Abstract
Redox imbalance is a primary cause for endothelial dysfunction (ED). Under oxidant stress, many critical proteins regulating endothelial function undergo oxidative modifications that lead to ED. Cellular levels of GSH, the primary reducing source in cells, can significantly regulate cell function via reversible protein thiol modification. N-Acetyl cysteine (NAC), a precursor for GSH biosynthesis, is beneficial for many vascular diseases; however, the detailed mechanism of these benefits is still not clear. From HPLC analysis, NAC significantly increases both cellular GSH and BH4 levels. Immunoblotting of eNOS and DUSP4, a dual-specificity phosphatase with a cysteine as its active residue, revealed that both enzymes are up-regulated by NAC. EPR spin-trapping further demonstrated that NAC enhances NO generation from cells. Long-term exposure to Cd2+ contributes to DUSP4 degradation and the uncontrolled activation of p38 and ERK1/2, leading to apoptosis. Treatment with NAC prevents DUSP4 degradation and protects cells against Cd2+-induced apoptosis. Moreover, the increased DUSP4 expression can redox regulate p38 and ERK1/2 pathways from hyper-activation, providing a survival mechanism against the toxicity of Cd2+. DUSP4 gene knockdown further supports the hypothesis that DUSP4 is an antioxidant gene, critical in the modulation of eNOS translation, and thus protects against Cd2+-induced stress. Depletion of intracellular GSH by BSO makes cells more susceptible to Cd2+-induced apoptosis. Pre-treatment with NAC prevents p38 over-activation and thus protects the endothelium from this oxidative stress. Therefore, the identification of DUSP4 activation by NAC provides a novel target for future drug design.
Keywords: Endothelial dysfunction (ED), Reactive oxygen species (ROS), Redox signaling, Phosphatase, Nitric oxide synthase, N-acetyl cysteine, DUSP4, MAP kinases (MAPKs)
INTRODUCTION
Elevated production of oxygen free radicals and reduced antioxidant activity contribute to cellular redox imbalance, the primary pathogenic factor of many oxidant-induced cardiovascular, aging-associated, and neurodegenerative diseases [1-3]. Under oxidant stress, many critical redox-sensitive proteins are either reversibly or irreversibly modified. Reversible modification, especially protein thiol oxidation, plays a key role in regulating cellular redox signaling and modulating cell function [2-4]. Glutathione (GSH), the primary reducing source in cells, is a substrate for many antioxidant enzymes, and GSH can also scavenge reactive oxygen species (ROS) through oxidation of its thiol to form oxidized GSH (GSSG) [5]. As such, an increase in the [GSSG]/[GSH] ratio is indicative of oxidative stress and leads to reversible thiol oxidation of many redox sensitive proteins, resulting in either their activation or inactivation [4, 6-8]. Thus, the regeneration of intracellular GSH can reverse this oxidative modification and restore protein function, and be of therapeutic significance for targeting oxidant-induced diseases.[9, 10].
NAC is a thiol-containing antioxidant and a precursor for GSH biosynthesis [11]. Because of its antioxidant nature and ability to elevate or restore the intracellular GSH, NAC has been used to treat various oxidant-derived diseases, such as chronic pulmonary diseases, cardiovascular diseases, neurodegenerative diseases, and cancer [12-14]. In cardiovascular diseases, NAC has been used in numerous clinical trials for ischemia/reperfusion injuries [15, 16]. However, the outcomes of these trials were inconsistent. Whereas some studies found NAC treatment to be beneficial, others found no significant difference between treatment groups. An underlying reason for this discrepancy lies in the complexity of NAC on cell redox modulation [11, 14]. As such, further and more detailed investigations are required to elucidate the redox mechanism of NAC treatment on oxidant-derived diseases, especially with regard to ED. Redox imbalance is a main contributing factor for ED. The resulting effects of elevated endothelial ROS are to increase cell surface expression of adhesion molecules through cytokine activation [14] and decrease the NO bioavailability [17-19]. NAC inhibits the expression of these adhesion molecules [14] and restores the level of NO generation by scavenging the endogenous ROS, thus benefiting vascular function. Further identification of NAC redox mechanisms will provide new therapeutic strategies targeting oxidant-derived diseases.
Apart from its antioxidant effect, NAC has been demonstrated to prevent apoptosis and enhance cell survival through activation of the extracellular signal-regulated kinase (ERK1/2) pathway [20]. NAC also inhibits the p38 [21] and c-Jun N-terminal kinases (JNKs) [14] of the mitogen-activated protein kinase (MAPK) signaling cascades that regulate differentiation, proliferation and apoptosis. The duration and extent of phosphorylation of these MAPKs are critical factors in determining their physiological effects [22, 23]. Kinetic control of the MAPK signal cascades is modulated by the process of kinase dephosphorylation, conducted by the MAPK phosphatase (MKPs) or dual-specificity protein phosphatases (DUSPs) [24-26]. Nonetheless, the mechanism of inhibition of these kinases by NAC is still unclear. A previous study showed that DUSP4/MKP2 is one of the genes up-regulated by NAC treatment using microarray gene expression analysis of endothelial cells treated with NAC [27], and we ourselves have seen similar effects. Therefore, in this study we hypothesize that NAC treatment results in MAPK inhibition via the activation of DUSP4, providing a feedback regulation of MAPKs, which are important in cell survival.
DUSPs, a subset of the protein tyrosine phosphatase superfamily, have an active cysteine for their catalytic function. The unique feature of DUSPs is to inactivate the terminal MAPKs via the dephosphorylation of both serine/threonine and tyrosine residues within the one substrate [24-26]. Each of the DUSP members has different substrate specificity (p38, ERK, or JNK) and different subcellular localization. Because DUSPs have an active cysteine in their catalytic domain, they are redox sensitive and can be inactivated by ROS or thiol modification [28].
In the endothelium, NO is a critical small molecule involved in regulating vascular tone, vascular growth, platelet aggregation, and modulation of inflammation [29-31]. The over-production of ROS, which reacts with NO in the vasculature, leads to a decrease in NO bioavailability and contributes to ED, including hypertension and atherosclerosis [17-19]. A study showed that chronic NAC treatment lowers the blood pressure of spontaneously hypertensive rats by increasing eNOS expression and activity [32]. However, the mechanism of eNOS activation by NAC treatment has not been determined. Recently, we demonstrated that eNOS is S-glutathionylated in hypertensive vessels and in endothelial cells with treatment by BCNU or Cd2+, leading to eNOS uncoupling and increased superoxide generation [6, 33]. This eNOS oxidative modification can be reversed by glutaredoxin1 but this reversal is dependent on the ratio of GSSG and GSH [34]. Thus, the restoration of intracellular GSH is expected to provide a beneficial effect for endothelial function by favoring eNOS in its reduced form.
Our results support the hypothesis that NAC treatment in endothelial cells up-regulates the expression of DUSP4 and eNOS, contributing to cell growth and augmenting NO generation. The increase in DUSP4 expression specifically prevents Cd2+-induced over-activation on both p38 and ERK1/2 pathways, thus promoting cell survival. This process is dependent on the intracellular [GSSG]/[GSH] ratio. Furthermore, the result of DUSP4 gene silencing demonstrates that DUSP4 plays a critical role against oxidant stress, and is important for eNOS expression via translational modulation. Therefore, the identification of DUSP4 activation in response to NAC treatment provides new insight into redox signaling and vascular function.
EXPERIMENTAL PROCEDURES
Materials
Anti-NOS3 (C-20) HRP, DUSP4, and β-actin antibodies were obtained from Santa Cruz (Santa Cruz, CA), GCH1 antibody from Abnova (Taipei, Taiwan), p-ERK1/2, ERK1/2, p-p38, p38, GAPDH, cleaved caspase-3, MEK1/2, and Histone antibodies from Cell Signaling (Cambridge, MA). NAC, GSH, GSSG, HEPES, and Tris were purchased from Sigma-Aldrich (St. Louis, MO). Secondary anti-rabbit and anti-mouse IgG-HRP antibodies were purchased from GE Life Sciences (Piscataway, NJ). RNA was isolated using Trizol reagent purchased from Ambion (Carlsbad, CA). cDNA was synthesized with a High-Capacity cDNA Reverse Transcription kit from Applied Biosystems (Foster City, CA) and real-time PCR conducted using LightCycler 480 SYBR GreenI from Roche (Mannheim, Germany). p38 inhibitor, SB 203580, was purchased from Cayman Chemical (Ann Arbor, MI).
Cell culture and NAC treatment
The maintenance of bovine aortic endothelial cells (BAECs) cultures was the same as previously described [6, 33, 34]. BAECs were treated overnight with various concentrations (1, 2, and 5 mM) of NAC. The activity of eNOS from BAECs was measured using EPR NO spin-trapping with Fe-N-methyl-d-glucamine dithiocarbamate (Fe2+-MGD) [33, 34] after 24 hours of NAC treatment. High-performance liquid chromatography (HPLC) was used to determine the cellular level of GSH and BH4 after treatment.
Measurement of NO generation from BAECs by EPR spectroscopy
Spin-trapping measurements of NO from BAECs were performed with a Bruker EMX spectrometer with Fe2+-MGD as the spin trap [6, 33, 34]. Spin-trapping experiments were performed on cells grown in six-well plates (106 cells per well). Before EPR spin-trapping measurements, control cells, and cells treated overnight with NAC were washed twice with PBS. Next, 0.8 ml of PBS containing glucose (1 g/L), CaCl2, MgCl2, the NO spin-trap Fe2+-MGD (0.5 mM Fe2+, 5.0 mM MGD) and calcium ionophore (1 μM) was added to each well, and the plates were incubated for 20 min at 37 °C in a humidified environment containing 5% CO2. After incubation, the medium from each well was removed, and the trapped NO in the supernatants was quantified by EPR. Spectra recorded from these cellular preparations were obtained with the following parameters: microwave power 20 mW, modulation amplitude 4.0 G, modulation frequency 100 kHz. 1 mM L-NAME was used to inhibit the intracellular eNOS activity. The quantification of NO generation from eNOS was determined from the L-NAME inhibitable EPR intensity.
HPLC measurement of cellular GSH and GSSG
The cellular level of GSH and GSSG in BAECs was determined using HPLC chromatography [35]. Cells were first lysed and sonicated in degassed PBS. The cellular level of reduced GSH was determined by addition of 50 μl of supernatant to 900 μl of 75 mM borate buffer pH 8.0 and 50 μl of 36.4 mM OPA (prepared in 75 mM borate buffer pH 8.0 and 10% ethanol) for 10 minutes in the dark. 50 μl of the reaction was separated using a Shimadzu LC-2010AT HPLC system with a C18 reversed-phase column (Tosoh Reversed-Phase C18 HPLC Columns, 4.6 mm × 150 mm) consisting of a mobile phase of 150 mM sodium acetate in 30% methanol and a flow rate of 0.5 mL/min. A Shimadzu RF-10A XL fluorescence detector with excitation wavelength of 338 nm and emission wavelength of 458 nm was used to detect GSH-OPA adduct and the integration of peak area used for GSH quantification. Bradford assay was used to determine the protein concentration. For comparison, the level of GSH was normalized to the total protein concentration.
GSSG content was determined separately by incubating 39 μl of cell lysate with 1 μl of 100 mM N-ethylmaleimide for 5 minutes to inactivate the reduced GSH. 10 μl of 100 mM DTT was then added to samples and incubated at RT for 10 minutes to reduce GSSG to GSH before the reaction with OPA to form a GSH-OPA adduct for HPLC analysis.
HPLC measurement of cellular BH4
The cellular level of BH4 in BAECs was determined using HPLC chromatography [36]. For BH4 measurement, cells were lysed and sonicated in cold extraction buffer (50 mM Tris-HCl, pH 7.4, 1 mM DTT, 1 mM EDTA). The total protein concentration was determined using a Bio-Rad protein assay. Total biopterin (BH4 + BH2 + biopterin) was quantified through acid oxidation, while BH2 and biopterin were quantified via alkaline oxidation. To determine total biopterin, 90 μl of cell extract was treated with 10 μl of a 1:1 mixture of 1.5 M HClO4 and 2 M H3PO4 and centrifuged for 30 min at 12,000 rpm. Acidic oxidation was performed by addition of 10 μl of 1% iodine, in 2% KI solution, to 90 μl of the acid-treated supernatant and incubated at room temperature for 1 hour in the dark. Alkaline oxidation was achieved by adding 10 μl of NaOH to 80 μl of cell lysate followed by addition of 10 μl of 1% iodine in 2% KI. The alkaline oxidation was carried out at room temperature for 1 hr in the dark and was then acidified with 20 μl of 1 M H3PO4. 50 μl of the acid or alkaline oxidized product was separated using a Shimadzu LC-2010AT HPLC system with a C18 reverse-phase column (Tosoh Reversed-Phase C18 HPLC Columns, 4.6 mm × 150 mm) using 5 % methanol as a mobile phase with a flow rate of 0.5 ml/min. A Shimadzu RF-10A XL fluorescence detector with excitation wavelength of 350 nm and emission wavelength of 450 nm was used to detect biopterin elution. The peak area was integrated and normalized to total protein for quantification.
Measurement of superoxide generated from BAECs using dihydroethidium (DHE) and HLPC analysis
The level of superoxide generation from control BAECs and BAECs treated with NAC, Cd2+, or Cd2+/NAC was measured using DHE HPLC analysis with a slight modification [37]. After overnight treatment, cells were washed once with PBS and incubated in Krebs-HEPES buffer. DHE was added to a final concentration of 25 μM and incubated at 37 °C for 30 min. The medium was then removed and replenished with fresh Krebs-HEPES buffer for additional 1 hr incubation. After incubation, cells were collected and lysed in 500 μL cold methanol, and centrifuged. 2-hydroxyethidium, dihydroethidium, and ethidium were separated using a gradient HPLC system (Shimadzu LC-2010A) with a Hypersil Gold column (250 × 4.6 mm, Thermo Scientific) and detected with a fluorescence detector using an emission wavelength at 580 nm and an excitation at 480 nm. A linear gradient at a flow rate of 0.5 mL/min was developed from mobile phase A (0.1% trifluoroacetic acid) to mobile phase B (acetonitrile) over 23 min from 37% to 47% acetonitrile.
Cells treatment with NAC, Cd2+, or BSO
To determine the effects of the various treatments on endothelial function, cells were treated overnight with 5 mM NAC, 100 μM Cd2+ alone, or 100 μM Cd2+ and 5 mM NAC. After treatment, the levels of GSH and [GSSG]/[GSH] ratio were measured using HPLC. Immunoblotting was used to determine the level of protein expression, and quantitative PCR (qPCR) was used to measure transcript levels. To further demonstrate the role of GSH on protein stability and cell viability, cells were pre-treated with 5 mM NAC or 1 mM buthionine sulfoximine (BSO) overnight, and followed with 100 μM Cd2+ exposure in fresh medium for 2 hr. Live cell imaging microscopy was used to determine cell viability. Cell apoptosis was determined using immunostaining of cleaved caspase-3. Immunoblotting was used to determine the level of protein expression. Non-reducing electrophoresis and immunoblotting of eNOS was used to determine eNOS protein thiol oxidation. Cellular level of GSSG and GSH was measured by HPLC as described in the previous section.
Immunoblotting analysis
The procedure for the immunoblotting was followed as previously described [6, 33, 34]. Samples were first separated on a 4–20% or 8% Tris-glycine polyacrylamide gel, and then electrophoretically transferred to a nitrocellulose membrane. Anti-eNOS, GCH1, and DUSP4 were used to determine the level of expression of these proteins. The extent of p38 and ERK1/2 phosphorylation was determined using anti-p-p38 and p-ERK1/2. The ratio of p-p38 and total p38 or p-ERK1/2 and total ERK1/2 was used to determine the extent of the activation of these kinases. β-actin or GAPDH served as loading control markers.
RNA isolation and quantitative PCR
After treatment with NAC, Cd2+, or Cd2+/NAC, cells were first washed with PBS and trypsinized, and pellet was then washed once with PBS before RNA isolation. In brief, total RNA was isolated from BAECs via the Trizol-chloroform extraction procedure. Extracted RNA was quantified via spectrophotometric analysis using absorption spectra at wavelengths of 230, 260 and 280 nm. A total of 1 g of RNA was reverse-transcribed using a High-Capacity cDNA Reverse Transcription Kit according to the kit’s instructions. Gene expression was detected via quantitative real-time PCR using a Roche 480 thermal cycler. Data were calculated using the 2−Cp method and are expressed as target gene transcript fold induction normalized to β-actin. The primers utilized are listed in Table 1.
Table 1. Primers for real-time PCR gene quantitation.
| Gene | Protein | Forward primer, 5’→3’ | Reverse primer, 5’→3’ |
|---|---|---|---|
|
Dusp4, Mkp2 |
DUSP4 | ATTCCGCCGTCATCGTCTAC | ATAGCCACCTTTCAGCAGGC |
| Nos3 | NOS3 | TACCAGCCGGGGGACCACATAGGC | CTCCAGCTGCTCCACAGCCACAGAC |
| Gch1 | GCH1 | GCCATGCAGTTCTTCACCAAA | CCGATATGGACCTTTCCAACAAA |
| Actb | β-actin | TGCCCATCTATGAGGGGTACG | GGACGATTTCCGCTCGGC |
Nuclear and Cytoplasmic Extraction
NE-PER nuclear and cytoplasmic extraction kit from Thermo Scientific (Rockford, IL) was used to determine the subcellular localization of proteins. Before the fractionation, BAECs were treated with NAC, Cd2+, and NAC/Cd2+ as previously described. After extraction, the protein localization was determined using immunoblotting for either nuclear or cytoplasmic fraction. Histone was used as the nuclear marker, and MEK1/2 as the cytoplasmic marker.
Live Cell Imaging Microscopy
BAECs were cultured on 22-mm2 sterile coverslips in 35-mm sterile dishes at a density of 104 cells per dish, subjected to overnight pre-treatment with NAC or BSO, then exposed for 2 hr to Cd2+. After treatment, the coverslips with cells were then mounted on a glass slide and viewed with the Zeiss Axiovert 135 microscope with Tucsen TCH-5.0ICE camera at 20× magnification, images were captured digitally and analyzed.
Immunostaining of cleaved caspase-3
BAEC cells were cultured on sterile coverslips coated with adhesion factor to a 50-60% confluence. Cells were pre-treated with either NAC or BOS overnight following with 2hr Cd2+ exposure. After treatment, cell media was removed and coverslips were washed 2× with PBS, fixed for 20 min at RT in 4% paraformaldehyde and subsequently washed 3× with PBS containing 0.1% BSA. Cells were then blocked for 45 min at RT in PBS containing 10% FBS and 0.3% TritonX-100 and then incubated overnight at 4°C in anti-cleaved caspase-3 diluted 1/400 in PBS with 0.3% TritonX-100. After overnight incubation, coverslips were rinsed 2× with PBS containing 0.1% BSA, and incubated with Alexa Fluor 594 secondary antibody (Cell Signaling) for 1 hr at RT. Finally, coverslips were washed 3× with PBS containing 0.1% BSA, mounted onto a glass slide with ProLong Gold antifade reagent with DAPI (Life technologies, Grand Island, NY), and fluorescent images (20×) obtained using a Zeiss Axiovert 135 microscope. To further demonstrate that the over-activation of p38 is one of contributing factors for apoptosis, BSO-treated cells were incubated with 10 μM SB 203580 30 min prior to and during 2 hr Cd2+ exposure.
DUSP4 gene silencing
The important role of DUSP4 on the modulation of p38 and ERK1/2, and eNOS expression in response to NAC treatment was demonstrated via DUSP4 gene silencing in rat aortic endothelial cells (RAECs) with rat DUSP4 siRNA kit (OriGene, Rockville, MD). For the control, cells only received DharmaFECT transfection reagent (GE Life Sciences, Pittsburg, PA) The efficiency of DUSP4 gene silencing was determined by qPCR analysis for DUSP4, eNOS, GAPDH, and β-actin 48 hr post transfection. Furthermore, immunoblotting of DUSP4 was also conducted 72 hr post transfection. The ratios of p-ERK1/2/ERK1/2 and p-p38/p38 were used to determine the effects of DUSP4 on ERK1/2 and p38 activities. The eNOS expression level was also measured using immunoblotting against eNOS. For the negative control, cells were transfected with Allstars Negative Control siRNA from Quiagen (Valencia, CA). Cellular apoptosis was determined using immunostaining of cleaved caspase-3 as detailed above, while cell death was determined by visually counting cells in at least 10 field of views/treatment group. Cell death was expressed as a percentage of dead cells (floating or rounded).
Statistics
Results were expressed as mean ± SEM, n ≥ 3. Statistical significance of difference between results was calculated using Anova single factor. A P value < 0.05 was considered statistically significant.
RESULTS
NAC treatment enhances endothelial NO production
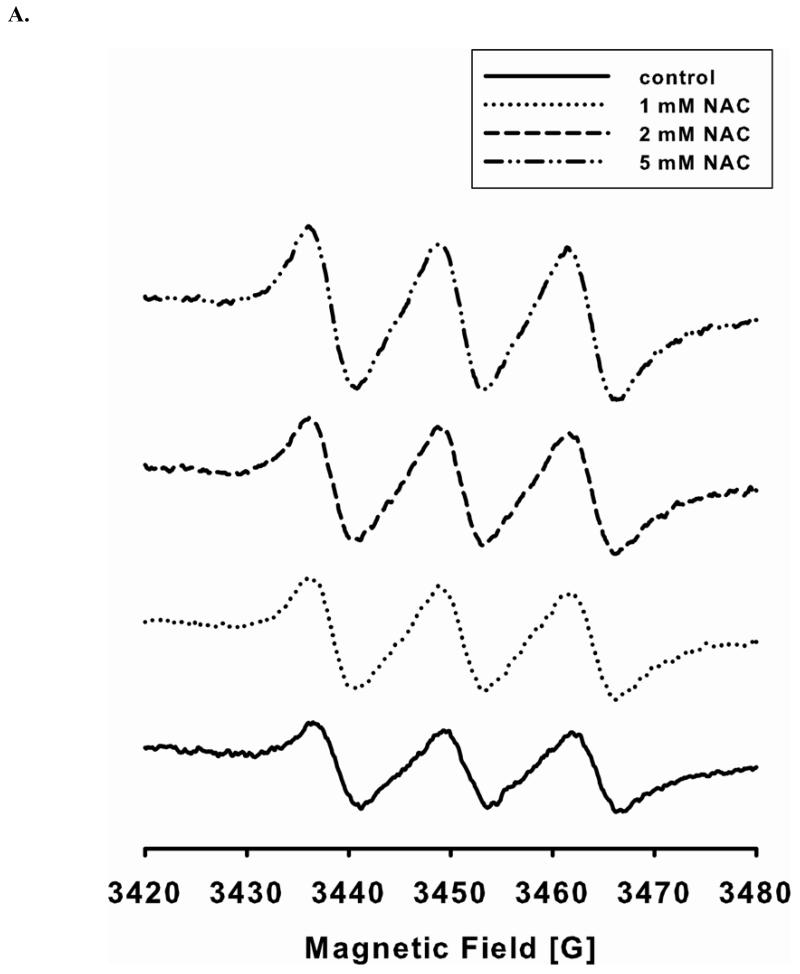
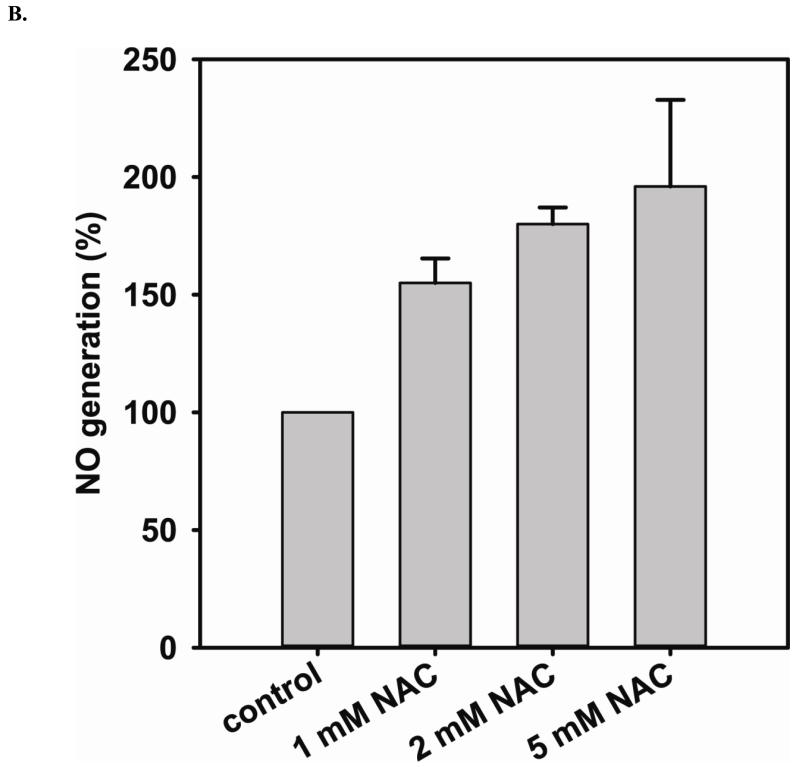
The level of NO generation from endothelial cells with or without NAC treatment is determined by EPR spin-trapping using Fe2+-MGD as the spin trap. Double integration of the EPR signals (Fig 1A) is used to determine the level of NO generation from cells. L-NAME inhibitable EPR intensity is further used to calculate the level of NO generation from eNOS. Long-term (24 hr) treatment of endothelial cells with various concentrations of NAC (1, 2, and 5 mM) leads to a dose-dependent increase in the NO generation from eNOS (Fig 1B). When cells are treated overnight with 5 mM NAC, the level of NO generation increases two fold. Thus, NAC treatment promotes NO production by eNOS in cultured endothelial cells.
FIGURE 1. NAC treatment enhances endothelial cell NO production.
A and B. NO generation from BAECs was measured by EPR spin-trapping using Fe2+-MGD. NAC treatment increases NO generation in a dose-dependent fashion. When cells are treated with 5 mM NAC, the level of NO generation from cells is increased by two-fold compared to untreated. 1 mM L-NAME is used to inhibit intracellular eNOS activity, and it is used as the zero point. Data are expressed as mean ± SEM (P < 0.05 vs. control), n=3.
NAC treatment promotes cellular GSH biosynthesis and prevents BH4 oxidation
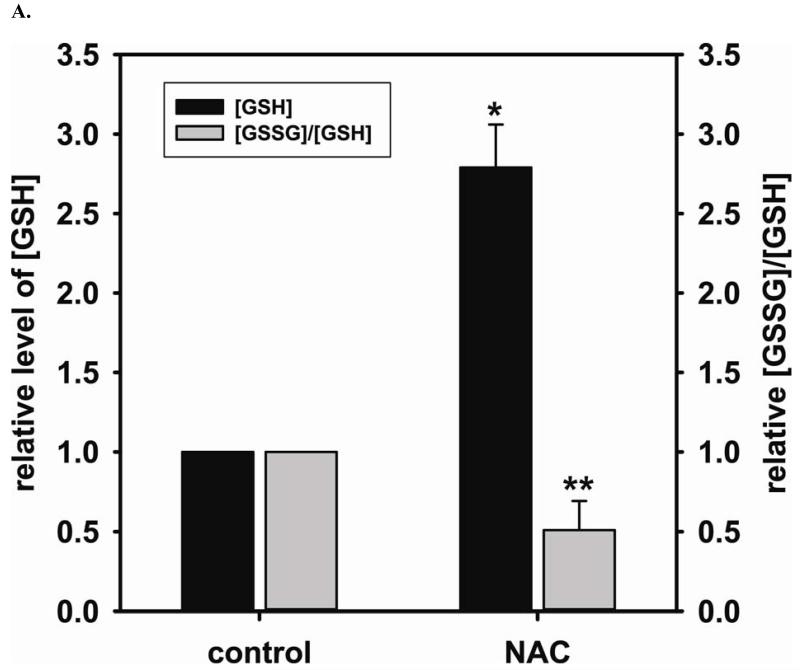
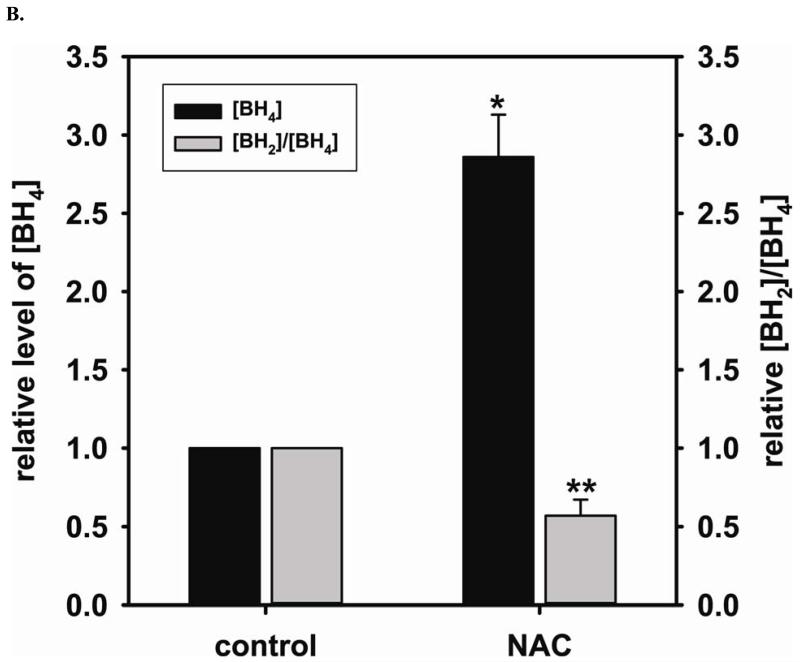
Using EPR spin-trapping we have demonstrated that NAC (5 mM) treatment significantly enhances NO generation from endothelial cells (196 % ± 36.8% compared to control) (Fig 1 A and B). We next measured the cellular level of GSH and the ratio of [GSSG]/[GSH] using HPLC to determine the effect of NAC treatment on the GSH biosynthesis. NAC is a precursor for GSH biosynthesis. It was expected that NAC treatment would augment the cellular level of GSH and, in turn, enhance the cellular reducing environment. Supplementation with 5 mM NAC overnight did indeed increase the intracellular GSH (2.7 ± 0.27 fold change compared to control (2.06 ± 0.68 nmol/mg protein)) and decreases the ratio of [GSSG]/[GSH] (0.51 ± 0.18 fold change compared to control) (Fig 2A), favoring a reducing intracellular environment. BH4 is a critical cofactor for eNOS coupling and NO generation by the enzyme. HPLC measurement of the cellular level of BH4 and the ratio of [BH2]/[BH4] is used to further determine the effect of the cellular level of GSH on the prevention of BH4 oxidation and maintenance of eNOS coupling. Overnight treatment with 5 mM NAC also increases intracellular BH4 (2.86 ± 0.27 fold change compared to control (11.20 ± 3.02 nmol/mg protein)) and decreases the ratio of [BH2]/[BH4] (0.57 ± 0.10 fold change compared to control) (Fig 2B).
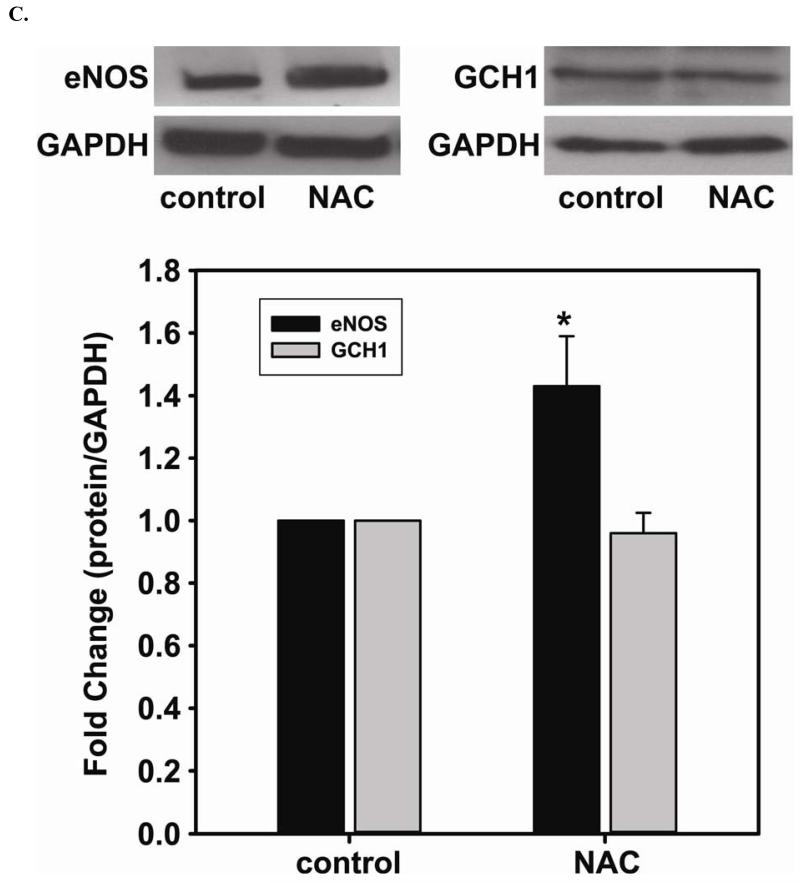
FIGURE 2. The beneficial effects of NAC treatment in BAECs.
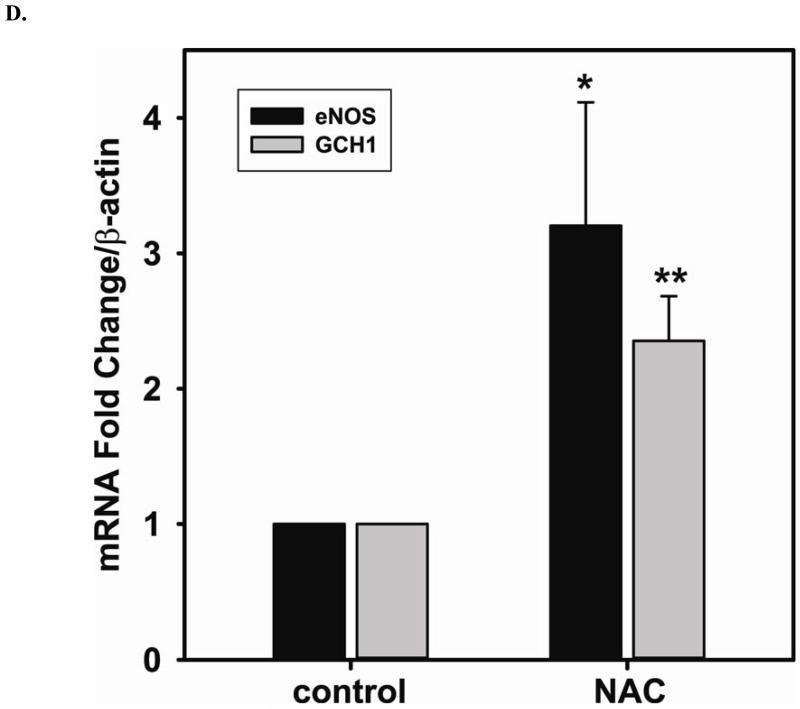
NAC (5 mM) treatment enhances NO generation from cells correlates with an increase in GSH and BH4, a critical cofactor of eNOS. A. 5 mM NAC treatment increases the level of GSH (2.7 ± 0.27 fold compared to the untreated cells (2.06 ± 0.68 nmol/mg protein)), and decreases [GSSG]/[GSH] ratio (0.51 ± 0.18 fold compared to the untreated cells) determined by HPLC (* P < 0.001 versus control and ** P < 0.05 versus control). B. 5 mM NAC treatment increases the level of BH4 (2.86 ± 0.27 fold compared to the untreated cells (11.20 ± 3.02 nmol/mg protein)) and decreases the ratio of [BH2]/[BH4] (0.57 ± 0.10 fold compared to the untreated cells) determined by HPLC. (* P < 0.001 versus control and ** P < 0.05 versus control) C. Left: Upper panel is immunoblot of eNOS. Lower panel is immunoblot of GAPDH as an internal standard. Right: Upper panel is immunoblot of GCH1. Lower panel is immunoblot of GAPDH as an internal standard. NAC treatment up-regulates eNOS expression, but not GCH1, as determined via immunoblotting analysis. 5 mM NAC increases eNOS expression by 1.43 ± 0.16 fold compared to the untreated cells (* P < 0.001 vs. control). D. Relative gene quantification of transcripts for eNOS and GCH1 are up-regulated by NAC treatment of BAECs. Using -actin as the reference gene, and normalizing data to the control cells, relative mRNA for eNOS and GCH1 are all increased by 5 mM NAC treatment (3.21 ± 0.91 fold and 2.35 ± 0.33 fold n ≥ 3;* and ** P < 0.05 versus control, respectively). Data are expressed as mean ± SEM, n ≥ 3.
NAC enhances protein and gene expression of eNOS, but not GCH1 protein expression
To further determine the mechanism underlying the augmented NO generation by NAC, immunoblotting is used to assess the level of eNOS expression before and after 5 mM NAC treatment. 5 mM NAC treatment increases eNOS expression by 1.43 ± 0.16 fold compared to that of the untreated cells (Fig 2C). However, NAC does not change the level of GCH1 protein expression, the rate-limiting enzyme for BH4 biosynthesis. Relative quantification of transcripts for eNOS and GCH1 are both up-regulated by NAC treatment of BAECs (Fig 2D). 5 mM NAC treatment more than doubled the transcription of both proteins (3.21 ± 0.91 fold for eNOS and 2.35 ± 0.33 for GCH1 versus control).
Elevated GSH by NAC treatment promotes DUSP4 expression, protecting against Cd2+-induced oxidative stress
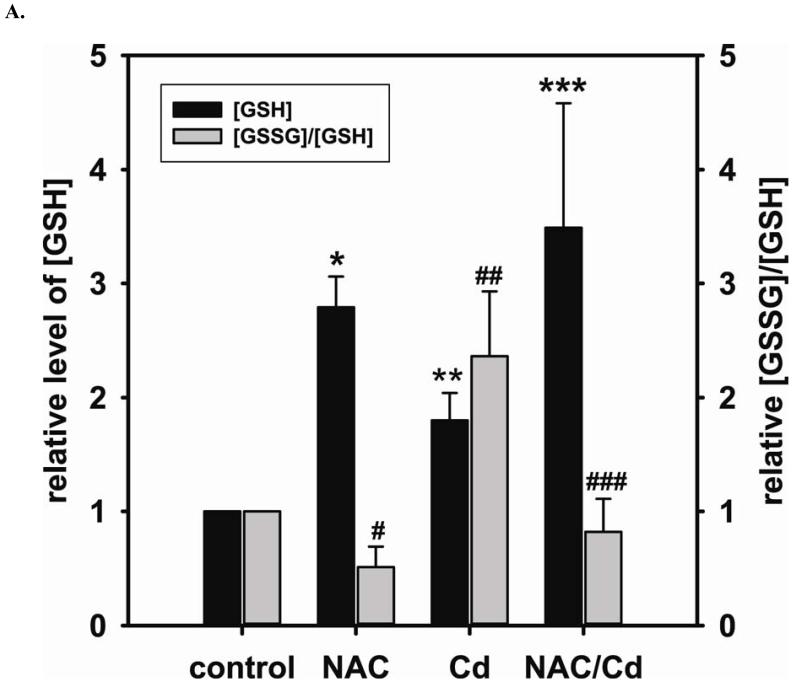
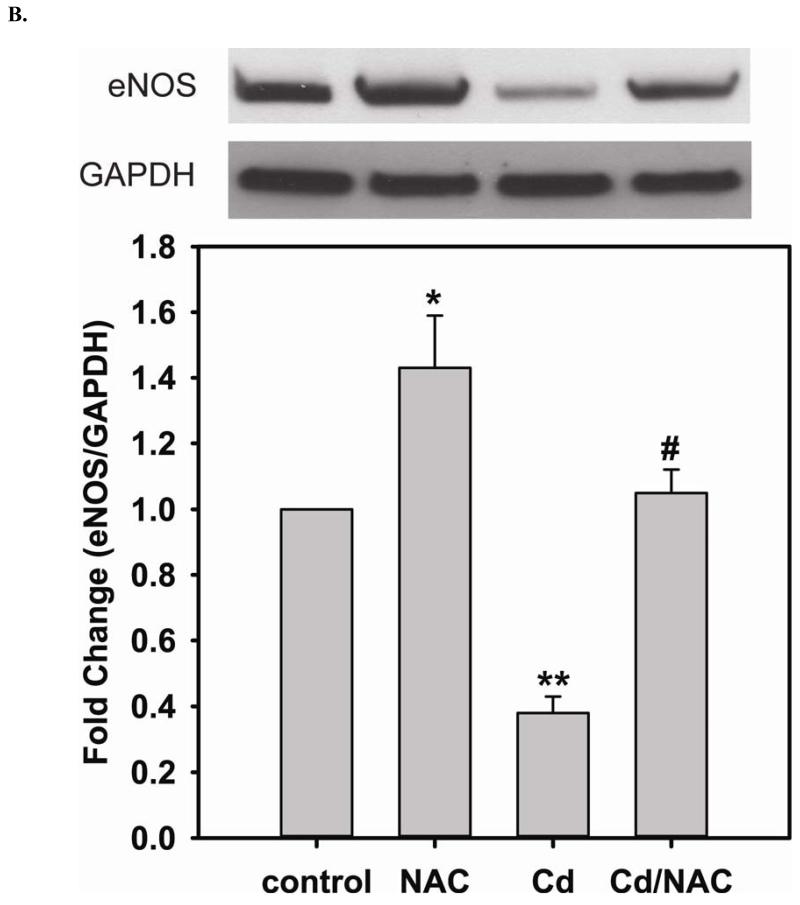
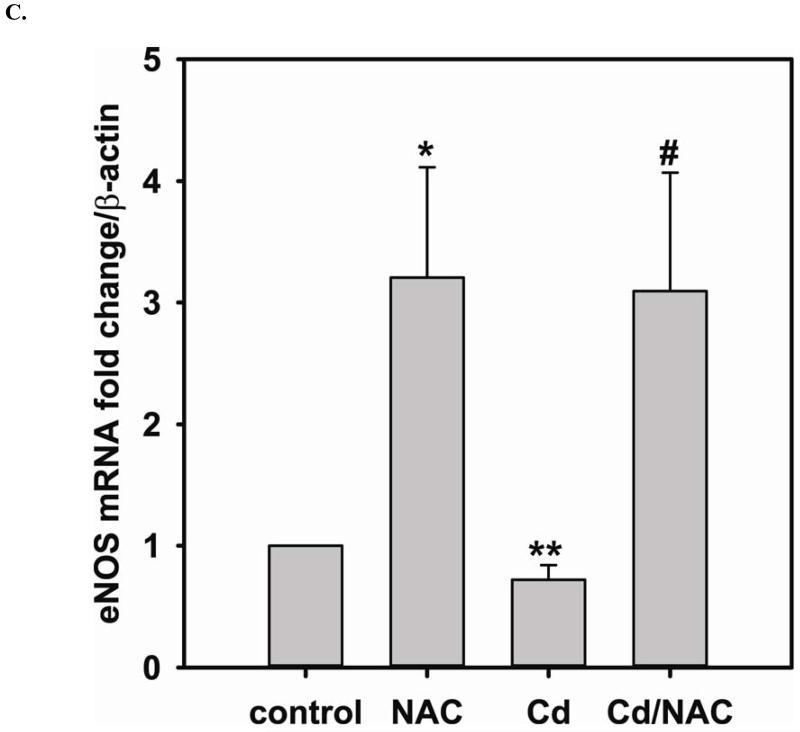
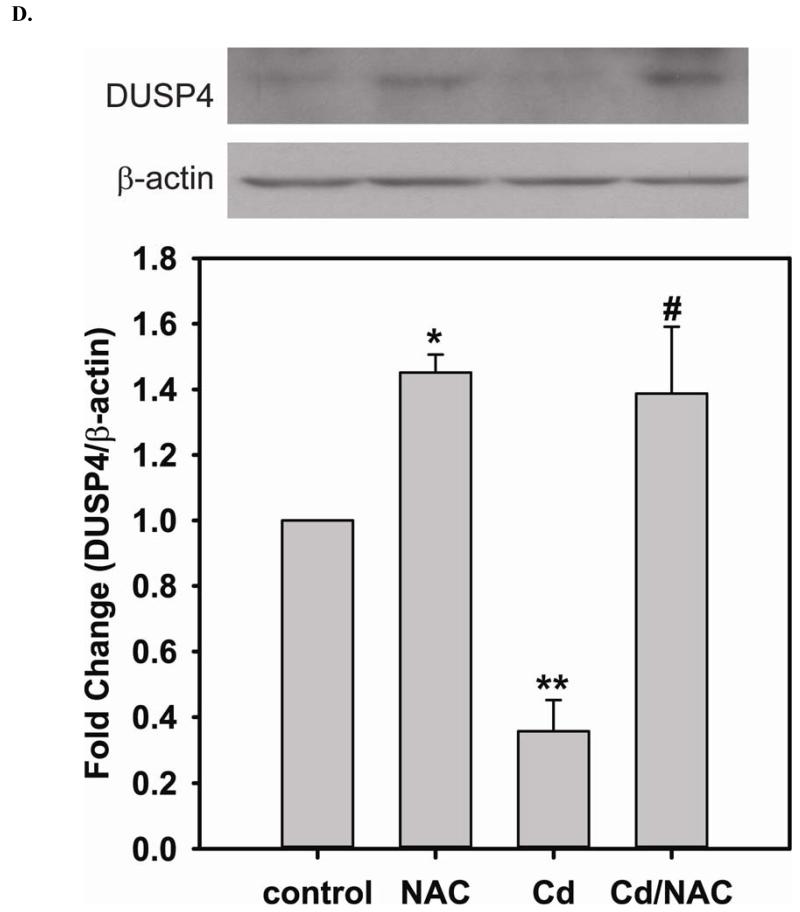
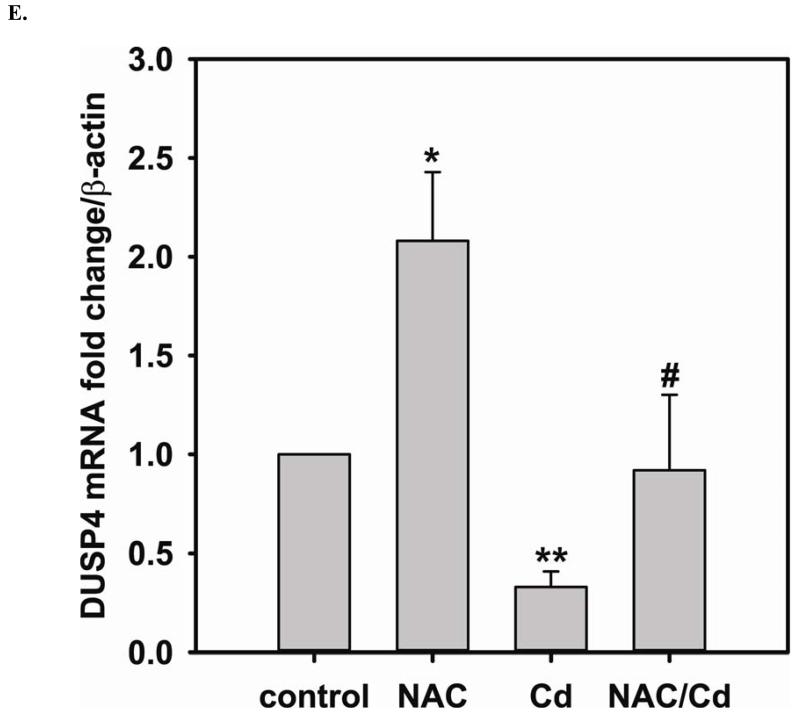
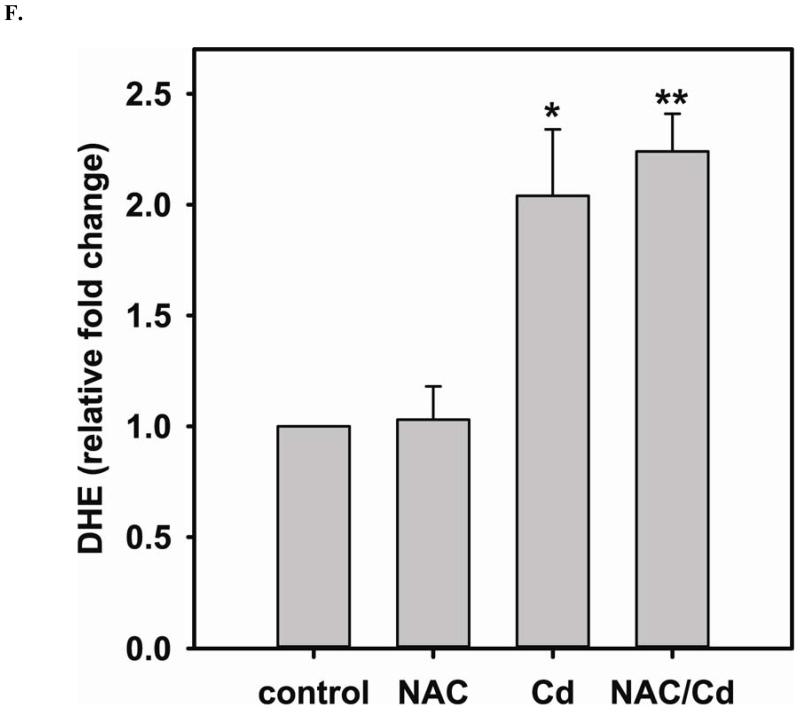
Long-term exposure (24 hr) to Cd2+ (100 μM) leads to an increase in oxidative stress and contributes to an elevated [GSSG] to [GSH] ratio (2.36 ± 0.57 fold change versus control; P < 0.05). Cells treated with 5 mM NAC are protected against Cd2+-induced oxidative stress (ratio of [GSSG]/[GSH] of NAC/Cd2+ versus Cd2+ is 0.82 ± 0.29 and 2.36 ± 0.57, respectively) (Fig 3A). The level of eNOS and DUSP4 expression determined by immunoblotting is assessed to identify the NAC protective mechanism against Cd2+ toxicity. When cells are treated with 5 mM NAC, the level of eNOS expression is increased by 1.43 ± 0.16 fold as seen in the previous section (Fig 3B). When cells are treated overnight with 100 μM Cd2+, the level of eNOS expression decreases (0.38 ± 0.05 fold change versus control). NAC treatment inhibits this Cd2+-induced eNOS degradation, and returns protein expression to basal levels. Transcription of eNOS is affected in a similar manner as protein expression (Fig 3C). Overnight treatment with NAC increases eNOS transcription (3.53 ± 1.1 fold increase versus control), and NAC co-treatment with Cd2+ (3.09 ± 0.98 versus control) is able to rescue the Cd2+-induced loss of eNOS transcript (0.72 ± 0.12 fold of control). With respect to DUSP4 expression, 5 mM NAC treatment up-regulates it, thus providing a beneficial effect (Fig 3D). Cells exposed to 100 μM Cd2+ overnight experienced a degradation of DUSP4 (0.36 ± 0.09 versus control). When cells are co-administered Cd2+ and NAC, DUSP4 is not just protected but actually increased (1.39 ± 0.2 versus control). The increase in DUSP4 expression provides a unique mechanism for cell survival against the toxicity of Cd2+. Similar to the effect on protein expression, NAC doubles DUSP4 mRNA (2.08 ± 0.35 versus control) and Cd2+ decreases it (0.33 ± 0.08 versus control) (Fig 3E). In contrast to the protein effect, co-treatment with NAC and Cd2+ returns DUSP4 mRNA to control level (0.8 ± 0.29 fold change versus control). It is interesting to note that long-term Cd2+ exposure indeed increases superoxide generation when DHE is used as a probe (Fig 3F). However, NAC co-treatment does not diminish Cd2+-induced superoxide generation.
FIGURE 3. Long-term exposure to Cd2+ leads to the degradation of eNOS and DUSP4 while NAC treatment promotes their transcription and prevents protein degradation, providing a protective effect in BAECs.
A. NAC treatment protects endothelial cells from Cd2+-induced oxidative stress via the increase intracellular GSH and the decrease in [GSSG]/[GSH] ratio. The intracellular GSH and the ratio of [GSSG]/[GSH] are determined by HPLC. Long-term Cd2+ exposure dramatically increased intracellular [GSSG]/[GSH] ratio. NAC treatment reversed this oxidative stress. Data are expressed as mean ± SEM, n ≥ 3; P < 0.05 versus control. B. Upper panel is the immunoblotting against eNOS. Lower panel is the immunoblotting for GAPDH, the loading control. Densitometric analysis of the immunoblots reveals that long-term exposure to 100 μM Cd2+ leads to eNOS degradation (0.38 ± 0.05 fold change versus control; ** P < 0.001). NAC treatment prevents this Cd2+-induced eNOS degradation. C. NAC increases eNOS transcription. Overnight treatment with NAC leads to a significant increase in eNOS transcription (3.21 ± 0.91 fold increase versus control; * P < 0.05), and NAC co-treatment with Cd2+ (3.09 ± 0.98 fold versus control; # P < 0.05) was able to rescue the Cd2+-induced degradation in eNOS mRNA (0.72 ± 0.12 fold of control; ** P < 0.01). D. Upper panel is the immunoblotting against DUSP4. Lower panel is the immunoblotting for β-actin, the loading control. Long-term exposure to 100 μM Cd2+ leads to DUSP4 degradation (0.36 ± 0.09 fold change versus control; ** P < 0.05). NAC treatment reverses this Cd2+-induced degradation. All experiments were performed at least in triplicate. E. NAC treatment promotes DUSP4 transcription in endothelial cells. The effect of NAC/Cd2+ treatment on DUSP4 transcription closely mirrored that seen in the protein blotting. NAC doubled DUSP4 mRNA (2.08 ± 0.35 fold versus control; * P < 0.01) whereas Cd2+ less than halved it (0.33 ± 0.08 fold; ** P < 0.001). However, unlike the protein effect, co-treatment with NAC and Cd2+ only returned DUSP4 mRNA to control level. F. Measurement of superoxide from endothelial cells. Cd2+ exposure induced cellular superoxide generation (2.04 ± 0.30 fold versus control; * P < 0.05); however, NAC treatment did not diminish Cd2+-induced superoxide generation (2.24 ± 0.17 fold versus control; ** P< 0.005).
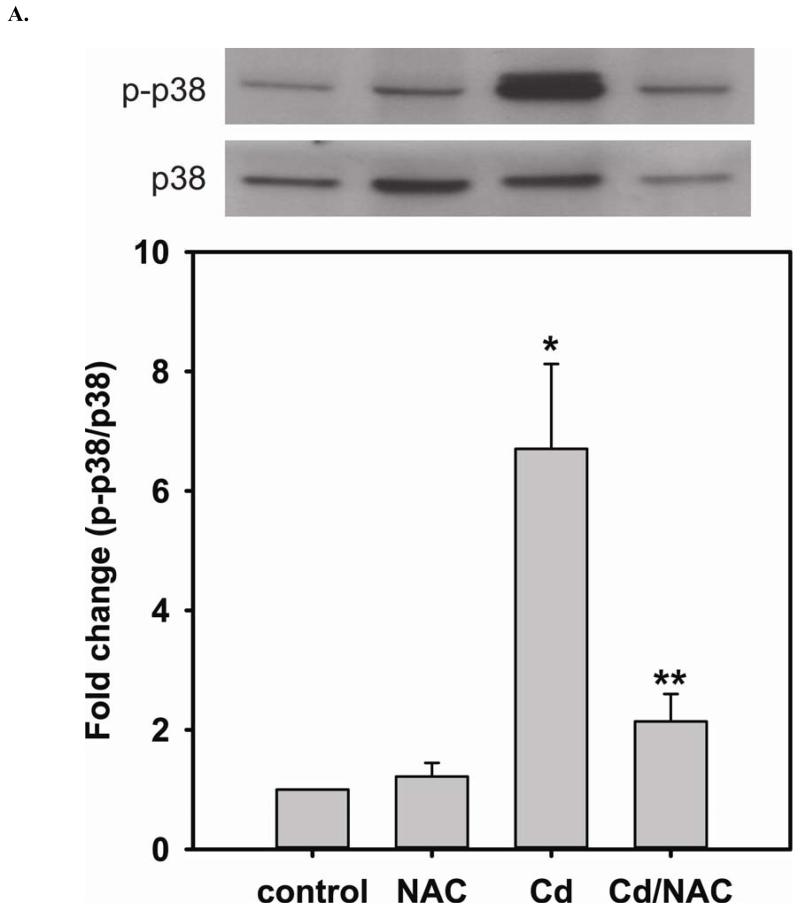
The increase in DUSP4 expression regulates p38 and ERK1/2 signal pathway as a survival mechanism against the toxicity of Cd2+
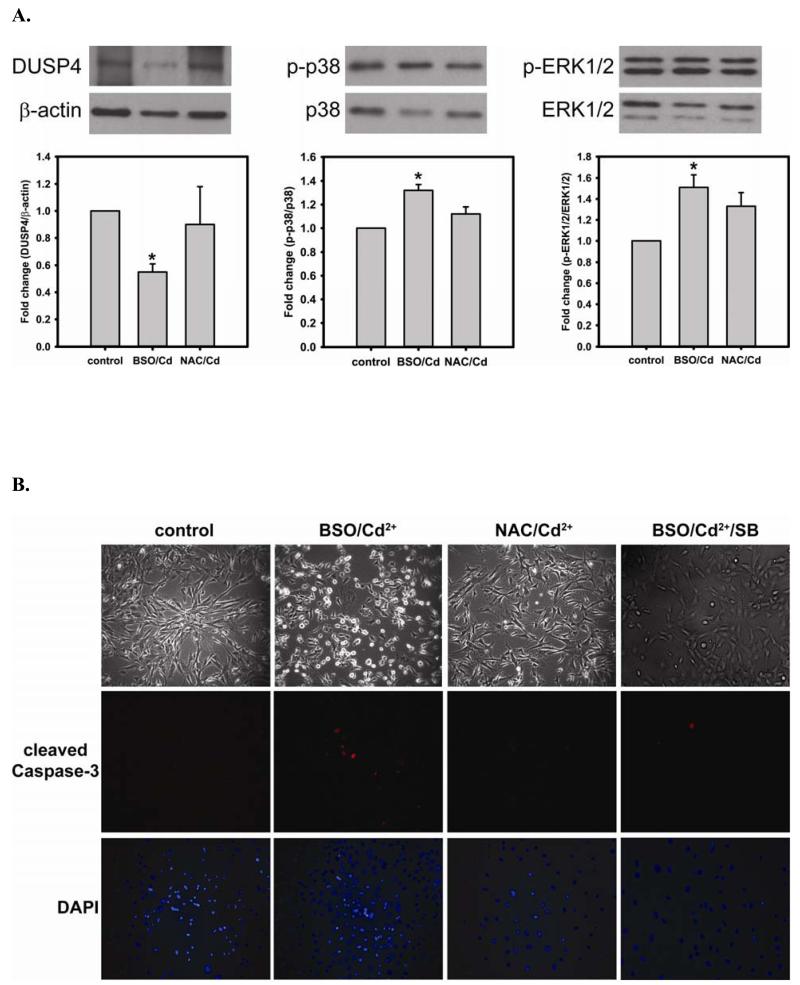
In the previous section, when cells are treated with NAC, the level of DUSP4 expression is increased, and this treatment protects DUSP4 from Cd2+-induced degradation (Fig 3D). DUSP4 has been shown to specifically modulate p38, ERK1/2, or JNK signal pathways depending on the cell type and thus determine the cell fate. When cells are treated with 100 μM Cd2+ overnight, the phosphorylation of p38 is dramatically increased (6.71 ± 1.42 fold change versus control) (Fig 4A). Treatment with NAC enhances the level of DUSP4 expression, as previously discussed. The increased DUSP4 expression, in turn, dephosphorylates p38 preventing it from over-activation and protects cells from Cd2+-induced oxidative stress.
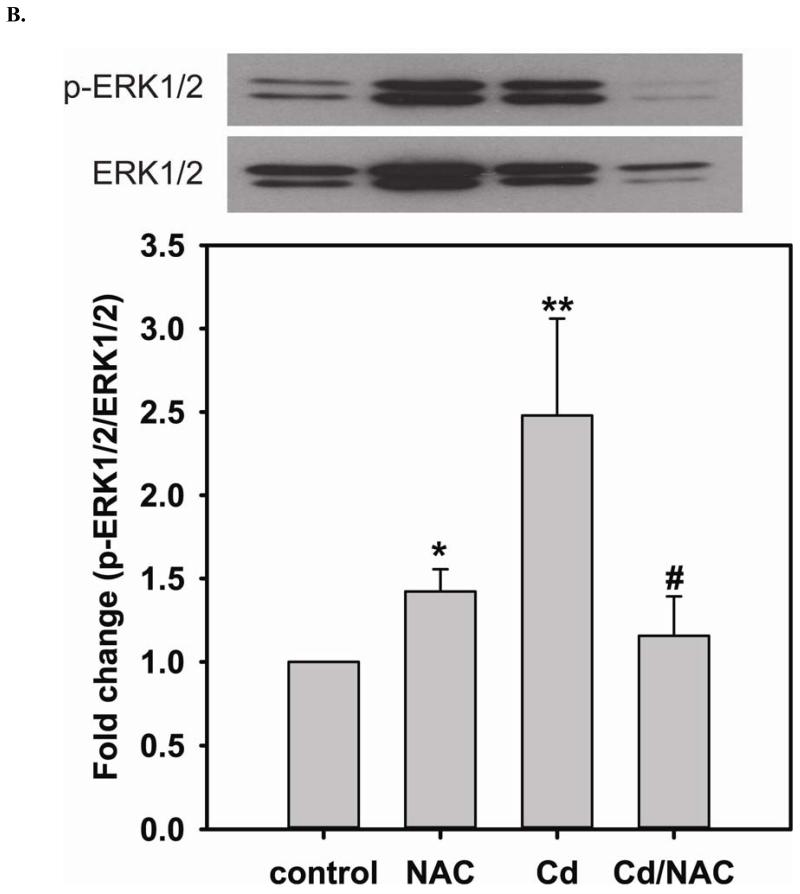
FIGURE 4. NAC treatment prevents Cd2+-induced hyper-phosphorylation of p38 and ERK1/2 in BAECs.
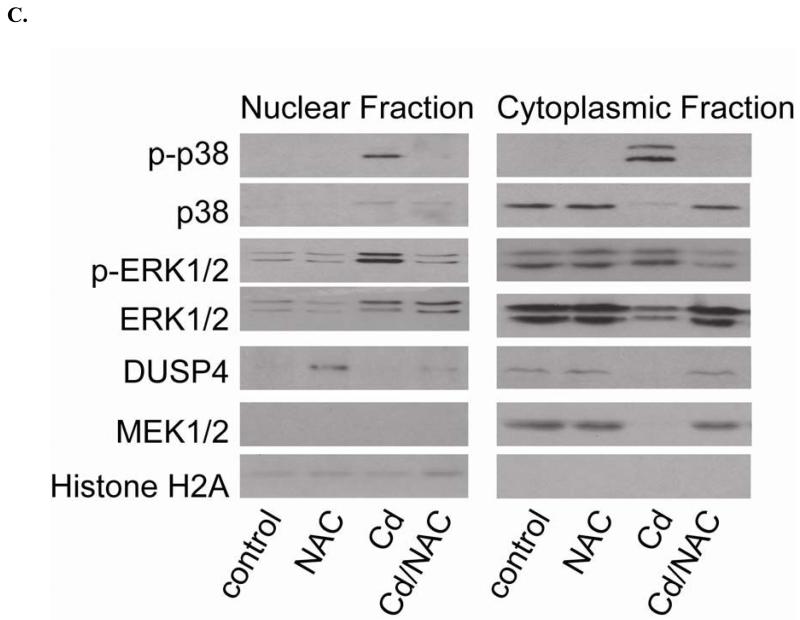
A. Upper panel is the immunoblotting against p-p38. Lower panel is the immunoblotting for p38. The ratio of p-p38/p38 is used to determine the extent of p38 phosphorylation. Cell exposure to 100 μM Cd2+ leads to the hyper-phosphorylation of p38. Co-treatment with 5 mM NAC reverses the phosphorylation of p38. * versus control; P < 0.05, and ** versus Cd2+ treatment; P < 0.05. B. Upper panel is the immunoblotting against p-ERK1/2. Lower panel is the immunoblotting for ERK1/2. The ratio of p-ERK1/2/ERK1/2 is used to determine the extent of ERK1/2 phosphorylation. 5 mM NAC treatment significantly enhances the phosphorylation of ERK1/2. With exposure to 100 μM Cd2+, the phosphorylation of ERK is further increased. Treatment with 5 mM NAC reverses the Cd2+-induced ERK1/2 phosphorylation. * and ** versus control; P < 0.05, and # versus Cd2+ treatment; P < 0.01. Data were expressed as mean ± SEM, n=3. C. Subcellular fractionation. NAC promotes DUSP4 expression in the nucleus, where it can redox regulate p38 and ERK1/2 signaling and serve as a protective mechanism. Left are immunoblots of nuclear fraction, and right are immunoblots of cytoplasmic fraction.
The phosphorylation of ERK1/2 has been considered a cell survival mechanism. When cells are treated with 5 mM NAC, the level of ERK1/2 phosphorylation is increased (1.42 ± 0.13 fold change compared to control) (Fig 4B). The increase in ERK1/2 phosphorylation contributes to cell proliferation, and ultimately enhances the level of eNOS and DUSP4 expression. However, the level of phosphorylation of ERK1/2 is further increased (2.48 ± 0.58 fold change compared to control) when cells are treated with 100 μM Cd2+. NAC treatment prevents DUSP4 degradation, and dephosphorylates ERK1/2, preventing over-activity. It is interesting to note that there is no effect on the JNK pathway when cells are treated with NAC, Cd2+, or Cd2+/NAC (results not shown). This suggests that the increase in DUSP4 expression by NAC regulates both p38 and ERK1/2 pathways and may serve as a protective mechanism against Cd2+-induced oxidative stress.
Subcellular fractionation
In the previous section, it is demonstrated that NAC up-regulates the expression of DUSP4, providing feedback modulation on ERK1/2 and p38 signaling pathways. After treatment, subcellular fractionation (Fig 4C) indicates that NAC induces DUSP4 expression in the nucleus. Nuclear localization of DUSP4 is important for regulation of ERK1/2 and p38, preventing them from over-activation.
DUSP4 gene silencing
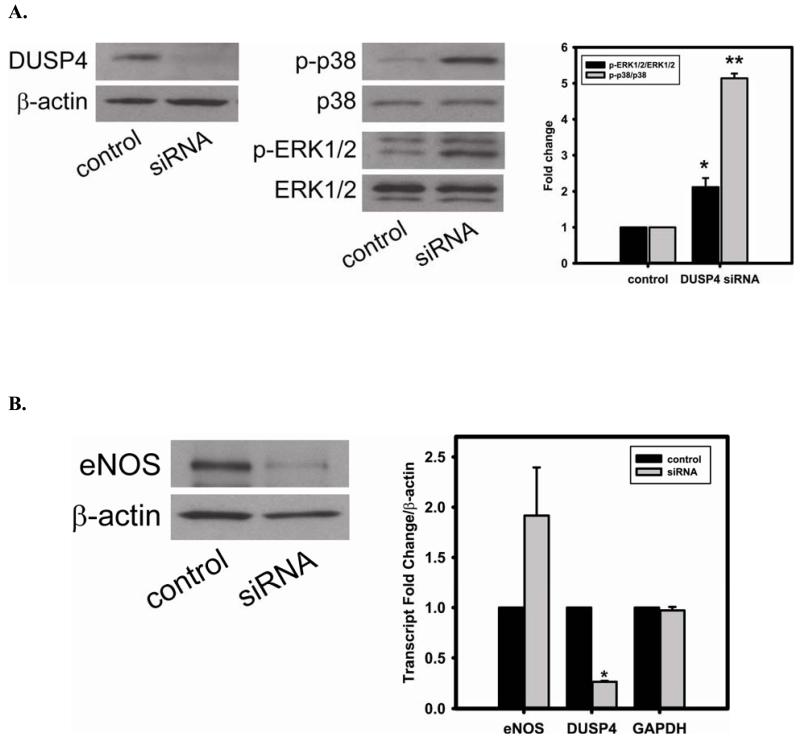
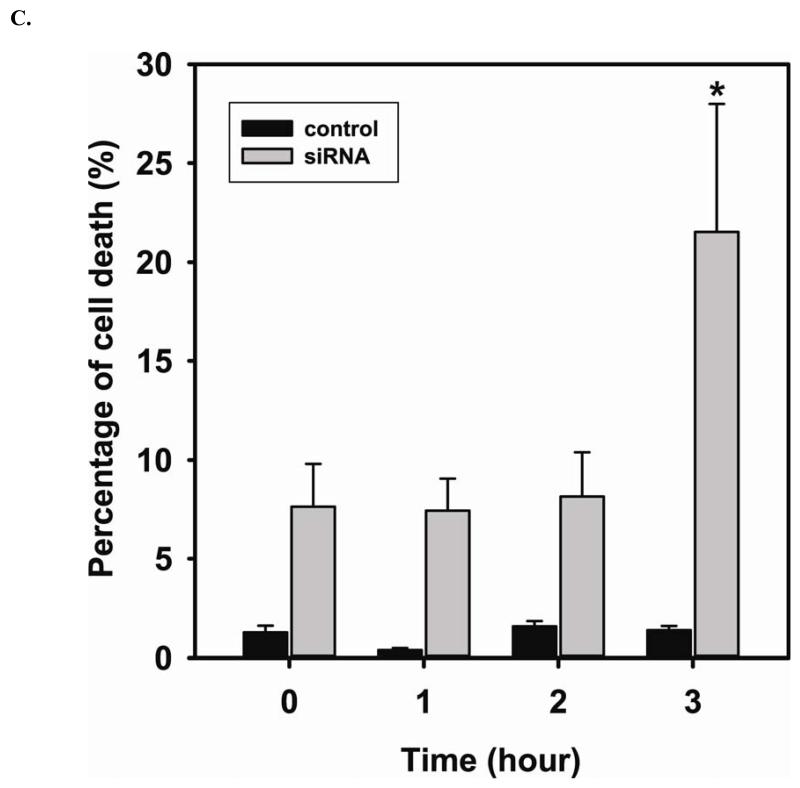
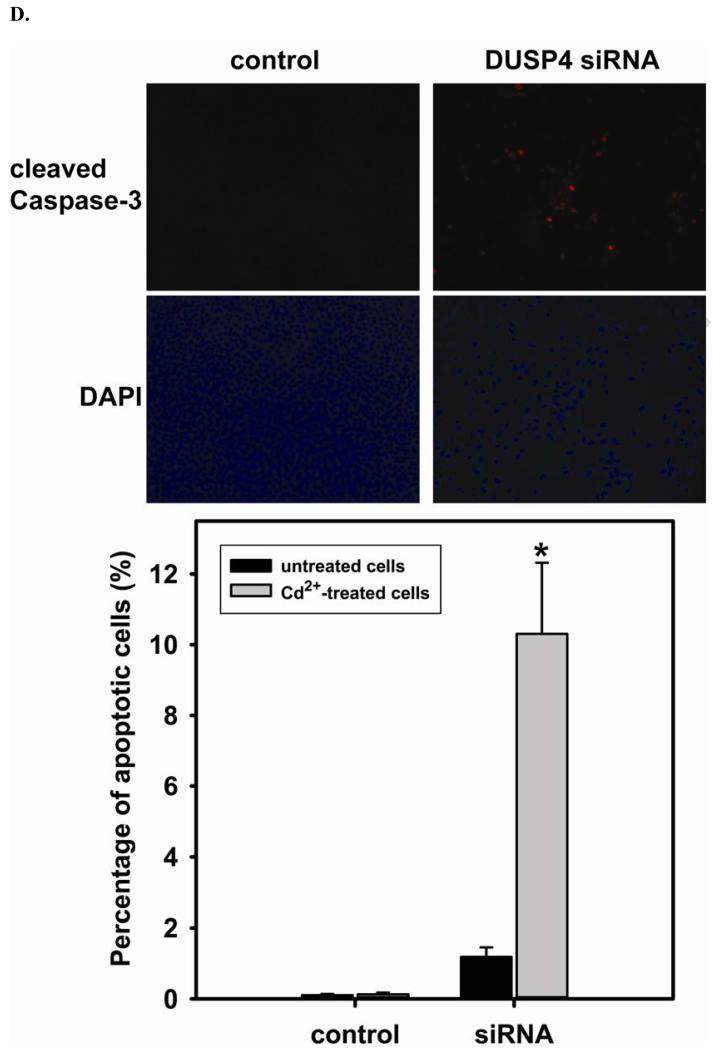
The efficiency of DUSP4 gene silencing is greater than 80%, as determined by immunoblotting with DUSP4 antibody (Fig 5A). This is further confirmed by quantitative mRNA measurement (DUSP4 mRNA of siRNA treated cells is 0.27 ± 0.01 versus control (Fig 5B)). DUSP4 gene silencing is closely associated with the over-activation of p38 and ERK1/2 (Fig 5A). The DUSP4 gene knockdown also leads to a dramatic decrease in eNOS expression (0.53 ± 0.12 versus control; P < 0.05) (Fig 5B). However, because there is no significant change in eNOS mRNA level, this siRNA effect is likely at the translational level. Moreover, endothelial cells are more sensitive to Cd2+-induced death (3 hr exposure) when DUSP4 is silenced (Fig 5C). There is an increase in cleaved caspase-3 positive immunoreactivity seen in cells with DUSP4 gene knockdown compared to control cells after 3 hr Cd2+ exposure (Figure 5D). In negative control, cells are transfected with scrambled RNA. No change in DUSP4 and eNOS expression is seen. This further demonstrates that DUSP4 is an important antioxidant gene, and plays a critical role on eNOS expression.
FIGURE 5. DUSP4-dependent eNOS expression, and the modulation of p38 and ERK1/2 signal cascades in RAECs.
A. DUSP4 gene silencing. Left: The efficiency of DUSP4 gene silencing is greater than 80% as determined by immunoblotting against DUSP4, with β-actin as a loading control. Middle: DUSP4 gene silencing contributes to the over-activation of both p38 (upper panel) and ERK1/2 (lower panel). Right: Fold change of the ratio of p-ERK1/2/ERK1/2 (2.12 ± 0.25 fold versus control; * P < 0.05) and p-p38/p38 (5.14 ± 0.13 fold versus control; ** P < 0.0001). B. DUSP4 gene silencing leads to a decrease in eNOS expression (0.55 ± 0.12 fold versus control; P < 0.05) via the translational modulation. Left: immunoblotting of eNOS and β-actin as a loading control. Right: Quantitative mRNA analysis of eNOS, DUSP4, and GAPDH using β-actin as an internal control. DUSP4 siRNA only affects DUSP4 gene expression (0.27 ± 0.01 fold versus control; * P < 0.0001), but not eNOS or GAPDH. C. Time course of cell death during 3 hr Cd2+ exposure. Rat endothelial cells with DUSP4 knockdown are more susceptible to Cd2+-induced death. Greater cell death is seen in cells with DUSP4 gene silencing compared to control cells after 3 hr Cd2+ exposure (21.54% ± 6.46% versus control; * P < 0.05). D. Immunostaining against cleaved caspase-3. Rat endothelial cells with DUSP4 knockdown are susceptible to Cd2+-induced apoptosis. More cleaved caspase-3 positive is seen in cells with DUSP4 gene silencing compared to control cells after 3 hr Cd2+ exposure (10.31% ± 2.01% versus control; * P < 0.05).
The role of GSH on the function of DUSP4
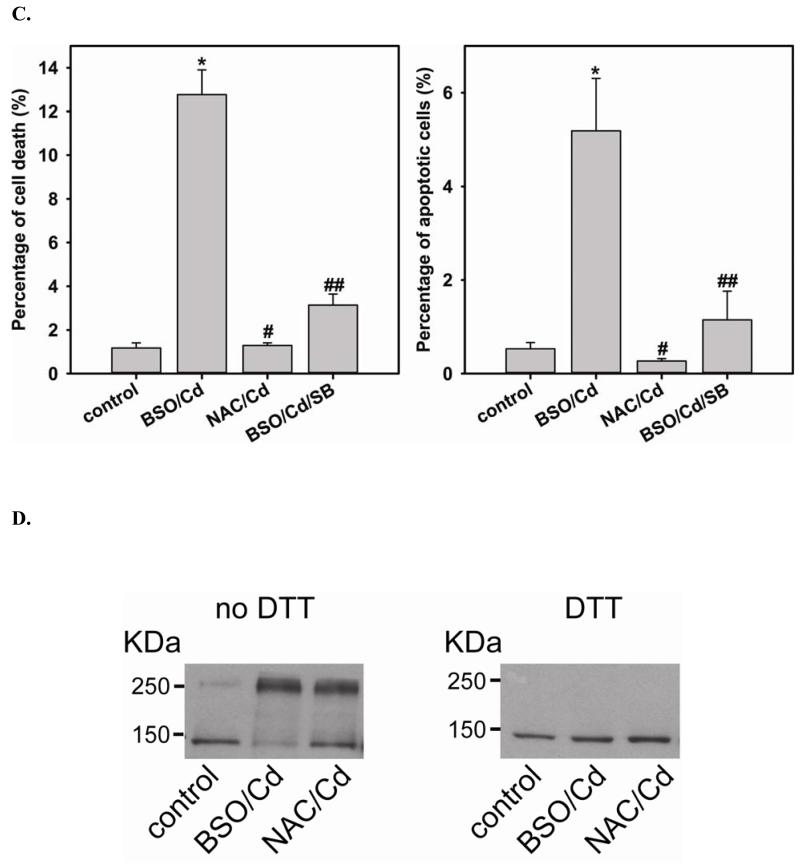
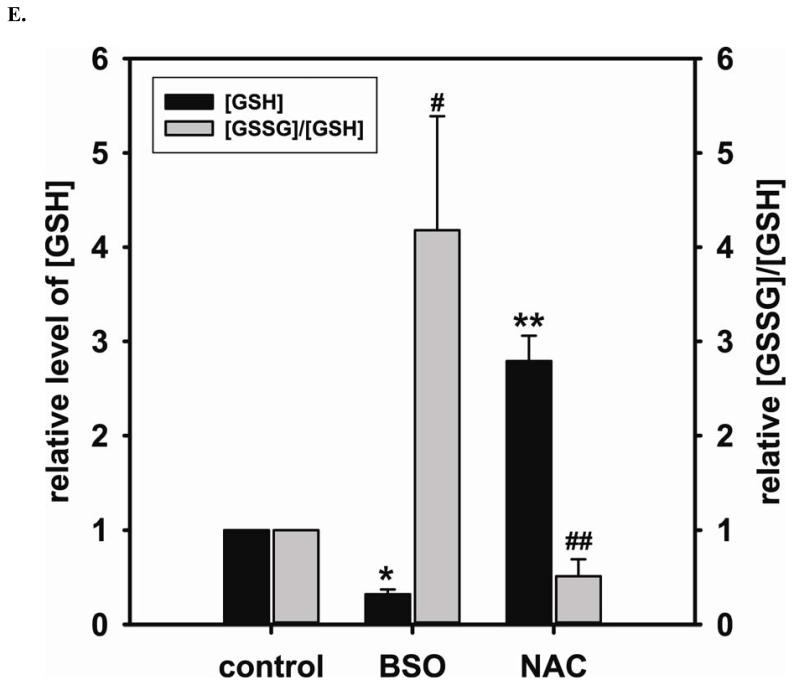
To further demonstrate the critical role of GSH on the function of DUSP4, cells are treated with either BSO or NAC to deplete or elevate intracellular GSH, respectively. After BSO or NAC pre-treatment, cells are then subjected to 2 hr Cd2+-induced oxidative stress. The depletion of cellular GSH leads to DUSP4 degradation and inactivation, which contributes to the over-activation of p38 and ERK1/2 when cells are exposed to Cd2+ (Fig 6A). However, the pre-treatment of NAC prevents DUSP4 inactivation and the over-activation of p38 and ERK1/2 (Fig 6A). Moreover, cells pre-treated with BSO are prone to Cd2+-induced apoptosis, which is demonstrated by immunostaining against cleaved caspase-3 (Fig 6B and C). p38 inhibitor, SB 20358, prevents the phosphorylation of p38 and significantly reverses the Cd2+-induced apoptosis demonstrating that the over-activation of p38 is one of contributing factors of apoptosis. Elevated cellular GSH by NAC also protects cells from Cd2+-induced apoptosis. Under the non-reducing condition of immunoblotting analysis of eNOS, the depletion of GSH by BSO contributes to the formation of the inter-disulfide bond of eNOS after 2 hr Cd2+ exposure (Fig 6D), which led to the inactivation of eNOS demonstrated in our previous study [34]. The formation of eNOS inter-disulfide bonds is further demonstrated by DTT treatment, resulting in the monomeric form. Furthermore, the pre-treatment of NAC can prevent the aggregation of eNOS via reversal of thiol oxidation, maintaining normal endothelial function. These results are correlated with the change in the ratio of [GSSG]/[GSH] after treatment (Fig 6E). BSO not only depletes cellular GSH, but also increases the [GSSG]/[GSH] ratio. However, NAC increases the total GSH level and decreases [GSSG]/[GSH], favoring DUSP4 and eNOS in their reduced conformation.
FIGURE 6. Intracellular GSH is important for DUSP4 stability and activity, and protects BAECs against short-term Cd2+-induced apoptosis.
A. Left: Immunoblots of DUSP4 and β-actin as a loading control. Depletion of GSH by BSO increases the Cd2+-induced DUSP4 degradation (0.55 ± 0.06 fold versus control; * P < 0.005). NAC pre-treatment prevents this. Middle: Immunoblots of p-p38 and p38. Depletion of GSH by BSO leads to the over-activation of p38 (1.32 ± 0.05 fold versus control; * P < 0.005). Right: Immunoblots of p-ERK1/2 and ERK1/2. Depletion of GSH by BSO also leads to the over-activation of ERK1/2 (1.51 ± 0.12 fold versus control; * P < 0.05). B. Upper panel is the live cell image using Zeiss Axiovert 135 microscope. When intracellular GSH is depleted by BSO, cells become hyper-sensitive to 2hr Cd2+-induced death. 5 mM NAC pre-treatment protects cells against Cd2+-induced death. Middle panel is the immunostaining against cleaved caspase-3. GSH depletion increases apoptosis. NAC pre-treatment prevents cells from Cd2+-induced apoptosis. Addition of 10 μ SB 203580, a p38 inhibitor, significantly reverses this Cd2+-induced apoptosis. Lower panel is DAPI nuclear staining. C. Left: Percentage of cell death. NAC pretreatment protects endothelial cells from Cd2+-induced death. Addition of SB 203580 significantly protects cells from this oxidative stress. (12.77% ± 1.13%; * P < 0.001 versus control; 1.28% ± 0.12% and 3.13% ± 0.51%, respectively; # and ## P < 0.001 versus BSO/Cd). Right: Percentage of apoptotic cells. Depletion of GSH by BSO increases apoptosis (5.19% ±1.1%; *P < 0.01, versus control). This process can be reversed by either the pre-treatment of NAC or addition of p38 inhibitor, SB 203580 (0.27% ± 0.05% and 1.15% ± 0.61%; # and ## P < 0.01, versus BSO/Cd). D. Depletion of GSH by BSO increases cellular oxidative stress contributing to eNOS inter-disulfide bond formation. Left: Non-reduced immnuoblotting against eNOS shows that BSO increases eNOS dimerization via inter-disulfide bond formation, and NAC pre-treatment prevents this thiol oxidation. Right: Incubation with DDT shows eNOS is in its monomer form. E. Cellular level of GSSG and GSH measured by HPLC. BSO inhibits GSH synthesis (0.32 ± 0.05 fold versus control, * P < 0.001), and enhances intracellular [GSSG]/[GSH] ratio (4.18 ± 1.21 fold versus control, # P < 0.05). NAC promotes GSH synthesis (2.79 ± 0.27 fold versus control, ** P < 0.001), and lowers the ratio of [GSSG]/[GSH] (0.51 ± 0.18 fold versus control, ## P < 0.05).
DISCUSSION
Glutathione is the most abundant small molecule and the primary reducing source in cells. An increase in GSSG contributes to redox imbalance leading to an increase in protein S-glutathionylation. This oxidative modification plays an important role in redox signaling, and is reversed by glutaredoxin using GSH as a reducing equivalent [38, 39]. We previously demonstrated that an increase in [GSSG]/[GSH] ratio under oxidative stress can switch the function of glutaredoxin from de-glutathionylation to glutathionylation, affecting the redox state which, in turn, regulates eNOS and endothelial function [34]. As such, restoring the cellular level of GSH is pivotal for maintaining endothelial function. In this study, we demonstrate that NAC supplementation in endothelial cells enhances the cellular level of GSH and up-regulates the expression of DUSP4, a redox-sensitive phosphatase, and modulates MAPK signaling cascades. MAPK signaling pathways have been shown to be involved in regulating several cellular functions, such as differentiation, proliferation, and apoptosis in response to extracellular stimuli [23, 40, 41]. Therefore, the identification of DUSP4 activation by NAC provides an initial step toward understanding the specific mechanism of the beneficial effects of NAC treatment against ED.
Previously, it was proposed that the mechanism of NAC-stimulated cell survival is through the stimulation of the ERK1/2 pathway, which activates multiple transcription factors and genes implicated in cell growth and survival [14]. More importantly, the extent and duration of phosphorylation of these MAPKs are the critical factors in determining their physiological effects [22, 23, 42]. Thus, the imperative question remains: what is the exact mechanism via which NAC treatment modulates signaling pathways to favor cell survival? In this study, we demonstrate that the administration of NAC stimulates the activation of ERK1/2 pathway, which in turn can elevate the expression of several important proteins involved in endothelial function such as eNOS and DUSP4. The increase in DUSP4 expression in endothelial cells can further modulate ERK1/2 signal, preventing it from prolonged activation and promoting cell growth [22, 23]. Because DUSP4 possesses an active cysteine in its catalytic site, it is believed to be a redox sensitive phosphatase regulated by GSH [28]. When endothelial cells were treated with NAC, the increase in the cellular level of GSH not only up-regulates DUSP4 expression through transcription but also redox-regulates DUSP4 activity, through which it can modulate the ERK1/2 pathway for cell survival.
The hallmark of ED is a decline in endothelial NO bioavailability. This decrease in NO is due to increased ROS formation that directly reacts with NO or indirectly affects NO generation by NOS [6, 17-19, 33, 34]. Short-term NAC treatment improved endothelial function possibly through its antioxidant effect by scavenging ROS formation [14, 43]. In this study, a long-term NAC treatment is conducted to determine the specific mechanism that is regulated by NAC and the beneficial effect for endothelial function. Long-term (24 hr) treatment with 5 mM NAC indeed increases the cellular level of GSH, and BH4, and up-regulates eNOS expression. This increase is correlated with an increase in endothelial NO production. We have previously demonstrated that, under oxidative stress, the increase in GSSG led to the formation of eNOS S-glutathionylation and inter-disulfide bond formation, thus affecting NO output [6, 33]. Therefore, the increase of intracellular GSH by NAC maintains the reducing state of eNOS, enhances its enzymatic activity and NO generation. It is interesting to note that NAC treatment does not alter expression of GCH1, a rate-determining enzyme for BH4 biosynthesis. However, HPLC analysis reveals that the cellular level of BH4 is dramatically improved when endothelial cells are treated with 5 mM NAC. Therefore, the elevation in the cellular BH4 level is primarily due to the increase in GSH and the antioxidant properties of NAC, which protects against BH4 oxidation, and ultimately improves endothelial function through enhanced eNOS coupling.
Cadmium (Cd2+) exposure is associated with many oxidant-induced diseases [44, 45]. Cd2+, a heavy metal, has high affinity for sulfhydryl groups in proteins, and can inactivate numerous thiol-containing enzymes, including glutaredoxin [46]. Our prior study demonstrated that in endothelial cells, short-term Cd2+ exposure led to direct inactivation of the glutaredoxin function and contributed to the accumulation of protein S-glutathionylation, including eNOS S-glutathionylation [34]. This oxidative modification is one of the major contributing factors for ED, and is reversible with the restoration of the cellular GSH level [47, 48]. Several studies showed that NAC administration can protect against Cd2+-induced toxicity [45, 49]. However, the mechanism of this protection is still unclear and appears to be multifactorial. Our current results demonstrate that NAC treatment in the endothelial cells protects DUSP4 and eNOS from Cd2+-induced thiol oxidation and degradation. It is interesting to note that NAC co-treatment does not diminish the Cd2+-induced superoxide generation, indicating that its mechanism of action is not merely as a direct antioxidant. Moreover, when endothelial cells are exposed long-term to Cd2+, the level of DUSP4 is significantly reduced, contributing to the hyper-phosphorylation of ERK1/2 and p38. Because DUSP4 becomes depleted, it is unable to modulate ERK1/2 and p38 signal cascades, and this is the primary factor leading to the sustained phosphorylation of both kinases. The prolonged phosphorylation of p38 and ERK1/2 ultimately contributes to cell death [50]. With NAC treatment, DUSP4 degradation is inhibited, and is thus able to kinetically modulate the extent and duration of phosphorylation of both kinases, preventing cell death. It is interesting to note that NAC induces DUSP4 expression in the nucleus, where DUSP4 can specifically regulate p38 and ERK1/2 phosphorylation and prevent hyper-phosphorylation. Interestingly, no DUSP1 protein degradation is seen in endothelial cells treated with Cd2+ (data not shown).
A previous study showed that overexpression of DUSP4 in human endothelial cells enhances adhesion molecule expression and protects against apoptosis [51]. Furthermore, DUSP4 gene deletion in mouse embryonic fibroblasts revealed that DUSP4 plays an important role in proliferation and cell survival [52]. A more recent study demonstrated that the combined disruption of DUSP1/4 promotes unrestrained p38 activity in both mouse embryonic fibroblast and heart [42]. Our results of DUSP4 gene silencing from rat endothelial cells reveals that DUSP4 plays a critical role in modulating activities of p38 and ERK1/2, especially p38. More importantly, DUSP4 gene silencing affects eNOS protein, but not mRNA, expression. This further suggests that DUSP4 is important in modulation of eNOS expression via translational control. Moreover, cells are significantly more sensitive to Cd2+-induced death, when DUSP4 is silenced. Together with these results, it supports our hypothesis that DUSP4 is an important anti-oxidant gene, and plays a critical role in regulating endothelial function.
The restoration of cellular GSH level is considered the primary beneficial effect of NAC against oxidant-derived diseases [15, 53]. This effect is further demonstrated via the pre-treatment with NAC and BSO, an inhibitor for GSH biosynthesis, in response to Cd2+-induced oxidative stress. Depletion of cellular GSH by BSO sensitizes cells to Cd2+-induced apoptosis via uncontrolled p38 phosphorylation, which is further supported by p38 inhibition. Pre-treatment with NAC protects cells from this Cd2+-induced apoptosis. The increase in the ratio of [GSSG]/[GSH] by BSO induces eNOS dimerization via the formation of inter-disulfide bonds in response to Cd2+ exposure. This Cd2+-induced eNOS dimerization is prevented by NAC pre-treatment. This further demonstrates that the ratio of [GSSG]/[GSH] is a critical factor affecting the stability and function of redox sensitive proteins, such as DUSP4 and eNOS. Thus, elevated cellular GSH and activation of DUSP4 by NAC pre-treatment can be a better therapeutic strategy against oxidant-derived diseases.
Conclusions
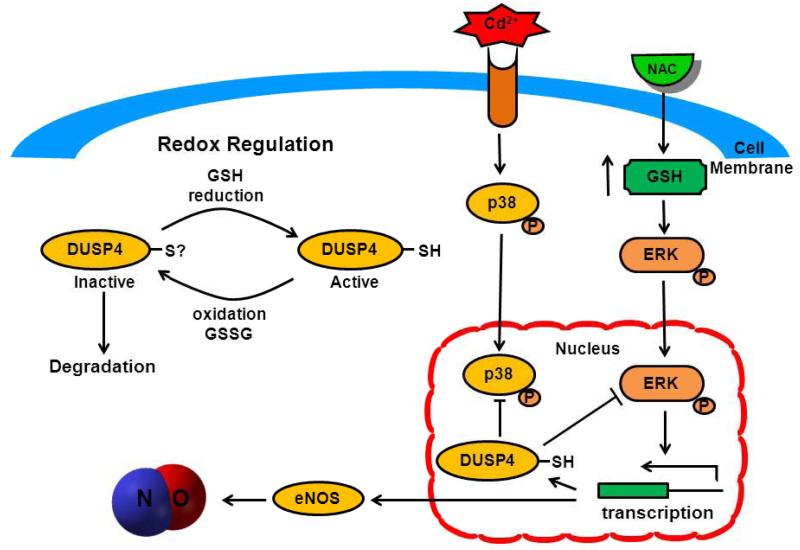
NAC treatment in endothelial cells first promotes ERK1/2 activation and subsequently activates several transcription factors, thus increasing eNOS and DUSP4 gene and protein expression (Fig 7). NAC treatment also augments intracellular GSH concentration, which redox modulates DUSP4 function, and maintains it in its active form. The active form of DUSP4 can further provide a feedback regulation of ERK1/2 signaling and prevent it from over-activation. In endothelial cells, however, Cd2+-induced oxidative stress coupled with a rise in GSSG can lead to the increase in oxidative modification of many critical proteins involved in vascular function. Without the restoration of GSH levels, these modified proteins, including eNOS and DUSP4, will be targeted for degradation. Moreover, long-term Cd2+ exposure also activates both p38 and ERK1/2 signal pathways. With the increase in oxidative stress, DUSP4 becomes depleted, resulting in an over-activation of p38 and ERK1/2 and deleterious effects on the cell. By restoring the cellular level of GSH, NAC is able to prevent DUSP4 inactivation and degradation, which contributes to the unrestrained activation of p38 and ERK1/2. Therefore, the identification of DUSP4 activation by NAC provides a novel target for future drug design based on NAC.
FIGURE 7. Mechanism of the beneficial effects of NAC treatment.
ERK1/2 activation by NAC promotes transcription leading to the overexpression of DUSP4 and eNOS. NAC also enhances intracellular GSH, which can maintain eNOS in its reduced state and DUSP4 in its active form, and prevent BH4 from oxidation. Therefore, NAC treatment increases NO generation from cells providing a beneficial effect. The increase in DUSP4 expression actively regulates ERK1/2 signaling, thus prevents it from over-activation. Cd2+ induces oxidative stress and with the increase in cellular level of GSSG, which can contribute to protein oxidative modification and degradation. The degradation of DUSP4 leads to the hyper-activation of p38 and ERK1/2, which ultimately induces apoptosis.
Acknowledgments
This work was supported by R00 Grant HL103846 (C.-A. C.) from the National Institutes of Health.
List of Abbreviations
- NAC
N-Acetyl cysteine
- BAECs
Bovine aortic endothelial cells
- BSO
Buthionine sulfoximine
- Cd2+
Cadmium
- DUSP4/MAPK2
Dual-specificity phosphatase 4 or MAPK phosphatase 2
- ED
Endothelial dysfunction
- eNOS
Endothelial Nitric Oxide Synthase
- EPR
Electron paramagnetic resonance
- GSH
Glutathione
- GSSG
Glutathione disulfide
- HPLC
High-performance liquid chromatography
- JNKs
c-Jun N-terminal kinases
- MAPK
Mitogen-activated protein kinase
- OPA
o-phthalaldehyde
- RAECs
Rat aortic endothelial cells
- ROS
Reactive oxygen species
- BH4
Tetrahydrobiopterin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Archiv : European journal of physiology. 2010;459:32–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- [2].Xiong Y, Uys JD, Tew KD, Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxidants & redox signaling. 2011;15:32–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sabens Liedhegner EA, Gao XH, Mieyal JJ. Mechanisms of altered redox regulation in neurodegenerative diseases--focus on S--glutathionylation. Antioxidants & redox signaling. 2012;16:32–566. doi: 10.1089/ars.2011.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxidants & redox signaling. 2011;14:32–1775. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- [6].Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:32–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:32–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- [8].Ghezzi P. Regulation of protein function by glutathionylation. Free Radic Res. 2005;39:32–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- [9].Qanungo S, Starke DW, Pai HV, Mieyal JJ, Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. The Journal of biological chemistry. 2007;282:32–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- [10].Gallogly MM, Starke DW, Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxidants & redox signaling. 2009;11:32–1081. doi: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830:32–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- [12].Millea PJ. N-acetylcysteine: multiple clinical applications. American family physician. 2009;80:32–269. [PubMed] [Google Scholar]
- [13].Nigwekar SU, Kandula P. N-acetylcysteine in cardiovascular-surgery-associated renal failure: a meta-analysis. The Annals of thoracic surgery. 2009;87:32–147. doi: 10.1016/j.athoracsur.2008.09.026. [DOI] [PubMed] [Google Scholar]
- [14].Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cellular and molecular life sciences: CMLS. 2003;60:32–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovascular research. 2000;47:32–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- [16].Sochman J. N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know?! Journal of the American College of Cardiology. 2002;39:1422–1428. doi: 10.1016/s0735-1097(02)01797-7. [DOI] [PubMed] [Google Scholar]
- [17].Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:32–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- [19].Wolin MS. Reactive oxygen species and vascular signal transduction mechanisms. Microcirculation. 1996;3:32–17. doi: 10.3109/10739689609146778. [DOI] [PubMed] [Google Scholar]
- [20].Li WQ, Dehnade F, Zafarullah M. Thiol antioxidant, N-acetylcysteine, activates extracellular signal-regulated kinase signaling pathway in articular chondrocytes. Biochemical and biophysical research communications. 2000;275:32–794. doi: 10.1006/bbrc.2000.3385. [DOI] [PubMed] [Google Scholar]
- [21].Hashimoto S, Gon Y, Matsumoto K, Takeshita I, Horie T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. British journal of pharmacology. 2001;132:32–276. doi: 10.1038/sj.bjp.0703787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Roskoski R., Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacological research : the official journal of the Italian Pharmacological Society. 2012;66:32–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- [23].Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiological reviews. 2010;90:32–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. Journal of cell science. 2006;119:32–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- [25].Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. The Biochemical journal. 2009;418:32–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- [26].Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nature reviews. Drug discovery. 2007;6:32–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- [27].Pfeffer U, Ferrari N, Dell’Eva R, Indraccolo S, Morini M, Noonan DM, Albini A. Molecular mechanisms of action of angiopreventive anti-oxidants on endothelial cells: microarray gene expression analyses. Mutation research. 2005;591:32–211. doi: 10.1016/j.mrfmmm.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [28].Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2803–2812. doi: 10.1073/pnas.1212596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rapoport RM, Draznin MB, Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306:32–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- [30].Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:32–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- [31].Hunley TE, Iwasaki S, Homma T, Kon V. Nitric oxide and endothelin in pathophysiological settings. Pediatr Nephrol. 1995;9:32–244. doi: 10.1007/BF00860758. [DOI] [PubMed] [Google Scholar]
- [32].Pechanova O, Zicha J, Kojsova S, Dobesova Z, Jendekova L, Kunes J. Effect of chronic N-acetylcysteine treatment on the development of spontaneous hypertension. Clin Sci (Lond) 2006;110:32–242. doi: 10.1042/CS20050227. [DOI] [PubMed] [Google Scholar]
- [33].Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. The Journal of biological chemistry. 2011;286:32–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen CA, De Pascali F, Basye A, Hemann C, Zweier JL. Redox Modulation of Endothelial Nitric Oxide Synthase by Glutaredoxin-1 through Reversible Oxidative Post-Translational Modification. Biochemistry. 2013;52:32–6723. doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Analytical biochemistry. 2000;280:80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- [36].Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, Heales S, Channon KM. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovascular research. 2002;55:32–849. doi: 10.1016/s0008-6363(02)00460-1. [DOI] [PubMed] [Google Scholar]
- [37].Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:32–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxidants & redox signaling. 2005;7:32–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- [39].Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxidants & redox signaling. 2004;6:32–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- [40].Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:32–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- [41].Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:32–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [42].Auger-Messier M, Accornero F, Goonasekera SA, Bueno OF, Lorenz JN, van Berlo JH, Willette RN, Molkentin JD. Unrestrained p38 MAPK activation in Dusp1/4 double-null mice induces cardiomyopathy. Circulation research. 2013;112:32–56. doi: 10.1161/CIRCRESAHA.112.272963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Fujita Y, Ali N, Ishizawa K, Yamauchi A, Tamaki T. Antioxidant effects of stereoisomers of N-acetylcysteine (NAC), L-NAC and D-NAC, on angiotensin II-stimulated MAP kinase activation and vascular smooth muscle cell proliferation. J Pharmacol Sci. 2004;95:32–486. doi: 10.1254/jphs.sc0040061. [DOI] [PubMed] [Google Scholar]
- [44].Waalkes MP. Cadmium carcinogenesis in review. Journal of inorganic biochemistry. 2000;79:32–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- [45].Houston MC. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Alternative therapies in health and medicine. 2007;13:S128–133. [PubMed] [Google Scholar]
- [46].Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:32–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- [47].Pimentel D, Haeussler DJ, Matsui R, Burgoyne JR, Cohen RA, Bachschmid MM. Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system. Antioxidants & redox signaling. 2012;16:32–542. doi: 10.1089/ars.2011.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. Journal of atherosclerosis and thrombosis. 2009;16:32–538. doi: 10.5551/jat.1255. [DOI] [PubMed] [Google Scholar]
- [49].Odewumi CO, Badisa VL, Le UT, Latinwo LM, Ikediobi CO, Badisa RB, Darling-Reed SF. Protective effects of N-acetylcysteine against cadmium-induced damage in cultured rat normal liver cells. International journal of molecular medicine. 2011;27:32–248. doi: 10.3892/ijmm.2010.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends in biochemical sciences. 2006;31:32–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- [51].Al-Mutairi M, Al-Harthi S, Cadalbert L, Plevin R. Over-expression of mitogen-activated protein kinase phosphatase-2 enhances adhesion molecule expression and protects against apoptosis in human endothelial cells. British journal of pharmacology. 2010;161:32–798. doi: 10.1111/j.1476-5381.2010.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lawan A, Al-Harthi S, Cadalbert L, McCluskey AG, Shweash M, Grassia G, Grant A, Boyd M, Currie S, Plevin R. Deletion of the dual specific phosphatase-4 (DUSP-4) gene reveals an essential non-redundant role for MAP kinase phosphatase-2 (MKP-2) in proliferation and cell survival. J Biol Chem. 2011;286:32–12943. doi: 10.1074/jbc.M110.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mates JM, Segura JA, Alonso FJ, Marquez J. Sulphur-containing non enzymatic antioxidants: therapeutic tools against cancer. Front Biosci (Schol Ed) 2012;4:32–748. doi: 10.2741/s296. [DOI] [PubMed] [Google Scholar]