Abstract
Objective
Macrophage migration inhibitory factor (MIF) is an important modulator of innate and adaptive immunity as well as local inflammatory response. We previously reported that MIF down-regulated osteoclastogenesis through a mechanism that requires CD74. In the current study, we examined if MIF modulates osteoclastogenesis through Lyn phosphorylation and if the down regulation of RANKL-mediated signaling requires the association of CD74, CD44 and Lyn.
Methods
CD74 knockout, CD44 knockout and Lyn knockout mouse models were used to investigate if Lyn requires these receptor and coreceptor. Effects of MIF on osteoclastogenesis were assessed using western blot analysis, siRNA targeted downregulation of Lyn and LynKO mice and real time imaging of Lyn molecules to surface proteins.
Results
MIF treatment induced Lyn expression and MIF down-regulated the RANKL-induced AP-1 and Syk-PCLγ cascade during osteoclastogenesis through activated Lyn tyrosine kinase. We also found by immunoprecipitation studies that MIF receptor(s) associated with Lyn in response to MIF treatment. Studies using siRNA specific for Lyn and LynKO mice confirmed our finding.
Conclusions
Our findings indicate that the tyrosine kinase, Lyn, is activated when MIF binds to its receptor, CD74 and co-receptor, CD44 and, in turn, down regulates the RANKL-mediated signaling cascade by suppressing NFATc1 protein expression through downregulation of AP-1 and calcium signaling components.
Osteoclasts are multinucleated giant cells that originate from hematopoietic stem cells (HSC) (1, 2). The mature osteoclast's specific role is to resorb bone matrix to maintain calcium levels in the blood and to initiate bone remodeling (3, 4). There are various characteristics unique to osteoclast, such as expressing tartrate-resistant acid phosphatase (TRAP), calcitonin receptors (CTR), vitronectin receptor (integrin avβ3) as well as matrix metalloproteinase (MMP) 9 (1, 5, 6). The differentiation and resorbing activity of osteoclasts are regulated by interaction between receptor activator of nuclear factor (NF)-κB (RANK) and its ligand, RANKL (7). Binding of RANKL to RANK induces the expression of the nuclear factor of activated T cells c1 (NFATc1), TRAP and cathepsin K during osteoclast development (3, 8-10).
MIF plays an important role in inflammation and immune responses. It is produced by a variety of cell types such as monocytes, endothelial cells, keratinocytes, anterior pituitary cells and osteoblasts (11-16). MIF acts as a classic pro-inflammatory cytokine that promotes innate and adaptive immune responses through the activation of macrophages and T cells (17). MIF has been reported to bind to the extracellular domain of CD74, also known as MHC class II associated invariant chain (18, 19). The mouse CD74 gene is known to encode 2 isoforms (p31: 31kD; p41: 41kD) by differential splicing. The p31 isoform is expressed at levels which are 5-10 fold greater than the p41 isoform (20, 21). The p41 isoform is thought to play an important role in T cell selection in the thymus (22). However, the function of CD74 isoforms in all tissues has not been clearly elucidated. CD74 is known to activate extracellular signal-regulated kinase (ERK) 1/2 MAP kinase cascade and requires simultaneous expression and activation of CD44 since CD74 lacks an intracellular sequences required for downstream signaling (23). In a separate report, the chemokine receptors, CXCR2 and CXCR4, have been implicated to function as additional MIF receptors (24). However, the mechanism by which MIF modulates osteoclastogenesis, has not been fully understood.
Lyn is a member of the Src family of tyrosine kinases and has been reported to have inhibitory effects in myeloid lineage proliferation (25). Lyn also plays a role in the transmission of inhibitory signals through phosphorylation of tyrosine residues within the immunoreceptor tyrosine-based inhibitory motifs (ITIM) in regulatory proteins such as CD22, PIR-B and FCγRIIb1 (26). It has been demonstrated recently that Lyn is a negative regulator of osteoclastogenesis through its ability to suppress NF-κB activation and inhibit NFATc1 expression by interfering with calcium signaling (27, 28).
We previously reported that MIF down-regulated osteoclast-like cell (OCL) formation and MIF deficient mice had decreased trabecular bone volume (29). In a subsequent report we also found that CD74KO mice had decreased bone volume and bone marrow cells from CD74KO mice formed a greater number of osteoclast-like cells in vitro compared to cells from WT mice. In addition, we recently found that MIF down-regulated NFATc1 expression through inhibition of RANKL induced AP-1 activation (30).
In the current study we demonstrate for the first time that upon binding, the MIF-CD74-CD44 complex activates the phosphorylation of Lyn during osteoclastogenesis. Subsequently, phosphorylated Lyn down-regulates RANKL-induced activation of the Gab2/JNK1/c-jun cascade and Syk/PLCγ pathway to suppress the transcription factor, NFATc1. These results indicate that MIF inhibits osteoclastogenesis by activating Lyn, which in turn down-regulates RANKL-mediated osteoclast differentiation by suppressing NFATc1 and AP-1.
Materials and Methods
Animals
All mice used in the experiments were seven to nine week old male WT, CD74KO (B6.129S6-Cd74tm1Liz/J), CD44KO (B6.129(Cg)-Cd44tm1Hbg/J) and LynKO (B6.129S4-Lyntm1Sor/J) mice in a C57BL/6J background (Jackson Laboratories, Bar Harbor, ME) (23, 31). Mice were housed in the Center for Comparative Medicine at the University of Connecticut Health Center. All animal protocols were approved by the animal care committee of the University of Connecticut Health Center.
Reagents and Antibodies
The following reagents were purchased: recombinant murine MIF and CD44 antibody (R&D Systems, Inc., Minneapolis, MN); inhibitor of κBα (IκBα), JNK, ERK, P38, β-actin and the phosphorylated forms of Lyn (Tyr507), Syk (Tyr525/526), ERK1/2 (Thr202/Tyr204), p38 (Thr180/Tyr182), JNK (Thr183/Tyr185), PLCγ2 (Tyr1217), c-jun (Ser 73) and Gab2 (Tyr452) antibodies and total form of PLCγ2, c-jun, Syk and Gab2 antibodies (Cell Signaling Technology, Danvers, MA); phosphorylated JNK1 (Thr183) Antibody (Abcam Inc., Cambridge, MA); c-Src and phospho-tyrosine (4G10) antibodies (Millipore, Temecula, CA); Lyn and NFATc1 antibodies (Santa Cruz Biotech. Santa Cruz, CA). Recombinant hM-CSF and hRANKL were gifts from Dr. Yongwon Choi (University of Pennsylvania, Philadelphia, PA).
Cell cultures and in vitro osteoclast formation assay
Mouse bone marrow (BM) cells were isolated from the femur and tibia by a modification of previously published methods (30, 32). Briefly, bone marrow cells were flushed, collected and washed twice with α-MEM. Cells were then cultured (5 ×104 cells/well in 96 well plate) with complete α-MEM medium (10% HIFBS, 2 mM L-glutamine, 100 U/ml penicillin–streptomycin) in the presence of M-CSF and/or RANKL (both at 30 ng/mL) and with or without rmMIF (25 ng/mL). Medium was replaced at day 3 of culture and MIF treatment was done daily for up to 5 days or at the time of medium change as indicated. Cells were fixed with 2.5% glutaraldehyde in PBS for 15 min at room temperature prior to TRAP enzyme histochemistry using a commercial kit (SIGMA, St. Louis, MO). TRAP-positive cells that contained more than 3 nuclei were counted as osteoclast-like cells.
Immunocytochemistry
Isolated BM cells from WT mice were cultured in the presence of M-CSF and/or RANKL (both at 30 ng/mL) for up to 5 days. Cells were fixed with 3.7% formalin in PBS for 10 min at room temperature. Cells were treated with ethanol/acetone (1:1) for 30 sec, washed 3 times before blocking with solution that contains 5% horse serum, 5% FBS and 1% BSA in PBS for 1h. Cells were then incubated with primary antibody Lyn and CD74 overnight at 4°C followed by incubation with FITC or rhodamine-conjugated secondary antibody respectively. F-actin was stained with rhodamine phalloidin (Molecular Probes, Grand Island, NY). Nuclei were stained with Hoechst 33342 (Eurogentec Group, Fremont, CA .)
Bone marrow macrophage preparation and immunoblotting
BMM cells were prepared as previously described (30). Briefly, bone marrow cells were cultured overnight, non-adherent cells were collected, layered on Ficoll–Hypaque (GE Healthcare, Piscataway, NJ) density gradient and centrifuged. The interface between Ficoll-Hypaque and medium, which contains mononuclear cells, was collected and used as bone marrow macrophage cells (30).
BMM cells (3 × 106 cells/6-well) were cultured with M-CSF alone for 3 days prior to the treatment with RANKL (30 ng/mL) with or without rmMIF (25 ng/mL) for the period indicated in each experiment. Cells were lysed using lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol) containing protease and phosphatase inhibitors. Cell extracts were collected, quantified, applied to 8-10% SDS-PAGE gels and transferred onto nitrocellulose membranes by immunoblotting. The membranes were blocked, and incubated with primary antibody overnight at 4°C followed by incubation with a secondary antibody conjugated to HRP. Reactive bands were detected by enhanced chemiluminescence using LumiGLO (Cell Signaling Technology, Danvers, MA).
Immunoprecipitation assay
BMMs (3 × 106 cells/60mm dish) were incubated with M-CSF (30ng/ml) in complete α-MEM for 3 days or RAW 264.7 cells (3 × 106 cells/100mm dish) were incubated in complete DMEM for 2 days before cells were starved in serum-free for 1 h. RANKL (30 ng/ml) and/or rmMIF (25 ng/ml) were added for up to 30 min before cells were lysed. An equal amount of extracted proteins (1/mg per sample) were incubated with specific antibody for 24h and samples were centrifuged to remove the debris. Supernatant was incubated with G-agarose for 24 h at 4 °C. The agarose was washed 5 times with washing buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% Triton X- 100, 1% glycerol, protease and phosphatase inhibitors). The precipitates were denatured by boiling at 95 °C for 10 min and quenched on ice for 2 min. Samples were centrifuged prior to immunoblotting.
Single molecule fluorescence video microscopy
Detailed experimental procedures are described (33). Briefly, template DNA sequences were obtained from the Human SH2 collection and the SH2 domain within Lyn was identified. SH2 domain sequences were cloned into pENTR/D-TOPO vector (Invitrogen, Carlsbad, CA) following the manufacture's TOPO cloning protocol to obtain entry clones. The chimeric fusion constructs were generated by recombining pDEST-tdEos-C with the corresponding entry clones using Gateway clonase (Invitrogen). The construct was then transfected into RAW 264.7 cells using Lipofectamine 2000 (Invitrogen). Total internal reflection (TIR) fluorescence microscopy was used to generate real time images of individual fluorescence molecules, which allows to visualization of molecules localized on the bottom membrane of cells (~200 nm from the glass surface). The excitation laser (DPSS diode, 532 nm emission, Crystal Lasers) for the small fraction of photo activated Eos fluorescence protein was expanded and filtered (532/10 nm line width bandpass filter, Chroma). Cells were stimulated with MIF (25 ng/ml) for 20 min and fluorescence snapshot images were taken before and after MIF treatment. For the single molecule tracking images were collected at 75 msec time intervals.
Lyn small interfering RNA (siRNA)
BMMs (106 cells/6-well) were incubated with M-CSF (30 ng/ml) for 3 days before transfection was performed using Lyn specific siRNA and Hiperfect transfection reagent (both from Qiagen, Valencia, CA) according to the manufacturer's instruction. Briefly, negative control or Lyn specific siRNA was incubated in Opti-MEM for 6 h and medium was replaced with complete α-MEM medium in the presence of M-CSF and/or RANKL (both at 30 ng/ml) with or without rmMIF (25 ng/ml) for up to 4 days. Cells were stained with TRAP or lysed for western blot analysis.
Statistical analysis
Statistical analysis was performed by Student's t-test or one way analysis of variance (ANOVA) and the Bonferroni post hoc test when ANOVA demonstrated significant differences. All experiments were repeated at least twice and representative experiment or pooled data are shown.
Results
RANKL down-regulates Lyn expression during osteoclastogenesis
BMM cells from WT mice were cultured with M-CSF and/or RANKL for up to 5 days and equal amounts of total proteins were immunoblotted to detect Lyn, c-Src or NFATc1. Lyn was expressed in cultures that were treated with M-CSF alone and RANKL treatment down-regulated Lyn expression for the time period we examined (Figure 1A). As shown figure 1B, expression of Lyn was reduced by 70% and 82%, respectively, on days 3 and 4 when compared to the corresponding M-CSF group. As previously reported (34-36), c-Src and NFATc1 expression was only detected in RANKL-treated groups (Figure 1A). We subsequently examined if Lyn protein is present in osteoclast by immunocytochemistry. Lyn protein (FITC labeled - green) was detected in mononuclear preosteoclasts while mature OC had minimal expression. However, F-actin (phalloidin-red) expression was strongly detected in both multi and mononuclear cells when BMM cells were cultured for 5 days. Hoechst stain was used to visualize nuclei (blue). This result indicates that the expression of Lyn was reduced by RANKL treatment as osteoclasts mature (Figure 1C).
Figure 1.
Lyn expression was down-regulated by RANKL during osteoclastogenesis. BMM cells were cultured with M-CSF and/or RANKL for the duration indicated on ‘Materials and Methods’. (A) The equal amount of total cell protein from BMM cultures were immunoblotted for Lyn, c-Src or NFATc1. β-actin was used as control. (B) The graph shows the density of Lyn/β-actin in immunoblotting (n=3). (C) Isolated BM cells from WT mice were cultured in the presence of M-CSF and RANKL (both at 30 ng/ml) for up to 5 days. Cells were stained with Lyn and FITC conjugated secondary specific antibody. F-actin was stained rhodamine phalloidin by immunocytochemistry. Nuclei were stained with Hoechst. Scale bar represents 50 µm. Values represent mean±SEM. *, Significant effect of RANKL treatment, p<0.05.
MIF down-regulated TRAP (+) OCL formation through induced Lyn expression
We next examined if MIF affects Lyn expression during osteoclastogenesis. BM cells from WT mice were cultured with M-CSF and RANKL (both at 30 ng/ml) with or without MIF (25 ng/ml) for up to 5 days. MIF treatment was applied once with medium change on day 3 or daily for 5 days. MIF treatment on day 3 down regulated OCL formation by 16% in BM cultures that were also treated with M-CSF and RANKL. Interestingly, daily treatment with MIF further down-regulated OCL formation by 38% compared to control cultures (Figure 2A and 2B). Concurrently, we examined the expression of Lyn, c-Src and NFATc1 by immunoblotting. BMM cells were cultured with the combination of M-CSF and RANKL (both at 30 ng/ml) and/or daily treatment with MIF (25 ng/ml) for up to 5 days. As in figure 1A, RANKL treatment up-regulated c-Src and NFATc1 expression in BMM cultures while Lyn expression gradually decreased as the cells differentiate into osteoclasts. In contrast, MIF treatment down regulated RANKL-induced NFATc1 and c-Src expression and up-regulated Lyn expression in these cultures (Figure 2C). We also examined CD74 expression in these cultures and found that it was not affected by MIF treatment.
Figure 2.
MIF down-regulated TRAP (+) OCL formation while up-regulating Lyn. (A) BM cells from WT mice were cultured with M-CSF and RANKL (both 30ng/ml) with or without MIF (25 ng/ml) for 5 days. MIF treatment was applied once with medium change on day 3 or daily for 5 days. (B) MIF treatment down regulated OCL formation in BM cultures that were treated with M-CSF and RANKL. (C) BMM cells were cultured with the combination of M-CSF and RANKL (both 30 ng/ml) and/or daily treatment of MIF (25 ng/ml) for up to 5 days. The equal amount of whole cell protein was immunoblotted for Lyn, c-Src, NFATc1 and CD74. β-actin was used as control. Original magnification: 40X. Scale bar represents 0.5 mm. Values represent mean±SEM. *, Significant effect of MIF treatment, p<0.05. # , Significant effect of daily treatment with MIF, p<0.05.
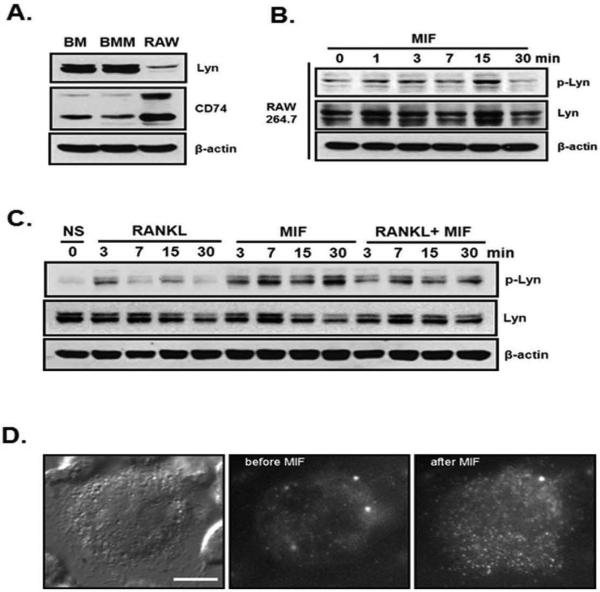
Binding of MIF to CD74 activates Lyn and recruits it to the cell membrane
First we examined the Lyn and CD74 expression in the bone marrow, bone marrow macrophages and RAW264.7, macrophage cell line by western blot analysis (Figure 3A). Total bone marrow, bone marrow macrophages and RAW264.7 cells were cultured for 3 days before cell lysates were prepared. All the cell types expressed CD74 and Lyn. In addition, MIF treatment rapidly activated Lyn phosphorylation in these cells (Figure 3B). To determine if tyrosine kinase, Lyn was activated by MIF treatment, we performed western blot analysis using BMM cultures. Cultures were treated with M-CSF for 3 days before RANKL and/or MIF was added for up to 30 minutes and activation of Lyn was then determined by western blot for phosphorylated Lyn (p-Lyn). RANKL activated Lyn weakly between 3-7 min while MIF treatment activated Lyn much more drastically (Figure 3C). Further, MIF up-regulated RANKL induced Lyn phosphorylation indicating that Lyn may be involved in MIF's inhibitory effect on RANKL induced osteoclastogenesis (Figure 3C).
Figure 3.
MIF activated the binding of Lyn to MIF receptor CD74. (A) Bone marrow, bone marrow macrophage from WT and RAW264.7 cells were lysed, the equal amount of whole cell protein from each sample was immunoblotted for Lyn and CD74. (B) RAW264.7 cells (3×106 cells/100 mm dish) were incubated for 2 days before MIF was added for up to 30 minutes. Equal amount of extracted proteins were immunoblotted using phospho-Lyn and Lyn antibodies. (C) BMM cells were cultured for 3 days with M-CSF alone before treated with RANKL (30 ng/ml) and/or MIF (25 ng/ml). BMM cells were treated with RANKL and/or MIF for 3, 7, 15 and 30 min and immunoblotted for phospho-Lyn and Lyn. (D) Real-time single molecule fluorescence images. Bright field image of RAW264.7 cell indicating that Lyn-tdEos fusion molecules are expressed (left panel). Fluorescence images of RAW264.7 cell before (middle) and after (right) MIF treatment. Lyn-tdEos fusion molecules are visible on the cell membrane after the stimulation compared to unstimulated cells. Individual bright dots correspond to a single Lyn-tdEos molecule on the membrane.
To determine whether MIF-induced activation of Lyn resulted in translocation of Lyn to the plasma membrane through interaction with CD74, we performed single molecule live cell imaging using total internal reflection (TIR) microscopy. We constructed a specific Lyn td-Eos fusion chimera and transfected to RAW264.7 cells, which were then treated with MIF. Figure 3D shows the expression and MIF induced membrane association of Lyn-td-Eos molecules, which were bright dots in live RAW 264.7 cells. Cells were immobilized on glass bottom dishes using a binding agent and imaged (bright field, left panel). We first confirmed that, in unstimulated cells the population of Lyn-td-Eos molecules in the cytoplasm is very high, as indicated by a high fluorescence intensity signal using epi illumination optics. However, the flurescence intensity was significantly decreased under TIR illumination optics, which only detects the molecules localized on the bottom membrane (middle panel). After stimulating cells for 20 min with MIF (25 ng/ml), the number of Lyn-td-Eos molecules on the bottom membrane was significantly induced (right panel). As shown in figure S1, live images were captured. These results indicate that binding of MIF activates Lyn protein in these cells and stimulates its transport from the cytoplasm to the cell membrane.
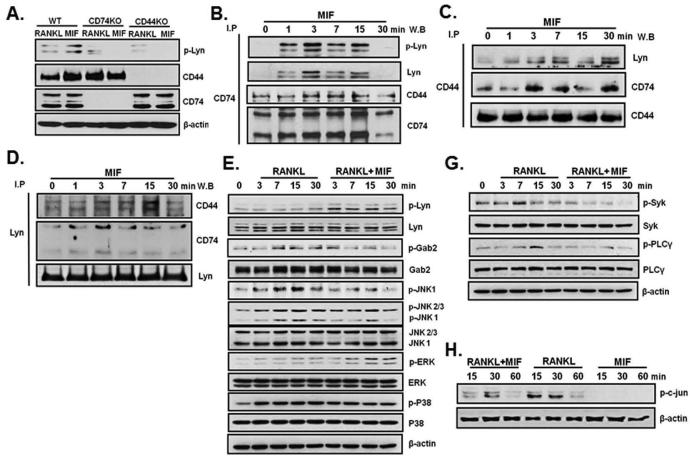
Both CD74 and CD44 appear necessary for MIF binding to cells and are believed to be putative MIF receptor proteins in certain cell types. To determine if CD74 and CD44 associate with Lyn in MIF signaling, MIF treated BMM cells were immunoprecipitated with CD74, CD44 or Lyn and immunoblotted with Lyn, CD44 or CD74. As shown in figure 4A, BMM cells from WT, CD74KO or CD44KO mice were cultured for 3 days with M-CSF before RANKL or MIF was added for 3 minutes. MIF up-regulated p-Lyn expression in WT cells but not in cells from either CD74KO or CD44 KO mice. Both p-Lyn and total Lyn were detected in BMM cells that were immunoprecipitated either with CD74 or CD44 antibody upon MIF treatment. This result implies that Lyn associated with CD74 and CD44 (Figure 4B and 4C). Consistent with this hypothesis, CD44 and CD74 were detected when BMM cells were immunoprecipitated with Lyn antibody (Figure 4D). Hence, it appears that a MIF-CD74-CD44 complex associates with Lyn after MIF treatment of BMMs.
Figure 4.
Lyn is associated with CD74-CD44 by MIF treatment and MIF modulated RANKL-induced AP-1 and calcium signaling via activated Lyn. BMM cells from WT, CD74KO and CD44KO mice were cultured for 3 days with M-CSF alone before treated with RANKL (30 ng/ml) and/or MIF (25 ng/ml). (A)The equal cells of BMM (3×106 cells/60mm dish) were lysed and the equal amount of whole cell protein from BMM cultures was immunoblotted for phospho-Lyn, CD44 and CD74. (B) To determine if CD74 and/or CD44 associates with Lyn for MIF signaling, BMM cells were treated with MIF (25 ng/ml) and immunoprecipitated with CD74 Ab and immunoblotted with phospho-Lyn, Lyn and CD44 Ab. (C) Immunoprecipitated with CD44 Ab and immunoblotted with Lyn Ab and CD74 Ab. (D) Immunoprecipitated with Lyn Ab and immunoblotted with CD44 Ab and CD74 Ab. (E-H) BMM cells were cultured with M-CSF alone for 3 days before treated with RANKL (30 ng/ml) and/or MIF (25 ng/ml) for up to 30 min. The each protein sample immunoblotted using specific antibodies for AP-1 pathway (E, F) and Syk-PLCγ cascade (G). β-actin was used as control.
MIF modulates RANKL-induced AP-1 and Syk-PLCγ cascade
We investigated if MIF modulates RANKL-induced signaling pathways. BMM cells were cultured with M-CSF alone for 3 days before being treated with RANKL (30 ng/ml) and/or MIF (25 ng/ml) for up to 30 min. We examined RANKL induced AP-1 signaling components (Gab2, JNK1, 2, 3 and c-jun) (Figure 4E). Since JNK1, not JNK2, has been known to modulate c-jun phosphorylation during osteoclastogenesis (37), we examined if MIF regulates osteoclast specific JNK1 (Figure 4E). We found that MIF inhibited RANKL-induced activation of JNK1 and Gab2. These results imply that MIF inhibited RANKL-induced phosphorylation of c-jun (Figure 4E and F) through suppressed activation of Gab2 and JNK 1. In addition, MIF treatment further activated RANKL induced ERK phosphorylation as previously reported in other cells types (23) . However, MIF did not alter p38 activation (Figure 4E). These results indicate that MIF down-regulates the RANKL-induced Gab2-JNK1-AP-1 signaling cascade but not MAPK. We also examined if MIF is involved in the RANKL-induced Syk-PLCγ cascade, which is known to stimulate NFATc1 expression. As shown in figure 4G , MIF indeed down-regulated RANKL-induced Syk-PLCγ cascade by decreasing the specific phosphorylation of Syk-PLCγ. These results indicate that MIF down-regulates osteoclastogenesis through Lyn by suppressing RANKL- induced AP-1 and Syk-PLCγ cascade.
MIF requires CD74 to activate Lyn
In order to determine if the CD74, MIF receptor, is required for Lyn expression and activation, we used BMM cells from WT and CD74KO mice. BMM from WT and CD74KO were cultured with M-CSF and RANKL (both at 30 ng/ml) with or without MIF (25 ng/ml) for 3, 4 or 5 days. Equal amounts of cell lysates from BMM cultures were immunoblotted for Lyn, c-Src and CD74. MIF treatment stimulated Lyn expression in WT BMMs but not in CD74KO BMM cultures (Figure 5A). c-Src expression was up-regulated in cells from CD74KO mice compared to in WT cells in response to RANKL treatment. MIF treatment down-regulated c-Src expression in WT but not in CD74KO cells (Figure 5A). To confirm whether CD74 is required for MIF effects on RANKL-induced signaling pathways, we used BMM cells from CD74KO mice (Figure 5B). Both RANKL-induced AP-1 and Syk-PLCγ pathways were not affected by MIF treatment in BMM cells from CD74KO mice. These data indicate that CD74 is required for MIF to modulate the activation of AP-1 and Syk-PLCγ pathways during osteoclastogenesis.
Figure 5.
MIF treatment did not alter Lyn expression in BMM from CD74 KO. (A) BMM from WT and CD74KO were cultured with M-CSF and RANKL (both 30 ng/ml) with or without MIF (25 ng/ml) for 3, 4 or 5 days. The equal amount of whole cell protein from BMM cultures was immunoblotted for Lyn, c-Src, and CD74. (B) BMM cells from CD74KO mice were cultured with M-CSF alone for 3 days before treated with RANKL and/or MIF for up to 30 min. The equal amount of whole cell protein from cultures was immunoblotted for Syk, PLCγ, Gab2, JNK1 and c-jun antibodies. β-actin was used as control. (C) Microphotographs of bone marrow (BM) or bone marrow macrophage (BMM) cells from WT or LynKO mice were cultured with or without MIF (25 ng/ml) in the presence of M-CSF and RANKL (both at 30 ng/ml) for 5 days. BM (upper panels) or BMM (lower panels) cells from LynKO mice formed increased number of TRAP(+) OCL compared to WT cells. In addition MIF treatment did not alter TRAP(+) OCL formation in cells from LynKO mice. (D) Number of TRAP(+) OCL/well was scored. (E) BMM cells from WT or LynKO mice were tested for MIF responsiveness by immunoblotting. BMM cells were stimulated with or without MIF for up to 30 minutes in the presence of M-CSF and RANKL. B-actin was used as control. Values represent mean±SEM. *, Significant effect of MIF treatment, p<0.05. #, Significant effect of LynKO mice, p<0.05.
Lyn is required for MIF signaling during osteoclastogenesis
In order to confirm whether Lyn is required for MIF to modulate osteoclastogenesis, BMM cells were transfected with Lyn specific siRNA to knockdown Lyn expression. As shown in figure S3, MIF down-regulated RANKL induced OCL formation in negative control (NC) siRNA transfected cells (Figure S3A, upper panels and 3B) while there is no significant reduction by MIF treatment in OCL formation in BMM cells that were transfected with Lyn specific siRNA (Figure S3A, low panels and 3B). Lyn knockdown cells formed more TRAP (+) OCL compared to NC cells. BMM cells that were transfected with NC or Lyn specific siRNA were also tested for MIF responsiveness by western blot analysis. MIF did not have effect on Lyn, NFATc1, and c-Src expression in cells that were transfected with Lyn specific siRNA compared to cells with negative control (Figure S3C). Additionally we examined if MIF modulated c-jun activation in Lyn knockdown cells. As shown in figure S3D, MIF down-regulated phosphorylation of c-jun in NC cells but not in Lyn knockdown cells. Subsequently, we examined similar experiments with bone marrow and bone marrow macrophage cells from Lyn KO mice. As shown in figure 5C and D, cells from LynKO mice formed more TRAP(+) OCL compared WT cells. In addition, cells from LynKO mice did not respond to MIF treatment. In contrast, cells from WT mice did decrease osteoclastogenesis in response to MIF. To confirm the MIF responsiveness in LynKO mice, western blot analysis was performed. As shown in figure 5E, c-jun activation was enhanced in cells from LynKO mice compared to WT cells. Further, c-jun activation was downregulated by MIF treatment in WT cells but not LynKO. Collectively, these results demonstrate that MIF treatment requires CD74, CD44 and Lyn activation to modulate RANKL-induced osteoclastogenesis.
Discussion
We and others have established that MIF plays an important role in bone under the normal and inflammatory conditions. We found that MIF is a negative regulator of osteoclastogenesis (29). In the current study we attempted to investigate the mechanisms that influence the negative regulation of osteoclastogenesis by MIF. We found that MIF binds to its receptors (CD74 and CD44) and activates Lyn tyrosine kinase to suppress the RANKL mediated downstream signaling pathways during osteoclast development. Among the three major pathways that mediate osteoclastogenesis, MIF alters two: RANKL induced AP-1 and the Syk-PLCγ cascade, which has been linked to calcium signaling (28, 38).
The current study is the first to report that the involvement of Lyn in MIF modulated osteoclastogenesis. Lyn is a member of Src family tyrosine kinase and known to be expressed in hematopoietic cells (39). It is physically associated with the B-cell receptor (BCR) in B cells and with FcγRI in mast cells. It is also activated upon cross-linking of the antigen receptors and modulates both positive and negative intracellular signaling (40). In addition, Lyn has been reported to be a negative regulator of osteoclastogenesis by modulating NF-κB and calcium signaling (27, 28). RANKL down-regulates Lyn expression as previously reported (28). We report here that Lyn expression was decreased by RANKL treatment while c-Src expression was induced during osteoclastogenesis as shown in figure 1A and B. We also showed that Lyn expression in mature osteoclast was minimal while intense expression was detected in the mononuclear cells in the cultures (Figure 1C). These results indicate that Lyn expression was down-regulated while c-Src expression was up-regulated as osteoclasts differentiate. During RANKL induced osteoclastogenesis, MIF down-regulated osteoclast formation as shown in our previous reports (29, 30). Interestingly, daily treatment of MIF further down-regulated osteoclast formation in vitro in the presence of RANKL. Based on the results in the current study, we conclude that MIF down-regulates the formation and resorption activity of osteoclast by suppressing the expression of NFATc1 and c-Src while up-regulating Lyn expression. To determine if CD74 is required for MIF induced Lyn expression, we used CD74 KO BMM cells, and found that CD74KO BMM cells did not respond to MIF treatment. Hence, it appears that CD74 is involved in the up-regulation of Lyn expression by MIF treatment.
In the current study, we attempted to determine the role of Lyn in MIF modulated down-regulation of osteoclastogenesis. MIF has been reported to induce ERK 1/2 MAP kinase activation through CD74 (41). MIF and JAB1 are co-localized in the cytoplasm, which inhibit the positive regulatory effects of JAB1 on the activity of JNK and AP-1 (42). Furthermore, the mechanism by which MIF interacts with CD74 during signaling has not been elucidated. CD44 has been implicated to play a role as a co-receptor for CD74 to induce intracellular signaling in response to MIF (23). In addition to CD74, There have been reports that CD44 could directly associate with Lyn in cancer cells (43, 44) and B cell (45). These studies have reported that the stimulation of CD44 using CD44 antibody or ligand increased tyrosine phosphorylation of intracellular substrates in several cell types (46, 47). In addition, the Src family kinases including Lck, Fyn, Lyn and Hck could couple to CD44 within sphingolipid-rich microdomains of the plasma membrane (48). These data imply that CD44 could interact with Lyn directly.
We demonstrated that both isoforms of CD74 were detected (p31 and p41) in primary BMM cells and RAW264.7 cells (Figure 3A) and Lyn could be activated in these cells (Figure 3B and C). Our real-time images show the activated Lyn was bound to the inner side membrane in response to MIF treatment (Figure S1). These results imply that either CD74 or CD44 could be the transmembrane protein that Lyn associates with in response to MIF. Consistent with this hypothesis, CD74 and Lyn were co-localized in response to MIF treatment as assessed by immunocytochemistry (Figure S2) and real time images (Figure S1). Our immunoprecipitation experiments indicate that CD74 and/or CD44 associate with Lyn, demonstrating for the first time that Lyn tyrosine kinase is involved in MIF, CD74 and CD44 signaling. Previously, CXCR4, one of the chemokine receptors, was found to form a complex with CD74, which mediated MIF- induced signaling (49) and CXCR4 was reported to associate with Lyn in lipid raft in chronic myeloid leukemia cell line (50). However, we failed to detect their association in our BMM cultures in response to MIF treatment (Figure S4), indicating that CXCR4 may not be involved in the MIF modulated downregulation of osteoclastogenesis.
It is possible that Lyn may associate directly with CD74 transmembrane protein in response to MIF treatment. In mice, CD74 has a short cytoplasmic tail, containing only 30 amino acids, and we were unable to find tyrosine residues in this intracellular domain. However, CD74 has several tyrosine residues in its transmembrane domain including at residue 33, (29-GALYTGVSVLVALL LAGQATTAYFLYQQ-58). The tyrosine residue Y33 of CD74 is located in proximity to the cytoplasmic domain of lipid raft. However, to date, this site has not been reported to be phosphorylated. Interestingly, Young et al found that Lyn was present in both the lipid raft and cytoplasmic compartments and the specific activity of Lyn was greater in the lipid rafts than in nonraft environments (51). When BMM and RAW264.7 cell lysates were immunoprecipitated with CD74 antibody, we were able to detect specific phosphotyrosine reactivity where CD74 (41kDa) isoform is detected (Figure S5). Conversely, p41 isoform was strongly detected when we immunoprecipitated with Lyn antibody. In contrast to CD74 transmembrane protein, tyrosine residue was not detected in CD44 transmembrane protein. However, Shi et al reported that Src kinase could phosphorylate the cytoplasmic domain of CD44 protein to mediate MIF initiated signaling and specifically the phosphorylation of 5 serine residues (23). Our immunoprecipitation studies indicated that there was tight association between CD74 and Lyn as well as CD44 and Lyn (Figure 4).
Lyn was reported to inhibit RANKL-induced Gab2, JNK, NF-κB or c-jun (27) and Syk and PLCγ, which are the component of Ca2+ signaling pathway (28). Lyn activation by MIF inhibited p-c-jun through suppressed JNK1 activation as well as RANKL-induced Ca2+ signaling through suppressed Syk and PLCγ activation. However, NF-κB activation was not altered by MIF treatment as we previously reported (30). We conclude that the involvement of Lyn in MIF modulated downregulation was affecting only AP-1 and calcium signaling through the binding of MIF to CD74. In either CD74KO or CD44KO cells, Lyn was not phosphorylated by MIF treatment (Figure 4). As expected, these results imply that CD74 alone or with CD44 interact with Lyn to modulate RANKL mediated osteoclastogenesis. In addition, Lyn knockdown cells showed increased c-Src and NFATc1 expression leading to induced OC formation even in the presence of CD74 (Figure S2C). These results indicate the activated Lyn through a MIF-CD74 or MIF-CD74-CD44 complex inhibit RANKL-induced expression of NFATc1 and c-Src by suppressing AP-1 and Ca2+ signaling during osteoclastogenesis.
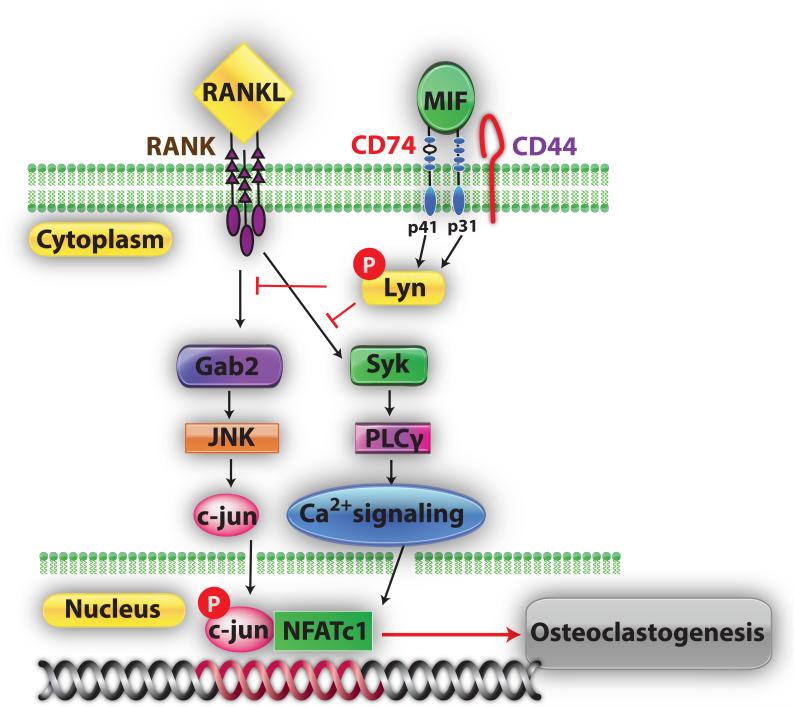
In summary, as shown in figure 6, in the current study we demonstrated for the first time that MIF up-regulates Lyn expression and activation, which in turn down-regulates RANKL-mediated NFATc1 activity by suppressing AP-1 and calcium signaling components.
Figure 6.
Model of MIF-CD74-Lyn signaling cascade during osteoclastogenesis. By binding MIF to CD74 can recruit CD44 transmembrane protein and activate Lyn tyrosine kinase to down-regulate RANKL mediated AP-1 and Syk-PLCγ cascade during osteoclast development.
Supplementary Material
Acknowledgments
The authors thank Dr. Joseph Lorenzo for critical review in the preparation of the manuscript and Dr. A. DeFranco (University of California San Fracisco) for providing LynKO mice. The authors also thank Drs. Bruce Mayer and Ji Yu (University of Connecticut Health Center) for helping in performing the live cell imaging. SHM, DO and SKL contributed to the study design, data collection and analysis. All authors participated in data interpretation. The report was drafted and edited by SHM and SKL. All authors have provided input to the manuscript and approved the final version. This study was supported by NIAMS/NIH grant (AR055143 to SKL).
Footnotes
Conflict of Interest: All authors have no conflict of interest.
References
- 1.Miyamoto T, Suda T. Differentiation and function of osteoclasts. Keio J. Medicine. 2003;52:1–7. doi: 10.2302/kjm.52.1. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M, Mackay AM, Beck SC, Jaiswal RKDR, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Lacey D, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, TJ. M. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocrine Reviews. 1999;20:345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 5.Roodman G. Advances in bone biology: the osteoclast. Endocrine Review. 1996;17:308–32. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 6.Suda T, Takahashi N, Martin T. Modulation of osteoclast differentiation. Endocrine Review. 1992;13:66–79. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 7.Asagiri M, Takayanagi H. The molecular understnading of osteoclast differentiation. Bone. 2007;40:251–64. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of National Academy of Sciences USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong B, Rho J, Arron J, Robinson E, Orlinick J, Chao M, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-jun N-terminal kinase in T cells. J Biological Chemistry. 1997;272:25190–4. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 10.Arai F, Miyamoto T, Ohneda O, Inada TST, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-fms and receptor activator of nuclear factor κB (RANK) receptors. J Experimental Medicine. 1999;190:1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David J. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proceedings National Academy Sciences U S A. 1966;56:72–7. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloom B, Bennet B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–2. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 13.Nishihira J, Koyama Y, Mizue Y. Identification of macrophage migration inhibitory factor in human vascular endothelial cells and its induction by lipopolysaccharide. Cytokine. 1998;10:199–205. doi: 10.1006/cyto.1997.0276. [DOI] [PubMed] [Google Scholar]
- 14.Abe R, Shimizu T, Ohkawara ANJ. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochimica Biophysica Acta. 2000;1500:1–9. doi: 10.1016/s0925-4439(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 15.Onodera S, Suzuki K, Matsuno T, Kaneda KKT, Nishihira J. Identification of macrophage migration inhibitory factor in murine neonatal calvariae and osteoblasts. Immunology. 1996;89:430–5. doi: 10.1046/j.1365-2567.1996.d01-751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernhagen J, Calandra T, Mitchell RA, Martin SBTK, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 17.Calandra T, Bernhagen J, Mitchell R, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Experimental Medicine. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF signal transduction initiated by binding to CD74. J Experimental Medicine. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leng L, Bucala R. Insight into the biology of macrophage migration inhibitory factor (MIF) revealed by the cloning of its cell surface receptor. Cell Research. 2006;16:162–8. doi: 10.1038/sj.cr.7310022. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Koch N, Steinmetz M, Hammerling GJ. One gene encodes two distinct Ia-associated invariant chains. J Immunol. 1985;134(5):3461–7. [PubMed] [Google Scholar]
- 21.Strubin M, Berte C, Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 1986;5(13):3483–8. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright RJ, Bikoff EK, Stockinger B. The Ii41 isoform of invariant chain mediates both positive and negative selection events in T-cell receptor transgenic mice. Immunology. 1998;95(3):309–13. doi: 10.1046/j.1365-2567.1998.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Leng L, Wang T, Wang W, Du X, Li J, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke AKR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Medicine. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 25.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, et al. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15(4):603–15. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 26.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8(4):497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Zhang K, Zhang L, Ross FP, Teitelbaum SL, Faccio R. The src family kinase, Lyn, suppresses osteoclastogenesis in vitro and in vivo. Proceedings of the National Academy of Sciences, USA. 2009;106:2325–30. doi: 10.1073/pnas.0806963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon S, Lee Y, Kim HJ, Lee ZH, Hyung SW, Lee SW, et al. Lyn inhibits osteoclast differentiation by interfering with PLCγ1-mediated Ca2+ signaling. FEBS Letters. 2009;583:1164–70. doi: 10.1016/j.febslet.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Jacquin C, Koczon-Jaremko B, Aguila HL, Leng L, Bucala R, Kuchel GA, et al. Macrophage migration inhibitory factor inhibits osteoclatogenesis. Bone. 2009;45:640–9. doi: 10.1016/j.bone.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Mun S, Won H, Hernandez P, Aguila H, Lee S. Deletion of CD74, a putative MIF receptor, in mice enhance osteoclastogenesis and decreases bone mass. J Bone and Mineral Research. 2013;28:948–59. doi: 10.1002/jbmr.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao J, Singleton PA, Majumdar S, Boudignon BBA, Kurimoto P, Wronski TJ, Bourguignon LYW, Halloran BP. Hyaluronan increases RANKL expression in bone marrow stromal cells through CD44. J. Bone and Mineral Research. 2005;20:30–40. doi: 10.1359/JBMR.041014. [DOI] [PubMed] [Google Scholar]
- 32.Jacquin C, Gran DE, Lee SK, Lorenzo, Aguila HL. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone and Mineral Research. 2006;21:67–77. doi: 10.1359/JBMR.051007. [DOI] [PubMed] [Google Scholar]
- 33.Oh D, Ogiue-Ikeda M, Jadwin JA, Machida K, Mayer BJ, Yu J. Fast rebuilding increases dwell time of Src homology 2 (SH2)-containing proteins near the plasma membrane. Proceedings National Academy of Science. 2012;109:14024–9. doi: 10.1073/pnas.1203397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong B, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF Family Member, Activates Akt/PKB through a Signaling Complex Involving TRAF6 and c-Src. Molecular Cell. 1999;4:1041–9. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biological Chemistry. 2004;279:26475–80. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 37.David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002;115(Pt 22):4317–25. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- 38.Negishi-Koga T, Takayanagi H. Misteries in Ca2+ signaling duing osteoclast differentiation. IBMS BoneKEy. 2009;6:301–6. [Google Scholar]
- 39.Yamanashi Y, Mori S, Yoshida M, Kishimoto T, Inoue K, Yamamoto T, et al. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc Natl Acad Sci U S A. 1989;86(17):6538–42. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med. 1998;187(8):1343–8. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell R, Metz C, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J Biological Chemistry. 1999;274:18100–6. doi: 10.1074/jbc.274.25.18100. [DOI] [PubMed] [Google Scholar]
- 42.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211–6. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 43.Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. 2001;61(13):5275–83. [PubMed] [Google Scholar]
- 44.Subramaniam V, Vincent IR, Gardner H, Chan E, Dhamko H, Jothy S. CD44 regulates cell migration in human colon cancer cells via Lyn kinase and AKT phosphorylation. Exp Mol Pathol. 2007;83(2):207–15. doi: 10.1016/j.yexmp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Rozsnyay Z. Signaling complex formation of CD44 with src-related kinases. Immunol Lett. 1999;68(1):101–8. doi: 10.1016/s0165-2478(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 46.Skubitz KM, Campbell KD, Skubitz AP. Tyrosine kinase activity is associated with CD44 in human neutrophils. FEBS Lett. 1998;439(1-2):97–100. doi: 10.1016/s0014-5793(98)01347-7. [DOI] [PubMed] [Google Scholar]
- 47.Sconocchia G, Titus JA, Segal DM. Signaling pathways regulating CD44-dependent cytolysis in natural killer cells. Blood. 1997;90(2):716–25. [PubMed] [Google Scholar]
- 48.Ilangumaran S, Borisch B, Hoessli DC. Signal transduction via CD44: role of plasma membrane microdomains. Leuk Lymphoma. 1999;35(5-6):455–69. doi: 10.1080/10428199909169610. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009;583(17):2749–57. doi: 10.1016/j.febslet.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T, et al. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib throughLyn/CXCR4 interactions in lipid rafts. Leukemia. 2012;26:883892. doi: 10.1038/leu.2011.291. [DOI] [PubMed] [Google Scholar]
- 51.Young RM, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem. 2003;278(23):20746–52. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.