SUMMARY
While imatinib and other tyrosine kinase inhibitors (TKIs) are highly efficacious in the treatment of chronic myeloid leukaemia (CML), some patients become refractory to these therapies. After confirming that interleukin-3 receptor (IL3R, CD123) is highly expressed on CD34+/CD38− BCR-ABL1+ CML stem cells, we investigated whether targeting IL3R with diphtheria toxin (DT)-IL3 fusion proteins SL-401 (DT388-IL3) and SL-501 (DT388-IL3[K116W]) could eradicate these stem cells. SL-401 and SL-501 inhibited cell growth and induced apoptosis in the KBM5 cell line and its TKI-resistant KBM5-STI subline. Combinations of imatinib with these agents increased apoptosis in KBM5 and in primary CML cells. In six primary CML samples, including CML cells harbouring the ABL1 T315I mutation, SL-401 and SL-501 decreased the absolute numbers of viable CD34+/CD38−/CD123+ CML progenitor cells by inducing apoptosis. IL3-targeting agents reduced clonogenic growth and diminished the fraction of primitive long-term culture-initiating cells in samples from patients with advanced phase CML that were resistant to TKIs or harboured an ABL1 mutation. Survival was also extended in a mouse model of primary TKI-resistant CML blast crisis. These data suggest that the DT-IL3 fusion proteins, SL-401 and SL-501, deplete CML stem cells and may increase the effectiveness of current CML treatment, which principally targets tumour bulk.
Keywords: SL-401, SL-501, chronic myeloid leukaemia, leukaemic stem cells, interleukin-3 receptor
INTRODUCTION
Chronic myeloid leukaemia (CML) is a malignant clonal disorder of haematopoietic stem cells that results in increased numbers of myeloid cells, erythroid cells and platelets in peripheral blood, as well as marked myeloid hyperplasia in the bone marrow. Eighty percent of newly diagnosed CML cases are in chronic phase, and imatinib, a BCR-ABL1 tyrosine kinase inhibitor (TKI), is efficacious as a front-line therapy in newly diagnosed patients. A long-term study of imatinib demonstrated a 7-year cumulative rate of complete cytogenetic response of 82%, an event-free survival rate of 81% and a freedom-from-progression rate of 93% (Guilhot et al., 2009). However, a subset of patients with chronic phase CML are resistant to imatinib, and more than 18% of cases that initially achieve a complete cytogenetic response develop secondary resistance to the drug (Radich et al., 2006). Patients whose disease progresses to accelerated or blast phase have shown rates of complete cytogenetic response to imatinib ranging from 16% to 50%, however, responses are generally brief and the estimated duration of overall survival of such patients is 7 months (Berman et al., 2006; Schiffer, 2007). Moreover, mutations that arise in the kinase domain of ABL1, such as the substitution of threonine at position 315 with isoleucine (T315I), limit the effectiveness of imatinib and other second-generation TKIs and often lead to resistance and disease relapse (Jamieson et al., 2004).
Cancer stem cells (CSCs) have been identified in virtually all major tumour types, including leukaemia and cancers of the brain, breast, colon, prostate and pancreas. In acute myeloid leukaemia (AML), the percentage of leukaemic stem cells (LSCs) represents a small fraction (0.5–5%) of the total number of cancer cells, yet these cells can give rise to, and are responsible for, the growth of the entire tumour. LSCs have also been shown to be highly resistant to standard cancer therapies, including chemotherapy, radiation and targeted therapies (Zhou et al., 2009). Therefore, the failure of therapeutics in our current armamentarium to target LSCs is widely believed to be partly responsible for overall treatment failure and relapse after standard therapies. CML progenitor cells have been shown to maintain BCR-ABL1 signalling, but they demonstrate insensitivity to TKIs. Indeed, quiescent CD34+ BCR-ABL1+ cells are not effectively killed in vitro by any of several BCR-ABL1–targeting TKIs, including imatinib, dasatinib and nilotinib (Copland et al., 2006; Graham et al., 2002; Jorgensen et al., 2007). Moreover, the persistence of LSCs as a cause of relapse may occur in as many as 75% of patients after treatment discontinuation (Copland et al., 2006; Corbin et al., 2011; Graham et al., 2002).
The interleukin-3 receptor (IL3R, also known as CD123) is normally expressed on certain haematopoietic cells, including maturing myeloid cells, B-cells and dendritic cells, but not on normal haematopoietic stem cells, and is involved in cell maturation, differentiation and survival. IL3R is also overexpressed in several haematological malignancies, including AML, myelodysplastic syndrome (MDS), CML, B-cell acute lymphoid leukaemia (ALL), hairy cell leukaemia, Hodgkin lymphoma, and certain aggressive forms of non-Hodgkin lymphoma (Aldinucci et al., 2005; Borthakur & Reddy, 2010; Djokic et al., 2009; Florian et al., 2006; Graf et al., 2004; Jordan et al., 2000; Lhermitte et al., 2006). Importantly, IL3R is also overexpressed on LSCs in AML, CML, MDS, and T-cell ALL (Borthakur & Reddy, 2010). Moreover, the expression of IL3R on leukaemic blasts correlates inversely with overall survival in AML (Graf et al., 2004; Testa et al., 2002). In particular, patients whose blasts expressed a high level of IL3R had a median complete remission time of 6 months, whereas those whose blasts expressed a normal IL3R level had a median complete remission time of greater than 24 months (Testa et al., 2002). The differential expression pattern of IL3R on normal stem cells and LSCs renders IL3R an attractive drug target in terms of specificity, overall toxicity and potency.
SL-401 and SL-501 are biological targeted therapeutics directed at IL3R. SL-401 (formerly known as DT388-IL3 (Cohen et al., 2004)) is a recombinant protein that is comprised of human IL3 joined by acid-labile amino acids to diphtheria toxin truncated at its receptor binding domain (amino acids 1–388), so that cytotoxicity results from binding of its IL3 domain to IL3R and subsequent internalization (Feuring-Buske et al., 2002; Frankel et al., 2000a; Kiser et al., 2001). Upon the interaction of the TAT-like domain of DT (residues 201–230) with cytosolic Hsp90 and thioredoxin reductase, the catalytic domain (A fragment) of SL-401 unfolds, is reduced and translocates to the cytosol. Once released into the cytosol, the A fragment refolds and catalytically inactivates cellular protein synthesis by ADP-ribosylating the diphthamide residue in domain IV of EF2. Global cellular inhibition of protein synthesis causes efficient and timely cell lysis or apoptosis.
In preclinical studies, SL-401 demonstrated prominent in vitro and in vivo activity against leukaemic blasts, AML colony-forming cells, AML long-term culture–initiating cells and AML cells engrafted into non-obese diabetic severe combined immunodeficient (NOD/SCID) mice, whereas it demonstrated negligible activity against normal bone marrow progenitor cells (Feuring-Buske et al., 2002; Frankel et al., 2000a; Kiser et al., 2001). The K116W variant DT388-IL3 molecule (DT388-IL3[K116W], or SL-501) exhibited greater IL3R binding affinity and cytotoxic activity against AML progenitors than SL-401, although SL-501 also possessed negligible activity against normal bone marrow progenitor cells (Hogge et al., 2006a; Testa et al., 2005). In a Phase 1–2 clinical trial in patients with advanced haematological cancers, a single cycle of SL-401 administered as a 15-min intravenous infusion daily for 5 days induced complete responses in patients with relapsed or refractory AML or blastic plasmacytoid dendritic cell neoplasm, an IL3R+ malignancy of plasmacytoid dendritic cells (Frankel et al., 2013), and a survival signal among AML patients who received this agent as third- or later-line therapy was noted (Konopleva et al., 2012). In these clinical trials, SL-401 administration was devoid of myelosuppressive effects.
Given the notable IL3R expression on the LSCs of CML, a population known to be resistant to TKIs, the lack of effectiveness of the TKIs against BCR-ABL1+ CML LSCs (Copland et al., 2006; Graham et al., 2002) and the differential expression of IL3R on the malignant cells of haematological cancers relative to normal marrow cells, IL3R is an attractive therapeutic target and both SL-401 and SL-501 are rational therapeutics to develop to selectively eradicate CML cells. In this study, we explored the consequence of targeting IL3R in CML LCSs, as well as the antitumour activity of SL-401 and SL-501 in this setting.
MATERIALS AND METHODS
Chemicals and reagents
SL-401 (DT388-IL3) was provided by Stemline Therapeutics, Inc. (New York, NY). The protein was diluted in culture medium immediately before use. DT388-IL3[K116W] (SL-501) was prepared and purified as previously described (Frankel et al., 1997; Frankel et al., 2000b; Su et al., 2010). Imatinib was purchased from LC Laboratories (Woburn, MA) and dissolved in dimethyl sulfoxide (DMSO) before use.
Cell lines and primary CML samples
KBM5, an imatinib-sensitive blast crisis CML cell line, and KBM5-STI, an imatinib-resistant KBM5 subline that harbours the ABL1 T315I mutation (Ricci et al., 2002), were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 50 µg/ml penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Bone marrow, peripheral blood and apheresis samples were obtained from patients with CML in various stages. All patient samples were collected at The University of Texas MD Anderson Cancer Center, and each patient signed a written informed consent document approved by the institution’s investigational review board. The clinical characteristics at the time of specimen collection of the patients whose samples were used for in vitro and in vivo studies are presented in Table I. Mononuclear cell fractions were obtained by Ficoll-Hypaque (Lymphocyte Separation Medium; Cellgro, Manassas, VA) density-gradient centrifugation and seeded at 1–2 × 106 cells/ml in RPMI-1640 medium containing 10% FBS and 50 µg/ml penicillin/streptomycin at 37°C. The cell lines and primary samples were treated with SL-401 (0.1–5 µg/ml), SL-501(0.1–5 µg/ml), imatinib (0.25–5 µM), or a combination of these for either 24 or 72 h.
Table I.
Clinical data for 21 CML patients who provided specimens
| Patient Number |
Source Blast% |
Status | Cytogenetics | ABL1 mutation | Resistance to TKI | Assay |
|---|---|---|---|---|---|---|
| 1* | BM, 85 | BP-L | 46,XX,t(9;22)(q34;q11.2)[7],47,XX,+ X,t(9;22)(q34;q11.2),del(11)(q13q23)[11],48,XX,+X,t(9;22)(q34;q11.2)x2,d el(11)(q13q23),+22[1],46,XX[1] |
T315I | Dasatinib | SL-401/501, DT+Im, LTC-IC |
| 3 | BM, 40 | BP-M | 45,XY,-7,t(9;22)(q34;q11.2)[20] | None | Imatinib | SL-401/501, LSC+ SL- 401/501, LTC-IC |
| 4 | BM, 1 | CP | 46,XX,t(9;22)(q34;q11.2)[20] | ND | N/A | SL-401/501, CFC, LTC-IC, LSC expression, FISH |
| 5 | BM, 49 | BP-M | 46,XY,t(3;17)(q26.2;q22),t(9;22)(q34;q11.2),del(11)(p11.2)[19] | H396R, T3151 | imatinib, dasatinib, nilotinib, DCC-2036 |
SL-401/501, SL-401/501+Im, CFC, LTC-IC |
| 6 | BM, 1 | CP | 46,XX,t(9;22)(q34;q11.2)[20] | None | N/A | SL-401/501, CFC |
| 7** | PB, 89 | BP-M | 46,XX,inv(3)(q21q26.2),del(6)(q21),d el(7)(p13p15),+8,t(9;22)(q34;q11.2), −10[10],46,XX,inv(3)(q21q26.2),del(6) (q21),del(7)(p15p13),+8,t(9;22) (q34;q11.2),-10[6] |
T3151 | imatinib, nilotinib | SL-401/501, CFC, LSC+ SL-401/501 |
| 8 | BM, 96 | BP-L | 45,XX,-9,del(22)t(9;22)(q34;q11.2)[19]; 46,XX,-9,der(22)t(9;22)(q34;q11.2)x2[1] |
ND | Imatinib | CFC |
| 9 | PH | AP | 46,XY,t(9;22)(q34;q11.2)[18], 46–47,XY,del(3)(p13p25),t(9;22)(q34;q11.2)[2] | M244V | N/A | SL-401/501, CFC |
| 10** | PB, 12 | BP-M | 47,XY,+8,t(9;22)(q34;q11.2),i(17)(q10)//[3], 48,sl,del(12)(p13.1p13.3),+der(22)t(9;22)//[15], 46,XX[2] |
T315I | imatinib, dasatinib | SL-401/501, LSC+ SL-401/501, LSC expression |
| 11 | PB, 30 | BP-M | 46,XY,t(9;22)(q34;q11.2)[20] | T315I | imatinib, bosutinib | SL-401/501 |
| 12 | PB-, 60 | BP-M | 47,XY, +8; 46,XY[17],46,XY[3]. FISH showed Ph+ signal (cryptic t(9;22)) in 94% of the interphases. |
None | imatinib, dasatinib | SL-401/501, SL-401/501+Im, LSC expression |
| 13* | BM, 66 | BP-L | 46,XX,t(9;22)q34;q11.2)[6]; 46,XX, del(6)(q21),t(9;22)q34;q11.2)[8];46, XX,t(4;19)(q13;p13),t(9;22)(q34;q11.2)[2], 46,XX,t(1;5)(p13;q21),t(9;22)(q34;q11.2)[4] |
Y253H | imatinib, dasatinib | SL-401/501, SL-401/501+Im, LSC+ SL-401/501, LSC expression |
| 14 | PB, 73 | BP-M | 50,XY,+8,+8,+14,+19[20] FISH showed Ph+ signal in 94% of the interphases. |
None | imatinib, dasatinib, nilotinib |
SL-401,/501 LSC+ SL-401/501 |
| 16 | PB, 23 | CP | 46,XY,t(9;22)(q34;q11.2)[20] | ND | N/A | SL-401/501 |
| 17 | PB, 1 | CP | 46,XX,t(9;22)(q34;q11.2)[20] | None | imatinib, nilotinib | SL-401/501 |
| 18 | BM, 1 | CP | 46,XY,t(9;22)(q34;q11.2) | ND | N/A | LSC expression FISH |
| 19 | PB, 12 | BP-M | 46,XY,t(9;22)(q34;q11.2)[20] | ND | N/A | LSC expression FISH |
| 20 | BM, 3 | AP | 46,XY,t(9;22)(q34;q11.2)[1]; 7,XY,t(9;22)(q34;q11.2),+der(22)t(9;22)[19] |
F317 L | imatinib, nilotinib, dasatinib |
LSC expression FISH |
| 21 | PB, 76 | BP-L | 46,XY,t(9;22)(q34;q11.2)[19] | F317 L | imatinib, dasatinib, nilotinib | LSC expression FISH |
| 22 | BM, 2 | CP | 46,XX,t(9;22)(q34;q11.2)[20] | ND | N/A | FISH |
| 23 | BM, 0 | CP | 46,XY,t(9;22)(q34;q11.2)[20] | None | N/A | FISH |
TKI, tyrosine kinase inhibitor; BM, bone marrow; BP-L, lymphoid blast phase; DT, diphtheria toxin; Im, imatinib; LTC-IC, long-term culture-initiating cells; BP-M, myeloid blast phase; LSC, leukaemia stem cells; CP, chronic phase; ND, not done; N/A, not applicable (no clinical resistance to TKIs); CFC, colony-forming cells; FISH, fluorescence in situ hybridization; PB, peripheral blood; PH, apheresis; AP, accelerated phase; Ph+, Philadelphia chromosome positive.
Cytogenetics showed three Philadelphia chromosome–positive clones
Cytogenetics showed two Philadelphia chromosome–positive clones
Cell viability and apoptosis
Trypan blue exclusion was used to assess cell viability. The induction of apoptosis was quantified by fluorescence-activated cell sorting (FACS) on treated cells stained with annexin V. Briefly, cells were washed, resuspended with annexin V binding buffer, stained with fluorescein isothiocyanate (FITC)–conjugated annexin V (Roche, Mannheim, Germany) for 15 min at room temperature in the dark, and then washed and counterstained with propidium iodide (PI). The analysis was performed by a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) at a wavelength of 488 nm using Cell QuestPro Software (Beckman-Coulter, Fullerton, CA).
Flow cytometry detection of CML stem cells and apoptosis
Mononuclear cell fractions derived from the bone marrow aspirates, peripheral blood and apheresis samples of CML patients were washed with phosphate-buffered saline (PBS) and then stained with anti-CD34, -CD38 and -CD123 antibodies (Becton Dickinson) for 30 min at room temperature to identify LSCs. To determine the fractions of viable and apoptotic cells, cells were also stained with annexin V-FITC (Roche) and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO). The frequency of CD34+/CD38−/CD123+/annexin V–positive cells was determined by multicolour flow cytometry. The percentage of non-apoptotic (annexin V–negative) stem cells was calculated after SL-401 or SL-501 treatment (number of stem cells in DMSO-treated cultures = 100%).
Long-term culture-initiating cell and colony-forming cell assays
Primary mononuclear cells used for the colony-forming cell (CFC) or long-term culture-initiating cell (LTC-IC) assays were first incubated (1×106 cells/ml) with or without SL-401 or SL-501 for 24 h. The viability of cultured cells was measured by trypan blue dye exclusion before plating for the assays.
The assays for CML CFCs were performed by plating cells at a density of 1.0×105 cells/ml in growth factor–enriched methylcellulose medium (Methocult; StemCell Technologies, Vancouver, BC, Canada) supplemented with 20 ng/ml IL6 (Invitrogen, Grand Island, NY). Plates were scored for the presence of colonies after 14 days as previously described (Ailles et al., 1997).
CML LTC-IC assays were established and maintained as previously described (Ailles et al., 1997). Briefly, CML cells (1.0 × 105) in myeloid long-term culture medium (Myelocult; StemCell Technologies) were co-cultured with feeder layers that consisted of MS-5 murine stromal cells treated with 0.75 µg/ml mitomycin C for 3 h. After culture for 6 weeks with weekly half-medium changes, adherent and nonadherent cells were harvested and assessed for their CML CFC proportion as described elsewhere (Ailles et al., 1997).
Fluorescence in situ hybridization analysis
CD34+ cells were isolated from primary mononuclear cells using a magnetic cell sorting kit (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Clinical characteristics of patients whose cells were used for this experiment are summarized in Table II. Briefly, primary mononuclear cells were washed twice with MACS buffer, stained with CD34 beads, and separated on a MACS column using positive selection. MACS-sorted CD34+-enriched cells were stained with anti-CD34-allophycocyanin (APC), anti-CD38-cyanin 5 (Cy5), and anti-CD123-phycoerythrin (PE) antibodies (BD Biosciences, San Jose, CA) for 30 min at room temperature. Unstained cells were used as a negative control. After incubation, 1×103 CD34+/CD38−/CD123+ or CD34+/CD38−/CD123− cells were FACS-sorted directly onto slides. Slides were fixed with methanol-acetic acid (V/V, 3:1) for 30 min, pretreated with pepsin (0.25 mg/1 ml 0.01N hydrochloric acid; Sigma) at 37°C for 10 min, and then fixed in 1% formaldehyde for 5 min at room temperature and washed with PBS. After the slides were dehydrated in an ethanol series, a standard fluorescence in situ hybridization (FISH) protocol was used (Engel et al., 1997; Wang et al., 1996). A BCR/ABL1 dual color probe was used for hybridization (Abbott Laboratories, Abbott Park, IL). Slides were analyzed under a fluorescence microscope (Photolab-2; Nikon Instruments, Melville, NY).
Table II.
Expression of IL3R (CD123) in the CD34+/CD38− population of primary CML cells
| Patient Number |
CML Phase | CD123+ cells within CD34+/CD38− population (%) |
|---|---|---|
| 4 | Chronic | 93.6 |
| 10 | Blast | 96.0 |
| 12 | Blast | 98.2 |
| 13 | Blast | 94.0 |
| 18 | Chronic | 49.1 |
| 19 | Blast | 70.8 |
| 20 | Chronic | 80.8 |
| 21 | Blast | 86.2 |
| Mean (SD) | 83.6 (16.7) | |
Mononuclear fractions of bone marrow or blood cells from chronic myeloid leukaemia (CML) patients were stained with CD34, CD38 and CD123 antibodies as described in Materials and Methods and analyzed by flow cytometry. Proportion of CD123+ cells was analysed within the gated CD34+/CD38− population of total mononuclear cells and data are expressed as the percentage of positive cells from the CD34+/CD38− cell population.
CML murine model and in vivo treatment with SL-401 and SL-501
All animal work was performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center. NOD/SCID/IL2Rγ-KO (NSG) mice were transplanted via tail vein injection with 3.5 × 106 or with 2 × 106 human primary leukaemic cells obtained from a patient with CML in blast crisis or an AML patient, respectively. In the CML model, once circulating human CD45 positive cells were detected by FACS (day 21), mice (six per group) received daily intraperitoneal injections of SL-401 or SL-501 at 0.2 mg/kg/mouse for 5 consecutive days. In the AML model, starting on day 26 after cell injection, mice were treated with SL-401 (at the same dose stated above), cytarabine (100 mg/kg body weight) or with a combination of these by daily intraperitoneal injections for 5 days. Control mice received daily intraperitoneal injections of 0.2 ml normal saline solution on the same schedule.
Statistical analysis
All experiments were conducted at least three times unless specified otherwise. The two-sided Student’s t-test was used to evaluate the statistical significance of differences between groups. Results are expressed as the mean ± standard error of the mean (SEM) or standard deviation (SD). Synergism, additive effects, and antagonism were assessed by the “Chou-Talalay method” (Chou & Talalay, 1984) using Calcusyn software (Biosoft, Ferguson, MO) whereby the combination index (CI) for each experimental combination was also calculated. A CI = 1 represents the conservation isobologram and indicates additive effects. CI values <1.0 indicated an additive effect characteristic of synergism. The Kaplan-Meier method was used to estimate overall survival with Prism software (GraphPad, La Jolla, CA). P-values were obtained by the log-rank test and were considered statistically significant when < 0.05.
RESULTS
CML cells express IL3R and are sensitive to SL-401 and SL-501
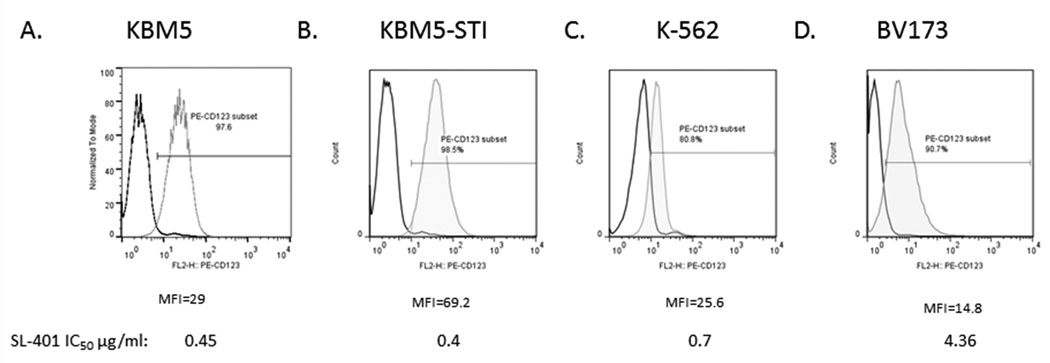
Supporting previous studies, which demonstrated that CD34+/CD38− CML cells express CD123 (Florian et al., 2006), the CML cell lines KBM5, K562 and BV173 were shown to express CD123 on the cell surface, albeit with varying intensities among the different lines (Fig. 1A–D). Notably, the TKI-resistant CML line KBM5-STI also expressed CD123, at similar or even higher levels than the parental line (Fig. 1A). Expression of CD123 by KBM5 cells was not affected by treatment with 2.5, 5 and 10 µM imatinib for up to 72 h (data not shown), suggesting that IL3R expression in CML is BCR-ABL1 independent.
Figure 1. CD123 is expressed on representative CML cell lines.
The (A) KBM5 and (B) TKI-resistant KBM5-STI, (C) K562, and (D) BV173 cell lines were assessed for CD123 expression by flow cytometry. An IgG isotype antibody was used as the control for gating. MFI, mean fluorescent intensity; IC50, 50% inhibitory concentration.
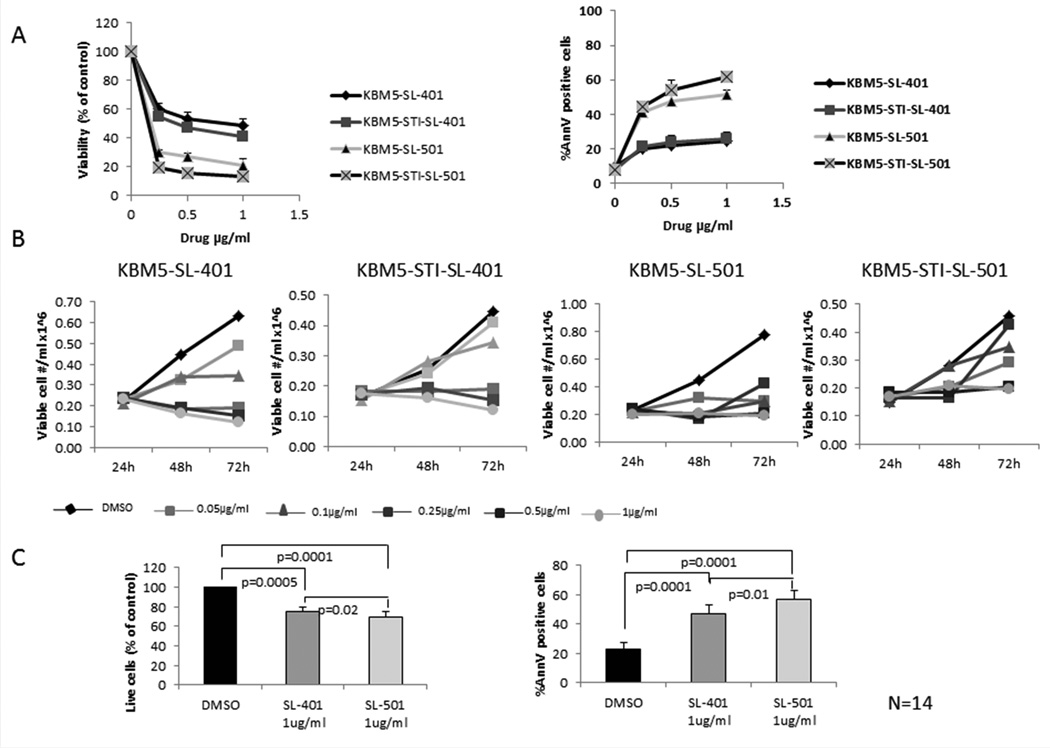
We next assessed the sensitivity of these CML lines to SL-401 and SL-501. Cells were incubated in the presence of each drug for 72 h and then assessed for viable cells and apoptosis using trypan blue and annexin V flow cytometry, respectively. As shown in Figure 2A, both KBM5 and the TKI-resistant KBM5-STI cell lines were sensitive to SL-401 in a dose-dependent manner, with 50% inhibitory concentration (IC50) values of 0.45 µg/ml and 0.4 µg/ml, respectively. SL-401 potently inhibited cell growth in a dose-dependent fashion and induced apoptotic cell death in CML cells. Similar results were obtained in CD123-expressing BV173 cells (Suppl. Fig 1). On the other hand, K562 cells, which have less CD123 expression than the other two cell lines, were less sensitive to SL-401; the IC50 value was 0.7 µg/ml (Suppl. Fig 1). SL-501 was significantly more cytotoxic than SL-401 in CML cells, as the agent induced apoptosis in more than 50% of the cells at the lowest concentration used in this assay (0.25 µg/ml). A time-course analysis demonstrated that SL-401 and SL-501 concentrations of at least 0.25 µg/ml and 0.1 µg/ml, respectively, completely arrested the growth of KBM-5 and KBM5-STI cells (Fig. 2B). Confocal microscopy of KBM-5 cells incubated with DyLight680-conjugated SL-401 revealed specific punctuated intracellular signal (Suppl. Fig. 2) consistent with endosomal localization upon internalization.
Figure 2. KBM5 and TKI-resistant KBM5-STI CML cell lines and primary CML cells are sensitive to SL-401 and SL-501.
(A) KBM5 and TKI-resistant KBM5-STI cells were incubated with 0.25, 0.5, 1 or 5 µg/ml of SL-401 or SL-501 for 72 h, after which cell viability was assessed by trypan blue staining and induction of cell death by annexin V flow cytometry. The left panel shows viable cell number per ml ×106, and the right panel shows the percentage of annexin V (AnnV)–positive cells relative to untreated controls. (B) Time-course analysis of KBM5 and KBM5-STI cells treated with SL-401 and SL-501. KBM5 and KBM5-STI cells were incubated with different concentrations of SL-401 or SL-501 (0.05, 0.1, 0.25, 0.5 or 1 µg/ml). At the indicated time points, cell viability was assessed as described above. DMSO, control (dimethyl sulfoxide). (C) Mononuclear cells from CML patients (N = 14, see clinical information for “SL-401” assay in Table I) were treated with SL-401 or SL-501 (1 µg/ml) for 72 h, after which cell viability was assessed by trypan blue staining (left) and induction of cell death by annexin V flow cytometry (right). Data represent means ± SEM.
We next tested the cytotoxic activity of SL-401 in primary leukaemia cells derived from CML patients. Of 14 primary CML specimens evaluated, nine were from CML patients in blast phase at sampling (seven myeloid and two lymphoid; Table I, assay “SL-401/501”), ten had disease that was resistant to one or more TKIs, and four harboured the T315I mutation. IL3R was ubiquitously highly expressed (>94% cells) within CD34+ cell compartment in four samples tested (1CP, 3 BP, data not shown). Treatment of these patient-derived leukaemic cells with 1 µg/ml SL-401 or SL-501 in vitro decreased viability and increased the percentages of cells undergoing apoptosis compared to control cells treated with vehicle only (average fractions of annexin V–positive cells were 47% [SL-401], 57% [SL-501], and 23% [control]; p <0.0001 for each drug compared to control; Fig. 2C), regardless of the patient’s clinical status or presence of the BCR-ABL1 mutation. These findings indicate that SL-401 and SL-501 induce apoptosis in CML cells ex vivo, including cells with acquired resistance to BCR-ABL1 TKIs.
SL-401 and SL-501 enhance imatinib-induced apoptosis of KBM5 and primary CML cells
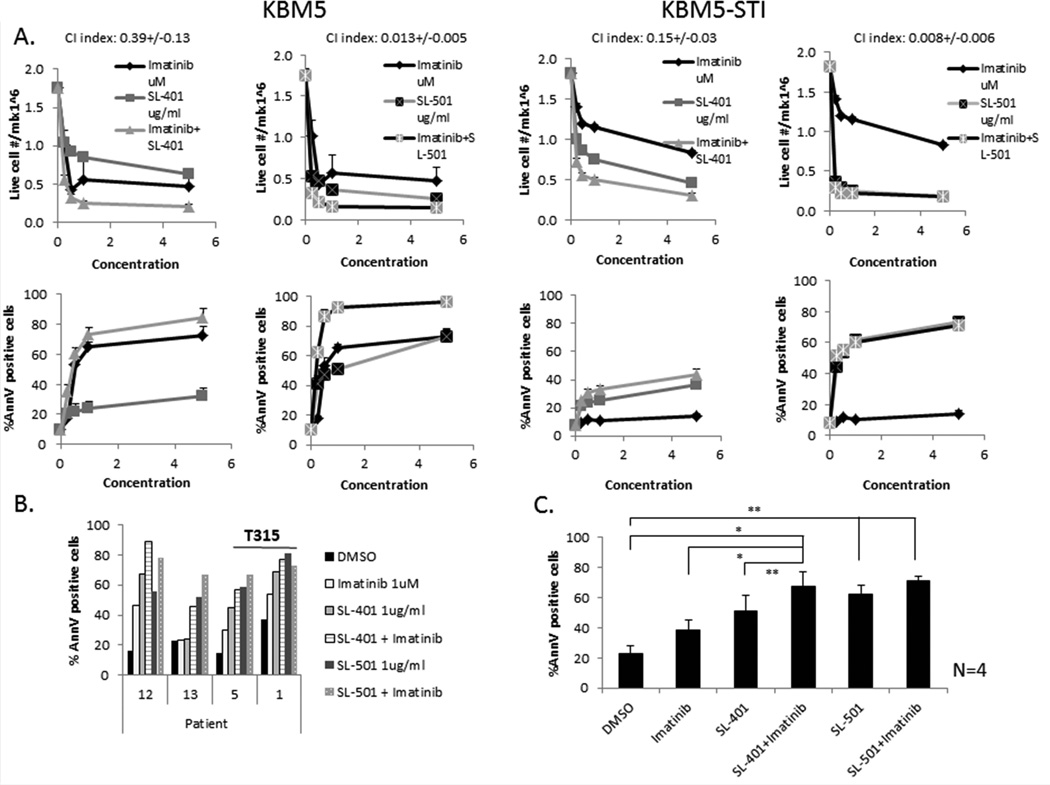
To determine whether SL-401 and SL-501 can enhance the cytotoxic activity of imatinib, we treated KBM5 and KBM5-STI cells or mononuclear cells from CML patients (N = 4) with imatinib (1 µM) in combination with SL-401 (1 µg/ml) or SL-501 for 72 h and measured apoptosis by FACS. Imatinib enhanced the cytotoxicity of both SL-401 and SL-501 against KBM5, as manifested as a dose-dependent increase in apoptosis and decrease in the numbers of viable cells (Fig. 3A). The combination of SL-401 and imatinib at a fixed ratio of 1:1 in KBM5 cells had a synergistic effect on viability, with a mean CI of 0.39 ± 0.13 at 72 h after treatment. TKI-resistant KBM5-STI cells were much less sensitive to imatinib than KBM5 cells but preserved their sensitivity to SL-401. Similar findings were observed with SL-501 combined with imatinib; the CI averaged 0.013 ± 0.005 for KBM5 cells. Furthermore, simultaneous treatment of CML cells with imatinib and either SL-401 or SL-501 increased cell death in samples obtained from four patients with TKI-resistant CML in blast phase, although the additive effect was restricted to samples without the T315 mutation (Patients 12 and 13). Two samples harbouring the T315I mutation (Patients 1 and 5) retained sensitivity to SL-401 and SL-501 (Fig. 3B,C).
Figure 3. Combination of imatinib with SL-401 or SL-501 enhances the apoptotic rate in KBM5 and primary BP-CML cells.
KBM5 and imatinib-resistant KBM5-STI cell lines (A) or mononuclear cells from four CML patients (B, C) were treated with SL-401 or SL-501 (1 µg/ml), imatinib (1 µM), a combination, or DMSO (control, dimethyl sulfoxide) for 72 h, after which apoptosis was measured by FACS after staining with annexin V (AnnV) and propidium iodide (PI). (A) Upper panels, live cell numbers; lower panels, percentage of annexin V–positive cells. (B) Percentage of annexin V–positive cells in four individual primary CML samples after treatment; (C) mean ± SEM annexin V–positive cells for all four samples; * p <0.05; p <0.01. For clinical information on patients, refer to Table I (patient numbers correspond to the numbers listed in Table I, assay “SL-401/501”).
CD123 is highly expressed in CD34+/CD38− progenitors of CML primary cells
CML was previously demonstrated to comprise a population of CD34+/CD38− LSCs that express IL3R and are resistant to standard chemotherapeutic agents and TKIs (Florian et al., 2006). In samples obtained from eight patients with CML in chronic or blast phase, IL3R expression on CD34+/CD38− progenitor cells ranged from 49% to 98% (mean 83.6 ± 16.7%; Table II). To determine whether the LSC population harboured oncogenic BCR-ABL1, we performed FISH on FACS-sorted CD34+/CD38−/CD123+ cells from seven CML samples (Patients 4 and 18–23). Interestingly, a significant percentage of the LSC population was BCR-ABL1 positive, and the proportion of BCR-ABL1 positivity was higher in CD123-expressing LSCs than in CD123-negative cells (mean 86.1% in CD34+/CD38−/CD123+ cells versus 57.1% in the CD34+/CD38−/CD123− fraction, p <0.05; Table 3).
Table 3.
Frequency of BCR-ABL1 expression in CD123+ and CD123− populations of primary CD34+/CD38− CML cells
| Patient Number |
CML Phase |
BCR-ABL1+ Cells in Unsorted Population (%) |
BCR-ABL1+ Cells in CD34+/CD38− /CD123+ Population (%) |
BCR-ABL1+ Cells in CD34+/CD38− /CD123− Population (%) |
|---|---|---|---|---|
| 4 | Chronic | 93.5 | 92.3 | 61.4 |
| 18 | Chronic | 95 | 71.8 | 32.5 |
| 19 | Blast, myeloid |
88.5 | 87.2 | 38.5 |
| 20 | Accelerated phase |
95.5 | 99.1 | 90.9 |
| 21 | Blast, lymphoid |
90 | 90.0 | 72.0 |
| 22 | Chronic | 97 | 84.8 | 61.8 |
| 23 | Chronic | 95.5 | 77.4 | 42.5 |
| Mean (SD) |
93.6 (3.1) | 86.1 (9.2) | 57.1 (20.7) | |
The CD34+-enriched fraction of mononuclear cells from bone marrow or peripheral blood was stained with CD34, CD38, and CD123 antibodies and FACS-sorted onto slides as CD34+/CD38−/CD123+ or CD34+/CD38−/CD123− populations. Slides were then analysed by FISH staining as described in Materials and Methods.
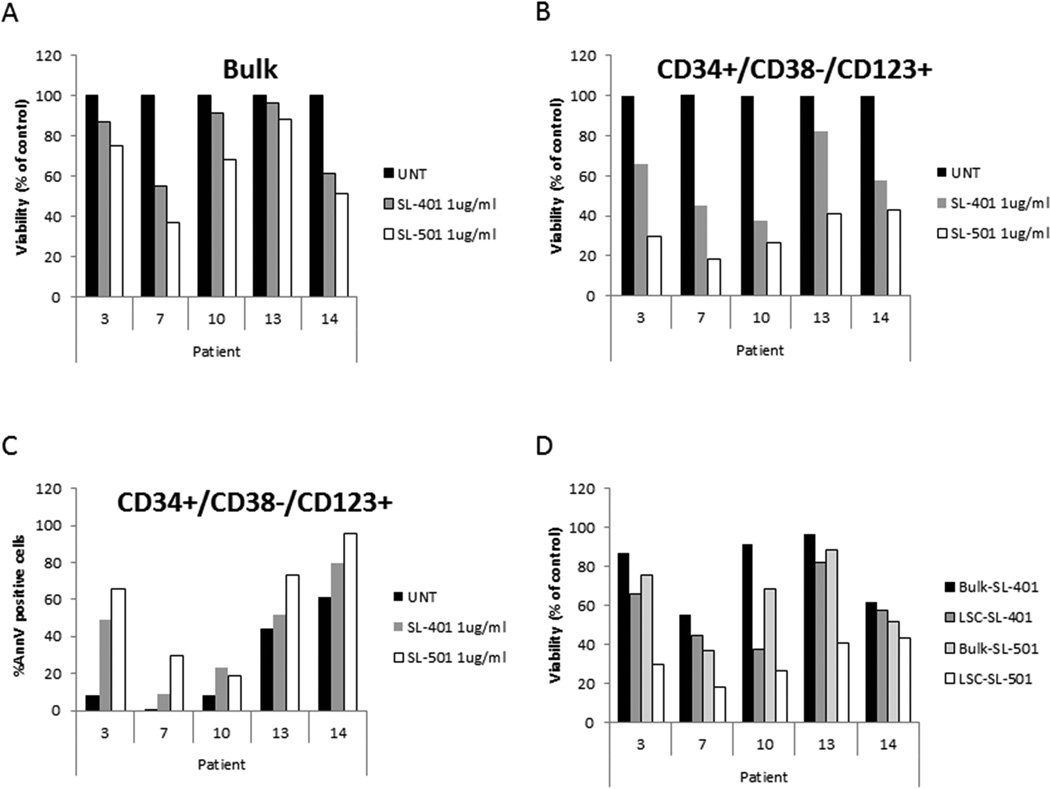
Based on the high CD123 expression in CML LSCs, we next determined whether this population of cells was sensitive to SL-401 and SL-501. CML cells were treated with either SL-401 or SL-501, and the number of CD34+/CD38−/CD123+ cells that expressed phosphatidylserine was determined by multiparametric flow cytometry. The absolute number of CD34+/CD38−/CD123+/annexin V–negative non-apoptotic stem cells was then calculated and normalized to the number of DMSO-treated controls. As shown in Figure 4, Figure 1 µg/ml of SL-401 caused a reduction in the numbers of viable bulk mononuclear CML cells (mean ± SEM, 85.3 ± 6.1% remaining viable cells; Fig. 4A), and decrease in viability of the putative LSC population compared to untreated controls (Fig. 4B, mean 54.8% ± 9.4% remaining viable LSCs). Additionally, SL-401 treatment induced apoptosis of the CD34+/CD38−/CD123+ CML cell population (percentage apoptosis in LSC, mean ± SEM: control, 27.6 ± 11.3%; SL-401, 44.8 ± 10.8%; Fig. 4C). In four samples (Patients 3, 10, 13 and 14), SL-401 treatment resulted in a greater loss of viability in the LSC fraction than in bulk CML cells, indicating selective sensitivity of CML LSCs to SL-401 (Fig. 4D). SL-501 was consistently more cytotoxic against CML mononuclear cells and CD34+/CD38−/CD123+ cells, causing further reduction in the fraction of viable LSCs (Fig. 4B, mean 31.7% ± 4.6% remaining viable LSCs). Both SL-401 and SL-501 caused LSC depletion in samples from patients whose disease was resistant to multiple TKIs, including those harbouring the T315I mutation (Patients 7 and 10).
Figure 4. SL-401 and SL-501 reduce viability and induce apoptosis in the CD34+/CD38−/CD123+ LSC population in CML patients.
Diphtheria toxin-interleukin 3 (DT-IL3) fusion proteins reduced viability of bulk CML cells (A; mean ± standard error of the mean [SEM], 85.3 ± 6.1% remaining viable cells) and of putative CD34+/CD38−/CD123+ leukaemic stem cells (LSCs) (B; mean 54.8% ± 9.4% remaining viable LSCs) after incubating samples at a dose of 1 µg/ml for 72 h; UNT, untreated. (C) Percentage of annexin V (AnnV)–positive cells in untreated or SL-401/SL-501–treated CML CD34+/CD38−/CD123+ LSCs percentage apoptosis in LSC, mean ± SEM: control, 27.6 ± 11.3%; SL-401, 44.8 ± 10.8%). (D) Fraction (percentage of untreated controls) of remaining viable cells (black columns, bulk; grey columns, CD34+/CD38−/CD123+ cells) after SL-401 and SL-501 exposure. For clinical information on patients, refer to Table I (patient numbers correspond to the numbers listed in Table I, assay “LSC+SL-401”).
SL-401 and SL-501 inhibit clonogenic growth and long-term colony formation of primary CML
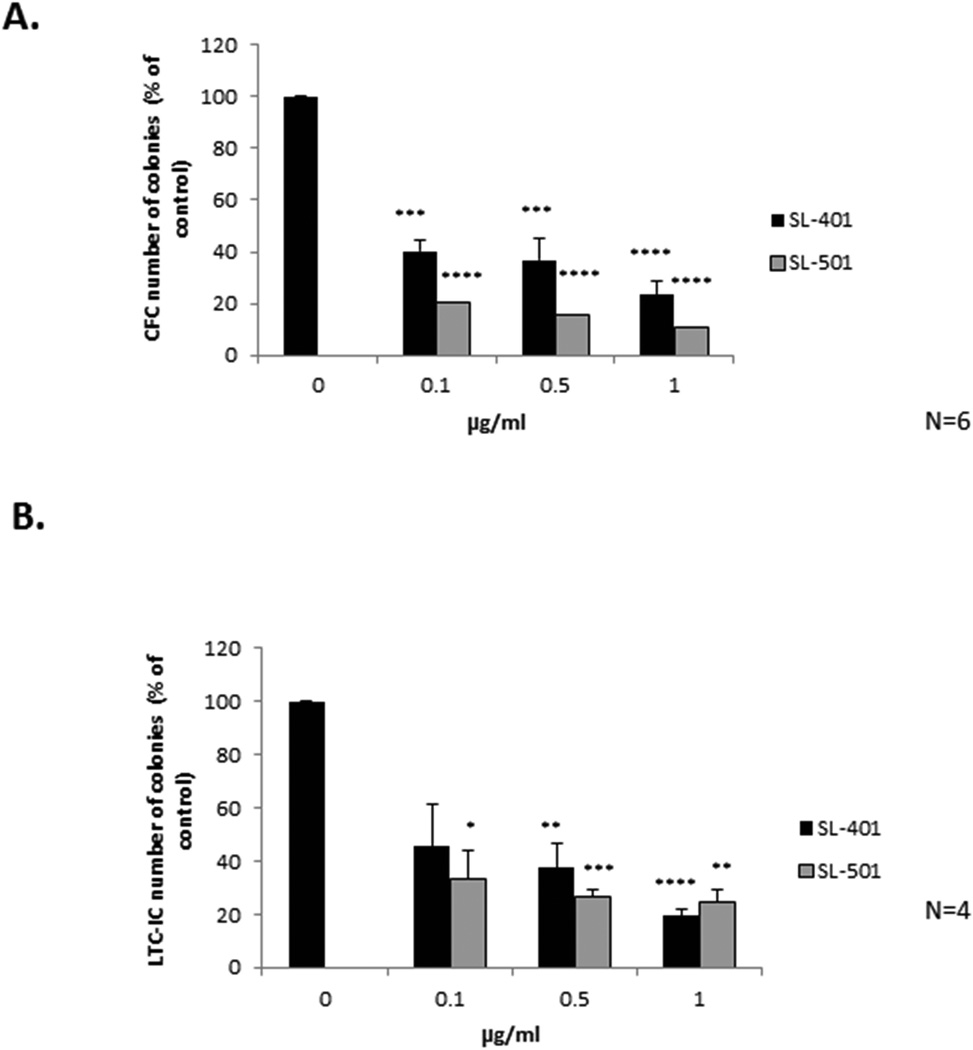
The effects of SL-401 and SL-501 on the viability of cells expressing CD34+/CD38−/CD123+ suggested that they may affect the growth of progenitors and the most primitive LSCs. Therefore, we first tested the effects of SL-401/SL-501 on colony forming ability by pretreating patient CML blasts with SL-401/SL-501 ex vivo and assessing their growth in a CFC assay for 14 days. As shown in Figure 5A, both SL-401 and SL-501 significantly reduced colony formation, by as much as 75% compared to vehicle-treated cells, in a dose-dependent manner (N = 6; p ≤0.0003), SL-501 again demonstrating greater inhibitory activity. To evaluate the effect of SL-401/SL-501 on the most primitive leukaemia-initiating cells, CML blasts from four primary samples were treated ex vivo and subjected to LTC-IC assay. IL3R inhibition by SL-401 or SL-501 significantly reduced the number of colonies, by approximately 80% over a 6-week period, in a dose-dependent manner (Fig. 5B; N = 4; p ≤0.009). Notably, the majority of the primary samples assessed in these assays were obtained from patients who harboured an ABL1 mutation and/or whose disease was resistant to TKIs (Table I, “CFC” and “LTC-IC” assays).
Figure 5. SL-401 and SL-501 inhibit colony formation and long-term colony-initiating cells in a dose-dependent manner.
Primary mononuclear cells (1×106 cells/ml) from CML patients were incubated with or without SL-401 or SL-501 for 24 h and then plated for (A) colony-forming cells (CFC; N = 6; 3 blast phase [BP], 2 chronic phase [CP] and 1 accelerated phase [AP]) or (B) long-term culture-initiating cells (LTC-IC; N = 4; 3BP and 1CP) assay. The CFC assay plates were analysed 14 days after plating and the LTC-IC assay plates were analysed 6 weeks after plating. Control vs. agents: * p ≤0.05**p ≤0.01, ***p ≤0.001, ****p <0.0001. For clinical information on patients, refer to Table I (patient numbers correspond to the numbers listed in Table I, assays “CFC” and “LTC-IC”).
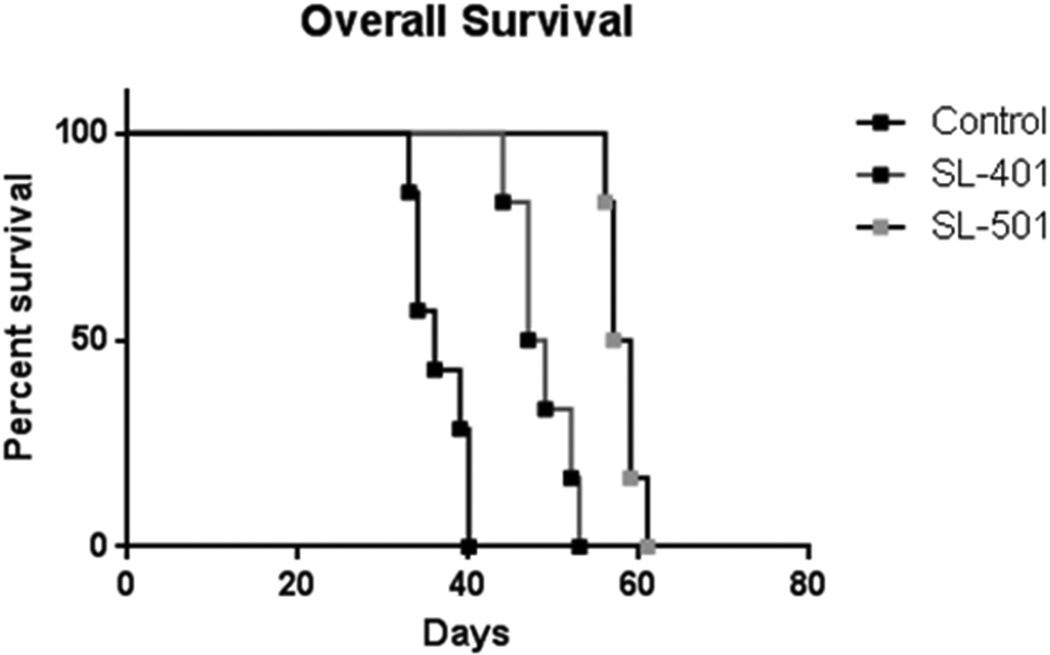
SL-401 and SL-501 prolong the survival of NSG mice with CML xenografts
The SL-401/SL-501–induced apoptosis of CML blasts and LSCs as well as the reduction of colony formation in the CFC and LTC-IC are consistent with in vitro features of drug-mediated killing of bulk tumour cells and LSCs, respectively. Thus, we next tested whether such responses were also evident in vivo and whether these fusion proteins would provide a survival benefit in a CML xenograft model. NSG mice were transplanted with CML cells from a patient in myeloid blast crisis whose leukaemia had developed resistance to both imatinib and dasatinib. At 3 weeks after inoculation, leukaemia engraftment was confirmed by detection of circulating human CD45+ cells (mean % of circulating human CD45 positive cells: 13.1+/−1.1[N=11]). The mice were then randomized and administered either 0.2 ml normal saline (control; N = 7) or 0.2 mg/kg body weight SL-401 or SL-501 (N = 6/each group) by intraperitoneal injection once daily for 5 days (one cycle). As shown in Figure 6, a single cycle of SL-401 or SL-501 significantly prolonged the survival of mice compared to control mice (48 days for SL-401 and 58 days for SL-501 versus 37 days in vehicle-treated mice; p < 0.0001). In concordance, the percentage of circulating human CD45 positive cells was lower in the treated mice than in the control (average percentage of circulating human CD45+ cells: 4.1±1.9% in IL3R antagonist treated mice [N=3] and 18.3±3.8% in controls [N=2]). To validate the in vivo anti-leukaemia activity of SL-40, a second model was developed in NSG mice injected with a primary AML sample (Suppl. Fig. 3). On day 26 after cell transplantation mice were randomized and treated by intraperitoneal injection once daily for 5 days (one cycle) with either 0.2 ml normal saline, 0.2 mg/kg body weight SL-401, 100 mg/kg cytarabine or their combination (N = 5/each group). Each single agent significantly prolonged survival but the combination was more effective at improving mice survival (median survival of 40 days in control arm versus 45, 47 and 55 days for cytarabine, SL-401 and the combination, respectively; p<0.0001). Taken together, these results suggest that both SL-401 and SL-501 demonstrate anti-leukaemia activity CML in vivo.
Figure 6. SL-401 and SL-501 significantly prolong the survival of NSG mice engrafted with CML blast crisis xenografts.
Mice were engrafted with cells obtained from a patient with CML in myeloid blast crisis with resistance to imatinib and dasatinib. After confirmed engraftment, mice received intraperitoneal injection of 0.2 ml normal saline solution (controls; N = 7), 0.2 mg/kg SL-401 or 0.2 mg/kg SL-501 daily for 5 days. Survival of the three groups was compared by Kaplan-Meier analysis.
DISCUSSION
The persistence and resistance of malignant CML LSCs against currently approved therapies continues to drive the need for new and novel therapeutic modalities that can eradicate this cell population. Although several TKIs that target the BCR-ABL1 fusion protein markedly improve the outcome of patients with CML, treatment invariably fails in a fraction of these patients as a result of minimal residual disease that consists of CML LSCs (Bhatia et al., 2003; Gorre et al., 2001). Recent studies showing that these cells are resistant to TKIs (Copland et al, 2006; Graham et al, 2002; Jorgensen et al, 2007), together with the finding that CML LSCs have survival mechanisms that are independent of BCR-ABL1, underscore the suggestion that non-TKI therapies capable of eradicating this population are needed to reduce relapse and improve overall survival rates.
In this study, we showed that SL-401 and SL-501, clinically active biological therapies directed to the IL3R overexpressed on CSCs and the tumour bulk of a wide range of haematological cancers, including CML, effectively induced apoptosis of CML blasts and LSCs both in vitro and in vivo. Previous data showed successful targeting of BCR-ABL1(+)ALL progenitor cells by SL-401 when combined with imatinib or dasatinib (Kim et al., 2010). We found that SL-401 and SL-501 specifically eradicated the CD34+/CD38−/CD123+ population of CML cells, inhibited colony and LTC-IC formation and significantly extended overall survival in a CML xenograft mouse model developed from CML-BP patient resistant to standard TKIs. Although samples from patients at different stages of the disease (blast crisis, chronic phase and accelerated phase) were tested by the CFC and LTC-IC assays, majority of the samples tested in this study were procured from patients with advanced phase CML. Together, these results indicate that SL-401 might be efficacious in eradicating both CML LSCs and leukaemia blasts, as well as against TKI-resistant advanced phase CML. Further detailed studies are needed to dissect its activity against CML LSCs at earlier stages of the disease. Our results are in agreement with recently published findings by Nievergall et al., (2014), who demonstrated effective targeting CML LSCs with CSL362, another agent directed against the IL3R that induces antibody-dependent cell-mediated cytotoxicity. Several studies have shown that, although BCR-ABL1 is important for the growth and survival of CML progenitors, this appears not to be the case for CML LSCs, and therefore TKIs most probably will have little effect on this subset of cells, which can persist for many years (Angstreich et al., 2005; Bedi et al., 1993; Holyoake et al., 1999; Jiang et al., 2003; Keating et al., 1994). Whether IL3R expression differs as disease progresses and genetic instability drives evolution of blast crisis has not been addressed in the present work and needs to be determined. In this regard, Gill et al. (2014) recently reported that, expression of IL3R increases with time in AML blasts, even in blasts with initially dim levels of the protein.
SL-401 was previously assessed in a completed Phase 1/2 clinical trial of patients with advanced haematological cancers, including AML, which also harbours blasts and LSCs overexpressing IL3R (Frankel et al., 2008). In that study, a single cycle of SL-401 administered alone demonstrated antitumour activity, including reductions in leukaemia blast cells or disease stabilization in more than half of all treated patients, the majority of whom had been heavily pretreated (Frankel et al., 2008). Reduction in leukaemia blasts or stabilization of disease was noted in 47% of patients with relapsed or refractory AML, 55% of patients with AML who were poor risk and not candidates for chemotherapy and 43% of patients with high-risk MDS. Moreover, durable complete responses were observed in two patients with relapsed/refractory AML. Importantly, in that study, CFC assay revealed 79% and 84% reductions in colonies in bone marrow sampled from two patients on day 30 following treatment with SL-401 (Giles et al., 2005; Keating et al., 1994).
Our increased understanding of LSCs, particularly the LSCs of CML, indicates that these cells can become quiescent in haematopoietic micro-niches, such as the bone marrow, for long periods of time during consecutive therapies (Bhatia et al., 2003). It has been shown that a population of residual CD34+ progenitor cells can survive in the bone marrow of CML patients in CP for several years during concomitant TKI therapy (Holyoake et al., 1999). Given that these cells appear to be resistant to TKI-mediated apoptosis, and residual BCR-ABL1+ stem cells are found in CML patients receiving long-term imatinib treatment, the effective eradication of this population will probably require BCR-ABL1– and TKI-independent treatment approaches. SL-401 can selectively target tumour blasts and LSCs overexpressing IL3R regardless of quiescence or cell cycle status (Kim et al., 2010). SL-401 and SL-501 induce receptor-mediated endocytosis and subsequent localization of the drug-toxin conjugate to early endosomes, where DT is cleaved from the larger protein. DT then disrupts ribosomal machinery, leading to global cellular inhibition of protein synthesis and subsequent efficient and rapid apoptosis independent of the cell cycle (Ratts et al., 2005; Ratts et al., 2003).
In conclusion, our results suggest that SL-401 and SL-501 may be an effective treatment modality for patients whose CML develops resistance to TKIs. The in vitro and in vivo results show that these agents are effective at inducing apoptosis of both CML cell lines and blasts from patients whose disease is TKI-resistant or harbours the T315I mutation. Importantly, they effectively killed CML stem cells, defined as CD34+/CD38−/CD123+, and provided a survival advantage to a cohort of mice harbouring a TKI-resistant CML xenograft after only a single cycle of treatment. Combined with the safety and efficacy profiles revealed by a human trial in patients with AML or MDS, these results warrant further exploration of SL-401 in the advanced phase CML. Our preclinical data further indicate that the variant fusion protein SL-501 has even greater anti-CML activity than SL-401, similar to findings reported in AML cells (Hogge et al., 2006b; Liu et al., 2004; Testa et al., 2005), and favor further clinical development of SL-501 for these indications.
Supplementary Material
ACKNOWLEDGEMENTS
Stemline Therapeutics Inc. provided research funding, SL-401, and SL-501. This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672. We are grateful to Wendy Schober, Jared Burks and Duncan Mak from the Flow Cytometry and Cellular Imaging Facility at MD Anderson Cancer Center (National Institutes of Health Cancer Center Support Grant CA016672) for their technical assistance.
Footnotes
CONFLICT OF INTEREST
Stemline Employment, Equity Ownership (CB, ER); Stemline Research Funding (MK, AF).
AUTHOR CONTRIBUTIONS
OF, JB, R-YW, BK performed experiments; OF, JB, CB, MA, AEF, MK designed the experiments and analysed the data; JB, CB, EKR, AEF, MK wrote the manuscript; JC, HK assisted with patient specimen experiments; MA, AEF assisted with experimental design.
Supplementary information is available at British Journal of Haematology’s website.
REFERENCES
- Ailles LE, Gerhard B, Hogge DE. Detection and characterization of primitive malignant and normal progenitors in patients with acute myelogenous leukemia using long-term coculture with supportive feeder layers and cytokines. Blood. 1997;90:2555–2564. [PubMed] [Google Scholar]
- Aldinucci D, Olivo K, Lorenzon D, Poletto D, Gloghini A, Carbone A, Pinto A. The role of interleukin-3 in classical Hodgkin's disease. Leukemia & lymphoma. 2005;46:303–311. doi: 10.1080/10428190400013712. [DOI] [PubMed] [Google Scholar]
- Angstreich GR, Matsui W, Huff CA, Vala MS, Barber J, Hawkins AL, Griffin CA, Smith BD, Jones RJ. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. British journal of haematology. 2005;130:373–381. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- Bedi A, Zehnbauer BA, Collector MI, Barber JP, Zicha MS, Sharkis SJ, Jones RJ. BCR-ABL gene rearrangement and expression of primitive hematopoietic progenitors in chronic myeloid leukemia. Blood. 1993;81:2898–2902. [PubMed] [Google Scholar]
- Berman E, Nicolaides M, Maki RG, Fleisher M, Chanel S, Scheu K, Wilson BA, Heller G, Sauter NP. Altered bone and mineral metabolism in patients receiving imatinib mesylate. The New England journal of medicine. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- Borthakur A, Reddy R. Imaging cartilage physiology. Topics in magnetic resonance imaging : TMRI. 2010;21:291–296. doi: 10.1097/RMR.0b013e31823dfe2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Cohen KA, Liu TF, Cline JM, Wagner JD, Hall PD, Frankel AE. Toxicology and pharmacokinetics of DT388IL3, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human interleukin 3 (IL3), in cynomolgus monkeys. Leukemia & lymphoma. 2004;45:1647–1656. doi: 10.1080/10428190410001663572. [DOI] [PubMed] [Google Scholar]
- Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, Barow M, Mountford JC, Holyoake TL. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML, but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. The Journal of clinical investigation. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokic M, Bjorklund E, Blennow E, Mazur J, Soderhall S, Porwit A. Overexpression of CD123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica. 2009;94:1016–1019. doi: 10.3324/haematol.2008.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel H, Goodacre A, Keyhani A, Jiang S, Van NT, Kimmel M, Sanchez-Williams G, Andreeff M. Minimal residual disease in acute myelogenous leukaemia and myelodysplastic syndromes: a follow-up of patients in clinical remission. British Journal of Haematology. 1997;99:64–75. doi: 10.1046/j.1365-2141.1997.3323151.x. [DOI] [PubMed] [Google Scholar]
- Feuring-Buske M, Frankel AE, Alexander RL, Gerhard B, Hogge DE. A diphtheria toxin-interleukin 3 fusion protein is cytotoxic to primitive acute myeloid leukemia progenitors but spares normal progenitors. Cancer Res. 2002;62:1730–1736. [PubMed] [Google Scholar]
- Florian S, Sonneck K, Hauswirth AW, Krauth MT, Schernthaner GH, Sperr WR, Valent P. Detection of molecular targets on the surface of CD34+/CD38-- stem cells in various myeloid malignancies. Leukemia & Lymphoma. 2006;47:207–222. doi: 10.1080/10428190500272507. [DOI] [PubMed] [Google Scholar]
- Frankel A, Liu JS, Rizzieri D, Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma. 2008;49:543–553. doi: 10.1080/10428190701799035. [DOI] [PubMed] [Google Scholar]
- Frankel A, Woo JH, Mauldin JP, Carraway HE, Frankfurt O, Forman SJ, Vasu S, Pemmaraju N, Konopleva M, Hogge DE, Garanache-Ottou F, Angelot-Delettre F, Brooks C, Szarek M, Bergstein I, Rowinsky E. An Update On The Robust Clinical Activity Of SL-401, a Targeted Therapy Directed To The Interleukin-3 Receptor On Cancer Stem Cells and Tumor Bulk, In Patients With Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) Blood (ASH Annual Meeting Abstracts) 2013:122. [Google Scholar]
- Frankel AE, Hall PD, Burbage C, Vesely J, Willingham M, Bhalla K, Kreitman RJ. Modulation of the apoptotic response of human myeloid leukemia cells to a diphtheria toxin granulocyte-macrophage colony-stimulating factor fusion protein. Blood. 1997;90:3654–3661. [PubMed] [Google Scholar]
- Frankel AE, McCubrey JA, Miller MS, Delatte S, Ramage J, Kiser M, Kucera GL, Alexander RL, Beran M, Tagge EP, Kreitman RJ, Hogge DE. Diphtheria toxin fused to human interleukin-3 is toxic to blasts from patients with myeloid leukemias. Leukemia. 2000a;14:576–585. doi: 10.1038/sj.leu.2401743. [DOI] [PubMed] [Google Scholar]
- Frankel AE, Ramage J, Kiser M, Alexander R, Kucera G, Miller MS. Characterization of diphtheria fusion proteins targeted to the human interleukin-3 receptor. Protein Eng. 2000b;13:575–581. doi: 10.1093/protein/13.8.575. [DOI] [PubMed] [Google Scholar]
- Giles F, O'Brien S, Cortes J, Verstovsek S, Bueso-Ramos C, Shan J, Pierce S, Garcia-Manero G, Keating M, Kantarjian H. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104:547–554. doi: 10.1002/cncr.21187. [DOI] [PubMed] [Google Scholar]
- Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA, June CH, Kalos M. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Graf M, Hecht K, Reif S, Pelka-Fleischer R, Pfister K, Schmetzer H. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): implications for future therapeutical strategies. European journal of haematology. 2004;72:89–106. doi: 10.1046/j.0902-4441.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Guilhot F, Druker B, Larson RA, Gathmann I, So C, Waltzman R, O'Brien SG. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009;94:1669–1675. doi: 10.3324/haematol.2009.010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge DE, Yalcintepe L, Wong SH, Gerhard B, Frankel AE. Variant diphtheria toxin-interleukin-3 fusion proteins with increased receptor affinity have enhanced cytotoxicity against acute myeloid leukemia progenitors. Clin Cancer Res. 2006a;12:1284–1291. doi: 10.1158/1078-0432.CCR-05-2070. [DOI] [PubMed] [Google Scholar]
- Hogge DE, Yalcintepe L, Wong SH, Gerhard B, Frankel AE. Variant diphtheria toxin-interleukin-3 fusion proteins with increased receptor affinity have enhanced cytotoxicity against acute myeloid leukemia progenitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006b;12:1284–1291. doi: 10.1158/1078-0432.CCR-05-2070. [DOI] [PubMed] [Google Scholar]
- Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. The New England journal of medicine. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Jiang G, Yang F, Li M, Weissbecker K, Price S, Kim KC, La Russa VF, Safah H, Ehrlich M. Imatinib (ST1571) provides only limited selectivity for CML cells and treatment might be complicated by silent BCR-ABL genes. Cancer biology & therapy. 2003;2:103–108. doi: 10.4161/cbt.240. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109:4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- Keating A, Wang XH, Laraya P. Variable transcription of BCR-ABL by Ph+ cells arising from hematopoietic progenitors in chronic myeloid leukemia. Blood. 1994;83:1744–1749. [PubMed] [Google Scholar]
- Kim HP, Frankel AE, Hogge DE. A diphtheria toxin interleukin-3 fusion protein synergizes with tyrosine kinase inhibitors in killing leukemic progenitors from BCR/ABL positive acute leukemia. Leuk Res. 2010;34:1035–1042. doi: 10.1016/j.leukres.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kiser M, McCubrey JA, Steelman LS, Shelton JG, Ramage J, Alexander RL, Kucera GL, Pettenati M, Willingham MC, Miller MS, Frankel AE. Oncogene-dependent engraftment of human myeloid leukemia cells in immunosuppressed mice. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2001;15:814–818. doi: 10.1038/sj.leu.2402084. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Hogge DE, Rizzieri DA, Cirrito TP, Kornblau SM, Borthakur G, Bivins C, Garcia-Manero G, Kadia TM, Ravandi F, Andreeff M, Cortes JE, Hoberman K, Szarek M, Rowinsky E, Bergstein I, Kantarjian HM, Frankel AE. SL-401, A Targeted Therapy Directed to the Interleukin-3 Receptor Present On Leukemia Blasts and Cancer Stem Cells, Is Active As a Single Agent in Patients with Advanced AML. Blood (ASH Annual Meeting Abstracts) 2012:120. [Abstr 3625] [Google Scholar]
- Lhermitte L, de Labarthe A, Dupret C, Lapillonne H, Millien C, Landman-Parker J, Hermine O, Baruchel A, Sigaux F, Macintyre E, Asnafi V. Most immature T-ALLs express Ra-IL3 (CD123): possible target for DT-IL3 therapy. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2006;20:1908–1910. doi: 10.1038/sj.leu.2404349. [DOI] [PubMed] [Google Scholar]
- Liu TF, Urieto JO, Moore JE, Miller MS, Lowe AC, Thorburn A, Frankel AE. Diphtheria toxin fused to variant interleukin-3 provides enhanced binding to the interleukin-3 receptor and more potent leukemia cell cytotoxicity. Experimental hematology. 2004;32:277–281. doi: 10.1016/j.exphem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Nievergall E, Ramshaw HS, Yong AS, Biondo M, Busfield SJ, Vairo G, Lopez AF, Hughes TP, White DL, Hiwase DK. Monoclonal antibody targeting of IL-3 receptor alpha with CSL362 effectively depletes CML progenitor and stem cells. Blood. 2014;123:1218–1228. doi: 10.1182/blood-2012-12-475194. [DOI] [PubMed] [Google Scholar]
- Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, Friend S, Linsley PS. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratts R, Zeng H, Berg EA, Blue C, McComb ME, Costello CE, vanderSpek JC, Murphy JR. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J Cell Biol. 2003;160:1139–1150. doi: 10.1083/jcb.200210028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratts R, Trujillo C, Bharti A, vanderSpek J, Harrison R, Murphy JR. A conserved motif in transmembrane helix 1 of diphtheria toxin mediates catalytic domain delivery to the cytosol. Proc Natl Acad Sci U S A. 2005;102:15635–15640. doi: 10.1073/pnas.0504937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci C, Scappini B, Divoky V, Gatto S, Onida F, Verstovsek S, Kantarjian HM, Beran M. Mutation in the ATP-binding pocket of the ABL kinase domain in an STI571-resistant BCR/ABL-positive cell line. Cancer research. 2002;62:5995–5998. [PubMed] [Google Scholar]
- Schiffer CA. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. The New England journal of medicine. 2007;357:258–265. doi: 10.1056/NEJMct071828. [DOI] [PubMed] [Google Scholar]
- Su Y, Li SY, Ghosh S, Ortiz J, Hogge DE, Frankel AE. Characterization of variant diphtheria toxin-interleukin-3 fusion protein, DTIL3K116W, for phase I clinical trials. Biologicals : journal of the International Association of Biological Standardization. 2010;38:144–149. doi: 10.1016/j.biologicals.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, Latagliata R, Mariani G, Rossini A, Battistini A, Lo-Coco F, Peschle C. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100:2980–2988. doi: 10.1182/blood-2002-03-0852. [DOI] [PubMed] [Google Scholar]
- Testa U, Riccioni R, Biffoni M, Diverio D, Lo-Coco F, Foa R, Peschle C, Frankel AE. Diphtheria toxin fused to variant human interleukin-3 induces cytotoxicity of blasts from patients with acute myeloid leukemia according to the level of interleukin-3 receptor expression. Blood. 2005;106:2527–2529. doi: 10.1182/blood-2005-02-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Troncoso P, Palmer JL, El-Naggar AK, Liang JC. Trisomy 7 by dual-color fluorescence in situ hybridization: a potential biological marker for prostate cancer progression. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996;2:1553–1558. [PubMed] [Google Scholar]
- Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nature reviews . Drug discovery. 2009;8:806–823. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.