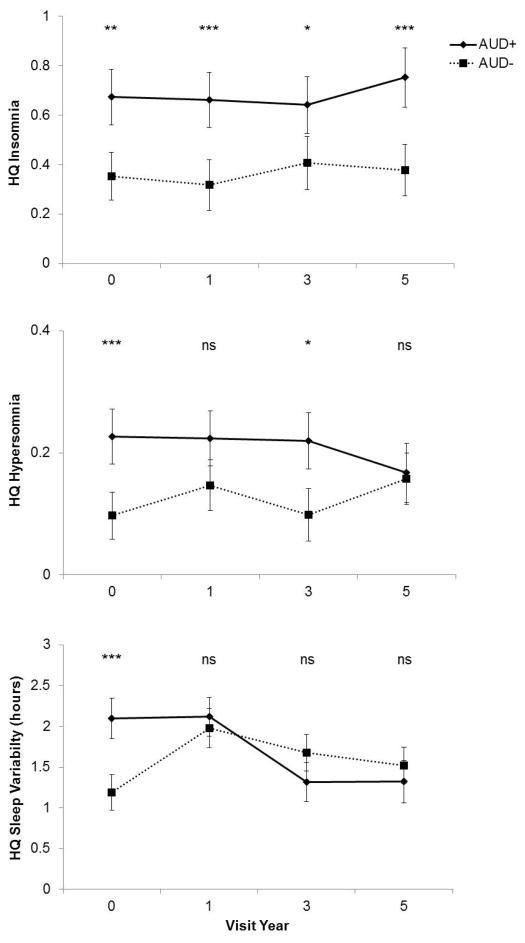
Between-group differences in sleep disturbance over time (Figure 1a–c)
Figure 1.
Longitudinal trajectories of insomnia, hypersomnia, and variability in sleep duration (absolute value) in individuals with and without AUDs at baseline. All individuals were adolescents (mean age = 16.23, range = 12–19 years) at the baseline visit. Data points reflect the estimated means, accounting for covariates including age, sex, and current alcohol symptoms, and error bars are the 95% confidence intervals. Significant group differences by year according to post-hoc comparisons are noted (ns: p > 0.5; *: p < 0.05; **: p < 0.01; ***: p < 0.001).
Solutions for fixed effects and type III tests of fixed effects for all the below analyses are provided in the Supplemental section.
Insomnia (Figure 1a)
Insomnia was lower among males (F1,686.1=7.65, p=0.006) but unrelated to baseline age (F1,725.7=0.18, p = 0.67). Baseline AUD status (F1,839=42.04, p<0.001) and current alcohol symptoms (F1,2103=7.19, p=0.007) were both associated with worse insomnia across visit years; that is, there were no interactions between baseline AUD status and visit year (F3,1728=0.67, p = 0.57), nor between current alcohol symptoms and visit year (F3,1951=0.34, p = 0.79). Post-hoc comparisons confirmed that the AUD+ group reported greater insomnia at baseline (t=3.64, adj-p=0.012) and the 1-year (t=4.78, adj-p<0.001), 3-year (t=3.11, adj-p=0.008), and 5-year (t=4.70, adj-p<0.001) follow-up visits relative to the AUD− group.
After accounting for the significant positive association between current depression symptoms and insomnia (F1,2061=243.62, p<0.001), however, the effects of sex and current alcohol symptoms dropped out and only baseline AUD status retained a significant association with insomnia (F1,852.7=15.00, p<0.001).
Hypersomnia (Figure 1b)
Hypersomnia was again lower among males (F1,818.6=21.50, p<0.001) but unrelated to baseline age (F1,823.7=0.20, p = 0.66). There was a significant interaction between baseline AUD status and visit year (F3,2135=0.84, p = 0.002). Current alcohol symptoms independently predicted greater hypersomnia overall (F1,2135=19.31, p<0.001) and this did not vary by visit year (F1,2135=0.66, p=0.58). Post-hoc comparisons revealed that the AUD+ group reported greater hypersomnia at baseline (t=4.75, adj-p<0.001) and the 3-year visit (t=3.45, adj-p=0.016), but not the 1-year (t=2.28, adj-p=0.663) or 5-year (t=0.40, adj-p=1.00) follow-up visits relative to the AUD− group. Based on within-group post-hoc comparisons, the only significant difference emerged between the AUD− group’s baseline and 5-year visits (t=−3.26, adj-p=0.032): hypersomnia in the AUD− group was higher during the 5-year visit relative to baseline. No significant within-group differences emerged for the AUD+ group.
The main effects of sex (F1,791.1=12.32, p<0.001) and current alcohol symptoms (F1,2121=8.40, p<0.004), and the baseline AUD status X visit year interaction (F3,2121=4.63, p=0.003) all remained significant after accounting for a significant association between current depression symptoms and hypersomnia (F1,2121=34.16, p<0.001).
Sleep variability (Figure 1c)
Variability in sleep duration was unrelated to sex (F1,631.3=0.11, p=0.74) or baseline age (F1,680.6=1.09, p=0.30). There was a significant interaction between baseline AUD status and visit year (F3.1467=10.02, p<0.001). Current alcohol symptoms were unrelated to sleep variability (F1,1758=1.15, p=0.28) and this did not vary by visit year (F1,1687=0.06, p=0.98). Post-hoc comparisons revealed that the AUD+ group reported greater sleep variability at baseline (t=5.46, adj-p<0.001), but not the 1-year (t=0.87, adj-p=1.000), 3-year visit (t=−2.28, p=0.644), or 5-year (t=−1.23, adj-p=1.000) follow-up visits relative to the AUD− group. Both groups showed within-group differences in sleep variability over time. In the AUD+ group, sleep variability was lower during the 3- and 5-year visits relative to the baseline (t=5.22,adj-p<0.0001; t=4.87, adj-p<0.0001) and 1-year (t=5.35,adj-p<0.0001; t=5.09, adj-p<0.000). In the AUD− group, sleep variability was higher during 1- and 3-year visits relative to baseline (t=−5.46,adj-p<0.0001; t=−3.53, adj-p<0.02).
In contrast to insomnia and hypersomnia, depression symptoms were not associated with sleep variability (F1,1679=0.35, p=0.55).
We ran several supplementary analyses to further explore the above models. First, we re-ran the models after restricting the sample to participants with data at all four visits, resulting in 141 AUD+ and 205 AUD− participants. All of the above findings remained, with only marginal changes in the specific estimated means, coefficients, and p-values. Second, we substituted past month’s alcohol use for the current alcohol symptoms covariate in the models, and we observed no change in the pattern of findings. Finally, we added daily tobacco use as an additional covariate in the models, and while daily tobacco use was positively associated with all three sleep outcomes, its inclusion did not change the pattern of findings. All results are available upon request.
Sleep disturbance and the emergence of alcohol symptoms at 1-year follow-up (Table 3)
Table 3.
Baseline sleep variables predict alcohol symptoms at a 1-, 3-, and 5-year follow-ups in adolescents without AUDs at baseline.
| 1-year follow-up | 3-year follow-up | 5-year follow-up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Predictor variable | β | t-value | p | ΔR2 | β | t-value | p | ΔR2 | β | t-value | p | ΔR2 |
| Step 1 | 0.08 | 0.25 | 0.06 | |||||||||
| Alcohol symptoms, baseline | 0.26 | 4.05 | <0.001 | 0.44 | 6.83 | <0.001 | 0.18 | 2.55 | 0.012 | |||
| Sex (Male) | 0.05 | 0.70 | 0.484 | −0.13 | −1.96 | 0.018 | −0.13 | −1.78 | 0.077 | |||
| Age | 0.10 | 1.55 | 0.123 | 0.15 | 2.40 | 0.051 | 0.08 | 1.09 | 0.278 | |||
| Step 2 | 0.00 | 0.00 | 0.01 | |||||||||
| Depression, baselinea | 0.04 | 0.51 | 0.610 | 0.05 | 0.71 | 0.477 | 0.10 | 1.31 | 0.192 | |||
| Step 3 | 0.04 | 0.03 | 0.03 | |||||||||
| Insomnia, baseline | 0.21 | 2.88 | 0.004 | −0.01 | −0.16 | 0.875 | −0.13 | −1.63 | 0.104 | |||
| Hypersomnia, baseline | 0.08 | 1.11 | 0.267 | 0.14 | 0.20 | 0.844 | −0.01 | −0.16 | 0.877 | |||
| Sleep variability, baseline | −0.03 | −0.42 | 0.672 | 0.18 | 2.67 | 0.008 | 0.16 | 2.13 | 0.035 | |||
Note:
Three participants (one AUD− and two AUD+) were missing BDI data, resulting in n=348 for AUD− and n=345 for AUD+
1-year follow-up. In participants without AUDs at baseline, more symptoms of insomnia at baseline predicted a significant increase in alcohol symptoms at the 1-year visit, accounting for 4% of the variance. Neither baseline depression, nor baseline hypersomnia or sleep variability was significantly associated with the change in alcohol symptoms over the 1-year interval.
3- and 5-year follow-ups. In participants without AUDs at baseline, greater variability in weekday-weekend sleep duration at baseline predicted a significant increase in alcohol symptoms at the 3- and 5-year visits, in each case accounting for 3% of the variance. Neither baseline depression, nor baseline insomnia or hypersomnia was significantly associated with the change in alcohol symptoms over the 3- and 5-year intervals.
DISCUSSION
Using a large longitudinal study, we examined the extent and trajectory of sleep problems in individuals with and without AUDs at baseline. Our results provide a unique complement to previous findings in other samples, indicating that insomnia and other sleep disturbance are chronic problems for adolescents with AUDs. Although the groups differed at baseline on all the sleep-related measures—including insomnia, hypersomnia, sleep duration, variability in sleep duration, and rhythmicity in sleep, meals, and daily habits—insomnia complaints emerged as the most chronic sleep problem in this sample. Similar to previous studies of younger children and adults (Brower, 2001, Drummond et al., 1998, Wong et al., 2004, Wong et al., 2010), our findings suggest that insomnia complaints put adolescents without alcohol problems at elevated risk of developing a new AUD. We also report a novel finding that weekday-weekend variability in sleep duration is a risk factor for future alcohol problems. Taken together, our findings indicate that insomnia and other sleep problems may be an important target for both prevention of new alcohol problems and treatment of existing alcohol problems.
Insomnia was a persistent problem in adolescents with AUDs at baseline. That is, as a group, those who had already developed AUDs during mid-adolescence continued to report worse sleep problems (for at least 5 years) than those who had not yet developed AUDs. Notably, these group differences in insomnia persisted despite within-group changes in AUD diagnostic status over the study period. In contrast, group differences in hypersomnia were less stable. The AUD+ group reported greater hypersomnia at the baseline and 3-year follow-ups, but sporadically increasing hypersomnia complaints in the AUD− group diminished the group differences at the 1- and 5-year follow-ups.
Major depressive disorder (MDD) often emerges during adolescence (Maughan et al., 2013). Coincident AUDs and MDD is relatively unusual in adolescents (e.g., 2% of the population: (Briere et al., 2014)). Among adolescents with AUD, however, an elevated rate of mood disorders has been observed (Clark et al., 1997). Consequently, it is plausible that those individuals with AUDs at baseline are characterized by depression or more general psychological dysregulation (Clark et al., 2008) that manifests in both sleep and alcohol-related symptoms. In contrast with this hypothesis, baseline AUD still predicted insomnia complaints over time even after accounting for depression. On the other hand, the association between current alcohol symptoms and insomnia complaints disappeared when we accounted for depression. Taken together, these findings suggest that an overlap between alcohol symptoms and depressive symptoms does exist, but that it does not completely account for the relationship between AUDs and self-reported insomnia. A different pattern emerged for hypersomnia complaints. Current alcohol symptoms predicted self-reported hypersomnia throughout the five years, even after accounting for depression, suggesting a distinct causal pathway linking alcohol problems and hypersomnia. Regardless of the mechanism, however, persistent sleep problems are a significant concern given that both insomnia and hypersomnia are well-documented risk factors for a growing list of physical and mental health problems (e.g., (McCall, 2011, Chien et al., 2010, Breslau et al., 1996, Baglioni et al., 2011, Rosekind and Gregory, 2010).
Similar to hypersomnia, variability in weekday-weekend sleep duration was greater in adolescents with AUDs only at the baseline visit. We speculate that developmental changes in sleep and circadian rhythms, coupled with shifting academic responsibilities, explain this pattern. Differences in weekday-weekend sleep timing and duration are common in adolescents, and driven in part by a mismatch between their preference for later sleep schedules and the early school start times of secondary education (Crowley and Carskadon, 2010, O’Brien and Mindell, 2005). That is, adolescents suffer sleep loss during the school week while trying to accommodate early school schedules, while on weekends, they stay up later at night and then sleep in the following morning. These weekend sleep habits expose them to later light/dark schedules, thereby maintaining their later sleep/circadian timing and resulting in chronic circadian misalignment sometimes termed “social jet lag” (Wittmann et al., 2006). In the current sample, this mismatch would likely be greatest at the baseline visit, when the average participant was approximately 16 years old and in high school. At later visits, more participants would have graduated from high school and gone on to employment or college where start times may be somewhat later and/or more flexible. Although we were unable to explore these hypotheses in the current dataset, such questions warrant directed investigations as they have policy implications (e.g., shifting school times later).
The group differences in rhythmicity of sleep, eating, and daily habits complement the sleep variability findings. Specifically, greater weekday-weekend differences in sleep duration among the adolescents with AUDs at baseline were accompanied by more variable sleep timing overall, as well as more variable timing of meals and other daily habits. Together, these variability and rhythmicity data suggest that adolescents with AUDs are experiencing circadian rhythm disturbance that may be a cause and/or consequence of their problems with sleep. Furthermore, sleep variability and circadian disturbance may be relevant to their alcohol problems. Multiple prior studies have reported that greater weekday-weekend differences in sleep timing and a greater extent of circadian misalignment are associated with more severe problems with alcohol and other drugs (e.g., (Pasch et al., 2010, Wittmann et al., 2006). These associations may be mediated in part by altered reward processing; we have shown that larger weekday-weekend differences in midsleep timing are associated with reduced prefrontal reactivity to reward in early adolescents (Hasler et al., 2012a).
Consistent with prior studies (Brower et al., 1998, Drummond et al., 1998, Wong et al., 2004, Wong et al., 2010), insomnia complaints at baseline predicted an increase of alcohol symptoms at the 1-year follow-up visit among AUD− participants. What distinguishes our findings is the apparent rapidity with which insomnia problems predicted alcohol problems; no prior studies have examined the risk trajectory of insomnia over a timeframe shorter than 3.5 years. This suggests the need for immediate attention to insomnia symptoms during adolescence. We also found first evidence that weekday-weekend differences in sleep duration during adolescence predict increased alcohol problems years later. This complements prior cross-sectional studies reporting that weekday-weekend sleep differences are associated with concurrent problems with alcohol and other drugs (Gau et al., 2007, O’Brien and Mindell, 2005, Pasch et al., 2010, Randler, 2008). It is not clear why insomnia predicted only 1-year alcohol symptoms, or why sleep variability predicted only the 3- and 5-year alcohol symptoms. One explanation could be that insomnia may represent more of a state factor that predicts short-term alcohol outcomes, while sleep variability may represent a more stable, trait-like factor related to overall dysregulation. However, this is inconsistent with our other findings that insomnia complaints were relatively stable among the AUD+ group while group differences in sleep variability disappeared after baseline. Future studies using more robust measures of insomnia and sleep duration will be required to resolve this apparent paradox. Furthermore, in our sample, hypersomnia did not emerge as a significant risk factor for the development of new AUDs. This conflicts with a prior report that hypersomnia is a risk factor for drug and alcohol problems (Breslau et al., 1996). Notably, that study’s hypersomnia assessment was based on DSM-III-R diagnostic criteria rather than our simple dichotomous “sleep too much—yes or no” without a specific time frame. Finally, due to data limitations, we were unable to examine whether sleep problems predicted relapse in adolescents with AUDs in remission as they do in adult samples, but this question deserves further inquiry.
Various mechanisms linking sleep and alcohol problems are plausible and are not mutually exclusive. Above, we have focused on pathways from sleep disturbance to alcohol symptoms—that sleep disturbance may precipitate psychological dysregulation in general, with specific affects on affective and motivational systems relevant to addiction. This pathway is unlikely to be operating in isolation. The reciprocal pathway—alcohol use impacting sleep—is supported by abundant evidence, mostly from adult samples (Ebrahim et al., 2013, Hasler et al., 2012b, Roehrs and Roth, 2001). Alcohol use, whether acute or chronic, has deleterious effects on sleep. Acute alcohol use speeds sleep onset and increases depth of sleep in first half of night, but leads to more overall sleep disturbance. The time-of-day of alcohol use impacts the biphasic stimulating and sedating properties of alcohol in ways particularly relevant to adolescents (Van Reen et al., 2013). Specifically, alcohol may be more stimulating (increasing onset latency to sleep) in the evening, and adolescents appear to be relatively less subject to alcohol’s sedating properties (Spear, 2000). Indeed, a recent study reported that, in contrast to adults, late adolescents did not demonstrate a more rapid onset of sleep following acute alcohol consumption (Chan et al., 2013). These effects are notable given that alcohol is a commonly-reported, if unwise, sleep aid (Johnson et al., 1998). Thus, a vicious cycle between sleep disturbance and alcohol problems may develop in some adolescents. Moreover, other substances may play a role in such cycles. Our finding that alcohol symptoms and daily tobacco use independently predicted sleep problems raises questions about potentially compounding effects of polysubstance use.
Our study has some notable limitations. The design does not allow us to look at the effect of premorbid sleep problems prior to the development of AUDs in the AUD+ group, nor does it allow us to test whether AUDs are causally contributing to the sleep problems. Also, a majority of AUD+ participants had a cannabis use disorder and a large minority had another Substance Use Disorder. More AUD+ participants dropped out, which could have influenced our findings. Next, our analyses do not consider the effect of ongoing treatment for AUDs and/or other problems, which likely varied widely in content and duration across individuals, and may have mitigated associations between alcohol problems and chronic sleep disturbance. As noted above, our assessments of sleep problems were retrospective and based on self-reported complaints, not diagnostic interviews. Diagnostic interviews and/or prospectively assessing sleep using daily diaries or wrist actigraphy may provide more reliable and valid measures of insomnia, hypersomnia, and variability in sleep duration. No one method of assessing sleep is inherently superior, but method discrepancies have potential implications for the findings (e.g., clinical insomnia diagnoses might be rarer in our sample, but also may demonstrate stronger associations with alcohol variables). Lastly, future studies should attend to the roles of puberty, sex, and family history of AUDs in sleep-alcohol pathways, given that these factors are known or plausible determinants to sleep, substance use, and mood disturbance.
In conclusion, our novel findings suggest that sleep problems are a persistent and under-addressed issue for adolescents with AUDs, and are even more prevalent than in non-AUD adolescents, who are already sleep-disturbed as a group. Our findings complement a larger evidence base supporting sleep problems as a precipitating factor for new AUDs, uniquely reporting that self-reported insomnia serves as a risk factor for adolescent alcohol problems over a relatively short 1-year timeframe, while variability in sleep duration is a risk factor for adolescent alcohol problems 3- and 5-years down the line. In addition to other treatment modalities, adjunctive sleep-based prevention and intervention efforts may be worthwhile, given their effectiveness, relative brevity, and potential of less associated-stigma than conventional drug/alcohol treatment programs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health, including K01DA032557 (Hasler), R01AA016482 (Clark), U01AA021690 (Clark), and P50DA005605 (Clark).
References
- Arria AM, Dohey MA, Mezzich AC, Bukstein OG, Van Thiel DH. Self-reported health problems and physical symptomatology in adolescent alcohol abusers. J Adolesc Health. 1995;16:226–31. doi: 10.1016/1054-139X(94)00066-N. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory manual. Boston, Harcourt, Brace: 1996. [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Briere FN, Rohde P, Seeley JR, Klein D, Lewinsohn PM. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Compr Psychiatry. 2014;55:526–33. doi: 10.1016/j.comppsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research & Health. 2001;25:110–25. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–71. [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JK, Trinder J, Andrewes HE, Colrain IM, Nicholas CL. The acute effects of alcohol on sleep architecture in late adolescence. Alcohol Clin Exp Res. 2013;37:1720–8. doi: 10.1111/acer.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Lynch KG, Donovan JE, Block GD. Health problems in adolescents with alcohol use disorders: self-report, liver injury, and physical examination findings and correlates. Alcohol Clin Exp Res. 2001;25:1350–9. [PubMed] [Google Scholar]
- Clark DB, Pollock N, Bukstein OG, Mezzich AC, Bromberger JT, Donovan JE. Gender and comorbid psychopathology in adolescents with alcohol dependence. J Am Acad Child Adolesc Psychiatry. 1997;36:1195–203. doi: 10.1097/00004583-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–85. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addict Biol. 2003;8:455–62. doi: 10.1080/13556210310001646439. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–92. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, Demodena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–802. [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: Effects on normal sleep. Alcohol Clin Exp Res. 2013;37:539–49. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms. 2007;22:268–74. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res. 2013;37:558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Phillips ML, Forbes EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012a;91:334–41. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev. 2012b;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117:247–256. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–86. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- Kenney SR, Labrie JW, Hummer JF, Pham AT. Global sleep quality as a moderator of alcohol consumption and consequences in college students. Addict Behav. 2012;37:507–12. doi: 10.1016/j.addbeh.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar AS, Santhi N, Hasan S, Lo JC, Johnston JD, Von Schantz M, Archer SN, Dijk DJ. Circadian period and the timing of melatonin onset in men and women: predictors of sleep during the weekend and in the laboratory. J Sleep Res. 2013;22:155–9. doi: 10.1111/jsr.12001. [DOI] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Depend. 2000;59:173–6. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Maughan B, Collishaw S, Stingaris A. Depression in childhood and adolescence. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2013;22:35–40. [PMC free article] [PubMed] [Google Scholar]
- Mccall WV. Insomnia is a risk factor for suicide-what are the next steps? Sleep. 2011;34:1149–50. doi: 10.5665/SLEEP.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3:113–33. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Pasch KE, Laska MN, Lytle LA, Moe SG. Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? Am J Health Behav. 2010;34:237–48. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman MR, Mahowald MW, Schenck CH, Bornemann MC. Alcohol-induced sleepwalking or confusional arousal as a defense to criminal behavior: a review of scientific evidence, methods and forensic considerations. J Sleep Res. 2007;16:198–212. doi: 10.1111/j.1365-2869.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- Randler C. Differences between smokers and nonsmokers in morningness-eveningness. Soc Behav Pers. 2008;36:673–680. [Google Scholar]
- Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31:1351–6. [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Rosekind MR, Gregory KB. Insomnia risks and costs: Health, safety, and quality of life. Am J Manag Care. 2010;16:617–26. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Hegedus AM. The Drug Use Screening Inventory: Its applications in the evaluation and treatment of alcohol and other drug abuse. Alcohol Health & Research World 1991 [Google Scholar]
- Van Reen E, Rupp TL, Acebo C, Seifer R, Carskadon MA. Biphasic effects of alcohol as a function of circadian phase. Sleep. 2013;36:137–45. doi: 10.5665/sleep.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Lerner RM. Reassessing the dimensions of temperamental individuality across the life span: The Revised Dimensions of Temperament Survey (DOTS-R) Journal of Adolescent Research. 1986;1:213–230. [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28:578–87. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcohol Clin Exp Res. 2010;34:1033–44. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Corley R, Stallings M, Rhee S, Crowley T, Hewitt J. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.