Abstract
Objective
Oxidative stress and oxidized high-density lipoprotein (oxHDL) are implicated as risk factors for cardiovascular disease (CVD) in systemic lupus erythematosus (SLE). Yet, how HDL is oxidized and rendered dysfunctional in SLE remains unclear. Neutrophil extracellular traps (NETs), which are elevated in lupus, possess oxidant-generating enzymes including myeloperoxidase (MPO), NADPH oxidase (NOX) and nitric oxide synthase (NOS). We hypothesized that NETs mediate HDL oxidation, impairing cholesterol efflux capacity (CEC).
Methods
Control and lupus plasma MPO levels and CEC activity were examined; 3-chlorotyrosine (MPO-specific) and 3-nitrotyrosine (derived from reactive nitrogen species) were quantified in human HDL. Multivariable linear models estimated and tested differences between groups. HDL was exposed to NETs from control and lupus neutrophils in the presence or absence of MPO, NOX, NOS inhibitors and chloroquine. Murine HDL oxidation was quantified after NET inhibition in vivo.
Results
SLE subjects displayed higher MPO levels and diminished CEC. SLE HDL had higher 3-nitrotyrosine and 3-chlorotyrosine content, with site-specific oxidation signatures on apoA1. Experiments with human and murine NETs confirmed that chlorination is mediated by MPO and NOX, and nitration by NOS and NOX. Lupus mice treated with the NET-inhibitor Cl-amidine displayed significantly decreased oxHDL. Chloroquine inhibited NET formation in vitro.
Conclusion
Active NOS, NOX and MPO within NETs significantly modify HDL, rendering the lipoprotein proatherogenic. As NET formation is enhanced in SLE, these findings support a novel role for NET-derived lipoprotein oxidation in SLE-associated CVD and identify additional proatherogenic roles of neutrophils and putative protective roles of antimalarials in autoimmunity.
Patients with systemic lupus erythematosus (SLE) exhibit enhanced morbidity and mortality from premature atherosclerotic cardiovascular disease (CVD), which is not explained by the Framingham risk equation (1-3). While immune dysregulation plays a prominent role in SLE-CVD, the mechanisms remain unclear. We proposed that altered innate immune responses play important roles in the development of endothelial damage and plaque formation in SLE (4-7). In addition, lipoprotein abnormalities triggered by oxidative stress may promote atherogenesis in systemic autoimmune diseases (3, 8-10).
Myeloperoxidase (MPO) is a major source of reactive oxidants within the human vasculature. Classically thought of as macrophage-derived, MPO localizes to atherosclerotic plaques and its activity is linked to plaque rupture (10-12) and oxidation of high density lipoprotein (HDL) (8, 13, 14). In its native form, HDL has vasoprotective properties due to its ability to remove excess cholesterol from arterial wall macrophages, via ATP-binding cassette transporter A1 (ABCA1) interactions, and pleiotropic anti-inflammatory and anti-oxidant effects (10, 15-17). When apolipoprotein A1 (apoA1), the most abundant protein within HDL, becomes oxidized via chlorination or nitration of tyrosine residues (Cl-Tyr and N-Tyr oxHDL, respectively), the lipoprotein loses vasoprotective capabilities and gains proinflammatory activity (8, 10, 13, 14, 17, 18). Patients with chronic inflammatory diseases, including SLE and rheumatoid arthritis (RA), have higher levels of oxidized HDL (8, 9). However, mechanisms and dominant cellular sources leading to HDL oxidation are still unclear.
Phagocytes possess the machinery to oxidize biomolecules and represent attractive candidates for HDL oxidation. In addition to MPO, phagocytes display NADPH oxidase (NOX) activity (19) which can lead to superoxide anion (O2•−) and hydrogen peroxide (H2O2) production, both damaging oxidative species. Indeed, increased NOX activity is significantly associated with CVD (19). MPO can transform NOX-generated H2O2 into hypochlorous acid (HOCl) if chloride is present, or generate nitrogen dioxide radical (NO2•), a reactive nitrogen species (RNS), if nitrite is present, both promoting vascular damage and HDL oxidation. Reports indicate associations between plasma MPO levels and CVD in the general population, although it remains unclear if this is more relevant to specific patient subsets (11, 20). As enhanced circulating MPO activity has been described in SLE, this raises the possibility that circulating sources of MPO play key roles in lipoprotein oxidation in lupus, as reported by our group in RA (8, 21).
The three isoforms of nitric oxide synthase (NOS) in humans can generate nitric oxide (NO•): endothelial (eNOS), neuronal (nNOS) and inducible (iNOS). While NO• is generally vasoprotective, it can combine with NOX-generated O2•− to produce the RNS peroxynitrite (ONOO−) or be dismutated into H2O2, leading to HOCl or NO2•, potential oxidizers of HDL. While the literature is mixed (22, 23), iNOS is the isoform most associated with CVD risk.
The oxidation of HDL, by NOS/NOX-produced ONOO− or MPO/NOX-generated NO2• and HOCl, transforms the lipoprotein into a proatherogenic form which can increase vascular damage and inflammatory cytokine production (10, 12, 14, 15, 17, 18). While the roles of NOS, NOX and MPO in CVD are largely associated with macrophages and endothelial cells, these enzymes are important for neutrophil respiratory bursts (12, 24, 25).
Neutrophil extracellular traps (NETs) are extracellular fibers composed of DNA, histones and microbicidal granular proteins that play important roles in antimicrobial responses (26, 27). NET formation is triggered by microbes, platelets, cytokines and immune complexes, and is enhanced in various autoimmune disorders including SLE (4, 6, 26-32). While NET formation pathways are not fully characterized, the process appears dependent on MPO, NOX, and peptidylarginine-deiminase-4 (PAD4) activity (26, 27, 32-34). Beyond innate defense mechanisms and autoimmunity, NETs have been implicated in CVD by promoting thrombosis and proatherogenic innate immune responses (1, 6, 35-37). Neutrophils and NETs have been detected in the vasculature tunica externa and in the lumen (35, 38, 39). Pertinent to SLE, we have identified a subset of aberrant peripheral blood lupus neutrophils, named low-density granulocytes (LDGs), which are primed to form NETs with enhanced oxidant externalization (4, 7). We hypothesized that NETs formed by lupus LDGs may represent a prominent source of extracellular NOS, NOX and MPO, leading to proatherogenic HDL oxidation.
MATERIALS AND METHODS
Patients
Plasma samples were collected from SLE patients fulfilling American College of Rheumatology SLE diagnostic criteria (40). Healthy controls were recruited by advertisement. Study was approved by the University of Michigan and NIH IRBs. Lupus disease activity was quantified by SLEDAI (41). Pregnant or lactating women and individuals with recent or current infections or liver dysfunction were excluded.
Mice
NZM2328 (NZM) breeding pairs were a gift from Dr. Chaim Jacob (5). Balb/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were bred and housed in a specific pathogen-free barrier facility at the University of Michigan. Female mice were euthanized at 24-weeks of age, before overt development of renal disease. Protocol was approved by University of Michigan’s Committee on Use and Care of Animals.
Plasma HDL Isolation
HDL was isolated from human and murine plasma by sequential ultracentrifugation (8). Protein concentration was estimated with Coomassie (Thermo Scientific, Rockford, IL). Samples were stored at −80 °C until analysis.
Quantification of Oxidized Amino Acids in Plasma, HDL, and apoA1 Peptides
Plasma proteins were precipitated and delipidated (8); oxidized amino acids were quantified using isotopically labeled internal standards, 13C tyrosine, 13 6 C6 3-chlorotyrosine and 13C6 3-nitrotyrosine, by liquid chromatography-electrospray ionization tandem mass spectrometry (LCESI-MS/MS) with multiple reaction monitoring (MRM) MS/MS positive ion acquisition mode (42).
MRM Analysis of Oxidized Tyrosine-Containing apoA1 Peptides with LC/ESI/MS/MS
Plasma HDL samples were delipidated, and reduced with dithitreitol (5 μM, Sigma-Aldrich, St. Louis, MO) before alkylation with iodoacetamide, (15 mM, Sigma-Aldrich) (8). Samples were trypsin-digested and purified using solid-phase extraction C18 Sep-Pak columns (Waters Corporation, Milford, MA). Isotopically labeled oxidized (nitrated and chlorinated) peptides and native apoA1 peptides were spiked into samples following trypsin digestion. MRM analysis was performed with an Agilent 6490 Triple Quadrupole MS system equipped with an Agilent 1200 Infinity UPLC (Agilent Technologies, New Castle, DE) in positive ion mode (8).
Cholesterol efflux capacity (CEC) and MPO Quantification
CEC assay and MPO quantification were performed as described (8). J774 cells were maintained in DMEM/10% FBS (Life Technologies, Carlsbad, CA) (43). To radiolabel cellular free cholesterol pool, cells were incubated with 1 μCi/ml [3H] cholesterol (Perkin Elmer, Waltham, MA) in DMEM/ 1 mg/ml fatty acid-free BSA (FAFA) and 5 μg/ml ACAT inhibitor Sandoz 58-035 (Sigma-Aldrich) overnight. Cells were incubated with 0.5 mM 8-Br-cAMP to induce ABCA1 expression for 20 hours, incubated with DMEM/FAFA with or without 2.8% apoB-depleted plasma for 4 hour at 37 oC, then chilled on ice. Medium was collected and filtered, [3H] cholesterol content of medium and cells was quantified, and fraction of total [3H] cholesterol released into the medium was calculated (8).
Induction and Purification of NETs and Co-culture Assays
Human control neutrophils and lupus LDGs were purified from peripheral blood (4), and murine neutrophils from bone marrow (6). Cells were plated at a density of 0.25 × 106 cells/cm2 on tissue-culture plates in RPMI-1640 without phenol red (Life Technologies) for Cl-Tyr quantification, or in Krebs Ringer’s Phosphate Glucose (KRPG, Sigma-Aldrich) for N-Tyr quantification (4). Neutrophils were cultured in the presence or absence of 20 nM PMA (Sigma-Aldrich) for 3 hours (human), or 100 nM PMA for 5 hours (mouse) to induce NET formation (27). As LDGs form NETs spontaneously, they were left unstimulated. To inhibit NET formation, 200 μM Cl-Amidine was added for the full incubation (6). As NOX and MPO are required for NET formation but are also targets for the conditions where oxidation was inhibited, cells were allowed to form NETs for 1 hour before adding the following inhibitors: diphenylene iodonium (DPI, blocks NOX activity, 100 μM, Tocris, Bristol, UK), L-NG-monomethyl-L-arginine (L-NMMA, blocks NOS activity, 200 μM, Abcam, Cambridge, UK) and 3-amino-1,2,4-triazole (3-AT, blocks MPO activity, 10 mM, Sigma-Aldrich) (26, 27, 32, 33, 44, 45). Inhibitors were replenished hourly during NET formation. NETs were isolated as described, using 100 U/mL DNase I (Roche, Branchburg, New Jersey) (31). Supernatants resulting from the final spin contain NET-bound proteins and DNA fragments. Control, non-oxidized HDL (50 μg/mL) was incubated with NETs in the presence or absence of L-NMMA, 3-AT and DPI for 30 minutes at 37° C. Relative abundance of HDL oxidation was calculated as fold change of each condition relative to HDL oxidation with NET formation.
Quantification of NOS and NOX in Human and Murine NETs
Immunoblot
To detect NOS and NOX externalization during NET formation, digested human and murine NETs and whole neutrophil pellets were harvested. NET proteins were precipitated with acetone; 50 μg of NET or whole cell pellet proteins were separated on a 10% SDS-PAGE gel. Proteins were transferred onto a nitrocellulose membrane, incubated in 5% BSA/ TBS/ 0.1% Tween-20 for 1 hour, and stained with goat anti-mouse p47 or p22 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-eNOS or iNOS (1:100, Abcam) or anti-tubulin (negative control, 1:500, Sigma-Aldrich), followed by horseradish peroxidase-conjugated rabbit anti-goat (1:1000, Millipore, Temecula, CA) or goat anti-rabbit (1:5000, Jackson ImmunoResearch, West Grove, PA) secondary antibodies. Densitometry was performed with Quantity One (Bio-Rad, Hercules, CA).
Fluorescence microscopy
Human control neutrophils and lupus LDGs and mouse neutrophils were seeded onto poly-L-lysine (Sigma-Aldrich) coated coverslips (4, 6), stimulated with PMA as above, or left unstimulated (for LDGs) before fixing in 4% paraformaldehyde and staining with goat anti-p47 or p22 (both 1:50 dilution, Santa Cruz), or rabbit anti-iNOS or eNOS (both 1:20 dilution, Abcam) for 1 hour at 4 °C, followed by secondary fluorochrome-conjugated antibodies (Jackson ImmunoResearch) and Hoechst 33342 (Life Technologies). Coverslips were mounted with ProLong Gold Antifade (Life Technologies). Images were acquired on a Zeiss LSM510 META confocal laser-scanning microscope (Carl Zeiss Microimaging, Thornwood, NY) with a ×63 lens and quantified (4, 6).
Effect of Chloroquine on Neutrophils
Whole blood was incubated with 250 ng/mL chloroquine (Sigma-Aldrich, CQ) for two hours before neutrophils/LDGs were purified (17). Effect of CQ on NET formation was quantified using Sytox assay (Life Technologies) (31), immunofluorescent staining and immunoblot. To determine the effect of CQ on MPO, NOS and NOX activity, 1 × 106 granulocytes/mL, in either RPMI 1640 or KRPG with protease inhibitor cocktail (Roche), were homogenized at 35,000 rpm (Omni International, Kennesaw, GA) for three 10 second intervals on ice before treatment with or without 20 nM PMA for 30 minutes, then in the absence or presence of 250 ng/mL CQ for 30 minutes, and finally with or without 50 μg/mL HDL for 30 minutes. Samples were frozen and analyzed for Cl- and N-Tyr oxidation.
In vivo Cl-Amidine Administration
PAD inhibitor Cl-amidine was synthesized (46). Twelve-week old NZM female mice were administered daily subcutaneous injections of Cl-Amidine (10 mg/kg/day) or PBS (Life Technologies) for 14 weeks (6). Plasma was isolated and HDL was purified (5).
Statistical Analysis
Pearson correlation coefficients were calculated between outcomes studied and patient characteristics. Multivariable linear models were used to explore significant predictors of the outcomes of interest. The method of best subsets with the R-squared selection criterion guided model selection process (8). These models were also used to estimate and test differences between control and SLE groups. Skewed variables were logarithm base 10 (log10) or natural log (ln) transformed to satisfy statistical assumptions. Normally distributed variables were not transformed. A p value <0.05 was considered significant. Analyses were conducted using SAS V.9.2 (SAS Institute Inc., Cary, North Carolina, USA) or GraphPad Prism version 5 (GraphPad, La Jolla, CA).
RESULTS
Patients Characteristics
Controls and lupus patients did not differ in demographic characteristics (Table 1). Levels of low-density lipoprotein were significantly lower in SLE, possibly associated with prevalent use of statins (22.5%). SLE statin use correlated with elevated plasma Cl-Tyr levels, whereas antimalarial use was associated with higher plasma N-Tyr content (Table 2). SLE patients displayed significantly higher levels of plasma MPO compared to controls (425.5 versus 326.7 fmol/mL, p <0.05). Elevated MPO levels significantly correlated with a higher erythrocyte sedimentation rate and low LDL levels. Oxidation levels did not correlate with SLEDAI (Table 2).
Table 1. Demographic and clinic characteristics of healthy controls and SLE patients studied.
Statistically significant differences between groups are shown in bold; p < 0.05. Normally distributed variables are displayed as mean ± standard deviation. Skewed variables are displayed as median (IQR). ACE = Angiotensin Converting Enzyme, HDL = High Density Lipoprotein, LDL = Low Density Lipoprotein, MPO = Myeloperoxidase, ESR = Erythrocyte Sedimentation Rate, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index
| Healthy Contro |
Lupus | |
|---|---|---|
| n=20 | n=40 | |
| Age (years) | 47.5 ± 10.9 | 48.2 ± 13.5 |
| Sex (males) | 10.0% (2) | 10.0% (4) |
| Body Mass Index (kg/m2) | 24.0 (6.6) | 27.8 (12.2) |
| Cholesterol (mg/dL) | 212.0 (58.0) | 150.0 (87.0) |
| Triglycerides (mg/dL) | 161.5 (89.0) | 124.5 (99.0) |
| HDL (mg/dL) | 57.5 (28.0) | 52.0 (18.5) |
| LDL (mg/dL) | 128.1 ± 29.7 | 92.9 ± 39.6 |
| ESR (mm/hr) | n/a | 13.0 (13.0) |
| C-Reactive Protein (mg/L) | n/a | 0.6 (0.7) |
| SLEDAI | 0 | 3.0 (6.0) |
| Steroid use | 0 | 42.5% (17) |
| Antimalarial use | 0 | 72.5% (29) |
| Statin Use | 0 | 22.5% (9) |
| Beta Blockers | 0 | 25.0% (10) |
| ACE inhibitor Use | 0 | 22.5% (9) |
| MPO (fmol/mL) | 326.7 (156.0) | 425.5 (212.0) |
| Cholesterol Efflux Capacity (%) |
9.2 ± 1.6 | 7.8 ± 1.5 |
Table 2. Correlations between lipoprotein characteristics and plasma oxidation with clinical features.
Pearson correlations of lipoprotein characteristics and plasma oxidation with clinical features in all subjects (lupus and control) or in lupus only. Statistically significant differences between groups are shaded and in bold; p < 0.05. Comparisons of lipoprotein profiles, MPO levels and cholesterol efflux capacity (CEC) between controls and SLE patients are shown. Differences in the estimated means between groups, standard error, and model R2 are displayed. Variables indicated with an “*” were transformed (natural log-scale) prior to correlation calculation. Values indicated with “**” are adjusted as follows: CEC (%): Adjusted for HDL (n=50, R2 =0.08); MPO (fmol/ml): Adjusted for Age, BMI, and LDL (n=49, r2 =0.33); HDL-Cl-Tyr: Adjusted for BMI (n=59, R2 =0.10); HDL-N-Tyr: Adjusted for cholesterol (n=54, R2 =0.34); Plasma-3-Cl-Tyr: Adjusted for LDL (n=50, R2 =0.21); Plasma-N-Tyr: Adjusted for LDL (n=50, R2 =0.07). CRP = C-Reactive Protein, ESR = Erythrocyte Sedimentation Rate, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index.
| Pearson Correlations Among All Patients (n=60) | ||||||
|---|---|---|---|---|---|---|
| MPO* | CE | HDL− Cl− Tyr* |
HDLN− Tyr* |
Plasma− Clxya Tyr * |
Plasma− N-Tyr |
|
| Age (years) | r = −0.23, p=0.08 |
r = 0.11, p=0.39 |
r = 0.03, p=0.83 |
r = 0.07, p=0.61 |
r = −0.05, p=0.71 |
r = −0.02, p=0.89 |
| Sex (males) | r = −0.21, p=0.11 |
r = −0.05, p=0.72 |
r = 0.21, p=0.12 |
r = 0.14, p=0.30 |
r = 0.08, p=0.54 |
r = 0.04, p=0.77 |
| Body Mass Index* (kg/m2) |
r = 0.20, p=0.13 |
r = −0.23, p=0.08 |
r = 0.31,
p=0.02 |
r = 0.05, p=0.72 |
r = 0.18, p=0.18 |
r = 0.07, p=0.61 |
| Cholesterol* (mg/dL) |
r = −0.13, p=0.33 |
r = 0.14, p=0.31 |
r = −0.04, p=0.77 |
r = 0.58,
p<0.01 |
r = −0.25, p=0.07 |
r = 0.23, p<0.10 |
| Triglycerides* (mg/dL) |
r = 0.11, p=0.44 |
r = −0.07, p=0.62 |
r = −0.14, p=0.32 |
r = 0.01, p=0.94 |
r = −0.02, p=0.90 |
r = −0.16, p=0.26 |
| HDL* (mg/dL) | r = −0.25, p=0.08 |
r = 0.28,
p<0.05 |
r = −0.15, p=0.28 |
r = 0.04, p=0.79 |
r = −0.17, p=0.22 |
r = 0.01, p=0.96 |
| LDL (mg/dL) |
r = −0.44,
p<0.01 |
r = 0.18, p=0.22 |
r = −0.29,
p=0.04 |
r = 0.12, p=0.41 |
r = −0.46,
p<0.01 |
r = −0.27, p=0.06 |
| Pearson Correlations Among Lupus Patients only (n=40) | ||||||
|---|---|---|---|---|---|---|
| MPO* | CE |
HDL− Cl−
Tyr* |
HDLN−
Tyr* |
Plasma− Cl−
Tyr * |
Plasma−
N-Tyr |
|
| CRP* (mg/L) | r = 0.05, p=0.76 |
r = 0.18, p=0.27 |
r = 0.17, p=0.31 |
r = 0.11, p=0.52 |
r = 0.24, p=0.15 |
r = −0.16, p=0.34 |
| ESR* (mm/hr) |
r = 0.36,
p=0.02 |
r = 0.06, p=0.71 |
r = −0.05, p=0.75 |
r = 0.19, p=0.26 |
r = 0.07, p=0.66 |
r = −0.14, p=0.40 |
| SLEDAI | r = 0.14, p=0.39 |
r = 0.08, p=0.63 |
r = 0.15, p=0.34 |
r = 0.06, p=0.71 |
r = 0.15, p=0.36 |
r = 0.05, p=0.76 |
| Steroid use | r= −0.09, p=0.59 |
r = 0.11, p=0.51 |
r = −0.20, p=0.22 |
r = −0.27, p=0.09 |
r = 0.11, p=0.49 |
r = 0.09, p=0.57 |
| Antimalarial use |
r = 0.03, p=0.83 |
r = 0.08, p=0.64 |
r = −0.08, p=0.62 |
r = 0.05, p=0.76 |
r = −0.20, p=0.23 |
r = 0.32,
p<0.05 |
| Statin use | r = −0.18, p=0.26 |
r = 0.13, p=0.42 |
r = −0.002, p=0.99 |
r = −0.01, p=0.95 |
r = 0.32,
p<0.05 |
r = −0.12, p=0.48 |
| Beta Blocker use |
r = −0.07, p=0.66 |
r = 0.22, p=0.17 |
r = −0.15, p=0.36 |
r = −0.26, p=0.11 |
r = 0.16, p=0.32 |
r = −0.004, p=0.98 |
| Ace inhibitor use |
r = −0.06, p=0.70 |
r = 0.16, p=0.33 |
r = 0.09, p=0.59 |
r = 0.12, p=0.46 |
r = 0.01, p=0.96 |
r = −0.06, p=0.71 |
| Unadjusted Comparison | Adjusted** Comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Lupus | p-value | Model R2 |
Controls | Lupus | p-value | Model R2 |
|
| CEC (%) | 9.2 ± 0.3 | 7.8 ± 0.2 | 0.0022 | 0.15 | 9.2 ± 0.3 | 8.0 ± 0.3 | 0.0073 | 0.21 |
| MPO (fmol/ml)* |
5.7 ± 0.1 | 6.0 ± 0.1 | 0.0005 | 0.19 | 5.8 ± 0.1 | 6.0 ± 0.1 | 0.0198 | 0.4 |
| HDL 3- chlorotyrosine* |
1.4 ± 0.4 | 5.4 ± 0.3 | <0.0001 | 0.51 | 1.5 ± 0.4 | 5.4 ± 0.3 | <0.0001 | 0.56 |
| HDL 3- nitrotyrosine* |
2.5 ± 0.4 | 4.2 ± 0.3 | 0.0003 | 0.2 | 2.6 ± 0.3 | 4.1 ± 0.2 | 0.0002 | 0.49 |
| Plasma 3- chlorotyrosine* |
0.4 ± 0.3 | 3.3 ± 0.2 | <0.0001 | 0.49 | 0.3 ± 0.3 | 3.3 ± 0.2 | <0.0001 | 0.62 |
| Plasma 3- nitrotyrosine* |
3.4 ± 0.2 | 5.7 ± 0.1 | <0.0001 | 0.64 | 3.3 ± 0.2 | 5.7 ± 0.2 | <0.0001 | 0.63 |
CEC is Impaired in SLE
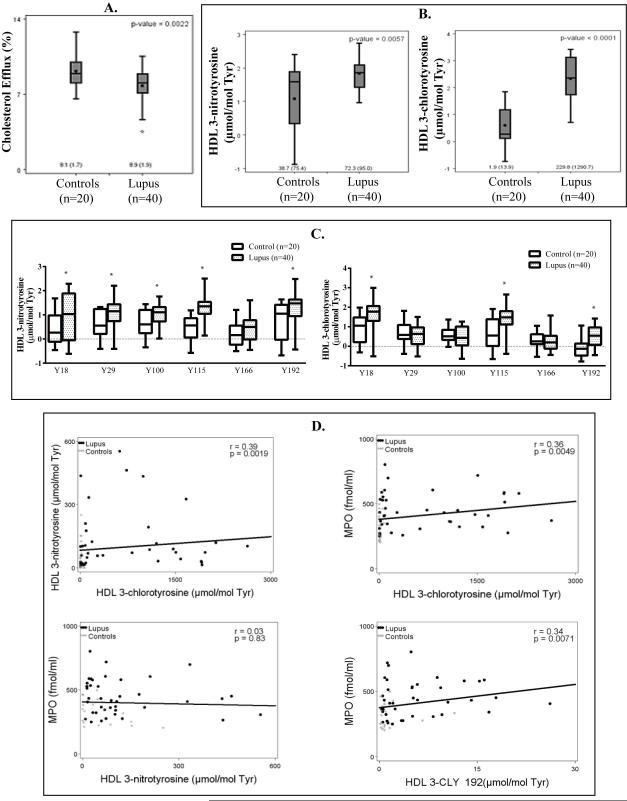
The ability of HDL to promote cholesterol efflux from macrophages is a metric of HDL function and has a strong inverse association with CVD (3, 14, 17). Plasma from patients with SLE displayed significantly diminished CEC when compared to control plasma (9.2 ± 1.6% vs. 7.8 ± 1.5%, p =0.001, Table 1). These results persisted after adjustment for significant predictors of CEC (Table 2) and support previous indications that dysfunctional HDL present in SLE leads to impaired CEC and may promote proatherogenic responses (3).
Chlorinated and Nitrated HDL is Increased in SLE
Impaired CEC has been associated with HDL modifications such as nitration and chlorination (14, 18). We quantified levels of Cl-Tyr, a highly specific product of MPO, and N-Tyr, a product of MPO and other RNS-producing enzymes in SLE and control isolated HDL and in total plasma (8, 42). SLE HDL displayed 1.9-fold higher median levels of N-Tyr (72.3 vs. 38.7 μmol/mol Tyr; p =0.0057) and 120.9-fold higher median levels of Cl-Tyr (229.8 vs. 1.9 μmol/mol Tyr; p <0.0001), compared to control HDL (Figure 1B) even after adjustment (listed in Table 2). Because a majority (72.5%, Table 1) of the SLE patients was on antimalarials, we determined the effect of antimalarial treatment in the study population. Adjusting for antimalarial use had no significant effect on the values in Table 1 or Table 2 (data not shown). As studies have identified that chlorination of tyrosine residue 192 (Tyr-192) within the apoA1 protein most directly associates with impaired CEC (14), with six other apoA1 tyrosine residues (Tyr-18, −29, −100, −115, −166, and −236) as other potential sites of oxidation (8, 13), we determined if regiospecific nitration and chlorination patterns occur in SLE. The highest levels of MPO-dependent Cl-Tyr HDL oxidation in SLE samples were observed at tyrosines 192, 115 and 18 (Figure 1C), when compared to control samples. Levels of N-Tyr HDL oxidation were highest at tyrosines 192, 115, 100, 29 and 18 in SLE (Figure 1C). Taken together, this data suggests that regiospecific modifications to HDL by MPO and RNS, specifically at Tyr-18, −115, and −192, may be of particular interest in the context of SLE-associated CVD.
Figure 1. HDL isolated from SLE patients is significantly oxidized and dysfunctional.
Plasma and HDL were purified from healthy controls (N=20), and SLE patients (N=40). (A) ABCA1-mediated cholesterol efflux capacity (determined in J774 cells loaded with radiolabeled [3H]-cholesterol and then incubated with SLE or control apoB depleted plasma). (B) MPO-specific 3-chlorotyrosine (Cl-Tyr) levels and RNS- and MPO-mediated 3-nitrotyrosine (N-Tyr) levels in lupus and control HDL (in log base 10). The length of the box defines the interquartile range (IQR). Medians (IQR) are on raw scale. (C) Box blots display distributions of isotopically labeled chlorinated or nitrated tyrosines. Native apoA-1 peptides were spiked into HDL samples from control (N=20, white) and SLE patients (N=40, shaded). Extracted ion chromatograms from specific fragment ions were used for quantitative analysis. Values are expressed in log base 10 and significant differences between controls and SLE are denoted by an asterisk (*p<0.05). The length of the box defines the IQR. (D) For all subjects, correlations between levels of N-Tyr and Cl-Tyr HDL oxidation, MPO and Cl-Tyr HDL oxidation, MPO and N-Tyr HDL oxidation, and MPO and chlorination levels at tyrosine 192 within apoA1 from patients. Figure D displays the scatter plot and least square regression line.
When we examined correlations between levels of N-Tyr and Cl-Tyr in SLE HDL, they positively correlated with each other (Figure 1D, r =0.39, p =0.0019), suggesting that these oxidative modifications are generated at similar sites. While levels of Cl-Tyr significantly correlated with plasma MPO levels (Figure 1D, r =0.36, p =0.0049), there was no significant correlation between N-Tyr and MPO levels (Figure 1D, r =0.03, p =0.83). This suggests that the RNS leading to HDL nitration are not primarily derived from MPO, but from another oxidative source. While tyrosines 115 and 18 displayed the highest levels of oxidation in lupus, Cl-Tyr 192 showed the most significant positive correlation with plasma MPO levels (Figure 1D). Overall, these results support the notion that in SLE, RNS and MPO-derived oxidants modify HDL, thereby impairing lipoprotein function.
Oxidation in total plasma proteins was also determined (Table 2). Similar trends were observed as for HDL: median lupus plasma N-Tyr levels were 8.8-fold (286.0 vs. 32.5 μmol/mol Tyr, p <0.0001) and Cl-Tyr levels 40.6-fold higher (33.3 vs. 0.82 μmol/mol Tyr, p <0.0001) than control plasma levels, which persisted after adjustment (Table 2, values in table are natural log transformed). As expected, the levels of plasma and HDL Cl-Tyr in SLE trended towards a negative correlation with CEC percentage, while HDL N-Tyr levels showed no significant association (data not shown) (14). Overall, this profile of enhanced HDL and total plasma protein oxidation is consistent with an environment of increased oxidative stress and impaired CEC in SLE.
NET-derived MPO, NOX and NOS Promote HDL Oxidation In Vitro
As levels of RNS- and MPO-derived oxidative modifications are markedly elevated in lupus and correlate with functional impairment of CEC, we attempted to identify the putative sources of enhanced RNS and MPO activity in SLE. MPO HDL oxidation is classically attributed to plaque macrophages (12, 24). Another possible source is peripheral blood MPO, reported enhanced in SLE (21) and which we were able to confirm (Tables 1 and 2). As a subset of peripheral blood lupus neutrophils (LDGs) has a significantly enhanced capacity to form NETs, a source of externalized NOX and MPO, we examined if the NETs could induce HDL oxidation (4, 27, 33). Additionally, because we found that MPO levels did not correlate with N-Tyr HDL levels (Figure 1D), we examined if an alternative producer of RNS (NOS and NOX formation of peroxynitrite) could be present in NETs and serve as a source of HDL nitration.
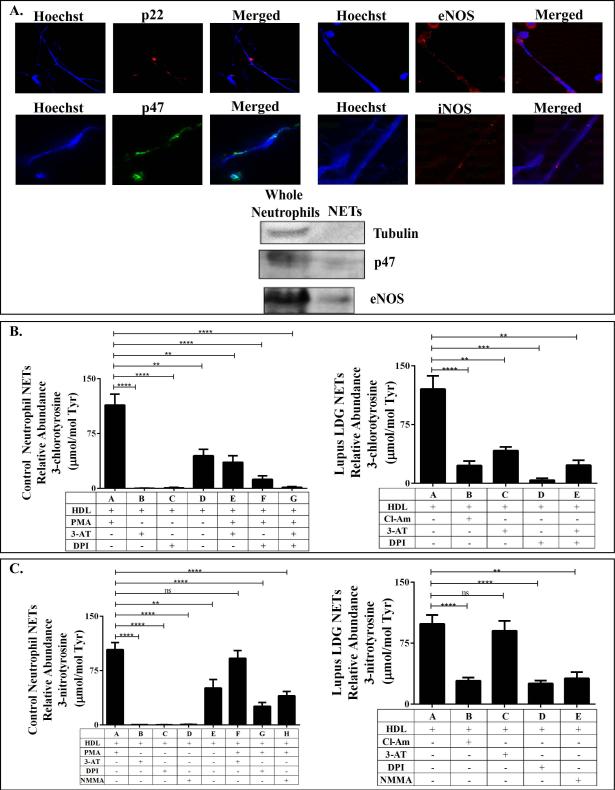
MPO (not shown), NOX and NOS were all detected on both control and LDG NETs by fluorescence microscopy and immunoblot (Figure 2A). Next, we exposed native HDL to NETs isolated from PMA-stimulated control neutrophils or spontaneously by lupus LDGs, in the absence or presence of MPO, NOX and NOS inhibitors (3-AT, DPI and L-NMMA, respectively), and examined HDL oxidation profile. As shown in Figures 2B and 2C, NETs from both lupus LDGs and control neutrophils significantly enhanced HDL oxidation. HOCl, which is synthesized by MPO downstream of NOX activity, is the major source of HDL chlorination in humans. When netting control neutrophils and lupus LDGs were treated with 3-AT and DPI to block MPO and NOX activity, respectively, HDL Cl-Tyr oxidation was abrogated (Figure 2B). These results support the hypothesis that MPO and NOX externalized on NETs can induce Cl-Tyr HDL oxidation in the periphery.
Figure 2. NET-derived MPO, NOS and NOX modify human HDL.
Immunofluorescent staining and immunoblotting was used to identify possible enzymatic sources of HDL nitration. (A) NOX subunits, p22 and p47, and two NOS isoforms, eNOS and iNOS, were detected in human NETs. Control neutrophil and lupus LDG NETs were added to control, unoxidized HDL. Resulting levels of (B) MPO-specific Cl-Tyr, and (C) RNS-mediated N-Tyr oxidation were quantified by mass spectrometry. 3-AT, NMMA and DPI were added to block MPO, NOS and NOX activity, respectively. The PAD inhibitor Cl-Am was added to LDGs to block spontaneous NETosis. For control neutrophils, absence of PMA stimulation was used as a negative control for NETosis. (N= 6/group; ** p<0.007, *** p=0.0002, **** p< 0.0001. Data are displayed as mean ± SEM).
Because both NOS and MPO are capable of producing the RNS required for HDL nitration, we used a KRPG solution with no added nitrite to examine the role of NOS alone in NTyr oxidation. Under these conditions, only NOS could generate the NO• required for peroxynitrite formation that could lead to HDL nitration. MPO, under these conditions, could not be the source of RNS. Indeed, the MPO inhibitor 3-AT was ineffective at blocking HDL nitration, while the NOS inhibitor L-NMMA significantly abrogated HDL N-Tyr oxidation for both LDGs and control neutrophils (Figure 2C). These results suggest that NET-bound NOS is a source of the RNS causing HDL nitration, and may explain why the levels of MPO did not correlate with the levels of N-Tyr oxidized HDL in SLE.
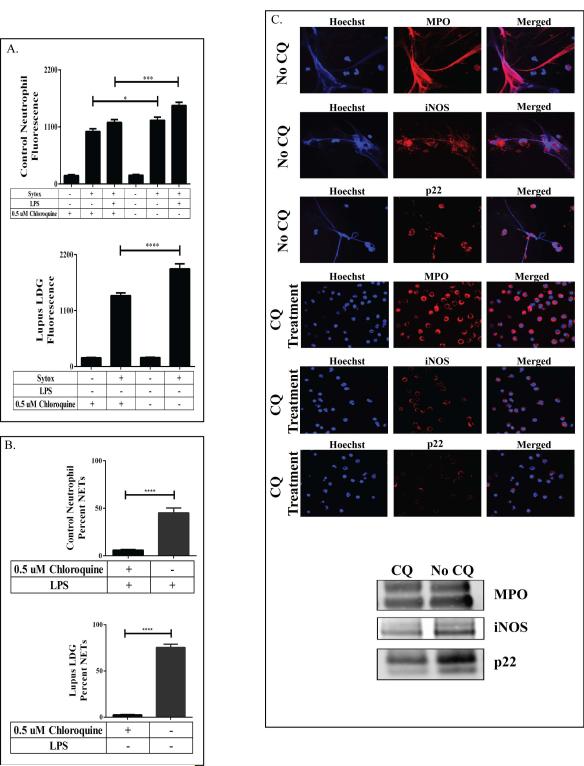
Chloroquine Blocks NET Formation In Vitro
We determined the effect of physiologically relevant concentrations of CQ (250 ng/mL, CQ) on NET formation (17). CQ significantly inhibited NET formation in control neutrophils and lupus LDGs (Figure 3A-C). To assess if CQ modified MPO, NOS and NOX activity, neutrophils were homogenized, treated with CQ, and incubated with HDL. CQ did not significantly block HDL Cl-Tyr or N-Tyr oxidation in lupus LDGs or control neutrophils (data not shown). Therefore, the inhibitory effects of CQ on NET formation do not occur by inhibition of oxidative enzyme activity.
Figure 3. Chloroquine abrogates NET formation.
(A) Control neutrophils or lupus LDGs purified from whole blood and treated with or without chloroquine (CQ) were assessed for DNA externalization via Sytox assay. Immunofluorescent staining of these treated cells was used to quantify (B) NET production and (C) MPO, iNOS and p22 externalization on the NETs. (N=5/group; * p=0.01, *** p=0.0001, **** p<0.0001. Data are displayed as mean ± SEM.)
NET-derived NOS and NOX are Sources of HDL Oxidation in Murine Systems
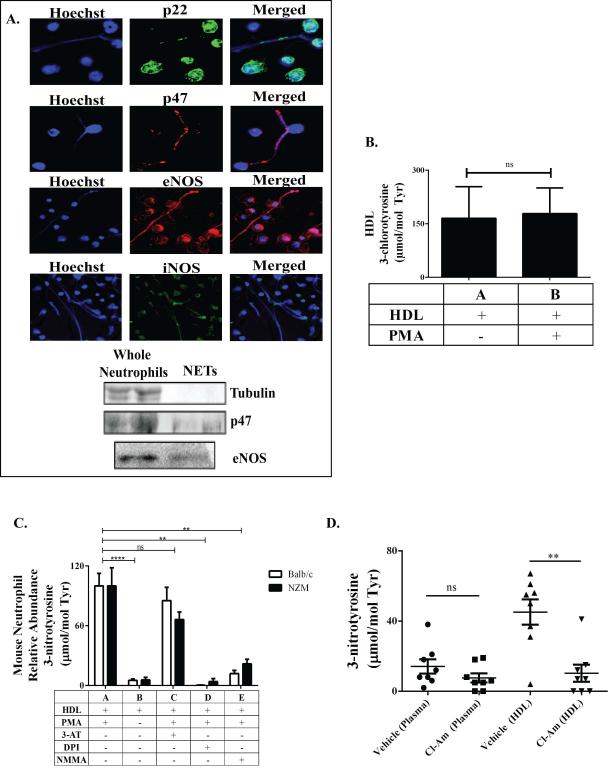
While present in murine NETs, MPO is not associated with murine atherosclerosis (24, 47). Lupus-prone NZM mice display striking elevations of N-Tyr HDL oxidation, but not Cl-Tyr, when compared to control mice (5), possibly because murine leukocytes contain less MPO, the sole known source of Cl-Tyr oxidation, than human leukocytes (12, 24, 47). We therefore examined if NOX and NOS were present in murine NETs and could produce RNS to form N-Tyr oxHDL (26, 33). We identified NOS and NOX machinery present in PMA-induced murine NETs (Figure 4A). To verify that these NET-bound enzymes could cause HDL oxidation, we exposed native HDL from non-lupus prone Balb/c to proteins purified from digested NZM or Balb/c NETs in the absence or presence of MPO, NOX and NOS inhibitors (3-AT, DPI and LNMMA, respectively) and examined HDL oxidation profiles (26, 27, 32, 33, 44, 45). Consistent with our previous report, we found no significant Cl-Tyr oxHDL patterns after HDL incubation with murine NETs (Figure 4B) (5). However, a significant oxidation pattern for N-Tyr oxHDL was observed (Figure 4C). While experiments were performed in nitrite containing media so that either NOS or MPO could potentially promote HDL nitration, only DPI and L-NMMA significantly blocked NET-induced N-Tyr HDL oxidation. These results support the idea that MPO is less active in mice and that NOX and NOS are the primary sources of the oxidative species required for HDL nitration in murine systems.
Figure 4. NET-derived NOS and NOX induce HDL oxidation in mice, while NET inhibition decreases HDL oxidation in vivo.
Immunofluorescent staining and immunoblotting was used to identify possible enzymatic sources of HDL nitration. (A) NOX subunits, p22 and p47, and two NOS isoforms, eNOS and iNOS, were detected in murine NETs. Control Balb/c and lupus NZM NETs were added to control, unoxidized murine HDL. (B) Resulting levels of MPO-specific Cl-Tyr oxidation in the absence or presence of isolated NETs, as quantified by mass spectrometry (N=4/group). (C) Resulting levels of NOS- and NOX-mediated N-Tyr oxidation as quantified by mass spectrometry (N=8/group). 3-AT, NMMA and DPI were added to block MPO, NOS and NOX activity, respectively. Absence of PMA stimulation was used as a negative control for NET formation. (D) Levels of plasma and HDL N-Tyr content in 26-week old NZM mice that received daily injections of PBS (N= 10) or Cl-Am (N=10) for 14 weeks. (For C, comparisons were made within the same mouse strain; ** p<0.01, **** p<0.0001. Data are displayed as mean ± SEM).
Finally, to verify that NETs are an important source of HDL oxidation in the periphery, we inhibited NET formation in NZM mice in vivo and examined their oxidative profile. NZM mice received the PAD inhibitor Cl-Am daily for 14 weeks, as PAD activity is necessary for NET formation (6, 34). Cl-Am-treated mice displayed significantly decreased N-Tyr content in HDL, but not total plasma, when compared to vehicle treated mice (Figure 4D). These results indicate that in vivo inhibition of NET formation in lupus-prone mice decreases HDL oxidation.
DISCUSSION
Part of the high risk for CVD observed in SLE may be related to increased levels and activity of oxidative enzymes that transform HDL into a dysfunctional proatherogenic lipoprotein (1, 3, 21). We found that lupus plasma exhibited impaired CEC and SLE patients had significantly increased plasma MPO levels and highly Cl- and N-Tyr oxidized plasma proteins, including HDL apoA1. HDL nitration did not correlate with MPO levels, indicating an alternate source of RNS. However, because Cl-Tyr and N-Tyr oxHDL levels correlated with each other, this suggests that both modifications occur at similar sites in the vasculature, though not through the same enzyme. Because of the link between SLE and enhanced NET formation, we hypothesized that the enzymatic sources of the proatherogenic oxHDL were derived from active NOS, MPO and NOX present in NETs. Indeed, previous literature suggest that these oxidative enzymes may have enhanced activity while bound on NETs (26). All three oxidative enzymes were detected and functional in NETs, as demonstrated when mass spectrometric analysis performed on HDL incubated with NETs revealed oxidative modifications (chlorination and nitration). Use of specific inhibitors for each of these enzymes reduced this oxidation, further confirming functional activity of these NET enzymes. Finally, we demonstrated that pharmacologic in vivo NET inhibition potently decreased levels of proinflammatory N-Tyr oxHDL in lupus-prone mice. These observations suggest a crucial role for NETs in the transformation of HDL into its proinflammatory form.
The connection between NET oxidation of HDL and plasma proteins is particularly interesting and novel in the context of chronic inflammatory conditions like SLE, RA, and vasculitis (8, 28). Since lupus patients exhibit both accelerated NET formation and impaired NET degradation, these structures represent a potent and chronic source of externalized oxidative material (4, 29, 30). Most of the literature to date has focused on atherosclerotic plaque macrophages as sources of MPO-driven HDL oxidation, yet circulating neutrophils contain higher levels of MPO than tissue-resident macrophages (12, 24) and neutrophils and NETs are present around thrombotic and atheroma plaques (35, 38, 39, 48). The high concentration of MPO in NETs may even specifically attract HDL for oxidation. Consistent with previous reports, we found that Cl-Tyr levels in HDL are higher than in circulating plasma proteins, suggesting specific targeting of HDL (8, 18). While others have demonstrated that neutrophils can cause protein oxidation, our observations are the first to specifically implicate active MPO, NOX, and NOS in NETs as sources of HDL oxidation (44), and to demonstrate NOS in NETs. Which NOS isoform is required for NET-derived HDL oxidation is not yet clear. Nonetheless, NOS activity in NETs and its association with HDL nitration opens potential avenues for CVD therapies.
To our knowledge, this is the first study to report that physiologic concentrations of antimalarials significantly inhibit NET formation in vitro. Previous evidence suggested that antimalarials are effective in SLE through modulation of Toll like receptors and antigen presentation (49, 50). We now describe an additional putative protective role of antimalarials in autoimmunity through modulation of NET formation. While use of antimalarials only associated with plasma N-Tyr oxidation, small sample size might have limited observing an effect. This should be further explored in future studies.
The study has some limitations. The effects on CEC reduction of 15% might appear modest, but we previously reported similar results in RA (8). Recent clinical studies have demonstrated smaller, though statistically significant, percent reductions in CEC with larger CVD and psoriasis cohort sample sizes (17, 43). Our results therefore are in agreement with these studies and even show a greater reduction in CEC than in traditional CVD. Further, while we focused on total HDL in this study, HDL subpopulations might be different in lupus subjects compared with control subjects and might account for altered susceptibility.
The proatherogenic effects of NETs include direct vascular cytotoxicity, activation of macrophage inflammasomes and induction of procoagulant pathways (1, 28-31, 37, 48). Overall, this study suggests an additional proatherogenic mechanism where NET-derived MPO/NOX-and NOS/NOX-generated oxidative species may generate dysfunctional HDL in SLE. HDL oxidation in SLE may take place in the periphery rather than the vascular wall (1, 31). Given its small particle size and active circulation during cholesterol efflux, it is conceivable that HDL is more readily trapped by NETs in the blood vessel lumen, endothelium, or sites of inflammation, rather than in subendothelial atherosclerotic lesions (17, 35, 36). NET-mediated modification of HDL may lead to a dysfunctional lipoprotein, impairing CEC and promoting proinflammatory responses in blood vessels and the periphery.
Acknowledgments
Funding: Funded by the Lupus Research Institute (to MJK), the Intramural Research Program, NIAMS/NIH, and, in part, by NIH through PHS grants HL088419 (to MJK), AI007413 (support of CKS), DK089503 and DK097153 (SP), GM079357 (PRT), and by the Rheumatology Research Foundation (SP and JSK). PRT holds the patent for Cl-amidine.
REFERENCES
- 1.Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med. 2013;64:249–63. doi: 10.1146/annurev-med-060911-090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73(3):609–15. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thacker SG, Zhao W, Smith CK, Luo W, Wang H, Vivekanandan-Giri A, et al. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum. 2012;64(9):2975–85. doi: 10.1002/art.34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123(7):2981–93. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–97. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis. 2013;72(10):1725–31. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon M, Grossman J, Skaggs B, Fitzgerald J, Sahakian L, Ragavendra N, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2428–37. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18(4):427–34. doi: 10.1097/MOL.0b013e3282364a17. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136–42. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 12.Karakas M, Koenig W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14(3):277–83. doi: 10.1007/s11883-012-0242-3. [DOI] [PubMed] [Google Scholar]
- 13.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012;287(9):6375–86. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280(7):5983–93. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 15.Assinger A, Koller F, Schmid W, Zellner M, Babeluk R, Koller E, et al. Specific binding of hypochlorite-oxidized HDL to platelet CD36 triggers proinflammatory and procoagulant effects. Atherosclerosis. 2010;212(1):153–60. doi: 10.1016/j.atherosclerosis.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Pritchard DK, Becker L, Hoofnagle AN, Tanimura N, Bammler TK, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122(19):1919–27. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(Suppl 1):S11–5. [PubMed] [Google Scholar]
- 18.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279(41):42977–83. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 19.Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart. 2004;90(5):491–3. doi: 10.1136/hrt.2003.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubala L, Lu G, Baldus S, Berglund L, Eiserich JP. Plasma levels of myeloperoxidase are not elevated in patients with stable coronary artery disease. Clin Chim Acta. 2008;394(1-2):59–62. doi: 10.1016/j.cca.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telles RW, Ferreira GA, da Silva NP, Sato EI. Increased plasma myeloperoxidase levels in systemic lupus erythematosus. Rheumatol Int. 2010;30(6):779–84. doi: 10.1007/s00296-009-1067-4. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui M, Shimokawa H, Otsuji Y, Yanagihara N. Pathophysiological relevance of NO signaling in the cardiovascular system: novel insight from mice lacking all NO synthases. Pharmacol Ther. 2010;128(3):499–508. doi: 10.1016/j.pharmthera.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33(11):1897–918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 24.McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation. 2005;111(21):2798–804. doi: 10.1161/CIRCULATIONAHA.104.516278. [DOI] [PubMed] [Google Scholar]
- 25.Cedergren J, Follin P, Forslund T, Lindmark M, Sundqvist T, Skogh T. Inducible nitric oxide synthase (NOS II) is constitutive in human neutrophils. APMIS. 2003;111(10):963–8. doi: 10.1034/j.1600-0463.2003.1111008.x. [DOI] [PubMed] [Google Scholar]
- 26.Munafo DB, Johnson JL, Brzezinska AA, Ellis BA, Wood MR, Catz SD. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J Innate Immun. 2009;1(6):527–42. doi: 10.1159/000235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522–31. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 30.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190(3):1217–26. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953–9. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchner T, Moller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm. 2012;2012:849136. doi: 10.1155/2012/849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Megens RT, Vijayan S, Lievens D, Doring Y, van Zandvoort MA, Grommes J, et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost. 2012;107(3):597–8. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777–83. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125(13):1673–83. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 38.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circulation research. 2012;110(6):875–88. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 39.Dorweiler B, Torzewski M, Dahm M, Kirkpatrick CJ, Lackner KJ, Vahl CF. Subendothelial infiltration of neutrophil granulocytes and liberation of matrix-destabilizing enzymes in an experimental model of human neo-intima. Thromb Haemost. 2008;99(2):373–81. doi: 10.1160/TH07-06-0387. [DOI] [PubMed] [Google Scholar]
- 40.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 41.Isenberg D, Bacon P, Bombardier C, Gladman D, Goldsmith CH, Kalunian K, et al. Criteria for assessing disease activity in systemic lupus erythematosus. J Rheumatol. 1989;16(10):1395–6. [PubMed] [Google Scholar]
- 42.Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. doi: 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergt C, Marsche G, Panzenboeck U, Heinecke JW, Malle E, Sattler W. Human neutrophils employ the myeloperoxidase/hydrogen peroxide/chloride system to oxidatively damage apolipoprotein A-I. Eur J Biochem. 2001;268(12):3523–31. doi: 10.1046/j.1432-1327.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 45.Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Activation of the cGMP signaling pathway is essential in delaying oocyte aging in diabetes mellitus. Biochemistry. 2006;45(38):11366–78. doi: 10.1021/bi060910e. [DOI] [PubMed] [Google Scholar]
- 46.Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, et al. The development of N-alpha-(2-carboxyl)benzoyl-N(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-F-amidine) and N-alpha-(2-carboxyl)benzoyl-N(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-Cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J Med Chem. 2011;54(19):6919–35. doi: 10.1021/jm2008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107(4):419–30. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox R. Anti-malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus. 1996;5(Suppl 1):S4–10. [PubMed] [Google Scholar]
- 50.Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794–804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]