Abstract
Objective
To examine the hypothesis that improving insulin sensitivity would improve vascular function in rheumatoid arthritis (RA).
Methods
We performed a 20-week, single center, randomized, double-blind, placebo-controlled, crossover study. Patients with RA (n=34) and moderate disease activity on stable disease modifying anti-rheumatic drug therapy were randomized to drug sequence, receiving either pioglitazone 45mg daily or matching placebo for 8 weeks, followed by a 4-week washout period and the alternative treatment for 8 weeks. We measured change in vascular stiffness (augmentation index and aortic pulse wave velocity), endothelial function (reactive hyperemia index), and blood pressure. High sensitivity C-reactive protein (CRP), and the homeostatic model assessment of insulin resistance (HOMA) were also measured. The treatment effect of pioglitazone on outcomes was analyzed using linear mixed effect models.
Results
Pioglitazone reduced augmentation index by −4.7% units (95% CI, −7.9, −1.5% units) P=0.004 and diastolic blood pressure by −3.0 mmHg (−5.7, −0.2 mmHg) P=0.03, but did not change aortic pulse wave velocity (P=0.33), or reactive hyperemia index (P=0.46) significantly. The improvements in augmentation index and diastolic blood pressure were not mediated by pioglitazone's effect on insulin resistance or inflammation.
Conclusion
Pioglitazone improved some indices of vascular function, including augmentation index and diastolic blood pressure, in patients with RA; this was not mediated by improved insulin sensitivity.
Keywords: rheumatoid arthritis, cardiovascular disease, insulin resistance, vascular function
Rheumatoid arthritis (RA) is a systemic inflammatory disease associated with increased cardiovascular (CV) risk and mortality; this increase is independent of traditional CV risk factors (1-3). One potentially modifiable novel risk factor that may contribute to excess CV risk in RA is insulin resistance. We have shown that the prevalence of insulin resistance is increased more than two-fold in patients with RA, compared to controls, and that this is associated with coronary atherosclerosis (4,5).
Insulin resistance is associated with vascular dysfunction measured by pulse wave reflection, arterial stiffness, and endothelium-dependent vasodilation in non-RA populations (6,7); this vascular dysfunction is thought to represent early, subclinical vascular disease that subsequently leads to increased atherosclerosis and CV mortality (8,9). We have shown that patients with RA have vascular dysfunction, measured as increased augmentation index, and that this is associated with coronary artery calcification, a measure of coronary artery atherosclerosis (10). Because of the relationship between insulin resistance, vascular dysfunction and atherosclerosis, improvement of insulin resistance is an attractive strategy to improve CV risk in RA.
Insulin resistance can be improved by treatment with a thiazolidinedione peroxisome proliferator activated receptor gamma (PPAR-γ) agonist, such as pioglitazone, an insulin sensitizing drug that has improved vascular function (11-16) and also reduced the risk of myocardial infarction and mortality among patients with diabetes (17,18). Whether improved insulin sensitivity in non-diabetic patients with RA would improve vascular function is not known. We have previously shown that pioglitazone improved insulin resistance in non-diabetic RA patients and decreased some measures of disease activity and inflammation (19). In the current study we examined the hypothesis that improvement in insulin resistance would improve vascular function in patients with RA. We postulated that improved insulin sensitivity resulting from treatment with pioglitazone in RA would significantly improve measures of vascular function including vascular stiffness (augmentation index and pulse wave velocity), endothelial function (reactive hyperemia index), and blood pressure.
Methods
Study design
The design of this study has been previously described (19). Briefly, this was a single center, randomized, double-blind, placebo-controlled, cross-over study with washout. Patients were randomly assigned to receive pioglitazone 45mg daily or matching placebo for 8 weeks in addition to their stable baseline disease modifying anti-rheumatic drug (DMARD) therapy at unchanged doses. This was followed by a 4-week washout, and then patients switched to the alternative treatment for an additional 8 weeks (Supplementary Figure). Study visits occurred at screening, baseline (week 0), and every 4 weeks through week 20, or withdrawal from the study. If a change in RA disease activity warranted a change in DMARD or corticosteroid therapy, the patient was withdrawn from the study. The study was approved by the Vanderbilt University Institutional Review Board and all subjects gave written informed consent.
Study population
We enrolled 34 patients meeting the 1987 ACR criteria for RA (20) who had moderate disease activity (≥3 tender and ≥3 swollen joints) on stable (no change in past one month) DMARD and anti-inflammatory therapy. Exclusion criteria included active cancer other than skin cancer, history of bladder cancer or precancerous bladder lesions, HIV, heart failure, severe edema, diabetes mellitus requiring drug therapy, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than twice the upper limit of normal, major surgery within 3 months, untreated osteoporosis, dialysis, pregnancy, unwillingness to use effective birth control if of child bearing potential, concomitant use of gemfibrozil or rifampin, allergy to pioglitazone, previous organ or bone marrow transplant, unwillingness or inability to cooperate with the study protocol, or severe comorbid conditions likely to compromise survival or study participation.
Study procedures
Study procedures were performed after at least 6 hours of fasting and 30 minutes of supine rest. At the baseline visit, and every 4 weeks thereafter, augmentation index, aortic pulse wave velocity, reactive hyperemia index and blood pressure were measured. Peripheral blood pressure was measured twice by automated sphygmomanometer (Dinamap Pro 110, GE Healthcare, Milwaukee, WI, USA), and the average blood pressure was recorded. At the beginning and end of each treatment arm venous blood was drawn for C-reactive protein (CRP) and fasting insulin and glucose concentrations.
Pulse wave analysis for augmentation index and aortic pulse wave velocity (also called carotid-femoral pulse wave velocity) was performed using applanation tonometry with the SphygmoCor system (AtCor Medical, Sydney, Australia) as we have previously described (10). For the augmentation index the tonometer was held lightly on the radial artery at the site of maximum impulse, and after at least 12 consecutive beats the measurements were recorded. Waveform quality was confirmed and the system software generated the central aortic pressure waveform normalized to a heart rate of 75 beats per minute (21). For the aortic pulse wave velocity, the distances from the suprasternal notch to the carotid and femoral arteries were measured. The tonometer was first applied to the carotid artery then to the femoral artery at the point of maximum impulse, each for at least 12 consecutive beats. The aortic pulse wave velocity was calculated as the distance travelled by the arterial pulse wave (meters) divided by the time delay (seconds) between the carotid and femoral arteries.
Endothelial function was measured as the reactive hyperemia index using the EndoPAT system (Itamar Medical, Caesarea, Israel) to assess endothelium-dependent vasodilator responses (22). Peripheral artery tonometry (PAT) probes were placed on a finger on each hand and were inflated. The baseline pulse amplitude was recorded for 10 minutes. Ischemia was induced in the test arm with a blood pressure cuff inflated 60 mmHg above systolic blood pressure or a maximum of 200 mmHg, whichever was lower, for 5 minutes. The blood pressure cuff was then deflated causing reactive hyperemia, and the post-ischemia pulse amplitude was recorded for 10 minutes. The pulse amplitude was measured simultaneously in the control arm without ischemia. Baseline pulse amplitude was recorded as the average PAT signal over 2.5 minutes before ischemia. The post-ischemia pulse amplitude was recorded as the average PAT signal 1.5 minutes after ischemia. Reactive hyperemia was calculated as the ratio of the pre to post-ischemia PAT signal of the test arm divided by the ratio of the pre to post-ischemia PAT signal of the control arm.
Hypertension was defined as a medical history of hypertension and currently on treatment or blood pressure greater than or equal to 140 mmHg systolic or 90 mmHg diastolic (23). Degree of insulin resistance was quantified by the homeostatic model assessment of insulin resistance (HOMA) and was calculated as fasting glucose [mmol/L] × fasting insulin [uU/ml]/22.5.
Statistics
For change in augmentation index, sample size was estimated using a paired t-test (calculated with PS Power v 3.0) assuming that the mean baseline augmentation index would be approximately 29.6±9.9 % units (10). A sample size of at least 32 patients contributing follow-up data would provide 90% power to detect a 20% decrease (24) (i.e., a decrease from 29.6% units to 23.7% units) in augmentation index comparing pioglitazone to placebo. Thus, 34 patients were enrolled to ensure at least 32 patients with follow-up data would be available. Because the outcomes were not independent and were pre-specified, we did not adjust for multiple comparisons.
Descriptive statistics are presented as frequencies and percentage (%) for categorical variables, and mean with standard deviations (mean ±SD) for continuous variables. Linear mixed-effects models were fitted separately for all outcomes to estimate the effect of pioglitazone on outcomes. The fixed effect of the baseline value of the outcome, treatment regimen, week, and product of week and treatment regimen, on outcomes was modeled. Random intercepts were used to model between-patient variability. We assessed for a carry-over effect by comparing the mean of baseline outcome measurements between patients who received pioglitazone in the first phase followed by placebo, and those who received placebo in the first phase followed by pioglitazone, using linear mixed-effects models with random intercepts for between-patient variation. Because this was a 2 arm cross-over design, data were pooled for the 2 treatment phases after finding no carryover effect, and we report between-group difference at the end of study time points (week 8 and week 20) using linear contrast of regression coefficients in the mixed effect models.
To assess if change in HOMA mediated pioglitazone's effect on vascular outcome measures, we used mixed effect models to examine for attenuation in the effect of the treatment by additional adjustment for the change in HOMA (25). Because we had previously found independent anti-inflammatory effects of pioglitazone on some RA disease activity measures (19), we similarly evaluated if pioglitazone's anti-inflammatory effect, measured as change in CRP, mediated its effect on vascular outcome measures. Values for CRP and HOMA were natural logarithm transformed to improve normality in the residuals. Thus, changes in these outcomes were back-transformed and presented as percent change.
Statistical analyses were performed using R version 2.15.1 (http:www.r-project.org) and IBM SPSS Statistics version 20. A two-sided significance level of 5% was considered statistically significant.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Patients were on average 51 years old, 82% were female, and 85% were Caucasian. Patients had moderate disease activity with a mean DAS28 CRP score of 4.58 (±1.10). Most patients (70.6%) were taking methotrexate, 52.9% a biologic DMARD, and 44.1% an anti-hypertensive drug.
Table 1.
Patient characteristics at baseline
| RA patients (n=34) | |
|---|---|
| Demographics and Anthropomorphic Measures | |
| Age, years | 51.0 (±14.2) |
| Sex, %female | 82% (28) |
| Race, %Caucasian | 85% (29) |
| Hypertension, % | 38% (13) |
| BMI, kg/m2 | 28.28 (±6.04) |
| Disease-related and Laboratory Indices | |
| DAS28 CRP, units | 4.58 (±1.10) |
| CRP, mg/dL | 12.7 (±24.6) |
| HOMA, units | 3.11 (±2.68) |
| Current Medication Use | |
| Methotrexate, % | 70.6% (24) |
| Leflunomide, % | 14.7% (5) |
| Sulfasalazine, % | 2.9% (1) |
| Hydroxychloroquine, % | 17.6% (6) |
| Biologic, % | 52.9% (18) |
| Corticosteroid, % | 52.9% (18) |
| Anti-hypertensive, % | 44.1% (15) |
Data are presented as mean (±SD) for continuous data and percent (number) for categorical data. BMI= body mass index, CRP= high sensitivity C-reactive protein, HOMA= homeostatic model assessment of insulin resistance.
Effect of pioglitazone on vascular function
The addition of pioglitazone to current RA treatment was associated with a −4.7% units (95% CI, −7.9, −1.5% units) decrease in augmentation index compared to placebo (P=0.004) (Table 2 and Figure). Aortic pulse wave velocity (P=0.33) and reactive hyperemia index (P=0.46) were not changed significantly by pioglitazone treatment (Table 2). Pioglitazone resulted in a non-significant decrease in systolic blood pressure by −2.1 mmHg (95%CI, −7.7, 3.4 mmHg) (P=0.45) and a significant decrease in diastolic blood pressure by −3.0 mmHg (95%CI, −5.7, −0.2 mmHg) (P=0.03).
Table 2.
Effect of pioglitazone on vascular function
| Pioglitazone phase | Placebo phase | Pioglitazone treatment effect | ||||
|---|---|---|---|---|---|---|
| Baseline (SD) | Mean △ (SD) | Baseline (SD) | Mean △ (SD) | Effect (95% CI) | P | |
| Augmentation index, % units | 29.1 (11.0) | −3.8 (10.6) | 27.2 (13.4) | 2.2 (10.4) | −4.7 (−7.9, −1.5) | 0.004 |
| Aortic PWV, m/s | 8.6 (2.3) | 0.10 (1.81) | 8.6 (2.8) | −0.46 (1.81) | 0.38 (−0.37, 1.13) | 0.33 |
| Reactive hyperemia index, units | 2.3 (0.7) | 0.09 (0.66) | 2.1 (0.75) | −0.20 (0.95) | 0.11 (−0.18, 0.40) | 0.46 |
| Systolic BP, mmHg | 129.3 (19.2) | −6.1 (12.9) | 125.2 (19.0) | −3.2 (12.2) | −2.1 (−7.7, 3.4) | 0.45 |
| Diastolic BP, mmHg | 72.5 (11.7) | −4.7 (9.3) | 70.4 (9.1) | 0.1 (10.5) | −3.0 (−5.7, −0.2) | 0.03 |
Data for each intervention phase (pioglitazone and placebo) are presented as mean (SD). Mean change (△) is the average change of the group. Pioglitazone treatment effect was calculated by linear mixed-effects models and is presented as change or % change (95% CI). Augmentation index is normalized to heart rate 75 beats per minute. PWV= pulse wave velocity, BP= blood pressure
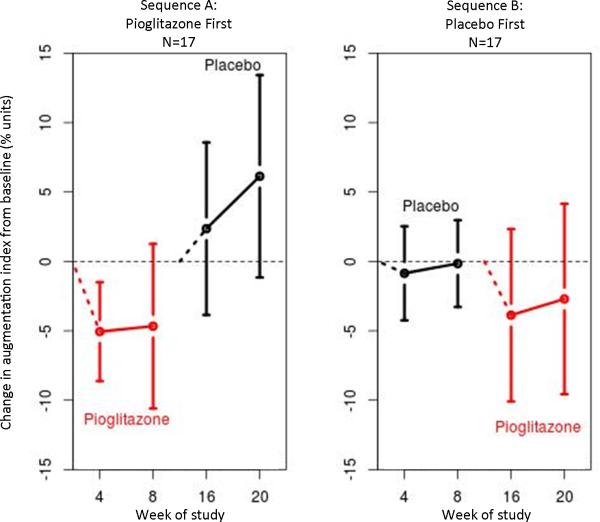
Figure. Augmentation index is decreased by pioglitazone.
The panel on the left shows the change in augmentation index among patients randomized to receive pioglitazone first, then placebo. The panel on the right shows change in augmentation index among patients randomized to receive placebo first, then pioglitazone. Red designates patients taking pioglitazone and black those taking placebo. Open circles represent mean change and bars represent 95% confidence intervals.
Relationship between improvement in vascular function and insulin resistance
We previously showed in this cohort that pioglitazone reduced DAS28 and insulin resistance, measured as the homeostatic model of insulin resistance (HOMA), by −26.4% (95% CI, −43.8, −3.7%), P=0.025 (19). To determine if improvement in insulin resistance mediated the improvement in augmentation index and diastolic blood pressure, we compared results of the models before and after additional adjustment for HOMA or fasting insulin. We found no significant attenuation of pioglitazone's effect on augmentation index (original analysis: −4.7% units (95% CI, −7.9, −1.5% units), P=0.004; compared to −5.26% unit (95% CI, −10.5, −0.04% units), P=0.048 after adjusting for HOMA; and −4.50% units (95%CI, −7.65, −1.36% units), P=0.005 after adjusting for fasting insulin). Similarly, there was little change in the magnitude of the effect of pioglitazone on diastolic pressure after additionally adjusting for HOMA (original analysis: −3.0 mmHg (95%CI, −5.7, −0.2 mmHg), P=0.03; compared to −3.67 mmHg (95% CI, −7.92, 0.578 mmHg), P=0.09 after adjusting for HOMA; and −2.95 mmHg (95%CI, −5.69, −0.20 mmHg), P=0.04 after adjusting for fasting insulin). Therefore, pioglitazone's effect on insulin resistance did not appear to mediate the change in augmentation index or diastolic blood pressure.
Relationship between improvement in vascular function and inflammation
Pioglitazone has an anti-inflammatory effect independent of its impact on insulin resistance (19,26,27). Therefore, we additionally sought to determine if improvement in augmentation index and diastolic blood pressure were mediated by pioglitazone's anti-inflammatory effect. We compared results of the statistical models before and after additional adjustment for CRP or DAS28 and found no attenuation of pioglitazone's effect on augmentation index (original analysis: −4.7% units (95% CI, −7.9, −1.5% units), P=0.004; compared to −4.84% units (95% CI, −7.21, −2.49% units), P<0.001 after adjusting for CRP; and −4.95% units (95%CI, −8.28, −1.62% units), P=0.004 after adjusting for DAS28). Similarly, there was no substantial change in pioglitazone's impact on diastolic pressure (original analysis: −3.0 mmHg (95%CI, −5.7, −0.2 mmHg), P=0.03; compared to −2.06 mmHg (95% CI, −4.23, 0.112 mmHg), P=0.06 after adjusting for CRP; and −2.62 mmHg (95%CI, −5.46, 0.21 mmHg), P=0.07 after adjusting for DAS28).
Discussion
The major new findings of this study are that pioglitazone improved augmentation index and diastolic blood pressure in patients with RA, but did not alter aortic pulse wave velocity, endothelial function or systolic blood pressure significantly. Additionally, pioglitazone appeared to improve augmentation index and diastolic blood pressure through mechanisms independent of improved insulin resistance or decreased inflammation.
Different techniques measure various components of vascular function. We measured large vessel stiffness (pulse wave velocity), large and small vessel stiffness (augmentation index) and endothelial function (reactive hyperemia index). Pulse wave velocity is the speed at which the pulse wave travels between two anatomic locations, indicating the stiffness of large blood vessels (28). Augmentation index is a measure of the reflected pressure wave produced when the pulse wave encounters resistance in the periphery; it is measured as the ratio (reported as percent, %) of the augmented pressure to the pulse pressure in the pulse wave form (29). Like pulse wave velocity, augmentation index measures stiffness of the large vessels, but it also encompasses resistance in smaller vessels. The reactive hyperemia index measures the endothelium-dependent increase in blood flow following brief ischemia, and is a validated measure of endothelial function (30). Several studies have reported that vascular function was impaired and associated with markers of CV risk in patients with RA.
We previously reported that augmentation index is increased in RA and is associated with coronary artery calcification, a measure of coronary artery atherosclerosis (10). Others have found an association between carotid intima-media thickness and both augmentation index and aortic pulse wave velocity (31) in RA. Endothelial dysfunction, measured by several modalities, is frequently present in patients with RA and is associated with carotid intima-media thickness in long-standing RA (32). In addition, all three measures of vascular function predict future CV risk in non-RA populations. Augmentation index and aortic pulse wave velocity are independent predictors of CV risk and mortality in several groups of patients, including those with diabetes (9), hypertension (28) and end stage renal disease (33). Additionally, the reactive hyperemia index is an independent predictor of cardiovascular events (34). Thus, an agent that improves vascular function may have beneficial CV effects in many patient populations, particularly in RA. Moreover, vascular dysfunction is strongly related to insulin resistance (35) and inflammation (36).
Pioglitazone improved vascular function in several different patient populations. In patients with type 2 diabetes mellitus, pioglitazone improved arterial stiffness (11) and endothelial function (12). It also improved endothelial function in patients with impaired glucose tolerance with (13) and without known coronary artery disease (14), and in women with polycystic ovary disease (15). Additionally, in patients with obesity, pioglitazone improved augmentation index and pulse wave velocity (16). Findings such as these provided a strong rationale for studying the effect of pioglitazone on vascular function in RA.
We found that pioglitazone improved augmentation index but not aortic pulse wave velocity, in RA. The finding of improved augmentation index but not pulse wave velocity is compatible with other studies reporting that changes in the two measures are not always concordant (37,38). One reason for such a differential change is that structural vascular changes may contribute more to pulse wave velocity than to augmentation index and may therefore change more slowly. Another reason is that the major vascular effect of pioglitazone in patients with RA may be to decrease resistance in small peripheral vessels, which would be reflected as a change in augmentation index but not pulse wave velocity. Potential mechanisms for such an effect of pioglitazone on the microvasculature are its PPAR-γ independent inhibition of vascular smooth muscle contraction (39) and ability to improve endothelial function (40).
Indeed, we postulated that pioglitazone would improve endothelial function in RA. Insulin resistance is frequently accompanied by endothelial dysfunction and reduced nitric oxide-mediated, endothelium-dependent vasodilation. Moreover, in patients with diabetes, pioglitazone improved endothelial function even before changes in insulin resistance occurred (40). However, we found that pioglitazone did not change endothelial function measured by the reactive hyperemia index in patients with RA. As previously shown (41), discordance in augmentation index and reactive hyperemia index can be seen as the two measure different aspects of vascular function. A study performed in patients with diabetes suggested that improvement in endothelial function after pioglitazone was mediated by the accompanying reduction in tumor necrosis factor- α (TNFα) concentrations (42). If correct, this could provide a mechanistic explanation for the lack of improvement in endothelial function in RA after pioglitazone, because we previously showed that although there was a robust decrease in interleukin-6 (IL-6) concentrations after pioglitazone in RA, there was no significant reduction in TNFα (19).
Changes in microvascular tone after pioglitazone might also be reflected by changes in blood pressure. We found that diastolic blood pressure was significantly reduced and systolic blood pressure trended lower with pioglitazone use. The effect of pioglitazone on blood pressure in other studies has been mixed, but several have reported a decrease. In patients with impaired glucose tolerance (43) and hypertension (44), pioglitazone lowered diastolic blood pressure robustly without major effects on systolic pressure, and in other studies it improved both systolic and diastolic pressure (45). However, in some studies there was no change in blood pressure (46). It is interesting to note that in a large meta-analysis metformin, another insulin sensitizer, did not affect blood pressure significantly (47). A potential mechanism is pioglitazone's PPAR-γ-independent inhibition of vascular smooth muscle contraction (39). Thus, changes in blood pressure resulting from pioglitazone are likely to be independent of its insulin sensitizing effects, as we also observed.
We used statistical models to examine if the vascular benefits of pioglitazone were mediated though improved insulin resistance or decreased inflammation, and found that these were not the primary mediators. Similar results were reported in subjects with impaired glucose tolerance in whom pioglitazone reduced insulin resistance and inflammation and progression of carotid intima-media thickness; however, improvements in insulin resistance and CRP did not account for the slower rate of progression of atherosclerosis (46).
Our study had several limitations. It was a small study, but the efficient cross-over design gave adequate power to evaluate the outcomes. We performed vascular function measures that predict long term CV outcomes in the general population, but it was a short study with no long-term outcome data. However, it is important to explore novel strategies to improve cardiovascular outcomes in RA, and this is most efficiently done by studying the effects of interventions on vascular function. Our finding that pioglitazone improves some measures of vascular function are interesting; however, long term pioglitazone treatment has several potential side effects, such as heart failure (18), osteoporosis (48), bladder cancer (49), and weight gain that could be particularly problematic in RA. Thus, although pioglitazone is not an ideal candidate for a novel approach to improve vascular function in RA, newer tissue-specific compounds in development may have similar CV, insulin sensitizing, and anti-inflammatory effects with fewer side effects (50).
Conclusion
Pioglitazone improves augmentation index and diastolic blood pressure in RA patients. This improvement, however, was not through reduction in inflammation or insulin resistance.
Supplementary Material
Acknowledgments
Funding: NIH Grants: P60 AR056116, T32 AR059039, and by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
This trial is registered with ClinicalTrials.gov, # NCT00763139.
Disclosures: None
References
- 1.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum. 2001;44:1234–6. doi: 10.1002/1529-0131(200106)44:6<1234::AID-ART213>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 4.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196:756–63. doi: 10.1016/j.atherosclerosis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58:2105–12. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JS, Nam JS, Cho MH, Yoo JS, Ahn CW, Jee SH, et al. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause. 2010;17:779–84. doi: 10.1097/gme.0b013e3181cd3d60. [DOI] [PubMed] [Google Scholar]
- 7.Samaras K, Viardot A, Lee PN, Jenkins A, Botelho NK, Bakopanos A, et al. Reduced arterial stiffness after weight loss in obese type 2 diabetes and impaired glucose tolerance: The role of immune cell activation and insulin resistance. Diab Vasc Dis Res. 2013;10:40–8. doi: 10.1177/1479164112443375. [DOI] [PubMed] [Google Scholar]
- 8.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 9.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 10.Avalos I, Chung CP, Oeser A, Gebretsadik T, Shintani A, Kurnik D, et al. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J Rheumatol. 2007;34:2388–94. [PubMed] [Google Scholar]
- 11.Harashima K, Hayashi J, Miwa T, Tsunoda T. Long-term pioglitazone therapy improves arterial stiffness in patients with type 2 diabetes mellitus. Metabolism. 2009;58:739–45. doi: 10.1016/j.metabol.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya K, Akaza I, Yoshimoto T, Hirata Y. Pioglitazone improves endothelial function with increased adiponectin and high-density lipoprotein cholesterol levels in type 2 diabetes. Endocr J. 2009;56:691–8. doi: 10.1507/endocrj.k08e-308. [DOI] [PubMed] [Google Scholar]
- 13.Rizza S, Cardellini M, Porzio O, Pecchioli C, Savo A, Cardolini I, et al. Pioglitazone improves endothelial and adipose tissue dysfunction in pre-diabetic CAD subjects. Atherosclerosis. 2011;215:180–3. doi: 10.1016/j.atherosclerosis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Quinn CE, Lockhart CJ, Hamilton PK, Loughrey CM, McVeigh GE. Effect of pioglitazone on endothelial function in impaired glucose tolerance. Diabetes Obes Metab. 2010;12:709–15. doi: 10.1111/j.1463-1326.2010.01224.x. [DOI] [PubMed] [Google Scholar]
- 15.Naka KK, Kalantaridou SN, Kravariti M, Bechlioulis A, Kazakos N, Calis KA, et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: a prospective randomized study. Fertil Steril. 2011;95:203–9. doi: 10.1016/j.fertnstert.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 16.Ryan KE, McCance DR, Powell L, McMahon R, Trimble ER. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194:e123–30. doi: 10.1016/j.atherosclerosis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 18.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. J Am Med Assoc. 2007;298:1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 19.Ormseth MJOA, Cunningham A, Bian A, Ayumi S, Tanner BS, Stein CM. PPAR-γ Agonist Effect on Rheumatoid Arthritis: a randomized controlled trial. Arthritis Res Ther. 2013;15:R110. doi: 10.1186/ar4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525 Pt1:263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, Barkris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 24.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–5. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 25.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 26.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 27.Pfutzner A, Hanefeld M, Dekordi LA, Muller J, Kleine I, Fuchs W, et al. Effect of pioglitazone and ramipril on biomarkers of low-grade inflammation and vascular function in nondiabetic patients with increased cardiovascular risk and an activated inflammation: results from the PIOace study. J Diabetes Sci Technol. 2011;5:989–98. doi: 10.1177/193229681100500422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–7. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Arend BJ, et al. Pulse-wave analysis: clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–52. doi: 10.1161/hq0102.101770. [DOI] [PubMed] [Google Scholar]
- 30.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 31.Soltesz P, Der H, Kerekes G, Szodoray P, Szucs G, Danko K, et al. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol. 2009;28:655–62. doi: 10.1007/s10067-009-1118-y. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Juanatey C, Llorca J, Gonzalez-Gay MA. Correlation between endothelial function and carotid atherosclerosis in rheumatoid arthritis patients with long-standing disease. Arthritis Res Ther. 2011;13:R101. doi: 10.1186/ar3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–8. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 34.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 35.Lukich E, Matas Z, Boaz M, Shargorodsky M. Increasing derangement of glucose homeostasis is associated with increased arterial stiffness in patients with diabetes, impaired fasting glucose and normal controls. Diabetes Metab Res Rev. 2010;26:365–70. doi: 10.1002/dmrr.1086. [DOI] [PubMed] [Google Scholar]
- 36.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 37.Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–92. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 38.Wong M, Oakley SP, Young L, Jiang BY, Wierzbicki A, Panayi G, et al. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:1277–84. doi: 10.1136/ard.2007.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinagra T, Tamburella A, Urso V, Siarkos I, Drago F, Bucolo C, et al. Reversible inhibition of vasoconstriction by thiazolidinediones related to PI3K/Akt inhibition in vascular smooth muscle cells. Biochem Pharmacol. 2013;85:551–9. doi: 10.1016/j.bcp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki M, Takamisawa I, Yoshimasa Y, Harano Y. Association between insulin resistance and endothelial dysfunction in type 2 diabetes and the effects of pioglitazone. Diabetes Res Clin Pract. 2007;76:12–7. doi: 10.1016/j.diabres.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Yang WI, Park S, Youn JC, Son NH, Lee SH, Kang SM, et al. Augmentation index association with reactive hyperemia as assessed by peripheral arterial tonometry in hypertension. Am J Hypertens. 2011;24:1234–8. doi: 10.1038/ajh.2011.132. [DOI] [PubMed] [Google Scholar]
- 42.Martens FM, Rabelink TJ, op't Roodt J, de Koning EJ, Visseren FL. TNF-alpha induces endothelial dysfunction in diabetic adults, an effect reversible by the PPAR-gamma agonist pioglitazone. Eur Heart J. 2006;27:1605–9. doi: 10.1093/eurheartj/ehl079. [DOI] [PubMed] [Google Scholar]
- 43.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–15. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 44.Schneider F, Vossler S, Franke S, Bar F, Konrad T. Impact of insulin sensitivity treatment with pioglitazone on endothelial function in non-diabetic patients with arterial hypertension. Int J Clin Pharmacol Ther. 2009;47:311–20. doi: 10.5414/cpp47311. [DOI] [PubMed] [Google Scholar]
- 45.Abe M, Okada K, Kikuchi F, Matsumoto K. Clinical investigation of the effects of pioglitazone on the improvement of insulin resistance and blood pressure in type 2-diabetic patients undergoing hemodialysis. Clin Nephrol. 2008;70:220–8. doi: 10.5414/cnp70220. [DOI] [PubMed] [Google Scholar]
- 46.Saremi A, Schwenke DC, Buchanan TA, Hodis HN, Mack WJ, Banerji M, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2013;33:393–9. doi: 10.1161/ATVBAHA.112.300346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F, Xiang H, Fan Y, Ganchuluun TA, Kong W, Ouyang Q, et al. The effects of sulfonylureas plus metformin on lipids, blood pressure, and adverse events in type 2 diabetes: a meta-analysis of randomized controlled trials. Endocrine. 2013 Dec;44:648–58. doi: 10.1007/s12020-013-9970-6. [DOI] [PubMed] [Google Scholar]
- 48.Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab. 2010;12:716–21. doi: 10.1111/j.1463-1326.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 49.Azoulay L, Yin H, Filion KB, Assayag J, Majdan A, Pollak MN, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012;344:e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–66. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.