Abstract
Background
While frequently assessed in trials and clinical practice, hemodynamic response to therapy has never been validated as a surrogate endpoint for clinical events in pulmonary arterial hypertension (PAH).
Methods and Results
We performed a patient-level pooled analysis of four randomized placebo-controlled trials to determine if treatment-induced changes in hemodynamic values at 12 weeks accounted for the relationship between treatment assignment and the probability of early clinical events (death, lung transplantation, atrial septostomy, PAH hospitalization, withdrawal for clinical worsening, escalation in PAH therapy). We included 1119 subjects with PAH. The median (interquartile range) age was 48 (37 – 59), and 23% were men. 656 (59%) received active therapy (101 [15%] iloprost, 118 [18%] sitaxsentan, 204 [31%] sildenafil, and 233 [36%] subcutaneous treprostinil). Active treatment significantly lowered right atrial pressure (RAP), mean pulmonary artery pressure (mPAP), and pulmonary vascular resistance and increased cardiac output and index (p < 0.01 for all). Changes in hemodynamic values (except for RAP and mPAP) were significantly associated with the risk of a clinical event (p ≤ 0.01 for all). While active treatment approximately halved the odds of a clinical event compared to placebo (p < 0.001), changes in hemodynamics accounted for only 1.2 – 13.9% of the overall treatment effect.
Conclusions
Treatment-induced changes in hemodynamics at 12 weeks only partially explain the impact of therapy on the probability of early clinical events in PAH. These findings suggest that resting hemodynamics are not valid surrogate endpoints for short-term events in PAH clinical trials.
Keywords: hemodynamics, pulmonary heart disease, trials
INTRODUCTION
Hemodynamic measures such as right atrial pressure (RAP), mean pulmonary artery pressure (mPAP), and cardiac index (CI) are the cornerstones of diagnosis and risk assessment in pulmonary arterial hypertension (PAH).1–3 As such, hemodynamics often serve as primary or secondary endpoints in Phase II trials of investigational PAH therapies as a signal for efficacy.4–8 While the United States Food and Drug Administration (FDA) does not consider hemodynamics as adequate surrogate or primary endpoints, many Phase III trials of currently approved PAH treatments have included hemodynamics as secondary endpoints.9–16 Given the absence of other well-established surrogate endpoints in PAH, the validation of hemodynamic markers as surrogate endpoints in PAH would be critical to improving the efficiency of drug evaluation.
Contrary to conventional wisdom, a recent study-level meta-analysis suggested that changes in hemodynamic measures with PAH therapy might not predict clinical events.17 The objective of our study was to determine whether changes in RAP, mPAP, cardiac output (CO), CI, and pulmonary vascular resistance (PVR) are valid surrogate endpoints in PAH clinical trials using a mediator analytic approach with patient-level data, the “gold standard” approach to synthesizing data across trials.18, 19 We pooled individual-level data from four randomized placebo-controlled trials submitted to the FDA for drug approval. We hypothesized that changes in hemodynamics at 12 weeks (adjusted for measures at baseline) would account for a significant portion of the relationship between treatment assignment and the odds of a clinical event, validating hemodynamics as surrogate endpoints in PAH.
METHODS
Study Population
We used de-identified individual patient data from placebo-controlled randomized trials of targeted PAH therapies submitted to the United States FDA through 2008. Eleven trials (ARIES-1 & -2, Bosentan-351, BREATHE-1, AIR, AIR II, SUPER, STRIDE-1, -2, and -4, and the subcutaneous treprostinil trial) comparing six active therapies (ambrisentan, bosentan, iloprost, sildenafil, sitaxsentan, and subcutaneous treprostinil, respectively) to placebo were considered. Details of these trials are provided elsewhere, but all had similar inclusion criteria and data collection processes.10–15, 20–23
We included patients from Phase III trials which collected baseline and 12-week hemodynamic values. We excluded patients with missing baseline hemodynamics and one study (PHIRST) because some subjects in this trial had been treated with background bosentan therapy.24 ARIES 1&2, BREATHE-1, STRIDE-2 and -4 were excluded because these trials did not assess hemodynamics at 12 weeks; Bosentan-351 and AIR II were excluded because they were small or not Phase III trials. The final study population included patients from four trials (AIR, SUPER, STRIDE-1, and subcutaneous treprostinil) of four therapies (iloprost, sildenafil, sitaxsentan, and subcutaneous treprostinil, respectively).
Clinical Events
Clinical events included the first occurrence of any of the following prior to the end of the randomized portion of the trials: death, lung transplantation, atrial septostomy, hospitalization due to worsening PAH, withdrawal for clinical worsening, or escalation in PAH therapy. We did not include change in six-minute walk distance (6MWD) as a clinical event in the primary analysis, because it has not been established as a patient-centered outcome and is itself an imperfect surrogate for clinical outcomes in PAH.25, 26 Sensitivity analyses including a decrement in 6MWD in the composite endpoint were performed (described below).
Hemodynamics
Hemodynamic values at baseline and at 12 weeks as reported to the FDA were used to calculate the absolute change in RAP (ΔRAP), mPAP (ΔmPAP), CO (ΔCO), CI (ΔCI), and PVR (ΔPVR). In addition to traditional hemodynamic measures, we included pulmonary artery (PA) compliance, calculated as follows: (CO/heart rate)/(systolic PAP – diastolic PAP).27–29 Subjects missing baseline hemodynamic values were excluded from all analyses (N = 20 for RAP, N = 4 for mPAP, N = 18 for CO, N = 60 for PVR, total N excluded = 102 [9%]).
Statistical Analysis
Continuous variables were expressed as median (interquartile range) and categorical variables were expressed as percentages. We employed a mediator analysis which is the preferred approach to validating potential surrogate endpoints.30, 31 Treatment assignment was designated as either active treatment or placebo. Regression analysis was used to evaluate four hypotheses. The rejection of all four null hypotheses was required to deem the change in a given hemodynamic measure a valid surrogate endpoint. The four alternative hypotheses included:
Treatment assignment has a significant effect on change in hemodynamics (ΔRAP, ΔmPAP, ΔCO, ΔCI, ΔPVR and ΔPA compliance) at 12-weeks;
Change in hemodynamics (ΔRAP, ΔmPAP, ΔCO, ΔCI, ΔPVR and ΔPA compliance) has a significant association with the odds of a clinical event;
Treatment assignment has a significant effect on the odds of a clinical event;
The effect of treatment assignment on the odds of a clinical event is attenuated when change in a given hemodynamic parameter (ΔRAP, ΔmPAP, ΔCO, ΔCI, ΔPVR and ΔPA compliance) is added to the model.
Logistic or linear regression was used for binary or continuous outcomes, respectively. All models were adjusted for the given hemodynamic measure at baseline and study.
Following the tests for mediation, we determined the proportion of the treatment effect (PTE) explained by ΔRAP, ΔmPAP, ΔCO, ΔCI, ΔPVR and ΔPA compliance, respectively.32, 33 Generalized linear modeling with a logit link was used to determine the effect estimate for treatment assignment and log odds of a clinical event, adjusted for baseline hemodynamic value and study. ΔRAP, ΔmPAP, ΔCO, ΔCI, ΔPVR and ΔPA compliance were then individually added to the initial models, such that the change in the effect estimates between baseline and those containing the hemodynamic mediator provided the amount of variability in clinical events explained by a change in the mediator at 12 weeks. Estimates of percent change were obtained for each resampled dataset, and the standard deviation of the estimates across 1000 resampled datasets were used as the standard error.34
A number of sensitivity and subset analyses were performed. First, the clinical event definition was expanded to include a 15% decrement in 6MWD during the randomized portion of the trials (in addition to death, lung transplantation, atrial septostomy, hospitalization due to worsening PAH, withdrawal for clinical worsening, or escalation in PAH therapy). Those missing 12-week 6MWD values were counted as having had an event. Second, we tested whether achieving certain established thresholds of hemodynamic improvement (i.e., CI > 2 L/min/m2 or > 3 L/min/m2, reduction in PVR > 30% at 12 weeks), using absolute values of hemodynamics (rather than the change values), and nonparametric modeling (using local smoothers) revealed alternative hemodynamic cutpoints which performed better as surrogates.1, 3, 35 Third, we adjusted our primary analysis for New York Heart Association (NYHA) functional class. Fourth, we included subset analyses limiting our study population to those with idiopathic PAH, connective tissue disease (CTD) and congenital heart disease associated PAH, respectively, as well as to the subset of patients who achieved a 12 week 6MWD of > 380 m, which is suggested as a treatment target in recent consensus guidelines.35, 36 Finally, for the AIR study, we substituted hemodynamic values 60 minutes post-iloprost inhalation (as opposed to those pre-inhalation for the main analysis) to calculate the change in hemodynamics.
Multiple imputation was used to derive missing 12-week hemodynamic values (N = 104 [9%] for RAP, N = 100 [9%] for mPAP, N = 100 for CO [9%], N = 134 [12%] for PVR).37 Age, sex, race, height, weight, diagnosis (idiopathic, CTD, human immunodeficiency virus infection/anorexigen use, or congenital heart disease), baseline 6MWD, baseline hemodynamic parameter, NYHA functional class, warfarin use, and baseline sodium level were included as predictors in the imputation models for the missing 12-week hemodynamic values. All analyses were performed using SAS version 9.2 and R, version 2.14.1. Statistical significance was defined as p < 0.05.
This study was determined to be exempt by the Institutional Review Board of the University of Pennsylvania (approval #818239). All co-authors had access to study data, take responsibility for the analysis, and have contributed to manuscript preparation and the decision to submit for publication.
RESULTS
The final study sample included 1119 patients from four trials (AIR, SUPER, STRIDE-1, and subcutaneous treprostinil) of four therapies (iloprost [N = 202, 18%], sildenafil [N = 269, 24%], sitaxsentan [N = 178, 16%], and subcutaneous treprostinil [N = 470, 42%], respectively). Characteristics of the study population and those receiving active treatment (N = 656, 59%) or placebo (N = 463, 41%) are shown in Table 1. The median age was 48 (range 37 – 59) years and 23% were men. Six hundred and thirty seven patients (60%) had idiopathic PAH, 247 (23%) had CTD associated PAH, and 482 (45%) were NYHA functional class III or IV. Demographics, anthropometrics, diagnoses, baseline 6MWD and baseline hemodynamics were similarly balanced by treatment allocation (as would be expected with randomization). A total of 110 patients (10%) had a clinical event between baseline and 12 weeks. Hospitalization for worsening PAH (N = 70) and premature withdrawal (N = 35) constituted the majority of these events; 29 deaths occurred during the 12 week randomized portion of the trials.
Table 1.
Characteristics of study participants
| Characteristic | Overall (n = 1119) | Active treatment (n=656) | Placebo (n=463) |
|---|---|---|---|
| Age, years | 48 (37–59) | 47 (36–58) | 49 (37–59) |
| Male, n (%) | 255 (23) | 145 (22) | 110 (24) |
| Race, n (%) | |||
| White | 943 (85) | 553 (86) | 390 (85) |
| Black | 42 (4) | 27 (4) | 15 (3) |
| Other | 134 (12) | 76 (12) | 58 (13) |
| Height, cm | 163 (157–170) | 163 (157–170) | 163 (157–170) |
| Weight, kg | 70.0 (59.1–81.5) | 69.1 (58.0–81.4) | 70.0 (60.3–81.6) |
| BMI, kg/m2 | 25.9 (22.5–30.0) | 25.7 (22.2–29.8) | 26.0 (22.8–30.3) |
| PAH diagnosis, n (%) | |||
| Idiopathic | 637 (60) | 374 (60) | 263 (60) |
| Connective tissue disease | 247 (23) | 147 (24) | 100 (23) |
| HIV/anorexigen use | 10 (1) | 5 (1) | 5 (1) |
| Congenital heart disease | 169 (16) | 98 (16) | 71 (16) |
| NYHA functional classification, n (%) | |||
| I/II | 599 (55) | 326 (51) | 273 (62) |
| III/IV | 482 (45) | 312 (49) | 170 (38) |
| Baseline hemodynamics | |||
| RAP, mm Hg | 8.0 (5.0–12.0) | 8.0 (5.0–12.0) | 8.0 (5.0–12.0) |
| mPAP, mm Hg | 55.0 (46.0–65.0) | 55.0 (46.0–64.0) | 56.0 (47.0–67.0) |
| Cardiac output, L/min | 3.8 (3.1–4.8) | 3.8 (3.2–4.9) | 3.8 (3.1–4.6) |
| Cardiac index, L/min/m2 | 2.1 (1.8–2.6) | 2.2 (1.8–2.8) | 2.1 (1.8–2.5) |
| PCWP, mm Hg | 9.0 (6.0–11.0) | 9.0 (6.0–11.0) | 9.0 (6.0–12.0) |
| PVR, Wood units | 11.9 (8.4–16.8) | 11.6 (7.9–16.5) | 12.4 (8.9–17.0) |
| PA compliance, mL/mmHg | 0.93 (0.68–1.33) | 0.95 (0.71–1.40) | 0.88 (0.65–1.23) |
| Sodium, mEq/L | 140 (138–142) | 140 (138–142) | 140 (138–142) |
| Warfarin use, n (%) | 582 (63) | 338 (61) | 244 (67) |
| Baseline 6MWD, m | 352 (281–409) | 355 (288–408) | 348 (273–410) |
| Study, n (%) | |||
| AIR | 202 (18) | 101 (15) | 101 (22) |
| SUPER | 269 (24) | 204 (31) | 65 (14) |
| STRIDE-1 | 178 (16) | 118 (18) | 60 (13) |
| Treprostinil | 470 (42) | 233 (36) | 237 (51) |
Summaries provided as median (Q1–Q3) unless otherwise indicated by n (%). BMI=body mass index; HIV=human immunodeficiency virus; NYHA=New York Heart Association; RAP=right atrial pressure; PAP=pulmonary artery pressure; PCWP=pulmonary capillary wedge pressure; PVR=pulmonary vascular resistance; PA=pulmonary artery; AIR=Aerosolized Iloprost Randomized; STRIDE=Sitaxsentan To Relieve Impaired Exercise; SUPER=Sildenafil Use in Pulmonary Hypertension.
The results for the four criteria necessary to establish changes in selected hemodynamic indices as mediators in the relationship between treatment allocation and clinical events are presented in Tables 2–4. Assignment to active treatment significantly reduced RAP, mPAP, and PVR and increased CO, CI, and PA compliance (Criterion #1; Table 2). Second, the relationship between the change in a given hemodynamic value at 12 weeks and the odds of a clinical event was tested (Criterion #2; Table 3). Decrease in PVR and increases in CO and CI were associated with significant reductions in the odds of a clinical event. Effect estimates appear small as they refer to the odds of a clinical event per 1 unit increase in the hemodynamic measure (e.g., odds ratio [OR] 1.07 for every 1 Wood unit increase in PVR, 95% CI 1.02 – 1.12, p = 0.006). No corresponding relationship was identified between ΔmPAP and ΔPA compliance and outcomes. Third, we assessed whether treatment allocation impacted the odds of a clinical event at 12 weeks in subjects with available respective hemodynamic measures. In all cases, active treatment substantially reduced the odds of a clinical event compared to placebo (ORs ranged from 0.43 – 0.49) (P < 0.001 for all) (Criterion #3; Table 4).
Table 2.
Treatment assignment has a significant effect on change in hemodynamics at 12 weeks (Criterion #1)
| Hemodynamic measure | Mean difference between treatment and placebo | 95% CI | P value |
|---|---|---|---|
|
| |||
| ΔRAP | −1.2 mm Hg | −1.8, −0.6 | < 0.001 |
| ΔmPAP | −2.4 mm Hg | −3.4, −1.3 | 0.003 |
| ΔCO | 0.37 L/min | 0.24, 0.50 | < 0.001 |
| ΔCI | 0.22 L/min/m2 | 0.14, 0.29 | < 0.001 |
| ΔPVR | −2.1 Wood units | −2.7, −1.5 | < 0.001 |
| ΔPA compliance | 0.15 mL/mm Hg | 0.09, 0.21 | < 0.001 |
All models include adjustment for baseline hemodynamic value and study. CI=confidence interval; ΔRAP=change in right atrial pressure; ΔmPAP=change in mean pulmonary artery pressure; ΔCO=change in cardiac output; ΔCI=change in cardiac index; ΔPVR=change in pulmonary vascular resistance; ΔPA compliance=change in pulmonary artery compliance.
Table 4.
Treatment assignment has a significant effect on the odds of a clinical event (Criterion #3) and the change in the odds of a clinical event when a given hemodynamic mediator is added to the model (Criterion #4)
| Criterion #3 | Criterion #4 | ||||
|---|---|---|---|---|---|
|
| |||||
| Baseline hemodynamic measure | OR active treatment vs. placebo | 95% CI | P value | OR active treatment vs. placebo when mediator added to model | Absolute change in OR when mediator is added to model |
|
| |||||
| RAP | 0.43 | 0.28, 0.66 | < 0.001 | 0.44 | 0.01 |
| mPAP | 0.48 | 0.31, 0.72 | < 0.001 | 0.48 | 0.00 |
| CO | 0.48 | 0.32, 0.74 | < 0.001 | 0.53 | 0.05 |
| CI | 0.49 | 0.32, 0.74 | < 0.001 | 0.53 | 0.04 |
| PVR | 0.47 | 0.30, 0.72 | < 0.001 | 0.52 | 0.05 |
| PA compliance | 0.48 | 0.31, 0.73 | < 0.001 | 0.49 | 0.01 |
All models include adjustment for the listed baseline hemodynamic value and study. OR=odds ratio; CI=confidence interval; RAP=right atrial pressure; mPAP=mean pulmonary artery pressure; CO=cardiac output; CI=cardiac index; PVR=pulmonary vascular resistance; PA=pulmonary artery.
Table 3.
Change in hemodynamics has a significant association with the odds of a clinical event (Criterion #2)
| Hemodynamic measure | OR clinical event per 1 unit increase of hemodynamic measure | 95% CI | P value |
|---|---|---|---|
|
| |||
| ΔRAP | 1.04 | 0.99, 1.09 | 0.124 |
| ΔmPAP | 1.01 | 0.98, 1.04 | 0.524 |
| ΔCO | 0.70 | 0.53, 0.93 | 0.011 |
| ΔCI | 0.54 | 0.33, 0.89 | 0.013 |
| ΔPVR | 1.07 | 1.02, 1.12 | 0.006 |
| ΔPA compliance | 0.67 | 0.21, 2.12 | 0.798 |
All models include adjustment for baseline hemodynamic value and study. OR=odds ratio; CI=confidence interval; ΔRAP=change in right atrial pressure; ΔmPAP=change in mean pulmonary artery pressure; ΔCO=change in cardiac output; ΔCI=change in cardiac index; ΔPVR=change in pulmonary vascular resistance; ΔPA compliance=change in pulmonary artery compliance.
Fourth, we assessed the change in the treatment ORs for clinical events after adjustment for the change in a given hemodynamic value at 12 weeks (Criterion #4; Table 4). There were no appreciable changes in the treatment ORs after serially accounting for ΔRAP, ΔmPAP, ΔCO, ΔCI, ΔPVR, and ΔPA compliance. For example, the OR for the relationship between treatment assignment and a clinical event only changed from 0.47 (95% CI 0.30 – 0.72) to 0.52 (95% CI 0.33 – 0.81) once ΔPVR was included in the model, suggesting that ΔPVR does not account for (or mediate) the effect of treatment on short-term clinical events in PAH. The fourth criterion was not met for any hemodynamic measure. Therefore, rejection of the null hypotheses was not possible, suggesting that no hemodynamic measure appeared to be a valid surrogate.
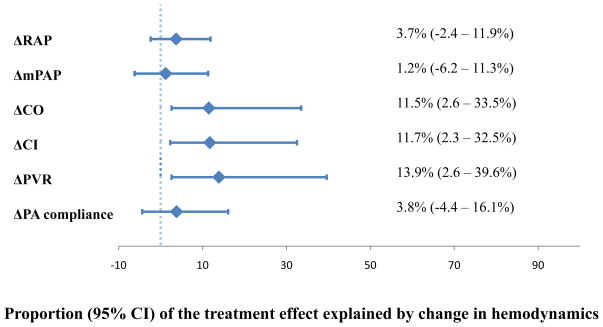
We next quantified the proportion of the treatment effect (PTE) explained by the hemodynamic change values (Figure 1). These mediators accounted for little (ΔCO, ΔCI, ΔPVR) to none (ΔRAP, ΔmPAP, ΔPA compliance) of the treatment effect. At most, ΔCI and ΔPVR accounted for 11.7% and 13.9% of the impact of treatment on clinical outcomes, well-below the proposed cutoff of 50–75% necessary to declare a surrogate valid.32
Figure 1.
Proportion of the treatment effect explained by change in hemodynamics. ΔRAP=change in right atrial pressure; ΔmPAP=change in mean pulmonary artery pressure; ΔCO=change in cardiac output; ΔCI=change in cardiac index; ΔPVR=change in pulmonary vascular resistance; ΔPA compliance=change in pulmonary artery compliance; CI=confidence interval
Sensitivity and Subset Analyses
The results were unchanged when a 15% decrement in 6MWD was included in the definition of a clinical event (Table 5). The ORs shown for Criterion #4 were not substantially attenuated with inclusion of the hemodynamic measures compared to the models for Criterion #3. While the PTEs were generally greater than in the main analysis (e.g., ΔPVR accounted for 26.9% of the treatment-clinical event relationship), they were still well below 50%, suggesting the hemodynamic change values were not acting as mediators. Similarly, analyses using predefined thresholds to indicate hemodynamic improvement, absolute values of hemodynamics (rather than change values), cutpoints derived from nonparametric modeling, and adjustment for NYHA functional class did not improve the performance of hemodynamics as surrogates (data not shown). Subset analysis including only idiopathic patients (N = 637) yielded results similar to our primary analysis (Supplemental Table 1). In the small subgroups of patients with CTD, congenital heart disease, and those patients who achieved a 6MWD > 380 m at 12 weeks (N = 469), findings were also unchanged although the estimates were imprecise due to a very low number of events (N = 21, 11, and 9 events, respectively) (data not shown). We repeated our analysis substituting hemodynamic values 60 minutes post-iloprost inhalation (in patients from the AIR study) in order to calculate the change values and the results were nearly identical (Supplemental Table 2).
Table 5.
Criteria to establish the change in a hemodynamic measure (ΔHD) as a mediator in the relationship between treatment assignment and clinical events including a 15% decrement in 6MWD at 12 weeks
| Hemodynamic measure | ||||||
|---|---|---|---|---|---|---|
| ΔRAP | ΔmPAP | ΔCO | ΔCI | ΔPVR | ΔPA Compliance | |
| 1. Treatment assignment has a significant effect on ΔHD | Mean difference (95% CI) between treatment and placebo −1.2 mm Hg (−1.8, −0.63) p < 0.001 |
Mean difference (95% CI) between treatment and placebo −2.4 mm Hg (−3.4, −1.31) p < 0.001 |
Mean difference (95% CI) between treatment and placebo 0.37 L/min (0.24, 0.50) p < 0.001 |
Mean difference (95% CI) between treatment and placebo 0.22 L/min/m2 (0.14, 0.29) p < 0.001 |
Mean difference (95% CI) between treatment and placebo −2.1 Wood units (−2.7, −1.5) p < 0.001 |
Mean difference (95% CI) between treatment and placebo 0.15 mL/mm Hg (0.09, 0.21) p < 0.001 |
| 2. ΔHD has a significant effect on the odds of a clinical event | OR (95% CI) per 1 mm Hg increase 1.06 (1.02, 1.09) p = 0.002 |
OR (95% CI) per 1 mm Hg increase 1.02 (1.00, 1.04) p = 0.036 |
OR (95% CI) per 1 L/min increase 0.65 (0.53, 0.78) p < 0.001 |
OR (95% CI) per 1 L/min/m2 unit increase 0.46 (0.32, 0.65) p < 0.001 |
OR (95% CI) per 1 Wood unit increase 1.10 (1.06, 1.15) p < 0.001 |
OR (95% CI) per 1 mL/mmHg increase 0.63 (0.40, 0.98) p = 0.034 |
| 3. Treatment assignment has a significant effect on the odds of a clinical event | OR (95% CI) for treatment vs. placebo 0.51 (0.38, 0.69) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.55 (0.41, 0.73) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.56 (0.42, 0.75) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.56 (0.42, 0.76) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.54 (0.40, 0.73) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.55 (0.41, 0.73) p < 0.001 |
| 4. The effect of treatment assignment on the odds of a clinical event is attenuated with the addition of ΔHD to the model (compare with #3 above) | OR (95% CI) for treatment vs. placebo 0.54 (0.40, 0.73) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.56 (0.42, 0.76) p < 0.001 |
OR (95% CI) for treatment vs. placebo 0.63 (0.47, 0.85) p = 0.003 |
OR (95% CI) for treatment vs. placebo 0.64 (0.47, 0.87) p = 0.004 |
OR (95% CI) for treatment vs. placebo 0.64 (0.47, 0.87) p = 0.005 |
OR (95% CI) for treatment vs. placebo 0.57 (0.42, 0.77) p < 0.001 |
| % variability explained by ΔHD (95% CI) | 7.6 (1.9, 19.0) | 5.4 (−1.6, 15.4) | 20.6 (9.5, 44.8) | 22.2 (10.7, 49.2) | 26.9 (12.9, 60.5) | 7.4 (0.6, 19.9) |
All models include adjustment for baseline hemodynamic value and study. ΔRAP=change in right atrial pressure; ΔmPAP=change in mean pulmonary artery pressure; ΔCO=change in cardiac output; ΔCI=change in cardiac index; ΔPVR=change in pulmonary vascular resistance; ΔPA compliance=change in pulmonary artery compliance; ΔHD=change in hemodynamic measure at 12 weeks as compared to baseline; OR=odds ratio; CI=confidence interval.
DISCUSSION
Based on this large pooled analysis of patient-level data we are unable to conclude that treatment induced changes in resting hemodynamics are valid surrogate endpoints for short-term outcomes in PAH. While 1) active treatment was significantly associated with modest improvement in hemodynamics at 12 weeks, 2) changes in some hemodynamics were significantly associated with the odds of clinical events, and 3) treatment assignment considerably reduced the odds of a clinical event, the relationship between treatment and clinical events was not mediated or “explained” by treatment-induced changes in these values. This held true in multiple secondary analyses which included the incorporation of 6MWD decrement into the clinical event definition and the restriction of the study sample to patients with idiopathic PAH.
The use of hemodynamic measures as surrogate endpoints in PAH clinical trials is appealing, given that these measures may in theory be standardized, are widely available, and are integral to our understanding and definition of PAH.38, 39 Observational studies have consistently shown associations between hemodynamics (especially those that pertain to right heart function, such as CO) and event-free survival (as seen in this study); however, such observational data are not sufficient to validate potential surrogates.1, 2, 40 Changes in hemodynamics with PAH treatment predict outcome, but the specific measures of import have been inconsistent across studies (e.g., mPAP and CI versus a reduction in PVR).2, 3 A useful surrogate endpoint should be reliable, valid, and account for most of the impact of a therapy on the ultimate clinical endpoint in multiple studies and settings.38
Our study demonstrates that treatment-induced changes in hemodynamics do not account for the treatment-associated reduction in events at 12 weeks, calling into question traditional assumptions about the mechanisms that underpin the effects of targeted PAH therapy on clinical outcomes. Treatment-associated changes in hemodynamics have been reported to be rather modest in short-term Phase III trials in PAH. In a randomized clinical trial of epoprostenol, the difference in the mean change for PVR between treatment and placebo groups was −4.9 Wood units (95% confidence interval −7.6 – −2.3 Wood units), for example.9
A recent study-level meta-analysis by Savarese and colleagues yielded similar results.17 Assignment to active treatment was associated with hemodynamic improvements and significantly reduced the odds of the composite outcome (OR 0.3, 95% confidence interval 0.3 – 0.5). However, there was no association between hemodynamic changes and the composite outcome at the study-level.17 This null finding could be explained by the analytic methods employed, as meta-analyses at the study-level are less robust and may suffer from aggregation bias (known as the ecological fallacy).18, 19 We have used patient-level data and the currently accepted approach to establishing surrogacy and found that short-term improvements in hemodynamics actually contribute very little (at most 13.9% for ΔPVR in our primary analysis) to the impact of treatment on early clinical events. It has been proposed that a valid surrogate endpoint should explain between 50 – 75% of the exposure-outcome relationship of interest.32
There are several possible explanations for these findings. Effective PAH therapies may act via heretofore unmeasured (or insufficiently measured) physiologic pathways to impact on outcome. For example, effects on the systemic circulation or other organ systems rather than changes in the pulmonary vasculature could contribute substantially to therapeutic benefit.41 Treatment could also affect right ventricular (RV) loading conditions in complex ways not adequately captured by invasive central measurements at rest. Small observational studies have used cardiac magnetic resonance imaging (MRI) to study the effects of PAH therapy on RV structure and function, and one has shown worsening RV performance is associated with poor outcomes independent of improvements in PVR.42–44 While it may not be feasible to incorporate cardiac MRI in a large-scale fashion in PAH clinical trials, other RV biomarkers may prove to be better surrogates. Advanced or novel RV imaging techniques, such as speckle-tracking Doppler and positron emission tomography, have been linked to clinical deterioration and RV metabolic changes in PAH, respectively, although more work is needed to establish clear correlations between these measures, PAH treatment response, and outcomes.45–48 While brain natriuretic peptide (BNP) is an easily obtained and reliable measure of RV strain, a recent study of changes in plasma BNP after treatment failed to discriminate two year survival in patients with PAH.49 Although we incorporated a measure of PA compliance in our study, there may be more sophisticated and comprehensive measures of the cardiopulmonary interaction which prove to be valid surrogates in PAH.
While short-term hemodynamic response may be a poor surrogate for short-term clinical events, these results may not apply to trials of longer duration. It is possible that changes in hemodynamics following longer-term therapy are adequate surrogates for long-term outcomes. We are not able to answer this question, since essentially all clinical trials including hemodynamic measures had a placebo-controlled period of only a few months (and long-term open labeled extension studies are not sufficient for the required analyses). Larger, time-to-event trials incorporating composite clinical outcomes have only recently been conducted in PAH NCT01106014, NCT01178073).50 While treatment-induced changes in hemodynamics were not adequate surrogates in patients without treatment at baseline, the validity in patients with background treatment, combination therapy, or in patients evaluated or reassessed sequentially is unknown.
This study has some limitations. The patient-level data were limited by design of the individual trials. Since the FDA accepts changes in short-term intermediate endpoints (6MWD) for registration, these Phase III trials were not surprisingly of short duration, limiting our conclusions regarding surrogacy for long-term outcomes. The exclusion of those subjects with missing baseline values could result in selection bias, however those excluded (< 10%) were similar to those in the final study sample, making this less likely. The imputation of 12-week hemodynamic values (at most 12% for PVR) may have been an additional source of bias. Four Phase III trials (of 11 in the dataset) included treatment naïve patients and had repeat hemodynamics available, although > 1000 patients were included as were all major therapeutic classes (i.e., prostacyclin analogues, endothelin receptor antagonists [ERA], and phosphodiesterase type 5 inhibitors), increasing generalizability. The ERA sitaxsentan was shown to be efficacious in STRIDE-1 and other trials, but was withdrawn from the market for hepatic toxicity. Sitaxsentan was the only ERA with follow-up hemodynamics in a Phase III trial.20 While two of the trials included, AIR and the subcutaneous treprostinil trial, met their respective primary endpoints for efficacy, effect sizes were smaller in these trials as compared to Phase III trials for other targeted PAH therapies.10, 11 An additional subset analysis including only the SUPER and STRIDE-1 trials did not alter our results, although precision was limited due to smaller sample size and lower event rate (data not shown). The results could differ with other treatments, in patients receiving background therapy, or for trials performed in other geographic regions or eras. Last, it is unknown whether the validity of hemodynamic measures would be enhanced by employing highly standardized measurement techniques and/or by capturing serial or dynamic (e.g., exercise or composite) values.
Treatment-induced changes in pulmonary hemodynamics measured at rest are not valid surrogates for short-term clinical outcomes in PAH trials. While active treatment was associated with significant hemodynamic improvement, this response to therapy mediates very little of the treatment effect on key clinical endpoints at 12 weeks. The impact of longer-term therapy on hemodynamics and the validity of these changes over time remain unknown. Future work should focus on validating commonly accepted surrogates in PAH prior to their incorporation into advanced phase trials, and on defining more robust and dynamic measures of disease burden in PAH.
Supplementary Material
Acknowledgments
The authors would like to thank Maximilian Herlim for his help with data preparation and analysis and Dr. Norman Stockbridge at the U.S. Food and Drug Administration for providing us with the data to make this study possible.
Funding Sources: This publication was made possible by grants from the American Heart Association 11FTF7400032 and National Institutes of Health K24 HL103844 and P20 GM103652.
Footnotes
Conflict of Interest Disclosures: Dr. Ventetuolo has received consulting fees from Maquet, served on advisory boards for United Therapeutics and Gilead, and has received grant support from Pfizer and Actelion. Dr. Gabler has participated in unrelated projects funded by Pfizer. Dr. Fritz has received advisory board fees from Gilead and institutional funding for the conduct of industry-sponsored, multi-center clinical trials from Actelion. Dr. Smith has received research funding from Gilead and United Therapeutics. Dr. Palevsky has received consulting fees, advisory board fees, speaking fees, and/or research funding from Actelion, Aires, Bayer, GeNO, Gilead, Pfizer, and United Therapeutics. Dr. Klinger has received consulting fees from United Therapeutics and Bayer, speaking fees from Gilead, and research funding from Actelion, Bayer, Gilead, and United Therapeutics. Dr. Halpern has received consulting fees from the World Bank. Dr. Kawut has served on Steering and Grant review committees for Gilead and Pfizer and received consulting fees from Ikaria and Insamed. His institution has received unrestricted educational grants from Actelion, Gilead, United Therapeutics, Lung Rx, Pfizer, Ikaria, and Merck, and grant funding from Gilead and Actelion.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: The impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: Prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 4.Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, Barst RJ. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension. Chest. 2006;129:683–688. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 5.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, Diehl JH, Crow J, Long W. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 6.Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlócai K, Galiè N, Degano B, Bonderman D, Kurzyna M, Efficace M, Giorgino R, Lang IM. Selexipag, an oral, selective IP receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J. 2012;40:874–880. doi: 10.1183/09031936.00137511. [DOI] [PubMed] [Google Scholar]
- 8.Voswinckel R, Enke B, Reichenberger F, Kohstall M, Kreckel A, Krick S, Gall H, Gessler T, Schmehl T, Ghofrani HA, Schermuly RT, Grimminger F, Rubin LJ, Seeger W, Olschewski H. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: Results from randomized controlled pilot studies. J Am Coll Cardiol. 2006;48:1672–1681. doi: 10.1016/j.jacc.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jobsis MM, Blackburn SD, Shortino D, Crow JW The Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 10.Simonneau G, Barst RJ, Galié N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz RJ, Frost AE, Blackburn S, Crow J, Rubin LJ. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 11.Olschewski H, Simonneau G, Galié N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W. Inhaled iloprost for severe pulmonary hypertension. New Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 12.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G the Bosentan Randomized Trial of Endothelin Antagonist Therapy Study Group. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 13.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebocontrolled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ for the Ambrisentan in Pulmonary Arterial Hypertension R, Double-Blind Placebo-Controlled Multicenter Efficacy Studies Group. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G the Sildenafil Use in Pulmonary Arterial Hypertension Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 16.Simonneau Gr, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 17.Savarese G, Musella F, D’Amore C, Losco T, Marciano C, Gargiulo P, Rengo G, Dellegrottaglie S, Bossone E, Leosco D, Perrone-Filardi P. Hemodynamics, exercise capacity and clinical events in pulmonary arterial hypertension. Eur Respir J. 2013;42:414–424. doi: 10.1183/09031936.00123712. [DOI] [PubMed] [Google Scholar]
- 18.Riley RD, Simmonds MC, Look MP. Evidence synthesis combining individual patient data and aggregate data: A systematic review identified current practice and possible methods. J Clin Epidemiol. 2007;60:431.e431–431.e412. doi: 10.1016/j.jclinepi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Thacker SB. Meta-analysis: A quantitative approach to research integration. JAMA. 1988;259:1685–1689. doi: 10.1001/jama.259.11.1685. [DOI] [PubMed] [Google Scholar]
- 20.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RAF, Frumkin LR. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 21.Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, Naeije R, Galie N. Treatment of pulmonary arterial hypertension with the selective endothelin-a receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Sandoval J, Torbicki A, Souza R, Ramírez A, Kurzyna M, Jardim C, Jerjes-Sánchez Díaz C, Teal SA, Hwang L-J, Pulido T. Safety and efficacy of sitaxsentan 50 and 100 mg in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther. 2012;25:33–39. doi: 10.1016/j.pupt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Olschewski H, Hoeper MM, Behr J, Ewert R, Meyer A, Borst MM, Winkler J, Pfeifer M, Wilkens H, Ghofrani HA, Nikkho S, Seeger W. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med. 2010;104:731–740. doi: 10.1016/j.rmed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 25.Gabler NB, French B, Strom BL, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012;126:349–356. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farber HW. Validation of the 6-minute walk in patients with pulmonary arterial hypertension: Trying to fit a square peg into a round hole? Circulation. 2012;126:258–260. doi: 10.1161/CIRCULATIONAHA.112.118547. [DOI] [PubMed] [Google Scholar]
- 27.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 28.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 29.Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, Shandas R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–174. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics. 1998;54:1014–1029. [PubMed] [Google Scholar]
- 31.MacKinnon D, Dwyer J. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–158. [Google Scholar]
- 32.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11:167–178. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Huang B. Evaluating the proportion of treatment effect explained by a continuous surrogate marker in logistic or probit regression models. Stat Biopharm Res. 2010;2:229–238. doi: 10.1198/sbr.2009.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 35.McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S, Palazzini M, Park MH, Tapson VF, Sitbon O. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D73–D81. doi: 10.1016/j.jacc.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 36.Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005;26:858–863. doi: 10.1183/09031936.05.00075305. [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff MA, Cole SR. Use of multiple imputation in the epidemiologic literature. Am J Epidemiol. 2008;168:355–357. doi: 10.1093/aje/kwn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming TR, DeMets DL. Surrogate end points in clinical trials: Are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 39.Ventetuolo CE, Benza RL, Peacock AJ, Zamanian RT, Badesch DB, Kawut SM. Surrogate and combined end points in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5:617–622. doi: 10.1513/pats.200803-029SK. [DOI] [PubMed] [Google Scholar]
- 40.Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra M, Beltran M, Guerrero M. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89:1733–1744. doi: 10.1161/01.cir.89.4.1733. [DOI] [PubMed] [Google Scholar]
- 41.Rich S. The effects of vasodilators in pulmonary hypertension: Pulmonary vascular or peripheral vascular? Circ Heart Fail. 2009;2:145–150. doi: 10.1161/CIRCHEARTFAILURE.108.805374. [DOI] [PubMed] [Google Scholar]
- 42.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, Westwood MA, Stefanidis A, Ng LL, Pennell DJ, Mohiaddin RH, Nihoyannopoulos P, Gibbs JS. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 44.Gan CT, Holverda S, Marcus JT, Paulus WJ, Marques KM, Bronzwaer JG, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest. 2007;132:11–17. doi: 10.1378/chest.06-1263. [DOI] [PubMed] [Google Scholar]
- 45.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–1309. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 46.Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS, Hsiao JF, McCully RB, Oh JK, Pellikka PA, Kane GC. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol. 2013;111:143–148. doi: 10.1016/j.amjcard.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 47.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, Archer SL. Lung (1)(8)f-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, Comhair SA, DiFilippo FP, Neumann DR, Davis L, Graham BB, Tuder RM, Dostanic I, Erzurum SC. Fasting 2-deoxy-2-[18f]fluoro-d-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc. 2013;10:1–9. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fritz JS, Blair C, Oudiz RJ, Dufton C, Olschewski H, Despain D, Gillies H, Kawut SM. Baseline and follow-up 6-min walk distance and brain natriuretic peptide predict 2-year mortality in pulmonary arterial hypertension. Chest. 2013;143:315–323. doi: 10.1378/chest.12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, Mittelholzer CM, Perchenet L, Sastry BK, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.