Abstract
Background
The course of posttraumatic stress disorder (PTSD) is frequently and severely complicated by co-occurring alcohol use disorder (AUD), yet there are few reports of pharmacologic treatments for these co-morbid conditions. The objective of this pilot study was to obtain a preliminary assessment of the efficacy and safety of topiramate in reducing alcohol use and PTSD symptoms in veterans with both disorders.
Methods
This was a prospective 12-week, randomized, double-blind, placebo-controlled pilot trial of flexible-dose topiramate up to 300mg/day in 30 veterans with PTSD and AUD. The primary outcome measure was frequency of drinking. Secondary outcomes consisted of other measures of alcohol use and PTSD symptom severity.
Results
Within-group analyses showed that topiramate treatment was associated with significant reductions in frequency and amount of alcohol use and alcohol craving from baseline through week 12. Between-group analyses showed that topiramate reduced frequency of alcohol use and alcohol craving significantly more than placebo and tended to reduce drinking amount. Topiramate treatment was also associated with decreased PTSD symptom severity and tended to reduce hyperarousal symptoms compared to placebo. Topiramate transiently impaired learning and memory, with significant recovery by the end of treatment.
Conclusions
These preliminary results indicate that in veterans with co-occurring PTSD and AUD, topiramate may be effective in reducing alcohol consumption, alcohol craving, and PTSD symptom severity – particularly hyperarousal symptoms.. Topiramate was associated with transient cognitive impairment but was otherwise well tolerated.
Keywords: Topiramate, Clinical Trial, Alcohol Use Disorder, PTSD, Cognition
Introduction
Among civilian and military personnel with Posttraumatic Stress Disorder (PTSD), up to 52% suffer from comorbid alcohol use disorders (AUD) (Kessler et al., 1995, Baker et al., 2009). The co-occurrence of AUDs and PTSD is associated with poor psychosocial and medical outcomes, high rates of hospitalization, and impaired psychosocial functioning (McCarthy and Petrakis, 2010), pointing to an urgent need to improve treatment options for the many veterans who suffer from these disorders.
To date, there have been few reported pharmacotherapy studies focused on these co-occurring conditions (Brady et al., 1995, Brady et al., 2005, Petrakis et al., 2006, Petrakis et al., 2012, Foa et al., 2013) and no consensus is readily available regarding the optimal use of medications (McCarthy and Petrakis, 2010, Sofuoglu et al., 2014). Evidence has recently emerged showing efficacy for topiramate in reducing problematic alcohol use (Johnson and Ait-Daoud, 2010) as well as independently showing topiramate's efficacy in reducing PTSD symptoms. Topiramate has been found to increase the proportion of days abstinent from alcohol use, and to reduce the number of heavy drinking days and drinks per drinking day (Johnson et al., 2003, Johnson et al., 2007, Baltieri et al., 2008, Rubio et al., 2009, Kranzler et al., 2014) and to reduce alcohol craving when compared to placebo (Johnson et al., 2003, Rubio et al., 2009) in AUD patients without PTSD. One exception to the generally positive findings was a controlled trial during residential detoxification treatment in which topiramate only showed a trend toward superiority to placebo, possibly due to the presence of intensive psychosocial interventions applied to both treatment groups (Likhitsathian et al., 2013).
Topiramate has also been proposed as a possible treatment for PTSD, based on its pharmacological GABA/glutamate profile; specifically its effects as a GABA agonist and its ability to block glutamate AMPA/kainite signaling (Berlant and van Kammen, 2002, Sofuoglu et al., 2014). Topiramate has shown partial effectiveness in reducing PTSD symptoms in patients without AUD in three open trials (Berlant and van Kammen, 2002, Berlant, 2004, Alderman et al., 2009) and three small-to-medium sized controlled trials (Lindley et al., 2007, Tucker et al., 2007, Yeh et al., 2011). In a placebo-controlled trial in veterans, topiramate treatment was associated with greater improvement in PTSD re-experiencing symptoms when used to augment standard PTSD pharmacotherapy, although with more adverse effects and higher dropout (Lindley et al., 2007). Topiramate also showed significantly greater reductions in PTSD re-experiencing symptoms than placebo in non-veterans with PTSD (Tucker et al., 2007). The most recent of the controlled trials in a civilian sample found that topiramate significantly reduced PTSD symptom severity as compared to placebo, with particular effectiveness in reducing re-experiencing and avoidance/numbing symptom clusters (Yeh et al., 2011).
There have been no controlled trials of topiramate to examine its effects in reducing alcohol consumption and PTSD symptom severity in patients with co-occurring AUD and PTSD, although a small open trial of topiramate in male combat veterans with PTSD showed a reduction in PTSD symptoms and a decrease in the proportion of patients with high-risk drinking (defined as >43 drinks/week) (Alderman et al., 2009). We therefore conducted a randomized, placebo-controlled pilot trial to provide a preliminary assessment of the efficacy, and safety of topiramate during a 12-week course of treatment in 30 veterans with PTSD and AUD whose treatment goals were to reduce and possibly stop alcohol consumption. We tested two a priori hypotheses: first, that the topiramate group would have a within-group reduction in percent drinking days over the course of the 12-week trial and second, that in a between-groups analysis, the topiramate group would have fewer percent drinking days when compared to the placebo group. We also planned to explore the efficacy of topiramate in reducing the amount of alcohol use, alcohol craving, and PTSD symptom severity.
Materials and Methods
Participants
All participants provided written informed consent prior to study and underwent procedures approved by the University of California, San Francisco, the San Francisco Veterans Affairs Medical Center (SF VAMC) and the Department of Defense. Participants were recruited, and all procedures took place at the SF VAMC in San Francisco, CA. Study participants were 30 veterans who met DSM-IV-TR (American Psychiatric Association, 2000) diagnostic criteria for both current alcohol dependence and PTSD. All participants also reported “at-risk” or “heavy” drinking in accordance with NIH/NIAAA criteria (at least 15 standard drinks per week on average over the 4 weeks prior to study entry for men and at least 8 standard drinks per week on average for women) (Willenbring et al., 2009) and all expressed a desire to reduce alcohol consumption with the possible long-term goal of abstinence. Participants included patients who were still actively drinking as well as those who had stopped in the days prior to random assignment. Participants were free to access any other standard psychological or pharmacologic treatments for PTSD and any psychosocial treatments for AUD, but they could not receive other AUD pharmacotherapy. Participants were excluded if they met diagnostic criteria for psychotic disorders, bipolar disorder, and dementia, were known to have any clinically significant unstable psychiatric or medical conditions, or had a suicide attempt or suicidal ideation in the six months prior to enrollment. Other exclusion criteria included acute alcohol withdrawal, history of either nephrolithiasis, narrow angle glaucoma or seizure disorder, current use of other anticonvulsant medications, topiramate use within the past four weeks and concurrent participation in other treatment studies.
Procedure
This was a randomized, double-blind, placebo-controlled, flexible-dose (25-300 mg/day) pilot trial of topiramate augmentation treatment. Screening consisted of 2-3 visits within one week during which participants completed the measures and interviews described below. Those who met entry criteria began the treatment phase of the study consisting of 12 weekly visits. Participants were randomly assigned in a 1:1 ratio to receive either topiramate or placebo treatment. Randomization was stratified by gender and balanced using computer-generated block randomization with permuted block sizes of six, created by a study statistician with no clinical involvement in the trial. The allocation list was given to an independent pharmacist who assigned participants to study group and dispensed study medication according to the randomization list. Participants and all research staff including raters were blinded to the assigned treatment. Study medication was provided in prepackaged bottles containing identical 25- or 100-mg capsules of either topiramate or placebo. Dosing followed the method of Johnson and coworkers (Johnson et al., 2007). The initial dose was 25 mg nightly for one week. The dose was increased to 50 mg per day in two divided doses in week 2; in week 3, the dose was increased to 100 mg per day; in week 4, to 150 mg per day; in week 5 to 200 mg per day, and in week 6, to 300 mg per day given as 100 mg in the morning and 200 mg in the evening. This final dose was maintained from week 6 through week 11. In week 12, study medication was tapered and discontinued. Dosing was flexible, in that the maximum daily dose was determined by tolerability – if participants experienced clinically significant adverse effects, then study medication dose would not be advanced, or, if needed, it would be decreased.
All participants also received weekly Medical Management counseling (Pettinati et al., 2005), a manual-driven, low-intensity supportive counseling method designed by the National Institute on Alcohol Abuse and Alcoholism to promote adherence to the medication regimen and reduction in alcohol use.
Measures
Demographics and Psychiatric Characteristics
All participants were administered the Substance Use Disorders sections of the Structured Clinical Interview for DSM-IV-TR (First et al., 2001). PTSD diagnosis was assessed with the Clinician Administered PTSD Checklist (CAPS) (Blake et al., 1995), a 30-item structured interview based on the DSM-IV. The CAPS instrument is divided into sections based on PTSD symptom clusters: Re-experiencing, Avoidance, and Arousal. A CAPS criterion was considered to be present if a participant endorsed a symptom with a score ≥ 1 in frequency and ≥ 2 in severity rating. All participants completed the Beck Depression Inventory [BDI-II] (Beck et al., 1961) and the Beck Anxiety Inventory [BAI] (Beck et al., 1988) at baseline and were assessed for PTSD symptom severity with the PTSD Checklist [PCL]; (Weathers and Litz, 1994) at baseline, week 4, 8, and 12.
Alcohol Consumption, Craving and Severity
Alcohol consumption frequency and amount were assessed using the Time Line Follow Back (TLFB; (Sobell, 1985, Sobell and Sobell, 1992)) interview which yields number of alcohol drinking days, number of heavy drinking days, and number of drinks per each day of drinking. The TLFB was administered at baseline to assess the 90-day period prior to the beginning of screening, and then weekly at each subsequent treatment visit. Obsessive thoughts and compulsions associated with alcohol craving were measured using the Obsessive Compulsive Drinking Scale (OCDS) (Anton et al., 1995) at baseline, week 4, 8, and 12. Severity of harmful and hazardous drinking was measured using the Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993) at baseline.
Auditory Verbal Learning and Memory
To assess areas of cognition known to be adversely affected by topiramate (Aldenkamp et al., 2000), participants completed the Hopkins Verbal Learning Test-Revised (HVLT-R (Brandt, 1991)) at baseline, week 6 and 12. HVLT-R includes total recall (learning) and delayed recall (memory).
Adverse Effects
Adverse effects (AEs) were collected weekly using a checklist of the 18 most common AEs associated with topiramate as indicated in the FDA-approved labeling for topiramate (Pharmaceuticals, 2012).
Statistical Analyses
Baseline characteristics for each group were compared using a t-test for continuous variables and Fisher's exact test for categorical variables. This pilot study was designed with adequate power to allow the primary outcome analysis of within-group change in percent drinking days from baseline through week 12 in the topiramate treatment condition. The study was also powered for a secondary, between-groups outcome analysis to detect a “signal” or statistical trend (p<0.10) for a difference in percent drinking days between the topiramate and placebo condition over the 12 weeks of the trial. Percent drinking days was over-dispersed, positively skewed count data. Our primary within-topiramate group analysis applied a random-intercept repeated subject negative binomial model, modeling week (baseline - week 12) as a continuous variable. Our secondary between-groups analysis examined the percent drinking days per week averaged over the treatment phase of the trial (weeks 1-12). The model included fixed effect for week, treatment group (topiramate and placebo), and the interaction between treatment group and week. The same approach was applied to the analyses of percent heavy drinking days, drinks per week, and average drinks per drinking day. Baseline alcohol consumption means were used as respective covariates in group comparisons to control for pre-study and study enrollment effects.
We used random-intercept linear mixed models to explore the efficacy for topiramate related reduction in PTSD symptomatology, craving, and effects on measures of learning and memory. We first looked for an effect of week within the topiramate treatment condition and then tested for a signal (trend) for a difference between treatment groups. Baseline scores for PTSD symptoms, craving, learning and memory were used as covariates in group comparisons. We calculated percent change for each outcome measure by comparing baseline to the respective average of weeks 1-12. All analyses were intent-to-treat and used all observations from all weeks. Given the preliminary nature of this study, all statistical tests were held to an alpha of 0.05 and completed with SPSS v21.
Results
Patient Characteristics
Baseline characteristics for the topiramate (TOP) and placebo (PLA) groups are shown in Table 1. Of the 30 participants, 14 were randomly assigned to TOP, 16 to PLA. All participants were veterans of Vietnam, the Gulf Wars, or Iraq and Afghanistan with war-zone and/or civilian related trauma exposure. There were no differences between treatment group characteristics at baseline. Of the 30 participants enrolled, four TOP and two PLA attended a 30-day community based residential rehabilitation treatment program that included a structured living environment with group therapy and individual case management. Participants were allowed to travel to and from the SF VAMC to participate in screening and study procedures. Medication was initiated when the participant passed our screening process and entered the active treatment phase, regardless of time spent in residential treatment.
Table 1. Participant Characteristics at Baseline (Means±Standard Deviation).
| TOP | PLA | |
|---|---|---|
|
|
||
| n (female) | 14 (1) | 16 (1) |
| Age [years] | 49.5±13.9 | 50.4±12.8 |
| Education [years] | 12.9±3.1 | 14.4±1.9 |
| Race | ||
| Caucasian (Hispanic/Latino) | 8 (2) | 8 |
| American Indian/Alaskan Native | 1 | 0 |
| Asian | 1 | 1 |
| African American | 2 | 5 |
| Pacific Island Native | 0 | 1 |
| Mixed Race | 2 | 1 |
| Combat Exposed, n (%) | 10 (71) | 12 (75) |
| Comorbid Substance Use Disorder, n (%) | 5 (36) | 5 (32) |
| AUD Residential TX, n (%) | 4 (29) | 2 (13) |
| AUD Outpatient TX, n (%) | 7 (50) | 8 (50) |
| PTSD Outpatient TX, n (%) | 9 (65) | 9 (56) |
| PTSD Pharmacotherapy TX, n (%) | 5 (37) | 9 (56) |
| Alpha Blocker [Prazosin] (%) | 1 (7) | 1 (6) |
| Antidepressants (%) | 4 (29) | 7 (44) |
| Buproprion (%) | 0 (0) | 1 (6) |
| Citalopram (%) | 2 (14) | 3 (19) |
| Fluoxetine (%) | 0 (0) | 1 (6) |
| Mirtazapine (%) | 1 (7) | 0 (0) |
| Setraline (%) | 0 (0) | 2 (13) |
| Venlafaxine (%) | 1 (7) | 0 (0) |
| Antipsychotic [Quetiapin] (%) | 1 (7) | 1 (6) |
| Anxiolytic (%) | 0 (0) | 2 (13) |
| Buspirone (%) | 0 (0) | 1 (6) |
| Hydroxyzine (%) | 0 (0) | 1 (6) |
| Beta Blocker [Propranolol] (%) | 0 (0) | 1 (6) |
| Sedative/Hypnotic (%) | 0 (0) | 3 (19) |
| Temazepam (%) | 0 (0) | 1 (6) |
| Trazodone (%) | 0 (0) | 2 (13) |
| Stimulant [Ritalin] (%) | 1 (7) | 0 (0) |
| BDI | 23.4±11.6 | 26.3±12.3 |
| BAI | 20.4±12.7 | 27.4±13.3 |
| AUDIT Score | 27.1±7.9 | 23.0±7.5 |
| Days Abstinent Between Last Drink and Initiation of Study Medication | 12.8±13.6 | 4.8±9.2 |
| Percent DD per Week | 73.3±30.3 | 80.4±21.5 |
| Percent HDD per Week | 58.5±33.7 | 72.6±28.5 |
| Avg. Drinks$ per Week | 52.4±34.2 | 58.2±25.4 |
| Avg. Drinks$ per DD | 11.1±6.1 | 10.9±4.7 |
| Baseline CAPS Total | 72.8±14.3 | 83.1±17.3 |
| Reexperiencing | 18.2±4.3 | 21.9±6.9 |
| Avoidance | 31.1±6.1 | 34.8±8.9 |
| Arousal | 23.5±6.7 | 26.4±4.1 |
Abbreviations: AUD, Alcohol Use Disorder; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; CAPS, Clinician Administered PTSD Scale; %DD, percent drinking day; HDD, heavy drinking day (>4 standard alcoholic drinks for men, >3 standard alcoholic drinks for women). Drink consumption was averaged over 90 days preceding study consent.
standard alcoholic drink is defined as containing 13.6 g of pure alcohol
Study retention
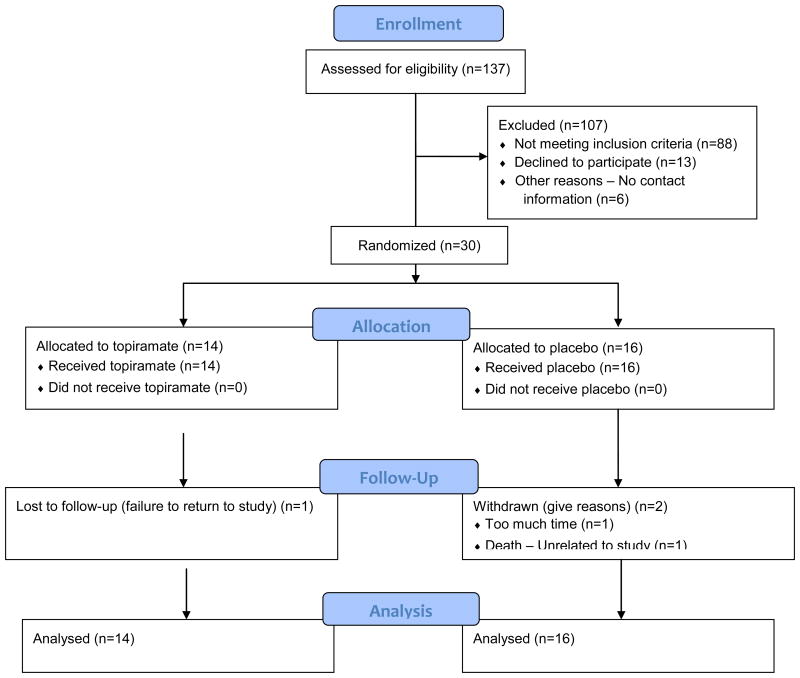
Of the 30 randomized patients, 27 [90%] (TOP: 13/14 [92.3%]; PLA: 14/16 [87.5%]) completed the trial, attending week 12 study visit. TOP attended a significantly higher percent of study visits (94.2%±23.5) than PLA (83.1%±37.5) during weeks 1-12 (p=0.002). Attrition was low in both groups over the course of the treatment phase (TOP=1/14, PLA=2/16). Subject flow is illustrated in Figure 1. Of the three participants who did not complete the study: one TOP participant was lost to follow-up (failed to return to study), one PLA participant withdrew due to lack of time, and one PLA participant died of myocardial infarction, judged to be unrelated to the study. No participants dropped out because of adverse effects related to study medication. Difference in total attrition between TOP and PLA at Week 12 was not statistically significant (p=0.556).
Figure 1. CONSORT Flow Diagram.
Maximum Medication Dose and Adherence
As described above, this was a flexible-dose study. The maximum study dose (300mg/day) was adjusted to participant tolerance. The average maximum study medication dose reached in each of the study conditions was 286±20mg/day for TOP and 281±45mg/day for PLA. The difference in maximum dose reached by TOP and PLA was not statistically significant (p=0.248).
Adherence was measured by self-report and verified by pill count. Medication adherence rate was the total dose (mg) self-reported taken ÷ total dose prescribed × 100. Mean adherence rate was 63.1%±20.3 for TOP and 60.2%±21.5 for PLA, with no significant difference between groups.
Primary and Secondary Analyses of Percent Drinking Days
Our primary analysis demonstrated a significant decrease in percent drinking days from baseline through week 12 within TOP (Table 2). Our secondary analysis, illustrated in Figure 2, showed a near-significant trend for a main effect of treatment [p=0.063, RR=0.430; 95% CI=(0.18-1.05)]. There was not a significant treatment-by-week interaction. Since we did not predict differential rates of change, we removed the insignificant interaction term and re-ran our between-group analysis, which revealed a significant main effect of treatment (p=0.036, Table 2), with TOP having 51% less drinking days than PLA averaged during weeks 1-12.
Table 2. TOP Within Group and Between Group Analyses for Alcohol Consumption, PTSD Symptoms and Alcohol Craving.
| Alcohol Consumption | TOP Within Group Analysis | Between Groups Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Baseline Mean±SD | Weeks 1-12 Mean±SD | p-Value | IRR (β) | 95% CI | %Δ | TOP Mean±SD | PLA Mean±SD | p-Value | IRR (β) | 95% CI | %Diff. | |
|
|
|
|||||||||||
| % DD | 73.3±30.3 | 19.5±34.2 | 0.019 | 0.89 | 0.82-0.98 | -74% | 19.5±34.2 | 39.7±36.5 | 0.036 | 0.38$ | 0.15-0.94 | 51% |
| % HDD | 58.5±33.7 | 11.1±27.1 | 0.035 | 0.87 | 0.76-0.99 | -81% | 11.1±27.1 | 16.8±26.3 | 0.342 | 0.56 | 0.17-1.87 | 34% |
| Std. Drinks per Week | 52.4±34.2 | 8.7±19.0 | 0.028 | 0.86 | 0.75-0.98 | -83% | 8.7±19.0 | 19.3±30.5 | 0.099 | 0.43 | 0.16-1.17 | 55% |
| Drinks per DD | 11.1±6.1 | 1.9±3.3 | 0.008 | 0.83 | 0.74-0.96 | -83% | 1.9±3.3 | 4.8±6.5 | 0.057 | 0.45 | 0.20-1.02 | 60% |
|
|
|
|||||||||||
| PTSD Symptoms | ||||||||||||
| PCL Total Score | 57.1±13.4 | 42.3±16.4 | 0.001 | (-1.22) | -1.84 to -0.62 | -26% | 42.3±16.0 | 49.0±16.5 | 0.100 | (-9.01) | -19.8-1.80 | 14% |
| PCL B-Reexperiencing | 15.9±5.2 | 12.3±5.5 | 0.026 | (-0.28) | -0.53 to -0.04 | -23% | 12.3±5.4 | 14.3±5.5 | 0.155 | (-2.75) | -6.57-1.08 | 14% |
| PCL C-Avoidance | 23.9±5.7 | 17.6±7.4 | 0.002 | (-0.46) | -0.73 to -0.20 | -26% | 17.6±7.2 | 19.9±6.9 | 0.272 | (-3.07) | -8.61-2.48 | 12% |
| PCL D-Arousal | 17.3±4.8 | 12.4±4.9 | 0 | (-0.48) | -0.71 to -0.25 | -28% | 12.4±4.9 | 14.9±5.0 | 0.071 | (-3.23) | -6.74-0.29 | 17% |
|
|
|
|||||||||||
| Alcohol Craving | ||||||||||||
| OCDS Total | 16.93±8.70 | 5.53±6.55 | 0.002 | (-0.95) | -1.48 to -0.43 | -67% | 5.53±6.55 | 11.08±8.12 | 0.025 | (-7.02) | -13.1 to -0.91 | 50% |
Abbreviations: %DD, percent drinking days; %HDD, percent heavy drinking days (>4 standard alcoholic drinks for men, >3 standard alcoholic drinks for women; standard alcoholic drink is defined as containing 13.6 g of pure alcohol); CI, confidence interval; IRR, incidence rate ratio (average relative change in outcome per week from baseline through week 12); %Δ, percent change (calculated by comparing baseline to the respective average of weeks 1-12); %Diff., percent difference (calculated by comparing weeks 1-12 averages between treatment groups).
p-value of negative binomial model when the interaction term is removed.
Figure 2. Mean and Median Percent Drinking Days Per Week.
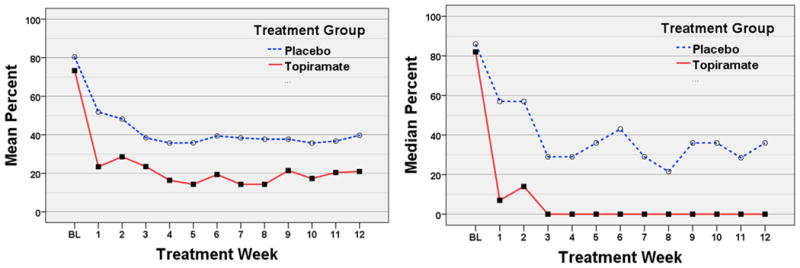
Abbreviation; BL, baseline weekly drinking average based on the 90 day period prior to the beginning of screening.
Exploratory Analyses
Percent Heavy Drinking Days, Drinks per Drinking Day, and Standard Drinks per Week
Each univariate analysis examining reductions of percent heavy drinking days, standard alcohol drinks consumed per week, and standard alcohol drinks consumed per drinking day within TOP found significant reductions and are summarized in Table 2. Between-group comparisons revealed a trend for a main effect of treatment on standard drinks per week (p=0.099, Table 2), with TOP having 55% fewer standard drinks during weeks 1-12 compared to PLA. We also observed a trend for a main effect of treatment on drinks per drinking day (p=0.057, Table 2) with TOP having 61% fewer drinks per drinking day than PLA during weeks 1-12. There were no between-group effects for percent heavy drinking days. There were also no significant treatment-by-week interactions for any of these exploratory analyses. Removing the insignificant interaction terms from their respective model did not markedly change the degree of significance in group comparisons.
PTSD Symptom Outcome
Univariate analysis revealed a significant reduction within TOP in PTSD symptom severity as measured by the PCL total score and all three subscale scores from baseline through week 12 (Table 2). When compared to PLA, there were trends for main effects of treatment on PCL-total [F(1,48)=2.81, p=0.100] and, as illustrated in Figure 3, arousal scores [F(1,52)=3.40, p=0.071] (Table 2). There were no significant treatment-by-week interactions for any PCL measure.
Figure 3. Mean PCL Arousal Scores.
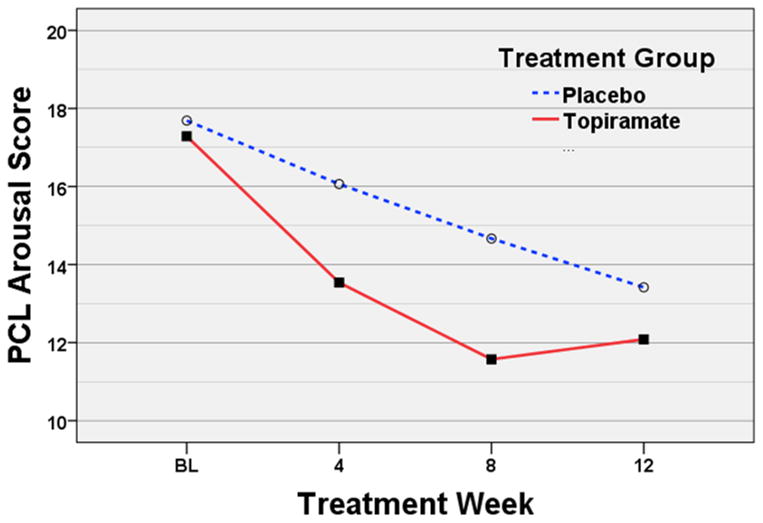
Abbreviation; BL, baseline weekly PCL average based on the 90 day period prior to the beginning of screening.
Alcohol Craving
As seen in Table 2, there was a significant reduction in OCDS scores from baseline through week 12 within TOP [F(1,14)=15.17, p=0.002]. When compared to PLA, there was a significant main effect of treatment [F(1,50)=5.33, p=0.025]. There was not a significant treatment-by-week interaction.
HVLT-R Total (learning)
There was a significant treatment-by-week interaction for HVLT-R total recall [F(1,21)=6.63, p=0.018] (Figure 4). Follow-up univariate analyses indicated that TOP decreased in performance between baseline and week 6 [F(1,13)=15.04, p=0.002] and then significantly regained some of that loss between week 6 and 12 [F(1,12)=17.59, p=0.001], whereas PLA did not show any change during these time intervals. Cross-sectional group comparisons showed no differences at baseline (TOP=42.3±10.3, PLA=41.5±13.8), significantly worse performance by TOP than PLA at Week 6 (p=0.013, TOP=31.6±8.4, PLA=43.4±15.3) and no significant differences at week 12 (TOP=41.0±7.8, PLA=44.8±13.8).
Figure 4. Mean HVLT-R Total and Delayed Recall Scores.
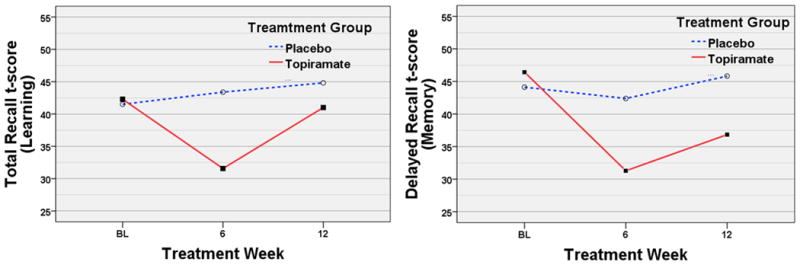
Abbreviation; BL, baseline weekly learning and memory scores based on the 90 day period prior to the beginning of screening.
HVLT-R Delayed Recall (memory)
There was a significant main effect of treatment [F(1,42)=5.01, p=0.031] and week [F(1,22)=6.23, p=0.021] suggesting differential treatment group performance between baseline and week 12 in HVLT-R delayed recall. There was no significant treatment-by-week interaction. Follow up univariate analysis indicated that TOP decreased in performance between baseline and week 6 [F(1,13)=17.76, p=0.001] and then significantly regained part of that loss between week 6 and 12 [F(1,12)=6.50, p=0.026], whereas PLA did not show any significant change during these same intervals (Figure 4). Cross-sectional group comparisons showed no differences at baseline (TOP=46.4±10.2, PLA=44.13±11.9), significantly worse performance of TOP compared to PLA at week 6 (p=0.028, TOP=31.3±11.2, PLA=42.4±16.8). At week 12, TOP still tended to have worse performance than PLA (p=0.096, TOP=36.8±8.8, PLA=45.8±15.0).
Adverse Events
Twelve (85.7%) TOP and 13 (81.3%) PLA participants experienced treatment-emergent adverse events during the trial. There were no significant differences between groups on any reported emergent adverse events. The most common reported emergent complaints were: sleepiness, in 36% of TOP and 13% of PLA; loss of appetite in 29% of TOP and 38% of PLA; change in sense of taste in 21% of TOP and 31% of PLA; itching in 21% of TOP and 6% of PLA; diarrhea in 29% of TOP and 19% of PLA; and abnormal vision in 21% of TOP and 19% of PLA. Four participants – all of them PLA – experienced a total of six serious adverse events (SAEs). Five SAEs were conservatively categorized as ‘possibly’ related to the study. Of the four particpants with SAEs, one was hospitalized for suicidal ideation; one participant had three hospitalizations for chest pain; another participant had one hospitalization for chest pain; and one participant died due to myocardial infarction, judged to be unrelated to the study.
Discussion
The study described here is the first prospective trial of topiramate for co-occurring alcohol use disorder and PTSD conducted in a cohort of veterans, whose goal was to reduce or stop alcohol use. The study was primarily powered to examine within-group changes in the topiramate condition, with secondary analyses intended to detect a between-groups signal of topiramate efficacy compared to placebo. As hypothesized, in the topiramate condition, treatment was associated with reduction in self-reported frequency and amount of alcohol use, alcohol craving and PTSD symptoms from baseline to week 12. Of greater interest, topiramate tended to be more efficacious than placebo in reducing these measures of alcohol use. Overall, topiramate was well tolerated, but was associated with transient reductions in learning and memory.
Topiramate's effects on reducing the frequency and amount of alcohol consumption and in reducing alcohol craving are in line with the findings of previously conducted studies of topiramate in AUD without PTSD (Johnson et al., 2003, Johnson et al., 2007, Baltieri et al., 2008, Rubio et al., 2009, Kranzler et al., 2014). Topiramate's effects on PTSD symptom severity are also supportive of the promising findings of prior studies that examined participants with PTSD but without AUD (Lindley et al., 2007, Tucker et al., 2007, Yeh et al., 2011).
In contrast with other controlled topiramate studies of PTSD, we observed a trend toward greater reduction in PTSD arousal symptoms in TOP compared to PLA. Only two other controlled studies have demonstrated efficacy for topiramate in the treatment of PTSD symptoms compared to placebo (Tucker et al., 2007, Yeh et al., 2011), both showing reductions in re-experiencing and avoidance symptoms. Neither of those studies found topiramate to reduce PTSD arousal symptoms. Our findings suggest that topiramate may target PTSD symptom clusters differently, dependent on the presence or absence of comorbid AUD. Topiramate may prove to be an especially useful treatment for those with comorbid AUD/PTSD who present with particularly troubling hyperarousal symptoms (e.g., irritability/anger, hypervigilance, exaggerated startle response). This conclusion remains tentative as we did not study a PTSD group without AUD for comparison.
Topiramate's tolerability was evidenced in several ways. Surprisingly, adverse events did not occur at a significantly higher rate in participants treated with TOP as compared to PLA. Also, TOP participants had higher retention rates and reached a similar rate of medication adherence and dose (286mg/day of a possible maximum target dose of 300 mg) compared to PLA. However, topiramate was associated with reductions in auditory/verbal learning and memory, although by week 12 there was recovery from the impairment in learning seen at mid-study. The topiramate-associated worsening of memory at week six also improved by end of study but continued to show impairment compared to placebo. Despite these test results, the TOP group did not report more subjective complaints of memory problems than the PLA group over the course of the trial. These findings are generally consistent with previously reported mixed observations on the effects of topiramate on learning and memory (Aldenkamp et al., 2000, Lee et al., 2003), but different from Likhitsathian and coworkers (Likhitsathian et al., 2012) who found no decrease in cognitive functioning in an open trial of topiramate in AUD patients. Given the limited sample size, we were unable to conduct any meaningful statistical analyses to definitively conclude that the cognitive impairment observed in this population was caused only by topiramate treatment and was unrelated to continued alcohol consumption. At the least, our findings support the need to further delineate the effects of topiramate treatment on cognition in both active drinkers and continuous abstainers. Of note, there were no differences between groups in central nervous system adverse events, which were associated with high dropout rates in a previous study for topiramate efficacy for PTSD (Lindley et al., 2007).
Strengths of this study included its double-blind, placebo-controlled, randomized design, intent-to-treat analyses, its focus on a veteran population, and the detailed measurement of both alcohol use and PTSD symptom severity. Moreover, while Likhitsathian and colleagues described cognitive changes in an open trial of topiramate in AUD (Likhitsathian et al., 2012), to our knowledge, we report the first placebo-controlled study of topiramate's neurocognitive adverse effects in a trial focusing on alcohol use. Limitations of the study include its sample size, consistent with the study's pilot nature, which may have decreased power to detect significant differences between topiramate and placebo despite there being large percent differences. Additionally, our small sample size did not allow for the examination of factors that may have influenced our outcomes, such as the moderating effects of concomitant treatment, genetics (Kranzler et al., 2014, Batki and Pennington, 2014), degree of motivation at study entry, or the presence of pretreatment abstinence. An additional limitation of this report is the reliance on self-report measures to assess drinking outcomes – although self-report at present remains the standard for alcohol use outcome measurement in clinical trials (Falk et al., 2010), e.g., Fertig et al., (2012) and Litten et al., (2012). Despite these limitations, our a priori hypothesis of detecting change within the topiramate group was confirmed, and signals for between group differences in alcohol use and PTSD symptom severity were found to favor topiramate.
In sum, topiramate's effects on reducing alcohol consumption and craving in veterans whose goal was to reduce or stop alcohol use were generally in line with larger trials in AUD patients without PTSD. Topiramate's effects on reducing PTSD symptoms provide further support to the evidence available from several previous small open and controlled trials. While topiramate appeared to be safe and well-tolerated, the benefits in alcohol use reduction and PTSD symptom improvements must be interpreted in the light of the apparent potential for transient cognitive decrements seen in the topiramate-treated participants. The results of this study warrant a larger investigation to more definitively assess the efficacy of topiramate treatment in reducing alcohol use and PTSD symptom severity in individuals with co-occurring AUD and PTSD.
Acknowledgments
This work was supported by grants from the Department of Defense W81XWH-05-2-0094, W81XWH-12-2-0137, and W81XWH-11-2-0245 (SLB), National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health UCSF-CTSI UL1 RR024131, which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California.
Footnotes
Financial Disclosures: DLP, BL, AW, KD, and EH have no disclosures to make. SLB has served as a consultant to Gilead Sciences and TN has consulted to Genentech. TN has received study medication from Actelion for a study funded by the Department of Defense and received study medication from Glaxo Smith Kline for a study funded by the Department of Veterans Affairs.
References
- Aldenkamp AP, Baker G, Mulder OG, Chadwick D, Cooper P, Doelman J, Duncan R, Gassmann-Mayer C, de Haan GJ, Hughson C, Hulsman J, Overweg J, Pledger G, Rentmeester TW, Riaz H, Wroe S. A multicenter, randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000;41:1167–1178. doi: 10.1111/j.1528-1157.2000.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Alderman CP, McCarthy LC, Condon JT, Marwood AC, Fuller JR. Topiramate in combat-related posttraumatic stress disorder. Ann Pharmacother. 2009;43:635–641. doi: 10.1345/aph.1L578. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Series Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Publishing; Washington, D.C: 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [Google Scholar]
- Andrus R, Gilbert E. Treatment of civilian and combat-related posttraumatic stress disorder with topiramate. The Annals of Pharmacotherapy. 2010;44:1810–1816. doi: 10.1345/aph.1P163. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A Self-Rated Instrument for the Quantification of Thoughts about Alcohol and Drinking Behavior. Alcoholism: Clinical and Experimental Research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Baker DG, Heppner P, Afari N, Nunnink S, Kilmer M, Simmons A, Harder L, Bosse B. Trauma exposure, branch of service, and physical injury in relation to mental health among U.S. veterans returning from Iraq and Afghanistan. Mil Med. 2009;174:773–778. [PubMed] [Google Scholar]
- Baltieri D, Daro F, Ribeiro P, de Andrade A. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103:2035. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Batki SL, Pennington DL. Toward personalized medicine in the pharmacotherapy of alcohol use disorder: targeting patient genes and patient goals. Am J Psychiatry. 2014;171:391–394. doi: 10.1176/appi.ajp.2014.14010061. [DOI] [PubMed] [Google Scholar]
- Beck A, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety: Psychometric properties. Journal of consulting and clinical psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63:15–20. doi: 10.4088/jcp.v63n0104. [DOI] [PubMed] [Google Scholar]
- Berlant JL. Prospective open-label study of add-on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. BMC Psychiatry. 2004;4:24. doi: 10.1186/1471-244X-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady K, Sonne S, Anton R, Randall C, Back S, Simpson K. Sertraline in the Treatment of Co-occurring Alcohol Dependence and Posttraumatic Stress Disorder. Alcoholism: Clinical and Experimental Research. 2005;29:395. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC, Roberts JM. Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence. J Clin Psychiatry. 1995;56:502–505. [PubMed] [Google Scholar]
- Brandt J. The hopkins verbal learning test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Falk D, Wang X, Liu L, Fertig JB, Mattson ME, Ryan M, Johnson B, Stout R, Litten R. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol: Clinical and Experimental Research. 2010;35:191–193. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, Rickman WJ, Scott C, Ciraulo D, Green AI, Tiouririne NA, Johnson B, Pettinati H, Strain EC, Devine E, Brunette MF, Kampman K, D AT, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:1421–1430. doi: 10.1111/j.1530-0277.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Series Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/PW/PSY SCREEN) American Psychiatric Press, Inc.; Washington D.C.: 2001. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/PW/PSY SCREEN) [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr, Oslin D, O'Brien CP, Imms P, Riggs DS, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. Jama. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Johnson B, Ait-Daoud N. Topiramate in the new generation of drugs: efficacy in the treatment of alcoholic patients. Current Pharmaceutical Design. 2010;16:2103–2112. doi: 10.2174/138161210791516404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM Topiramate for Alcoholism Advisory B, Topiramate for Alcoholism Study G. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate Treatment for Heavy Drinkers: Moderation by a GRIK1 Polymorphism. Am J Psychiatry. 2014;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sziklas V, Andermann F, Farnham S, Risse G, Gustafson M, Gates J, Penovich P, Al-Asmi A, Dubeau F, Jones-Gotman M. The effects of adjunctive topiramate on cognitive function in patients with epilepsy. Epilepsia. 2003;44:339–347. doi: 10.1046/j.1528-1157.2003.27402.x. [DOI] [PubMed] [Google Scholar]
- Likhitsathian S, Saengcharnchai P, Uttawichai K, Yingwiwattanapong J, Wittayanookulluk A, Srisurapanont M. Cognitive changes in topiramate-treated patients with alcoholism: a 12-week prospective study in patients recently detoxified. Psychiatry Clin Neurosci. 2012;66:235–241. doi: 10.1111/j.1440-1819.2012.02326.x. [DOI] [PubMed] [Google Scholar]
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12-week, randomized, placebo-controlled trial. Drug Alcohol Depend. 2013;133:440–446. doi: 10.1016/j.drugalcdep.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Carlson EB, Hill K. A randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27:677–681. doi: 10.1097/jcp.0b013e31815a43ee. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NA, Kampman K, Stout R. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy E, Petrakis I. Epidemiology and management of alcohol dependence in individuals with post-traumatic stress disorder. CNS Drugs. 2010;24:997–1007. doi: 10.2165/11539710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Poling J, Levinson C, Nich C, Carroll K, Ralevski E, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biological Psychiatry. 2006;60:777–783. doi: 10.1016/j.biopsych.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology. 2012;37:996–1004. doi: 10.1038/npp.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati H, Weiss R, Dundon W, Miller W, Donovan D, Ernst D, Rounsaville B. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. Journal of studies on alcohol. 2005:170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- Pharmaceuticals J. Full Prescribing Information for TOPOMAX 2012 [Google Scholar]
- Rubio G, Martinez-Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. Journal of Clinical Psychopharmacology. 2009;29:584–589. doi: 10.1097/JCP.0b013e3181bfdb79. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption, Measuring Alcohol Consumption. The Humana Press Inc.; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Maisto SA, Cooper AM. Time-line follow-back assessment method National Institute on Alcoholism and Alcohol Abuse. Washington, D.C.: 1985. [Google Scholar]
- Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: Recent progress. Addict Behav. 2014;39:428–433. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Trautman RP, Wyatt DB, Thompson J, Wu SC, Capece JA, Rosenthal NR. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:201–206. doi: 10.4088/jcp.v68n0204. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT. Psychometric properties of the Clinician-Administered PTSD Scale, CAPS-1. PTSD Research Quarterly. 1994;5:2–6. [Google Scholar]
- Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician. 2009;80:44–50. [PubMed] [Google Scholar]
- Yeh M, Mari J, Costa M, Andreoli S, Bressan R, Mello M. Double-blind randomized controlled trial to study efficacy of topiramate in a civilian sample of PTSD. CNS Neuroscience Therapy. 2011;17:305–310. doi: 10.1111/j.1755-5949.2010.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]