Abstract
The multiple beneficial effects of calorie restriction (CR) on several organs, including the heart, are widely known. Recently, the plant polyphenol resveratrol has been shown to possess beneficial effects similar to that of CR. Among the host of effects on cardiac muscle, a cellular self-eating process called autophagy has been shown to be induced by both CR and resveratrol. Autophagy is vital in removing dysfunctional organelles and damaged proteins from the cell, thereby maintaining cellular quality control. In this study, we explored whether short-term moderate CR (20%), either alone or in combination with resveratrol, can induce autophagy in the hearts of 26-month old Fischer 344 × Brown Norway (FBN) rats. Autophagy stimulation was investigated by measuring protein expression levels of autophagy proteins Beclin-1, Atg5, p62, and LC3-II/LC3-I ratio. We found that 20% CR or resveratrol alone for 6 weeks could not induce autophagy, but 20% CR in combination with 50 mg/kg/day resveratrol resulted in an induction of autophagy in the hearts of 26 month old rats. Although oxidative stress has been proposed to be an inducer of autophagy, treatment with the chemotherapeutic drug doxorubicin was unable to stimulate autophagy. The enhanced autophagy due to CR + resveratrol was associated with protection from doxorubicin-induced damage, as measured by cardiac apoptotic levels, serum creatine kinase (CK) and lactate dehydrogenase (LDH) activity. We propose that a combinatorial approach of low-dose CR and resveratrol has the potential to be used therapeutically to induce autophagy and provides protection against doxorubicin-mediated toxicity.
Keywords: Autophagy, Calorie Restriction, Resveratrol, Heart, Doxorubicin, Oxidative stress
Introduction
Autophagy is a cellular self-digestion process whereby cells degrade dysfunctional proteins and organelles, thereby playing a major role in mediating cellular quality control. This vital housekeeping process has been shown to protect against several pathological conditions such as infections, neurodegenerative disorders, inflammatory diseases, and cancer [1]. Cardiac-specific loss of autophagy, by the conditional deletion of AuTophaGy protein 5 (Atg5), has confirmed that this housekeeping process is essential for optimal cardiac functioning and survival [2, 3]. Abrogation of the autophagic pathway in the adult heart by conditional inactivation of autophagy proteins Atg5 or Atg7, causes rapid onset of cardiac abnormalities characterized by cardiac hypertrophy, left ventricular dilatation, and decreased cardiac output [2, 3]. In addition, an age-related decline in the efficiency of the autophagic process can lead to the accumulation of damaged cellular components, which can further lead to cardiac functional deterioration [4]. Although several inducers of autophagy in cell culture conditions have been discovered, few studies have investigated interventions to induce autophagy in vivo, especially starting at an older age. Kanamori et al. reported that starving transgenic mice expressing GFP fused to microtubule associated light chain 3 (GFP-LC3) for 12 hours led to an increase in fluorescent autophagic puncta, which was further confirmed by electron microscopic visualization of autophagic vacuoles [5]. Starvation periods longer than 12 hours led to a more robust autophagic stimulation and enhanced the expression of downstream lysosomal enzymes, such as cathepsin D, suggesting an overall enhancement of autophagic flux [5]. Consistent with Kanamori et al.'s study, our group has previously shown that lifelong 40% calorie restriction (CR) can increase the appearance of autophagic vacuoles in the hearts of Fischer 344 rats and enhance the expression of autophagy proteins Atg7 and Atg9 and lysosomal enzyme procathepsin D [6]. Similar effects also have been observed in the skeletal muscle of 40% CR animals [7]. However, in spite of the autophagy-enhancing capability of lifelong 40% CR, a severe and prolonged dietary restriction is not practical in humans. In addition to infeasibility, it can lead to undesirable and potentially harmful changes if started at a vulnerable stage of life cycle such as during adolescence, at a very old age, or during pregnancy. Hence, in the current study, we investigated whether a moderate CR (20%) regimen, beginning in late middle age, could have a similar effect of inducing autophagy in Fischer 344 × Brown Norway (FBN) rat hearts.
In addition to a mild CR regimen, we investigated the natural polyphenol resveratrol as a potential inducer of autophagy. Resveratrol is produced by a wide variety of plants, such as grapes, berries, and peanuts, in response to environmental stress and has been extensively investigated in numerous clinical studies [8]. Resveratrol has been implicated in the French paradox, an observation that French people, in spite of their high consumption of saturated fats, have low incidence of age-associated cardiovascular disorders. It is believed that this protective effect is due to their moderately high consumption of red wine, which contains resveratrol. In animals, resveratrol was shown to have numerous beneficial effects, such as promoting vasodilation in models of coronary heart disease and enhancing the expression of antioxidant enzymes [9]. Additionally, resveratrol can protect the heart against ischemia-reperfusion injury [10], improve endothelial function [11], and prevent platelet aggregation [12]. Notably, resveratrol has been shown to be a potent inducer of autophagy in several cell culture models, including cardiomyocytes [13-16]. Studies investigating whether resveratrol can induce autophagy in animals, however, are limited. In this study, we investigated whether CR or resveratrol alone, or a combination of the two, can stimulate autophagy in the hearts of 26-month-old FBN rats.
In addition to exploring interventions to induce autophagy in rodent hearts, we examined whether such autophagy interventions can protect against cardiac damages induced by the oxidative stressor doxorubicin. While doxorubicin is a highly effective chemotherapeutic agent used in the treatment of solid tumors and hematologic malignancies [17], its cardiotoxic effects severely limit its dosage and hence, the chemotherapeutic efficacy [17, 18]. Although a complete understanding of the mechanisms involved in doxorubicin's toxicity remains elusive, the generation of reactive oxygen species (ROS) in cells has been proposed to be a major player [19, 20]. The generation of ROS by doxorubicin can lead to cellular oxidative damage, resulting in cytotoxic effects [21] and the housecleaning process of autophagy can be hypothesized to be beneficial under such circumstances.
Our study showed that 50 mg/kg/day of resveratrol combined with 20% CR for 6 weeks can enhance autophagic flux in the hearts of 26-month-old FBN rats. Interestingly, CR or resveratrol alone was found to have no effect on autophagy. Induction of autophagy using the combinatorial approach helps protect rat hearts against doxorubicin-mediated toxicity. We therefore propose that induction of autophagy in late middle-aged rat hearts could potentially be developed as a therapeutic target for mitigating oxidative stress-induced damage.
Materials and methods
Animals and dietary intervention
Male FBN rats at 25 months of age were purchased from the National Institute on Aging (NIA) and were singly housed at the University of Florida animal facility in a temperature- (20 ± 2.5 °C) and light-controlled (12:12 hour light-dark cycle) environment with unrestricted access to water. After arrival, animals were acclimated for a period of 3 weeks and were then randomly assigned into one of six experimental groups: 1) Ad libitum (AL), 2) 20% CR (CR), 3) 5 mg/kg/day resveratrol (Resv-5), 4) 50 mg/kg/day resveratrol (Resv-50), 5) CR with 5 mg/kg/day resveratrol (CR + Resv-5) and 6) CR with 50 mg/kg/day resveratrol (CR + Resv-50). Animals were maintained on the interventions for 6 weeks. Animals on the CR, CR + Resv-5, and CR + Resv-50 groups received 20% less food from a 125% fortified diet to ensure that all groups received equal amounts of proteins, vitamins and minerals. Both AL and CR diets were based on the AIN-93M chow, formulated for the maintenance of mature rodents, with lower levels of proteins and fats to reduce the incidence of kidney stones in older rodents [22]. Table S1 summarizes the composition of AL and CR diets. Resveratrol (Sigma, St. Louis, MO) was given as highly palatable supplementation pellets. Control supplementation tablets (without resveratrol) were given to AL rats. The supplementation pellets were similarly formulated with the AL or CR diet as the base chow, with or without resveratrol, and were prepared by Custom Animal Diets (Bangor, PA) using an effective bacon-flavor masking capability. The pellets were made as 1 g tablets containing either 0.5 mg or 5 mg resveratrol. To ensure that every rat received the proper dose of resveratrol, the number of pellets given on each day was calculated based on the body weight of individual animals. The consumption of tablets was visually inspected daily. Throughout the entire study, the amount of food given to the CR rats was adjusted weekly based on the food consumed by the AL rats. Body weights of rats were recorded at least once per week.
Induction of oxidative stress by doxorubicin
At the end of the intervention period, animals received a single intraperitoneal (IP) injection of 10 mg/kg doxorubicin (Sigma, St. Louis, MO) or saline control. The dose was chosen based on our previous observation that 10 mg/kg/day of doxorubicin can activate caspase-3 and induce apoptosis in the hearts of Fischer 344 rats [23]. Twenty-four hours after injection, animals were sacrificed by rapid-decapitation. Serum and plasma were collected by trunk blood processing and stored at -80 °C until further analysis. Hearts were dissected out, separated into individual compartments, weighed and saved at -80 °C. All experiments and procedures were approved by the Institute on Animal Care and Use Committee at the University of Florida.
Body composition analysis
Fat and lean mass percentages of rats were determined at baseline levels (before the start of interventions) and after 6 weeks of CR and resveratrol interventions using time-domain nuclear magnetic resonance (TD-NMR) analyzer (Minispec, Bruker Optics, The Woodlands, TX). TD-NMR provides an accurate, fast, and easy-to-use method for determining fat and lean tissue in rodents without the need for anesthesia. The validation of the TD-NMR methodology has been provided elsewhere [24].
Plasma resveratrol and resveratrol conjugate(s) concentration analysis
Resveratrol and resveratrol conjugate(s) were quantified in plasma using LC/MS/MS assay at the Biomedical Mass Spectrometry Core at the University of Florida. Rat serum samples (30 μL) were loaded onto an Accela Open Autosampler (Thermo Fisher Scientific, San Jose, CA) connected to an Accela 600 UPLC and LTQ Velos mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Separation was achieved on a BetaBasic C18 HPLC column (150 × 2.1 mm, 5 um, Thermo Fisher Scientific, San Jose, CA).
Preparation of tissue extracts
For the preparation of tissue extracts, approximately 100 mg of the left ventricle was pulverized in liquid nitrogen. The powder was subsequently resuspended in 1 mL ice-cold buffer containing 20 mM HEPES, pH 7.4, 2 mM EGTA, 50 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM Na3VO4, 10% glycerol, 1% Triton X-100 and Halts Protease Inhibitor (Thermo Scientific, Fair Lawn, NJ). Resuspended tissue was further homogenized using a mechanically driven Potter-Elvehjem glass-glass homogenizer with approximately 14 up and down strokes. The homogenates were subsequently rotated for 1 hour at 4 °C for efficient cell lysis. Finally, the homogenates were centrifuged at 10,000 g for 10 minutes at 4 °C and the supernatant was transferred to clean tubes. Protein concentrations were determined using Bradford Assay [25], and aliquots were stored at −80 °C until further analysis.
Western blotting
Protein samples were prepared in Laemmli buffer (62.5 mM Tris-HCl, 2% SDS, 25% Glycerol, 0.01% Bromophenol Blue, pH 6.8; Bio-Rad, Hercules, CA) with 5% β-mercaptoethanol and were boiled at 95 °C for 5 minutes prior to loading in gels. Equal amounts of proteins were loaded in pre-cast Tris-HCl polyacrylamide gels (Criterion system, Bio-Rad, Hercules, CA). After electrophoretic separation, proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA) and subsequently blocked for 1 hour in Starting Block (Thermo Scientific, Fair Lawn, NJ), followed by overnight incubation with primary antibodies at 4 °C. Membranes were subsequently washed with Tris-buffered saline with 0.1% Tween 20 (TBST) and incubated with the appropriate secondary antibodies in Starting Block for 1 hour. Membranes were washed again with TBST, chemiluminescent signals developed using ECL Plus reagent (Amersham Biosciences, Buckinghamshire, UK), and captured using ChemiDoc XRS System (Bio-Rad, Hercules, CA). Digital images were analyzed for densitometry using ImageLab software (Bio-Rad, Hercules, CA). A complete list of primary antibodies and their catalog numbers is provided in Table S2.
Quantitative real-time PCR
Quantitative real time PCR was performed according to protocol described before [26]. Briefly, 20-40 mg of tissue was homogenized in 1 mL of TriReagent (Sigma-Aldrich, St. Louise, MO) using a glass on glass mortar and pestle. The homogenate was cleared by centrifugation and the RNA was isolated from the supernatant according to manufacturer's instructions. Total RNA was subsequently dissolved in nuclease-free water and any contaminating DNA was removed via DNase digestion. RNA quality was evaluated using the 2100 Nano Labchip Kit on an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA). cDNA was synthesized from 2 μg of RNA using the high capacity cDNA reverse transcription kit (ABI, Foster City, CA), according to manufacturer's instructions. Samples were incubated at 25°C for 10 minutes, 37°C for 120 minutes and finally, enzyme activity was terminated by heating to 85°C for 5 minutes. Q-PCR analysis was performed using Taqman Master Mix, 0.2 nM primers and nuclease-free water in a 25 μL reaction. Relative expression was determined using the ABI 7500 real-time PCR system (ABI, Foster City, CA) with universal cycling conditions. All samples were examined in triplicate. Analysis of data was performed using the 2-ΔΔCt method, using 18s RNA as the internal control, as described before [26]. Rat primer and probe sequence for p62 was commercially available from Applied Biosystems (Assays-on-Demand).
Apoptotic analysis
Apoptotic analysis on left ventricular homogenates was performed using Cell Death Detection Plus ELISA kit (Roche Diagnostics, Indianapolis, IN) following manufacturer's instructions. The kit is designed to detect mono- and oligonucleosomes in the cytoplasmic fraction of heart lysates and is based on a sandwich-enzyme immunoassay principle using mouse monoclonal antibodies directed against DNA and histones. Absorbance was read at 405 nm with a Biotek Synergy plate reader (Biotek, Winooski, VT), and the values were normalized to protein concentrations.
Creatine kinase (CK) assay
CK levels in serum were determined using Enzychrom Creatine Kinase Assay kit (Bioassay Systems, Hayward, CA) following manufacturer's instructions. The kit uses an enzyme-coupled reaction in which creatine phosphate and adenosine diphosphate (ADP) are converted to creatine and adenosine triphosphate (ATP) by CK. The ATP generated in the process is used by hexokinase in phosphorylating glucose to glucose-6-phosphate. The latter is subsequently oxidized by nicotinamide adenine dinucleotide phosphate (NADP), in the presence of glucose-6-phosphate dehydrogenase and produces reduced NADP (NADPH). The produced NADPH is proportional to the CK activity in the samples and was quantified by measuring the absorbance at 340 nm using Biotek Synergy plate reader (Biotek, Winooski, VT).
Lactate dehydrogenase (LDH) assay
LDH levels in serum were measured using Quantichrom Lactate Dehydrogenase Assay kit (BioAssay Systems, Hayward, CA) according to manufacturer's instructions. The assay is based on LDH-dependent and NADH-catalyzed reduction of the tetrazolium salt (3-(4,5 Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to a reduced form. Absorbance was measured at 565 nm using a Biotek plate reader (Biotek, Winooski, VT), and the values were directly proportional to the enzyme activity.
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 4.0 (GraphPad Software, San Diego, CA). Student's t-test and one-way analysis of variance (ANOVA) were performed, wherever applicable. Whenever required, post-hoc analysis was performed using Bonferroni multiple comparison test. Statistical significance was set to p < 0.05. Results are expressed as mean ± SE.
Results
CR diet was associated with lower body weights
Six weeks of 20% CR, with or without resveratrol, resulted in significantly lower body weights in comparison to AL (p < 0.001 vs. AL) (Table 1). Heart weights of rats in CR + Resv-5 and CR + Resv-50 groups were significantly lower in comparison to that of AL rats (p < 0.001 vs. AL). There was no statistical difference between groups when heart weight was normalized to body weight (Table 1).
Table 1. Body weight (BW) and heart weight (HW) analysis.
| AL | CR | CR + Resv-5 | CR + Resv-50 | |
|---|---|---|---|---|
| BW (g) | 581 ± 39 | 529 ± 45** | 519 ± 31*** | 521 ± 35*** |
| HW (g) | 1.36 ± 0.08 | 1.33 ± 0.13 | 1.25 ± 0.06*** | 1.24 ± 0.09*** |
| HW/BW | 2.34 ± 0.17 | 2.52 ± 0.18 | 2.43 ± 0.22 | 2.38 ± 0.16 |
| RA (g) | 0.041 ± 0.011 | 0.060 ± 0.028 | 0.050 ± 0.060 | 0.036 ± 0.009 |
| RV (g) | 0.205 ± 0.029 | 0.216 ± 0.029 | 0.187 ± 0.026 | 0.187 ± 0.034 |
| LA (g) | 0.040 ± 0.009 | 0.044 ± 0.019 | 0.034 ± 0.007 | 0.035 ± 0.007 |
| LV (g) | 0.523 ± 0.068 | 0.555 ± 0.053 | 0.505 ± 0.079 | 0.477 ± 0.058 |
RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
p < 0.01,
p <0.001 vs. AL. n = 9 to 23.
CR diet alone or in combination with resveratrol attenuates body composition changes over time
Paired t-test analysis showed that fat mass % and fat/lean ratio significantly increased (p < 0.01) and lean mass % decreased (p < 0.05) in animals on an AL diet over a 6-week period (Fig. S1). However, fat mass %, lean mass % and fat/lean ratio remained unchanged in the CR, CR + Resv-5 and CR + Resv-50 groups pre- and post-intervention.
Plasma resveratrol conjugate(s) concentrations increase in a dose-dependent manner in resveratrol-fed rats
LC/MS/MS analysis showed a dose-dependent increase in resveratrol conjugate(s) concentration in resveratrol-fed animals, with the parent resveratrol molecule not being detected. The mean plasma concentration of resveratrol conjugate(s) in the CR + Resv-5 and CR + Resv-50 groups were 7.4 ± 3.7 and 41 ± 19 ng/mL, respectively (Table 2). Resveratrol conjugate(s) could not be detected in the plasma of AL rats.
Table 2.
Plasma concentrations of resveratrol conjugate(s).
| Intervention groups | Resveratrol conjugate(s) concentration (ng/mL) |
|---|---|
| AL | ND |
| 20% CR + Resv-5 | 7.4 ± 3.7 |
| 20% CR + Resv-50 | 41 ± 19 |
ND, not detected. n = 5.
CR in combination with 50 mg/kg/day resveratrol increases autophagic flux in the left ventricle
We analyzed the expression levels of key autophagy proteins (LC3B, p62, Beclin-1 and Atg5-Atg12 conjugate) in the left ventricle of saline-treated rats, to determine whether any of the interventions tested can induce cardiac autophagy in 26-month-old FBN rats. Interestingly, we observed no changes in the levels of the abovementioned autophagy proteins with 5 mg/kg/day or 50 mg/kg/day resveratrol alone, compared to saline-treated AL rats. These results are summarized in the supplemental information (Fig. S2). Autophagy protein expression analysis in the intervention groups AL, CR, CR+Resv-5 and CR+Resv-50 are summarized as follows:
LC3B
Autophagy induction converts cytosolic LC3-I to autophagosomal membrane-associated and PE-conjugated LC3-II. The LC3-II/LC3-I ratio is therefore a widely used indicator of steady- state autophagic levels. We could not detect an increase in the LC3-II/LC3-I ratio in any of the intervention groups (Fig. 1 A). However, although the LC3-II/LC3-I ratio remained unchanged with the interventions, we cannot rule out the possibility that an increase in the rate of their degradation in the lysosomes, i.e., an enhancement in autophagic flux, contributed to their unaltered levels.
Fig. 1. Effect of interventions on autophagy markers in the left ventricle.
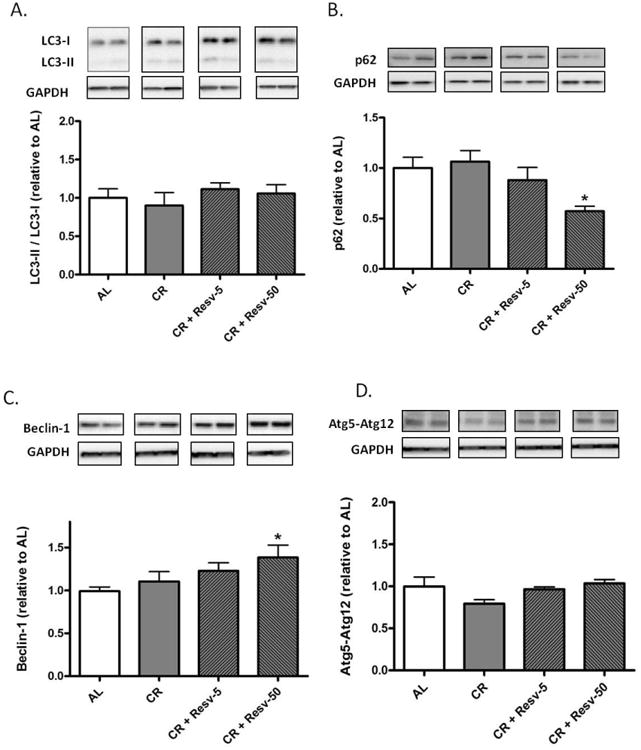
(A–D) Protein expression levels of autophagy markers LC3B (A), p62 (B), Beclin-1 (C) and Atg5–Atg12 conjugate (D) were measured by immunoblotting in the left ventricular homogenates of saline-treated AL, CR, CR + Resv-5 and CR + Resv-50 animals. GAPDH was used as a loading control. *p < 0.05 vs. AL. n = 4 to 12.
p62/sequestosome 1 (SQSTM1)
To determine autophagic flux, we measured the abundance of p62/SQSTM1 in left ventricular homogenates. p62 binds to ubiquitinated proteins through its UBA domain and can interact with LC3 through its LC3 binding motif, thereby directing the degradation of ubiquitinated proteins through the autophagic pathway [27]. In the process, p62 is degraded through autophagy; therefore, a decrease in the abundance of p62 is considered indicative of enhanced autophagic flux [27, 28]. We observed a decrease in p62 abundance in the left ventricle of CR + Resv-50 rats (p < 0.05 vs. AL) (Fig. 1B), indicating an increased autophagic flux. No significant changes were observed in the CR or CR + Resv-5 groups in comparison to AL. To rule out the possibility that a decline in p62 levels is due to a decrease in the level of its transcription (and eventually protein expression), we performed RT-PCR on left ventricular homogenates. Our results indicate no difference in the p62 mRNA levels between the AL and CR + Resv-50 groups (Fig. S3).
Beclin-1
Beclin-1 (mammalian homolog of yeast Atg6) is required for the induction phase of autophagy [29]. Specifically, Beclin-1 associates with a pre-autophagosomal complex comprising of Atg14, Class III PI3K/Vps34 and Vps15 and this complex is required for the localization of autophagic proteins to the pre-autophagosomal structure [29-31]. Autophagic triggers have been shown to upregulate Beclin-1 [32]. We observed that the expression of Beclin-1 was increased in left ventricular homogenates of CR + Resv-50 rats in comparison to AL (p < 0.05) (Fig. 1C).
Atg5–Atg12
In addition to the Atg8-PE (LC3-PE) conjugation system, a second ubiquitin-like conjugation system (comprising Atg7, Atg12 and Atg10) links the carboxy-terminal glycine residue of Atg12 to a lysine of Atg5 [33]. The Atg5–Atg12 conjugate further complexes with Atg16, which is associated with the growing phagophore [34]. We observed no differences in Atg12–Atg5 levels in left ventricular homogenates between AL rats and the intervention groups (Fig. 1D).
Treatment with doxorubicin does not induce autophagy in the left ventricle
We have previously shown that treatment with doxorubicin leads to an induction of oxidative stress in the myocardium [18]. Since recent studies have reported a potential role of oxidative stress as an inducer of autophagy [35, 36], we investigated whether treatment with doxorubicin can also induce autophagy. The results from autophagy protein expression analysis in the intervention groups are summarized as follows:
LC3B
Treatment with doxorubicin-enhanced LC3-II/LC3-I ratio, in comparison to saline-treated AL rats (p < 0.005) (Fig. 2A), indicating either an enhanced generation of autophagic vacuoles or a decrease in their clearance.
Fig. 2. Effect of doxorubicin on LC3-II/LC3-I ratio and p62 accumulation in the left ventricle.
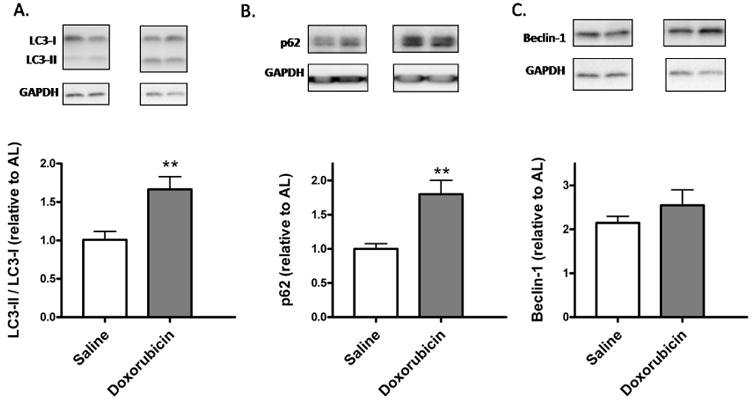
(A–C) Protein expression levels of autophagy markers LC3B (a), p62 (B), and Beclin-1 (C) were measured by immunoblotting in the left ventricular homogenates of saline or doxorubicin-treated animals. GAPDH was used as a loading control. **p < 0.01 vs. saline. n = 5 to 12.
p62/sequestosome 1 (SQSTM1)
We observed an increase in p62 accumulation in the doxorubicin-treated hearts (Fig. 2B). This suggests an impairment of autophagic flux with doxorubicin treatment (p < 0.01 vs. saline-treated AL rats).
Beclin-1
There were no changes in Beclin-1 protein levels between saline and doxorubicin treatment groups (Fig. 2C).
CR in combination with 50 mg/kg/day resveratrol attenuates doxorubicin-mediated increases in cardiac apoptotic levels
Apoptotic levels were determined by measuring mono- and oligo-nucleosomes released in the cell cytoplasm. Doxorubicin increased apoptotic index in the left ventricle of AL rats (p < 0.05 vs. saline) (Fig. 5A) and CR + Resv-50 attenuated the apoptotic induction (p < 0.05 vs. AL + doxorubicin). No significant changes in cardiac apoptotic levels were observed between AL and CR or CR + Resv-5 groups (Fig. 3A).
Fig. 5. Proposed pathways through which CR and resveratrol induces autophagy.
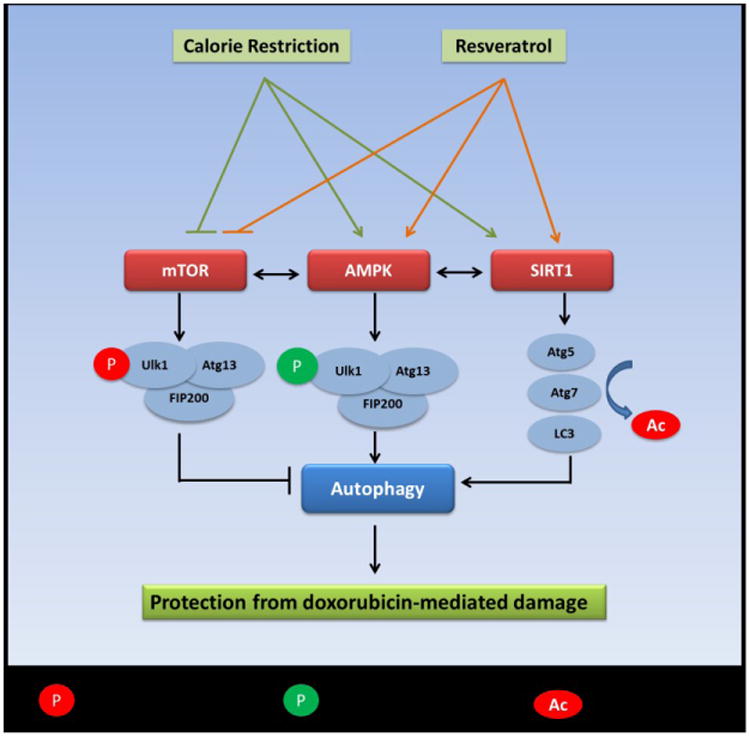
Both CR and resveratrol can activate autophagy by inhibiting the mTOR pathway and/or by activating of the AMPK and SIRT1 pathways. AMPK induces activating phosphorylation on the ULK1 – Atg13 – FIP200 complex, thereby stimulating autophagy. SIRT1 can decetylate and activate key autophagy genes Atg5, Atg7 and LC3, thereby inducing autophagy.
Fig. 3. Effect of interventions on doxorubicin-mediated cardiac damage.
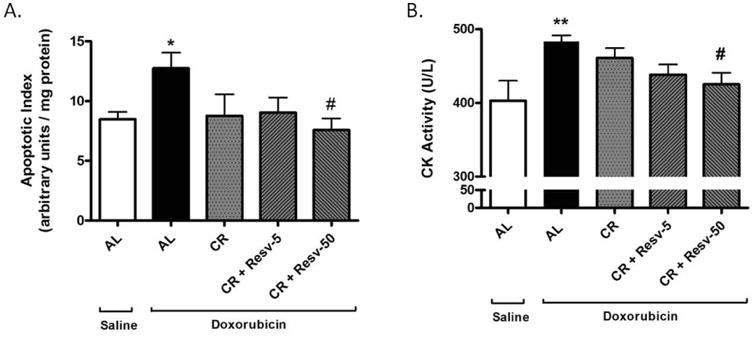
(A) Apoptotic induction in left ventricular homogenates of saline-treated AL rats and doxorubicin-treated AL, CR, CR + Resv-5 and CR + Resv-50 groups was determined by measuring the release of mono- and oligo-nucleosomes in the cytosolic fraction. Values for the assay have been normalized to the total amount of protein. *p < 0.05 vs. AL + saline; #p < 0.05 vs. AL + doxorubicin; (B) Serum CK levels were measured in the same groups as in (A). **p < 0.01 vs. AL + saline, #p < 0.05 vs. AL + doxorubicin. n = 5 to 12.
CR in combination with 50 mg/kg/day resveratrol attenuates doxorubicin-mediated increases in serum CK levels
CK, an enzyme predominantly expressed in muscles, is involved in the conversion of creatine to phosphocreatine with the consumption of ATP [37]. An elevated CK level in blood is a widely used indicator of muscle damage. We observed that treatment with doxorubicin-increased serum CK levels (p < 0.01 vs. saline) and that CR + Resv-50 significantly ameliorated the increase (p < 0.05 vs. AL + doxorubicin) (Fig. 3B).
CR in combination with 5 or 50 mg/kg/day resveratrol attenuates doxorubicin-mediated increases in serum LDH activity
LDH is an oxidoreductase enzyme that catalyzes the inter-conversion of lactate and pyruvate. Since it is usually sequestered within cells and is released into the bloodstream under conditions of tissue injury, serum LDH activity represents a marker of general tissue damage [38]. We observed that treatment with doxorubicin significantly increased serum LDH levels (p < 0.01) and that both CR + Resv-5 and CR + Resv-50 significantly ameliorated the increase (p < 0.05 vs. AL + doxorubicin) (Fig. 4A). No differences were observed in serum LDH activity among AL and any of the intervention groups in saline-treated rats (Fig. 4B). Importantly, resveratrol alone (5 mg/kg/day or 50 mg/kg/day) did not attenuate the increase in serum LDH levels associated with doxorubicin injection (Fig. S4).
Fig. 4. Serum LDH activity in saline- and doxorubicin-treated AL, CR, CR + Resv-5 and CR + Resv-50 FBN rats.
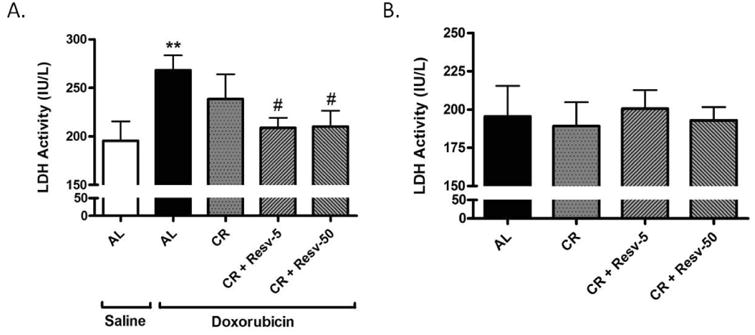
(A) Serum LDH activity was determined in saline-treated AL and in doxorubicin-treated AL, CR, CR + Resv-5 and CR + Resv-50 animals. **p < 0.01 vs. AL + saline; #p < 0.05 vs. AL + doxorubicin. n = 5 to 12; (B) Serum LDH activity was determined in saline-treated AL, CR, CR + Resv-5 and CR + Resv-50 animals. n = 4 to 12.
Proposed mechanisms of autophagy activation by the combinatorial approach of CR and resveratrol
Resveratrol is a known CR mimetic and is proposed to have a mechanism of action similar to that of CR [39, 40]. It is possible that when present together, CR and resveratrol reinforce and activate certain signal transduction pathways, which can lead to an induction of autophagy. For example, both CR and resveratrol can inhibit the mTOR pathway, which has inhibitory effects on autophagic induction (Fig. 5) [41-43]. Both interventions can activate the 5′ AMP-activated protein kinase (AMPK) pathway [44], which has been shown to enhance autophagic induction by phosphorylating ULK1 and activating the ULK1-Atg13- Atg17 complex (Fig. 5) [45]. Finally, the NAD+-dependent deacetylase sirtuin 1 (SIRT1) can deacetylate essential autophagy proteins Atg5, Atg7 and Atg8 and activate autophagy [46] and both CR and resveratrol have been shown to activate Sir2 (homolog of mammalian SIRT1) in lower organisms [47, 48] and SIRT1 in mammals (Fig. 5) [49, 50].
The activation state of mTOR was investigated in the AL, CR + Resv-5 and CR + Resv-50 groups, by measuring the phosphorylation levels of ribosomal protein S6 (rpS6), one of the downstream effectors of mTOR activation [51]. Western blot analysis revealed that both the CR + Resv groups tended to have lower levels of phosphorylated rpS6, normalized to that of total rpS6 (Fig. S5A). Although the data was not statistically significant, there was a strong trend for a decrease in phospho-rpS6, and hence for an inhibition of mTOR activity in the CR + Resv groups (p=0.07). SIRT1 protein levels, on the other hand, were unchanged in the AL, CR + Resv-5 and CR + Resv-50 groups (Fig. S5B).
Discussion
The self-cleanup process of autophagy recycles damaged and dysfunctional cellular components, thereby contributing to organelle and protein quality control. Upregulation of this process could therefore be hypothesized to be beneficial against oxidative stress-induced damage. However, despite its beneficial effects, few studies have focused on its stimulation in vivo. Although lifelong 40% CR has been reported to enhance autophagy in different tissues [6, 7], severe CR is also associated with slower wound healing, osteoporosis, cold sensitivity, and psychological challenges, such as food obsession, depression, and irritability [52]. Furthermore, adolescents practicing chronic severe CR may experience several adverse events, such as loss of strength and stamina, menstrual irregularities, infertility and loss of libido [52]. Hence a moderate CR starting late in life could offset some of the limitations associated with life-long CR. Additionally, combining a mild CR regimen with a so-called CR mimetic can potentially reinforce the beneficial effects of CR, without the potential harmful effects of a drastic food reduction. Resveratrol is a known CR mimetic that has shown to produce CR-like extension of lifespan in lower organisms and has a proposed mechanism of action very similar to that of CR [40, 47, 53].
In the present study, we have shown that 20% CR in combination with 50 mg/kg/day resveratrol can enhance autophagic flux in the hearts of late-middle-aged (26-month-old) FBN rats. It is important to note that FBN rats are excellent models for gerontology research because they have fewer incidences of age-associated pathological lesions and a longer disease-free life span than other rat strains [54, 55]. In the FBN rats, changes in cardiomyocyte volume density occur as early as 24 months of age, and percentage fractional shortening (as a measure of cardiac function) shows significant differences starting at 27 months of age [56, 57]. These observations suggest that for the FBN rat strain, 26 month may represent an optimum life stage to start late-life interventions, i.e. a time when structural changes begin to appear in the heart, without drastic functional consequences. Additionally, 26 months of age in FBN rats correspond to roughly 60 years in humans, a stage in life when moderate dietary restriction can be assumed to have little or no deleterious effects [58].
Rodent models using resveratrol as an intervention have used broad concentrations, ranging from 0.1 mg/kg to 1000 mg/kg with numerous beneficial effects in several organs [59]. Cardioprotection with the plant polyphenol has been observed with doses as low as 2.5 mg/kg/day or 5 mg/kg/day [60], the latter being the lower dose used in our study. However, most of the reported studies have been done in a younger cohort of animals and the dosage required to see similar effects in aged animals remains largely unknown. This is the first time to our knowledge that resveratrol have been tested in older animals. With the assumption that doxorubicin may cause more damage in an age-stressed heart, the current study uses an additional dose of resveratrol, 10 times higher (50 mg/kg/day) than the low dose used in the study.
Although showing numerous CR-like effects, a major drawback of resveratrol is its poor bioavailability, since resveratrol undergoes rapid metabolism to glucoronide and sulphate conjugates in the liver [61]. However it has recently been shown that resveratrol is delivered to target tissues in a sulfate-conjugated form, where the sulphate conjugates can regenerate the parent compound [62]. Importantly, such conjugates induced autophagy in human cells [62].
Autophagy assessments in the present study were conducted by measuring protein expression levels of LC3B, p62, Beclin-1 and Atg5-Atg12 conjugate. We observed no changes in the LC3-II/LC3-I ratio in the left ventricle of AL or any of the interventions, which could either mean: 1) no autophagic stimulation by the treatments or 2) an enhanced autophagic flux, such that an increased amount of LC3-II is rapidly degraded in the lysosomes, resulting in unaltered LC3-II /LC3-I ratios. The ubiquitin-binding protein p62/SQSTM1 targets cytosolic cargo for autophagic degradation, being selectively incorporated into autophagosomes and efficiently degraded in the autolysosomes [63]. Therefore, the cellular abundance of p62 inversely correlates with autophagic flux [64]. In our study, CR + Resv-50 rats showed a decrease in the abundance of p62 in the left ventricle, suggesting an increase in autophagic flux. We further confirmed that the decrease in p62 protein levels was not due to a decrease in its transcription. In addition, we observed an increase in Beclin-1 levels in the same group, pointing to an enhanced induction of autophagy. Finally, there were no differences in Atg5-Atg12 conjugate levels between AL and any of the intervention groups. Although the conjugation of Atg5 to Atg12 is essential for autophagy, Atg5– Atg12 dissociates from the autophagosome after it completely engulfs the cargo [34]. Hence, the observation that the Atg5-Atg12 levels remain unaltered is consistent with LC3-II/LC3-I ratios observed in the study.
The mechanism by which the combined regimen of 20% CR and 50 mg/kg/day resveratrol (but not either intervention alone) can induce autophagy in rodent hearts will need further investigation. Our results suggest a possible role of mTOR, but additional analysis is required to confirm this hypothesis. Although our results revealed no changes in SIRT1 protein levels in any of the intervention groups, it is possible that the deacetylate activity of SIRT1 is controlled at the post-translational level, such as by phosphorylation at Thr522 (thereby activating the monomeric, active state [65]) or by influencing its binding to other protein partners for deacetylation [66]. Experiments investigating such pathways might better answer the question as to whether SIRT1 also plays a role in the autophagy-inducing properties of CR + Resv.
Finally, whether autophagy induction occurs through the AMPK pathway will also need further investigation.
Our results suggest that doxorubicin per se increases the LC3-II/LC3-I ratio. An increase in this ratio by doxorubicin treatment has previously been reported [67-69]. Autophagic flux analysis, however, was not conducted in these studies. We observed that doxorubicin increased p62 abundance in the left ventricle, suggesting that the increase in LC3-II/LC3-I levels is due to an impairment of autophagic flux. Consistent with this hypothesis, a decrease in the activity of lysosomal enzyme cathepsin D has been reported in the hearts of doxorubicin-treated animals [70]. It is also possible that the autophagic flux impairment is a characteristic of the relatively older population of rats used in the study, as all previous studies with doxorubicin were conducted in a younger cohort [67]. For instance, it is possible that doxorubicin induces autophagy as a protective mechanism in all age groups, but that complete degradation of autophagic cargo is affected or attenuated only in older rats.
In the clinical setting, a significant proportion of patients given doxorubicin for long periods go on to develop cardiac pathologies. However, as of yet, there is no specific treatment for preventing such chemotherapy-derived cardiomyopathy [71]. The development of cardioprotective agents that can intervene against doxorubicin-mediated toxicity is therefore highly desirable. Notably, in the present study, we observed that induction of autophagy protects the myocardium from doxorubicin-mediated injury, which is confirmed by the attenuation of drug-mediated increases in myocardial apoptotic index and serum CK levels (Fig. 5). While skeletal muscle injury cannot be ruled out, it is likely that the increases in CK levels in doxorubicin-treated rats is primarily due to cardiac damage, since the heart is especially vulnerable to anthracycline-mediated toxicity [72]. Additionally, CR in combination with 50 mg/kg/day resveratrol also attenuated doxorubicin-mediated increases in serum LDH levels, which is considered a general marker of tissue damage. Importantly, a previous study has shown that a 35% CR for 10 weeks can be protective against doxorubicin-mediated cardiotoxicity in male Sprague-Dawley rats [73], which is in contrast to our current finding that no protective effect could be observed by 20% CR for 6 weeks. The difference could be attributed to the relatively mild CR regimen and the shorter duration of interventions used in our study.
Importantly, it is possible that the cardioprotective effects of CR + Resv-50 are caused by additional effects on the cardiomyocyte, in addition to autophagy. For example, the vasodilatory, anti-inflammatory and anti-oxidative properties of both CR and resveratrol cannot be ruled out in the current study. However, we propose that autophagy plays a vital role. Several observations led to such a hypothesis: first, autophagy induction and maximum cardioprotection was both observed in the same intervention group; second, our experiments in cultured cardiomyocytes have shown that an induction of autophagy can protect cells from oxidative stress-induced injury, selectively remove damaged mitochondria, improve mitochondrial respiration and inhibit apoptosis [74]. Importantly, our preliminary analysis in permeabilized cardiac muscle fibers showed increased State 3 (ADP-stimulated) respiration in CR + Resv-50 group, in comparison to AL (data not shown).
It is worth noting that our moderate, late-age-onset CR is similar to most clinical trials of dietary restriction in humans [75, 76], where a high-severity CR can be challenging and potentially deleterious. For example, a 36-year follow-up study showed that mortality rate in healthy non-smoking Japanese-American men was increased when 50% CR was practiced and that a moderate 15% CR showed a trend for lower mortality [75]. In another study, a 12-month 20% CR has been shown to improve glucose tolerance [77] and reduce DNA and RNA oxidation in the white blood cells of healthy normal and overweight persons aged 50 to 60 years [78]. Studies have shown that such low-intensity dietary restriction can also result in significant improvements in traditional cardiovascular risk factors, such as blood pressure, blood glucose, circulating lipids and body composition, in both overweight [76, 79, 80] and lean individuals [81, 82]. With respect to resveratrol, although animal studies are plenty, clinical trials investigating the cardiovascular effects of resveratrol have been comparatively limited. Most of trials have investigated the pharmacodynamics and safety issues associated with the drug [83]. However, at least one clinical trial in obese men has shown that short-term (30 day) supplementation with 150 mg/kg/day resveratrol increased AMPK and SIRT1 levels in muscles and led to metabolic changes in blood similar to that of CR [84]. In conclusion, both late-age-onset mild CR regimen and the plant polyphenol resveratrol have shown numerous cardioprotective effects in experimental animal models and in controlled clinical trials. Therefore, combining these two interventions might provide additional cardioprotective effects. Although their exact mechanism is still a matter of debate, our results suggest that an induction of myocardial autophagy is at least partially responsible for the beneficial effects.
Supplementary Material
Highlights.
Autophagy is a self-cleanup process whereby damaged cellular components are recycled
20% CR combined with 50 mg/kg/day resveratrol induce autophagy in the hearts of 26-month-old Fischer 344 × Brown Norway rats
Induction of autophagy by a combination of CR and resveratrol protects against doxorubicin-induced toxicity in the heart
A combinatorial approach of low dose CR and resveratrol can be potentially exploited therapeutically for protection against doxorubicin-mediated cardiac damage in the aged heart.
Acknowledgments
This work was supported by The Claude D. Pepper Older Americans Independence Center (OAIC) Metabolism and Translational Science Core. The OAIC is supported by a grant from the National Institutes of Health/National Institute on Aging (1P30AG028740). The study is also supported by the American Heart Greater Southeast Affiliate Fellowship (10PRE4310091) to D. Dutta. We thank Dr. Timothy Garrett at the Biomedical Mass Spectrometry Core of the University of Florida for his help in plasma resveratrol analysis. We are grateful to Gauthami Balagopal for her help with general laboratory work.
Abbreviations
- AL
Ad libitum
- AMPK
5′ AMP-activated protein kinase
- Atg
AuTophaGy
- CK
creatine kinase
- CR
calorie restriction
- FBN
Fischer 344 × Brown Norway
- LC3
microtubule-associated light chain 3
- LDH
lactate dehydrogenase
- ROS
reactive oxygen species
- rpS6
ribosomal protein S6
- SIRT1
sirtuin 1
- SQSTM1
sequestosome 1
- TD-NMR
time-domain nuclear magnetic resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 2.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 3.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Shirasawa T, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6 doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 4.Terman A, Brunk UT. The aging myocardium: roles of mitochondrial damage and lysosomal degradation. Heart Lung Circ. 2005;14:107–114. doi: 10.1016/j.hlc.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Kanamori H, Takemura G, Maruyama R, Goto K, Tsujimoto A, Ogino A, Li L, Kawamura I, Takeyama T, Kawaguchi T, Nagashima K, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. Am J Pathol. 2009;174:1705–1714. doi: 10.2353/ajpath.2009.080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 7.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullin GE. Red wine, grapes, and better health--resveratrol. Nutr Clin Pract. 2011;26:722–723. doi: 10.1177/0884533611423927. [DOI] [PubMed] [Google Scholar]
- 9.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 10.Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Forstermann U. Resveratrol: a multifunctional compound improving endothelialfunction. Editorial to: “Resveratrol supplementation gender independently improves endothelialreactivity and suppresses superoxide production in healthy rats” by S. Soylemez et al. Cardiovasc Drugs Ther. 2009;23:425–429. doi: 10.1007/s10557-009-6209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramprasath VR, Jones PJ. Anti-atherogenic effects of resveratrol. Eur J Clin Nutr. 2010;64:660–668. doi: 10.1038/ejcn.2010.77. [DOI] [PubMed] [Google Scholar]
- 13.Lv XC, Zhou HY. Resveratrol protects H9c2 embryonic rat heart derived cells from oxidative stress by inducing autophagy: role of p38 mitogen-activated protein kinase. Can J Physiol Pharmacol. 2012 doi: 10.1139/y2012-051. [DOI] [PubMed] [Google Scholar]
- 14.Scarlatti F, Maffei R, Beau I, Ghidoni R, Codogno P. Non-canonical autophagy: an exception or an underestimated form of autophagy? Autophagy. 2008;4:1083–1085. doi: 10.4161/auto.7068. [DOI] [PubMed] [Google Scholar]
- 15.Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Kang HS, Kim JS, Kang SJ, Park SY. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res. 2012;73:99–105. doi: 10.1016/j.neures.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 19.Mordente A, Meucci E, Silvestrini A, Martorana GE, Giardina B. Anthracyclines and mitochondria. Adv Exp Med Biol. 2012;942:385–419. doi: 10.1007/978-94-007-2869-1_18. [DOI] [PubMed] [Google Scholar]
- 20.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 22.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 23.Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–490. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 24.Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 2010;18:1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Manini TM, Vincent KR, Leeuwenburgh CL, Lees HA, Kavazis AN, Borst SE, Clark BC. Myogenic and proteolytic mRNA expression following blood flow restricted exercise. Acta Physiol (Oxf) 2011;201:255–263. doi: 10.1111/j.1748-1716.2010.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 28.Larsen KB, Lamark T, Overvatn A, Harneshaug I, Johansen T, Bjorkoy G. A reporter cell system to monitor autophagy based on p62/SQSTM1. Autophagy. 2010;6:784–793. doi: 10.4161/auto.6.6.12510. [DOI] [PubMed] [Google Scholar]
- 29.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 30.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947–948. doi: 10.4161/auto.6787. [DOI] [PubMed] [Google Scholar]
- 33.Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 35.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 37.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–767. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 38.Glick JH., Jr Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase values in health and disease, and clinical evaluation of these tests by means of discriminant analysis. Am J Clin Pathol. 1969;52:320–328. doi: 10.1093/ajcp/52.3.320. [DOI] [PubMed] [Google Scholar]
- 39.Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem. 285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 48.Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 49.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 50.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 52.Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med. 2009;25:715–732. ix. doi: 10.1016/j.cger.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 54.Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46:B87–88. doi: 10.1093/geronj/46.3.b87. [DOI] [PubMed] [Google Scholar]
- 55.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 x Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2006;290:H304–311. doi: 10.1152/ajpheart.00290.2005. [DOI] [PubMed] [Google Scholar]
- 57.Walker E, Jr, Nillas MS, Mangiarua EI, Cansino S, Morrison RG, Perdue RR, Triest WE, Wright GL, Studeny M, Wehner P, Rice KM, Blough ER. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci. 2006;36:427–438. [PubMed] [Google Scholar]
- 58.Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol. 2008;43:859–866. doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2010;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel KR, Andreadi C, Britton RG, Horner-Glister E, Karmokar A, Sale S, Brown VA, Brenner DE, Singh R, Steward WP, Gescher AJ, Brown K. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med. 2013;5:205ra133. doi: 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- 63.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo X, Kesimer M, Tolun G, Zheng X, Xu Q, Lu J, Sheehan JK, Griffith JD, Li X. The NAD(+)-dependent protein deacetylase activity of SIRT1 is regulated by its oligomeric status. Sci Rep. 2012;2:640. doi: 10.1038/srep00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milner J. Cellular regulation of SIRT1. Curr Pharm Des. 2009;15:39–44. doi: 10.2174/138161209787185841. [DOI] [PubMed] [Google Scholar]
- 67.Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol. 2011;111:1190–1198. doi: 10.1152/japplphysiol.00429.2011. [DOI] [PubMed] [Google Scholar]
- 68.Manov I, Pollak Y, Broneshter R, Iancu TC. Inhibition of doxorubicin-induced autophagy in hepatocellular carcinoma Hep3B cells by sorafenib--the role of extracellular signal-regulated kinase counteraction. FEBS J. 2011;278:3494–3507. doi: 10.1111/j.1742-4658.2011.08271.x. [DOI] [PubMed] [Google Scholar]
- 69.Pan Y, Gao Y, Chen L, Gao G, Dong H, Yang Y, Dong B, Chen X. Targeting autophagy augments in vitro and in vivo antimyeloma activity of DNA-damaging chemotherapy. Clin Cancer Res. 2011;17:3248–3258. doi: 10.1158/1078-0432.CCR-10-0890. [DOI] [PubMed] [Google Scholar]
- 70.Gebbia N, Leto G, Gagliano M, Tumminello FM, Rausa L. Lysosomal alterations in heart and liver of mice treated with doxorubicin. Cancer Chemother Pharmacol. 1985;15:26–30. doi: 10.1007/BF00257289. [DOI] [PubMed] [Google Scholar]
- 71.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol. 2006;41:389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2009;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitra MS, Donthamsetty S, White B, Latendresse JR, Mehendale HM. Mechanism of protection of moderately diet restricted rats against doxorubicin-induced acute cardiotoxicity. Toxicol Appl Pharmacol. 2007;225:90–101. doi: 10.1016/j.taap.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 74.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110:1125–1138. doi: 10.1161/CIRCRESAHA.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willcox BJ, Yano K, Chen R, Willcox DC, Rodriguez BL, Masaki KH, Donlon T, Tanaka B, Curb JD. How much should we eat? The association between energy intake and mortality in a 36-year follow-up study of Japanese-American men. J Gerontol A Biol Sci Med Sci. 2004;59:789–795. doi: 10.1093/gerona/59.8.b789. [DOI] [PubMed] [Google Scholar]
- 76.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11:793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: are there differences between men and women or between weight loss and maintenance? Int J Obes Relat Metab Disord. 1995;19:67–73. [PubMed] [Google Scholar]
- 80.Sung MM, Soltys CL, Masson G, Boisvenue JJ, Dyck JR. Improved cardiac metabolism and activation of the RISK pathway contributes to improved post-ischemic recovery in calorie restricted mice. J Mol Med (Berl) 2010;89:291–302. doi: 10.1007/s00109-010-0703-5. [DOI] [PubMed] [Google Scholar]
- 81.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 82.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health--a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 84.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


