Abstract
Depression is a significant comorbid condition in diabetes. Individuals with type 2 diabetes (T2DM) are 2 times more likely to experience depression or elevated depressive symptoms compared to those without T2DM. The aims of this state of the science review were to summarize the putative links between diabetes and depression and review empirically supported treatments of depression in diabetes. Findings suggest that a bidirectional association between depression and T2DM exists and that several biological and psychosocial mediators underlie these conditions. Available data indicate that conventional treatments (antidepressant medication, cognitive behavioral therapy, and collaborative care) reduce depression and symptoms of depression; however more controlled studies and development of novel therapies are needed. Glycemic outcomes have most frequently been examined, but findings have been mixed. Self-care and adherence outcomes have been less well studied. Emerging evidence suggests that these outcomes may be important targets for future depression research in T2DM.
Keywords: depression, diabetes, antidepressants, cognitive behavioral therapy, collaborative care
Impact of Depression and Diabetes
Depression is a significant comorbid condition in diabetes (Anderson, Freedland, Clouse, & Lustman, 2001). It is estimated that by 2050, one in four persons will have diabetes if present trends continue (Boyle, Thompson, Gregg, Barker, & Williamson, 2010). Individuals with type 2 diabetes (T2DM) are two times more likely to experience depression or elevated depressive symptoms compared to individuals without diabetes (Pan et al., 2010). Having both diabetes and depression significantly impacts adherence to diabetes self-care which is associated with worse clinical outcomes (Ciechanowski, Katon, & Russo, 2000; Katon, 2008) and a greater severity of diabetes complications (de Groot, Anderson, Freedland, Clouse, & Lustman, 2001; Lin et al., 2010). All cause mortality for individuals with diabetes who are depressed is reported to be 46% higher than those who are not depressed (van Dooren et al., 2013).
Association between Depression and Diabetes
The causality dilemma between diabetes and depression has been a controversial topic. Initially, researchers hypothesized that the depression observed in persons with diabetes may have been the result of the stress and psychosocial demands of having a chronic illness (Talbot & Nouwen, 2000; see Figure 1). However, research has also suggested that depression may be a risk factor for new onset diabetes, likely as a result of the biochemical changes which occur in depression as well as reduced self-care behaviors associated with depression (Golden et al., 2008). While the exact nature and directionality of the association between these comorbidities remains unknown, findings from several systematic reviews and meta-analyses generally suggest that this association is bi-directional rather than uni-directional (Mezuk, Eaton, Albreicht, & Golden, 2008; Nefs, Pouwer, Denollet, & Pop, 2012; Nouwen et al., 2010).
Figure 1.
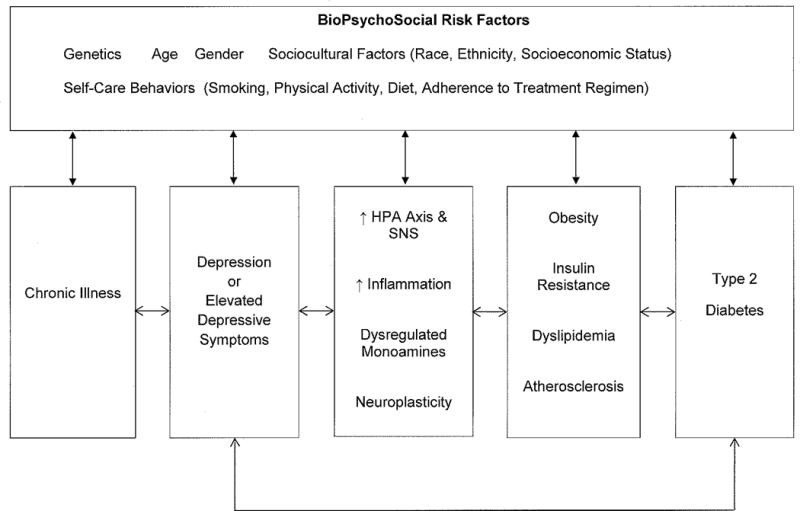
Diabetes and Depression: Potential Mechanisms
Note: HPA Axis = Hypothalamic-Pituitary-Adrenal Axis; SNS = Sympathetic Nervous System
Depression Predicting Incident Diabetes
In the past few years, several prospective design studies (Demakakos, Pierce, & Hardy, 2010; Golden et al., 2008; Nefs et al., 2012; Pan et al., 2010) and at least three meta-analyses (Cosgrove, Sargeant, & Griffin, 2008; Knol et al., 2006; Mezuk et al., 2008) have examined the association between depression and incident diabetes. Meta-analytic findings have generally shown that depression is associated with an increased risk of incident diabetes, with relative risk estimates ranging from 20% (Cosgrove et al., 2008) to 60% (Mezuk et al., 2008). Consistent with these findings, ten year follow-up data from 55,000 women participating in the Nurses’ Health Study cohort showed that depressed mood symptoms were associated with a 42% increased risk of diabetes (Pan et al., 2010). However, it was also found that the relative risk was attenuated (Relative Risk = 1.17; 95% CI, 1.05 – 1.30) following adjustment for covariates such as body mass index and physical activity level. It was concluded that these variables were likely important mediators between depression and incident diabetes and that the effects of depression on incident diabetes were independent of adiposity and inactivity.
Depression also appears to be linked to greater insulin resistance prior to diabetes onset. In a recent meta-analysis, Kan and colleagues (2013) found a small, but significant association between depression and insulin resistance (d = 0.19). However, this association varied according to the methods of depression assessment and insulin resistance measurement that were used. They also reported that larger effect sizes were observed for diagnostic interviews compared to self-report measures of depression (d = 0.46 vs. d = 0.13) and insulin sensitivity compared to Homeostasis Model Assessment Estimated Insulin Resistance (HOMA-IR, d = 0.32 vs. d = 0.17). To further support the link between depression and insulin resistance, treatment of depression using antidepressant medications has been associated with lower insulin resistance in adults at risk for T2DM. Wagner, Allen, Swalley, Melkus, and Whittemore (2009) showed that depressed participants taking an antidepressant medication demonstrated levels of insulin sensitivity similar to non-depressed participants. Both of these groups had significantly higher levels of insulin sensitivity compared to depressed patients who were not prescribed an antidepressant medication.
However, contrary to these findings, antidepressant medication use also appears to be linked to an increased risk of incident T2DM even after controlling for potential mediators such as fasting glucose levels and BMI (Ma et al., 2011; Pan et al., 2012; Rotella & Mannucci, 2013; Rubin et al., 2008). Although the etiologic role of antidepressant medication use in T2DM continues to be debated, it may be that use of this medication serves as a proxy for depressive symptom severity, such that participants taking antidepressants were more severely depressed in the past or have a history of recurrent depressive symptoms. For example, Rubin and colleagues (2008) examined the association between antidepressant medication use (defined as intermittent use, continuous use, & no use at baseline) and risk of developing T2DM among n=3,187 men and women enrolled the Diabetes Prevention Program; participants were randomized to three treatment arms (i.e., lifestyle intervention [ILS], metformin [MET], & placebo [PLB]). Baseline antidepressant medication use was associated with incident T2DM in the ILS (Hazard Ratio = 3.48; 95% CI, 1.93 – 6.28) and PLB (HR = 2.25; 95% CI, 1.38 – 3.66) arms after controlling for baseline depression. Continuous antidepressant use (versus no use) was also associated with incident T2DM in the ILS (HR = 3.39; 95% CI, 1.61 – 7.13) and PLB (HR = 2.6; 95% CI, 1.37 – 4.94) arms after controlling for baseline depression. These associations were not observed among participants in the MET arm. At least two recent empirical investigations (Ma et. al., 2011; Pan et al., 2012) and one meta-analysis by Rotella and Mannucci (2013) have provided additional support for these findings. However, further research is needed to more fully understand the mechanisms underlying these associations as well as the influence of antidepressant use on glucose tolerance and the risk of T2DM. Nonetheless, it appears as though depression and antidepressant use are risk factors for incident T2DM, and health care professionals will need to consider the negative effects of prescribing psychotropic medications for depression, particularly among patients at elevated risk for T2DM. Non-pharmacological treatments for depression in T2DM, such as cognitive behavioral therapy, could be used as safe and efficacious alternatives and should be pursued further.
Diabetes Predicting Incident Depression
In a systematic review of seven studies of diabetes predicting incident depression, which included N = 6,414 cases of depression, Mezuk et al. (2008) found that diabetes was associated with a modest 15% increased relative risk of depression. A more recent meta-analysis by Nouwen and colleagues (2010) showed a marginally higher relative risk of depression (i.e., 24%). Consistent with these findings, Icks et al. (2013) found a 22% increased risk of elevated depressive symptoms in patients with diabetes compared to those without diabetes following adjustment for age and gender. One potential explanation for the lower directional effect of diabetes on risk of incident depression may be related to variability in the use of oral hypoglycemic agents. Wahlqvist and colleagues (2012) showed that patients taking both sulfonylurea and metformin therapy had a 60% reduced risk of depression (which was defined in their study as both uni- and bipolar depression) compared to diabetes patients who were not taking oral hypoglycemic agents. Future investigations examining the effects of diabetes on risk of incident depression will need to consider accounting for oral hypoglycemic agent use as a covariate since its inclusion may account for underestimations in relative risk values.
Potential Mechanisms Linking Depression and Diabetes
To date, no evidence-based explanatory models exist to account for the temporal associations between diabetes and depression. However, several mechanisms could plausibly explain the bi-directional associations between these comorbidities. Figure 1 provides a conceptual overview of the multifaceted and complex interactions between these genetic, biological, and psychosocial factors. Although the role of chronic illness in the development of depression is noted; factors beginning with depression and moving to the development of diabetes are emphasized. This is to remain consistent with emerging evidence which suggests that the strength of the association between depression and risk of incident diabetes is relatively robust, in comparison to the association between diabetes and risk of depression, even though researchers acknowledge that a bi-directional link exists (Mezuk et al., 2008).
First, an accumulating amount of literature suggests that diabetes and depression are independently associated with numerous biopsychosocial risk factors (e.g., genetics, age, gender, sociocultural factors, and self-care behaviors; see Figure 1) which may all play a mediating role between these comorbidities (Golden et al., 2008; Silva, Atlantis, & Ismail, 2012). For example, Egede and Zheng (2003) found that perceptions about worsening health affected the development of depression in diabetes as well as other psychosocial factors such as age, gender, education, socioeconomic status. It has also been suggested that poor self-care behaviors (e.g., physical inactivity &/or diets high in carbohydrates) and presence of obesity (or greater body mass index) may play a role in the association between diabetes and depression (Golden et al., 2008; Katon et al., 2004; Knol et al., 2006). Cigarette smoking is independently associated with depression and diabetes (Egede & Zheng, 2003). This may reflect the tendency of nicotine dependent smokers to report more depressive symptoms compared to never-smokers (Jamal, Willem van der Does, Cuijpers, & Penninx, 2012) or that use of nicotine can be a form of self-medication for depressive symptoms. Finally, diabetes-related distress has been linked to self-care behaviors, glycemic control, and quality of life (Fisher et al., 2007). Although numerous psychosocial risk factors are outlined in Figure 1, this is not an exhaustive list, and instead highlights the important role these factors appear to play in the etiology of diabetes and depression.
Second, traditional models of depression have suggested that alterations in the function(s) of the hypothalamic-pituitary-adrenal axis, monoamine regulation, and neuroplasticity play a prominent role in the development and progression of depression. Although these models are fairly heterogeneous and only partially inter-related, a nascent amount of literature suggests that inflammation and pro-inflammatory cytokines play a significant overlapping role in these models of depression (Irwin & Miller, 2007; Miller, Maletic, & Raison, 2009; Raison, Capuron, & Miller, 2006; Wichers & Maes, 2002). For example, pro-inflammatory cytokines such as IL-6 or TNF-α are known to be strongly associated with depression (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Raison et al., 2006; Wichers & Maes, 2002) and recent findings by Doyle and colleagues (2013) have also shown that inflammation is associated with elevated depressive symptoms in T2DM. These cytokines correlate with increased HPA axis activity and sympathetic nervous system activity, glucocorticoid production (e.g., cortisol & catecholamines), and glucocorticoid resistance (Pace, Hu, & Miller, 2007). The resulting chronically elevated glucocorticoid concentrations which occurs through inhibition of negative feedback mechanisms on glucocorticoid receptors can be harmful and eventually influence the development and progression of diabetes risk factors such as visceral fat accumulation, insulin resistance, dyslipidemia, and hypertension (Champaneri, Wand, Malhotra, Casagrande, & Golden, 2010; Pickup, 2004). Lastly, depression has been positively associated with visceral obesity, insulin resistance, and hypertension which are underlying disease processes associated with metabolic syndrome and diabetes. Taken together, these various mechanisms are complex and may involve several shared physiological bi-directional associations. More studies are needed to explore the mechanisms underlying these reciprocal associations, which will be necessary for the prevention and treatment of both comorbidities.
Treatments for Depression among Individuals with Diabetes
There have been a number of well written contemporary reviews addressing the positive effects of anti-depressant medications, cognitive behavioral therapy (CBT), and collaborative care for depression treatment in individuals with T2DM (Table 1). However, there is limited evidence to demonstrate significant improvements in glycosylated hemoglobin (HbA1C) and even less regarding the impact on diabetes self-care behaviors.
Table 1.
Contemporary Reviews of Depression Treatments for Persons with Diabetes
| Authors | Year | Summary |
|---|---|---|
| Balhara & Verma | 2013 | A review of psychosocial interventions in management of depression among diabetics addressed 2 RCTs, 2 open label RCTs, 2 ongoing studies, and 5 systematic reviews. Concluded that psychosocial interventions, particularly CBT, improve depression, but were not consistently noted to improve glycemic control. |
| Baumeister, Hutter, Bengel | 2012 | Cochran review of 19 trials for psychological and pharmacologic interventions for depression in patients with diabetes and diabetes. Concluded that psychological and pharmacological intervention have a significant beneficial effect on depression outcomes with moderate improvements in glycemic outcomes in pharmacologic trials and inconclusive for psychological interventions. |
| Markowitz, Gonzalez, Wikinson, Safren | 2011 | A review of 17 studies to evaluate psychosocial, anti-depressant medications and collaborative care for treatment of depression in patients with diabetes. Concluded that psychosocial interventions, particularly CBT, anti-depressant medications, and collaborative care were effective for treatment of depression however, for improving glycemic control, the interventions had mixed outcomes. |
| Van der Feltz-Cornelius, Nuyen, Stoop, Chan, Jacobson, Katon et al. | 2010 | Systematic review and meta-analysis of 14 RCTs for effect psychotherapy, pharmacology, or collaborative care for treatment of major depressive disorder and significant depression symptoms in patients with diabetes. For all treatments, there was a moderate effect size (ES: −0.512) in reducing severity of depression symptoms. A large ES (−0.581) was noted from psychotherapeutic interventions which were often combined with diabetes education (ES=−0.581), moderate ES for pharmacologic treatment (ES= −0.467), and smaller ES for collaborative care (−0.292). Overall treatment effect for improving glycemic control was noted (ES= −.274), but may be due to diabetes education. Pharmocotherapy and collaborative care had little to no effect on glycemic control other than Sertraline which improved this outcome. |
| Petrak & Herpertz | 2009 | Review of 11 RCTs that evaluated treatments of depression in diabetes according to pharmacological, psychological or mixed (pharmacological and psychological) interventions. Best results for psychological interventions were counseling and CBT. Antidepressants were effective for treatment of depression with no benefit to diabetes outcomes conclusively. Mixed interventions provide best outcomes for depression, but no positive effects observed on glycemic control and difficult to determine the effective component of treatment. |
Antidepressant Medication
Early researchers presumed that depression was a natural response to the hardships imposed by diabetes, consequently this view tended to dampen interest in depression treatment. However, recent meta-analyses support cautiously the efficacy of antidepressant medication for depression in diabetes (Baumeister, Hutter, & Bengel, 2012; van der Feltz-Cornelis et al., 2010). Over eight placebo-controlled antidepressant medication trials, Baumeister et al. (2012) found a moderate beneficial effect of antidepressant medication on depression severity ratings (standardized mean difference, SMD =−0.61, confidence interval, CI = −0.94 to −0.27; N=377). The effect was significant albeit smaller in the subset of studies of serotonin reuptake inhibitors (SSRIs, SMD=−0.39, CI −0.64 to −0.13, N=241). The likelihood of remission was significantly greater with antidepressant medication (odds ratio, OR =2.5, 95% CI, 1.2 to 5.1) although this analysis included only three studies having a combined total of 136 subjects. van der Feltz-Cornelis et al. (2010) found similar moderate beneficial effect reducing depression symptom severity in an aggregate analysis of 14 randomized controlled trials (−0.51, 95%CI −0.63 to −0.39). The pooled effect of seven pharmacological trials was also judged to be moderate (−0.47, 95% CI −0.67 to −0.27).
The strongest evidence regarding medication efficacy emanates from randomized double blind placebo controlled trials. Despite significant interest in comorbid depression and diabetes including research funded by federal (e.g., the National Institutes of Diabetes, Digestive, and Kidney Diseases of the National Institutes of Health) and private agencies (e.g., the American Diabetes Association); few such studies are available in diabetes populations. A total of six studies have tested the efficacy of pharmacotherapy vs. placebo in the context of a randomized controlled trial (Echeverry, Duran, Bonds, Lee, & Davidson, 2009; Lustman, Freedland, Griffith, & Clouse, 2000; Lustman et al., 1997; Paile-Hyvarinen, Wahlbeck, & Eriksson, 2003, 2007; Xue, 2004). These six studies enrolled and randomized a total of 296 patients, 85% of enrolled patients completed acute-phase treatment, the estimate based on the subset (n=5) of studies reporting treatment completion data. Caution should be exercised in aggregating information from these trials. Generalizability of the findings to the general population is suspect in light of the small numbers and considering the potential substantial effect of publication bias (Turner, Matthews, Linardatos, Tell, & Rosenthal, 2008). In three of six of these studies, the primary endpoint was changes in glycemic control during the treatment trial. In terms of antidepressant efficacy, both studies reported by Paile-Hyvarinen et al. (2003, 2007) and the study by Echeverry et al. (2009) failed to find a significant beneficial effect of treatment on depression symptom severity. However, significant mean differences favoring medication treatment over placebo were reported by Lustman et al. (1997), Lustman et al. (2000), and by Xue (2004). Thus, the effectiveness of antidepressant medication in patients with diabetes remains uncertain based on the limited data from the six available, randomized, placebo controlled trials.
Maintaining good glycemic control is the cornerstone of diabetes therapy. Studies intent on determining the benefits of depression treatment in patients with comorbid diabetes typically included glycemic measures. All six randomized, placebo-controlled trials reported the impact of treatment on glycemic control measured via determinations of glycosylated hemoglobin levels. All six trials reported some significant beneficial effect of antidepressant treatment (i.e., lower A1C levels vs. placebo) although the comparisons reached the level of a trend in two studies (Lustman et al., 2000; Paile-Hyvarinen et al., 2003) and was apparent at the three month follow-up but not at six (Echeverry et al., 2009). The results are consistent with the Cochrane meta-analysis reported by Baumeister et al. (2012; −0.4%; 95% CI −0.6 to −0.1; P =0.002; 238 participants; five trials). Other meta-analyses or reviews judged the results as mixed (Markowitz, Gonzalez, Wilkinson, & Safren, 2011) or the beneficial effects limited to sertraline (van der Feltz-Cornelis et al., 2010).
Adequate care of diabetes is critically dependent on the patient exhibiting self-care behavior that includes proper diet and levels of physical activity that support good diabetes outcomes. Gonzalez et al. (2008) reported a meta-analysis of 47 independent studies that had measured the association of depression with measures of diabetes self-care behaviors. Depression was associated with poorer adherence, the weighted effect size being small to moderate (r = 0.21, 95% CI 0.17–0.25). Effect sizes were largest for missed medical appointments and composite measures of self-care (r values = 0.31, 0.29). Less is known about the impact of depression treatment on diabetes self-care. The beneficial effect of nortriptyline observed by Lustman et al. (1997) could not be attributed to changes in adherence to depression treatment measured by medication dispensers equipped with electronic memory. This group reported a separate but relevant study designed to reveal mechanisms that may underlie reported adverse effects of depression on glycemic control (Lustman, Williams, Sayuk, Nix, & Clouse, 2007). Enrollment was limited to patients with major depressive disorder and comorbid T2DM. All subjects received open label treatment with bupropion and changes in glycemic control were examined in relation to changes in depression symptoms, anthropometrics, and measures of diabetes self-care. In the short term, improvement in glycemic control during bupropion treatment is predicted independently by improvements in mood and body composition. Longer-term improvements in glycemic control are predicted primarily by sustained improvement in mood via mechanisms independent of anthropometric and self-care modifications.
Cognitive Behavioral Therapy (CBT)
This is a collaborative, goal-oriented form of therapy that helps clients overcome difficulties by changing their thinking, behavior, and emotional responses (Beck, 1995). It has been reported that CBT is an effective treatment for depression with lower relapse rates than antidepressant medications (Driessen & Hollon, 2010). For adults with T2DM, CBT targeted at treatment of depression has demonstrated positive outcomes relative to depression remission, however, studies are limited. Evidence from CBT depression trials suggests some improvements in glycemic control, yet findings regarding changes in self-care behaviors are limited (Table 1).
Lustman, Griffith, Freedland, Kissle, & Clouse (1998) conducted the earliest study using CBT to treat depression in patients with comorbid depression and diabetes. This was a randomized controlled CBT trial for major depression in 51 patients with T2DM. One group (n=25) received CBT for 10 weeks (administered by a licensed psychologist) and the other group received usual care (n=26). Both groups received diabetes education to control for the effects of supportive attention. Follow-up measurements were at the end of treatment and six months later. Post treatment, 85% of the treatment group and 27.3% of the control group achieved remission (p<.001). By six months, this was 70% and 33.3% respectively (p=.03). All patients were shown glucose monitoring prior to randomization with no differences at baseline. However, at the end of treatment those who completed CBT demonstrated less adherence to self-monitoring (p=.01). The authors suggested that because CBT had homework and other activities, this may have affected adherence to diabetes management. Interestingly, however, although immediate post treatment did not indicate differences in HbA1C between groups, at six months follow-up, the CBT group had significantly lower HbA1C compared to the controls (9.5% vs. 10.9%; p=.03), suggesting a delayed benefit of CBT on glycemic control. Since both groups received diabetes education, it is unclear whether the improvement in HbA1C was related to depression improvement or the benefit of diabetes education.
Penckofer et al. (2012) reported a randomized clinical trial for depressed women (n=70) with T2DM. One group (n=34) received an eight week nurse delivered, group CBT therapy followed by two booster sessions while one group (n=36) received usual care. The Study of Women’s Emotions and Evaluation of a Psychoeducational (SWEEP) program focused on recognizing depression and other moods (e.g., anxiety or anger), relationship of moods to metabolic control, and management of moods. Women were followed over a period of six months. Although 100% of the women were categorized as depressed at baseline, 48% of the women in the SWEEP program as compared to 70% of those in usual care remained depressed at three months (p=.08). At six month follow-up, 35% of those in the SWEEP program were depressed compared to 80% of those in usual care (p<.001). It was reported that the SWEEP program significantly improved depression outcomes as well as anxiety, anger, and quality of life. Even though diabetes education was not a component of the SWEEP program, there was a clinical improvement in HbA1C (−0.4%) compared to usual care (−0.1%) from baseline to three months in the treatment group but this difference was not significant between groups. Findings regarding diabetes self-care behaviors were not reported, however, participants recommended that diabetes education be provided after the SWEEP program. Thus, these authors recommended that research to systematically examine whether treating depression followed by diabetes education could improve HbA1C and self-care behaviors should be conducted.
Most recently Safren and colleagues (2013) published findings on an integrative approach to depression treatment which included CBT for adherence and depression (CBT-AD) among (n=87) adults with T2DM. Participants were randomized to either an enhanced treatment as usual group (ETAU), which included medication adherence, self-monitoring blood glucose, and lifestyle counseling or 9 to 11 session of CBT for adherence and depression counseling (CBT – AD). Outcomes included changes to adherence, depression, and HbA1C. Following four months of treatment, participants in the CBT-AD group showed significantly higher rates of medication adherence (20.7 percentage points, p=.000), greater self-monitoring of blood glucose (30.2 percentage points, p=.000), and lower self-reported depressive symptoms (6.44 lower depression score on Montgomery-Ashberg Depression Rating Scale, p=.002), and better glycemic control (.72 difference in HbA1C, p=.001) compared to participants in the ETAU group. These improved outcomes were maintained at 8- and 12-month follow-up periods. The authors concluded that CBT-AD was an effective intervention for improving medication adherence, reducing depressive symptoms, and improving glycemic control.
CBT Combined with Exercise
Exercise in combination with CBT for depression was studied in a single-arm, repeated measures trial called Program ACTIVE (Appalachians Coming Together to Increase Vital Exercise). de Groot and colleagues (2012) tested the effectiveness of a novel combination of community-based exercise and CBT treatment on depression, glycemic control, and exercise outcomes at baseline, post-intervention and three month follow-up in rural Appalachian patients with T2DM and comorbid depression (n=50). Patients completed ten sessions of manualized CBT, 12 concurrent weeks of aerobic exercise, and six exercise classes. Depression remission was found in 66% of patients at post intervention (p<.001) and 63% at three months (p<.001). Patients completed 193 minutes per week of aerobic activity (range: 76–478 minutes) during the intervention. Further, self-reported physical activity improved significantly from baseline to post intervention (p<.01) and three months (p<.01). The authors concluded that this novel combination treatment approach using CBT and exercise was both feasible and effective in improving depression and diabetes outcomes.
Other investigators are currently examining physical activity as part of depression treatment among individuals with T2DM (Petrak et al., 2010). In the MIND-DIA trial, elderly persons (age 65 to 85) will be randomized into one of three arms: (1) group CBT delivered by trained psychologists who focus on strategies to overcome depression and diminish diabetes-related distress in addition to the use of pedometers to increase physical activity, (2) manualized guided self-help intervention or (3) treatment as usual. Diabetes self-care activities will be measured; however, given that physical activity is being emphasized in the treatment arm, an improvement in self-care might be expected.
CBT Delivered via Telephone and Web
Other nontraditional methods of delivering depression treatment using CBT among individuals with diabetes have also been reported. Piette et al. (2011) reported a study of patients with T2DM and depression (n=291) who were randomized to a 12 week nurse delivered telephone-based CBT program for depression plus walking followed by nine monthly booster sessions. The CBT program focused on depression at first, then pedometer-based walking program (after week 5) and then on the links of depression, activity, and diabetes. The usual care group received a book based on CBT for depression called the “Feeling Good Handbook.” After one year, the intervention group had a greater reduction in depression symptoms (58% remitted at 12 months versus 39%, p=.002), a greater decrease in systolic blood pressure (4.26 mmHg, p=.05), and a greater increase in steps for walking (mean difference = 1,131 steps day, p=.0002) compared to the control group. However, there was no change in HbA1C. The intervention patients also experienced significant improvements in their self-efficacy. The use of a telephone delivered CBT program for depression treatment with walking was successful in improving health outcomes but not HbA1C in these depressed individuals with T2DM.
The use of web-based depression treatment in diabetes has been examined, though limited findings are available to support this mode of treatment. van Bastelaar, Pouwer, Cuijpers, Riper, & Snoek (2011) reported a clinical trial of adult diabetes patients (more than half with T2DM) with depression who were randomized to an eight week web-based, therapist supported diabetes-specific depression intervention (n=125) or a 12 week waiting list control group (n=130). Intention to treat analysis indicated that the web-based CBT depression treatment was effective in reducing depression (p<.04) as well as diabetes-specific emotional distress (p<.03). Stronger improvements occurred for completers of the web-based course compared to the control group. Overall, however, there was no beneficial effect on glycemic control. Self-management behaviors were not reported as part of this investigation.
Overall evidence suggests that CBT for depression treatment for individuals with T2DM demonstrates successful improvements in depression outcomes regardless of the mode of administration (i.e., individual or group sessions, telephone, or web-based). Although some improvements in HbA1C were reported (e.g., Lustman et al., 1998; Safren et al., 2013) these improvements may have been due to caring for their diabetes (e.g., diabetes education). Few studies reported self-care behavior activities at baseline and whether these improved as a result of depression remission. It should be noted that for some self-care behaviors like physical activity, an improvement was most likely due to exercise prescription as a component of the depression treatment program (de Groot et al., 2012). Thus, future research examining the impact of CBT for treatment of depression and its impact on diabetes self-care behaviors is warranted.
Collaborative Care
There has been much emphasis on collaborative care delivery models for individuals with diabetes who have depression. Collaborative care for depression is structured care using a multimodal intervention with generally two components which include (1) trained health professionals to support primary care providers in the education of patients with depression, monitoring for improvement, and scheduling follow-up visits as needed, and (2) psychiatry services to provide consultation to primary care providers and supervisory assistance for depression care managers (Katon & Unutzer, 2006). Collaborative care has been reported to be more effective than standard care in improving long and short-term depression outcomes (Gilbody, Bower, Fletcher, Richards, & Sutton, 2006). The IMPACT (Improving Mood Promoting Access to Collaborative Treatment) Trial was a large depression trial using collaborative care in older adults (Unutzer et al., 2002). In this study, Medicare patients with depression, aged 60, and over were randomized to a stepped-care algorithm for depression treatment (antidepressant medications or a course of Problem Solving Treatment in Primary Care [PST-PC] delivered by a psychologist or a nurse trained in depression for six to eight sessions) or usual care. If after ten weeks there was no improvement in depression, the addition of a second therapy (either antidepressant or PST-PC) was recommended. The IMPACT sub-study of individuals with T2DM who were depressed (n=417) reported that for those individuals who received collaborative care, they showed significantly less depression (p<.001) and increased their weekly exercise more (p=.001) when compared to usual care after 12 months. However, HbA1C and other self-care behaviors were not affected (Williams et al., 2004).
Shortly after the IMPACT Trial, the Pathways Study was conducted, which was another RCT using collaborative care for patients with diabetes who also had depression. However, participants were not limited to older adults (Katon et al., 2004). Individuals were randomized to the Pathways case management intervention (n=164) delivered by depression care manager nurses who used stepped-care algorithm similar to the IMPACT study (n=165). Findings indicated less depression severity (p=.004) and greater adherence to medication (odd ratios 2.18 to 3.20) over time for the treatment compared to the usual care group. However, no significant differences between groups in glycemic control (mean study decrease in HbA1C of 0.35% after one year) were noted.
Another collaborative care study randomized Hispanic participants (n=387) to either a stepped-care algorithm for depression treatment delivered by a bilingual social worker or enhanced usual care which included a depression education pamphlet. This study reported a greater reduction in depression in the treatment group when compared to the usual care group (p<.001). However, no significant differences between treatment and usual care on HbA1C (−0.67% vs. −0.55) or diabetes self-management was found over a period of 18 months (Ell et al., 2010).
In the TEAMcare Trial (www.TEAMcarehealth.org), persons with poorly controlled diabetes, coronary heart disease or both and coexisting depression were randomized to either collaborative care (n=106) or usual care (n =108) for depression (Katon et al., 2010). Nurses experienced with diabetes education completed training in depression and lifestyle modification and then delivered the treatment intervention using coaching and motivational interviewing to assist patients in problem solving and goal setting for 12 months. The decrease in depression was significantly higher for the treatment than the usual care group (p<.001). In addition, the intervention group had greater improvements in HbA1C (difference 0.58%), cholesterol (difference 6.9 mg/dl), systolic blood pressure (difference 5.1 mmHg), and were more likely to have medication adjustments than the usual care group. A similar study used integrated care to improve adherence to medications in depressed individuals with T2DM (Bogner, Morales, de Vries, & Cappola, 2012). Patients (n=180; 56% African American) who were being medically treated with antidepressants and oral hypoglycemics in primary care were randomized to either integrated care (care managers trained in pharmacotherapy for depression & diabetes management who collaborated with physicians) or usual care. Results were that the integrated care group had better HbA1C (−0.7% vs. +.5%, p<.001) and depression improvement (58.7% vs. 30.7%, p<.001) than the usual care group. Both of these studies suggest that healthcare providers who have diabetes expertise and training in depression may be most successful in improving metabolic control and depression outcomes.
Most recently, a modified TEAMcare implementation pilot called the “Synergy Model” was conducted at an urban academic center which was designated as an accountable care organization for patients with depression and chronic medical illness (n=61; Chung et al., 2013). The team (nurse, behavioral health manager, social worker, consultant psychiatrist) worked with the primary care physicians and nurses who were trained in the TEAMcare model. In the diabetes subgroup (n=21) who had depression and HbA1C>8% at baseline, 33% had depression response with at least 0.5% HbA1C reduction after a minimum of 8 weeks. Although no self-care behaviors for this study were reported, this pilot study demonstrated feasibility and promise for future work in this area.
Collaborative care for depression treatment for individuals with diabetes has been effective. The effects of depression treatment on glycemic control in collaborative care seem to be larger than the effects seen in traditional CBT studies. Consistent with CBT studies, information regarding its impact on diabetes self-care has been limited. However its success may be dependent upon the experience and skills of the individuals delivering the treatment.
Summary of Depression Treatment Findings
Evidence suggests that antidepressants, CBT, and collaborative care for depression, either alone or in combination, are effective treatment regimens for individuals with T2DM. Glycemic control outcomes (e.g., HbA1C) have most frequently been examined with studies having mixed results with improvements reported by some (Bogner et al., 2012; Katon et al., 2010; Lustman et al., 1998; Lustman et al., 2007; Safren et al., 2013) and not others (Katon et al., 2004; Williams et al., 2004). For those studies where an improvement in HbA1C was reported, it may have been due to caring for their diabetes (e.g., diabetes education as part of treatment). For some studies, the baseline HBA1C demonstrated fairly good control (e.g., Penckofer et al., 2012). This could reduce the observed variability in glycemic control change scores at follow-up as well as underestimate the effects of the interventions on glycemic control. One forthcoming study will be comparing depression treatments in persons with diabetes and targeting those with poor glycemic control (Petrak et al., 2013).
Another finding from the literature is that only several depression treatment studies have reported aspects of self-care or treatment adherence with significant improvements (e.g., Lustman et al., 2007; Safren et al., 2013) despite evidence that depression is related to self-care. For example, Lin et al. (2004) reported that major depression was associated with less physical activity, unhealthy diet, and lower adherence to medications (i.e., oral hypoglycemic, antihypertensive, & lipid lowering meds) in a study of over 4000 persons with diabetes. Park, Hong, Lee, Ha, and Sung (2004) reported that for individuals with T2DM, depression was associated with poor participation in diabetes education programs as well as poor diet and medication adherence.
More recently, Wagner, Tennen, and Osborn (2010) reported that even for individuals with T2DM who had a lifetime history of major depressive disorder but were not currently depressed, there was poorer diabetes self-management when compared to those who had never been depressed. Chen, Ruppert, Charron-Prochownik, Noullet, and Zgibor (2011) examined the influence of depression on goal setting and barriers to diabetes self-care for patients with T2DM. They reported that while goal setting may be similar between depressed and non-depressed individuals with diabetes, the skill acquisition and motivation for self-care may be less for those who are depressed, which may consequently lead to worse self-care behaviors. Overall, studies on the treatment of depression using antidepressant medication, CBT, and collaborative care have not focused on whether treatment of depression can improve self-care behaviors (Gonzalez, Kupperman, & Vileikyte, 2010).
Treating both depression and diabetes is a challenge for the healthcare provider and the patient. Of concern is that health disparities exist for the treatment of diabetes and depression. African Americans and Hispanics have the greatest risk for diabetes with significantly greater risk of death, renal disease, and extremity amputation (Office of Minority Health, 2013a, 2013b). Disparities in depression care include poor access to mental health treatment, cultural barriers to quality care including language translation and cultural understanding, as well as limited healthcare providers (de Groot et al., 2010). It has been reported that visits to primary care physicians result in less antidepressant prescription and counseling referral for blacks and Hispanics (Lagomasino, Stockdale, & Miranda, 2011). However, research also indicates a lack of comfort for patients with diabetes in discussing depression with their healthcare provider particularly if they are African American (Wagner, Perkins, Piette, Lipton, & Aikens, 2009) or Hispanic (Caplan & Whittemore, 2013). This may account for the lack of depression treatment observed in these groups and thus research addressing these issues is necessary.
Recommendations for Future Research
Future depression studies should explore whether an improvement of depression provides an opportunity to engage patients in better self-care behaviors. Evidence indicates that depression interferes with learning, the performance of learned skills, and self-care behaviors (Brand, Reimer, & Opwis, 2007). Whether treatment of depression increases ones receptivity to diabetes education as depression subsides is not understood, however, it is logical that a “window of opportunity” may exist. With less anxiety and improved mood, patients may be more receptive to diabetes education which may allow for better engagement in diabetes self-management, improved glycemic control, and greater overall health. Although some studies have suggested that this may be possible, a systematic evaluation of this has not been done.
Relapse of depression is common in persons with T2DM and interventions to prevent relapse are needed. Nefs et al. (2012) reported that in patients with T2DM (n=630) who were followed over a period of two years, 26% had depression (Edinburg Depression Score ≥ 12) at baseline. For those who were not depressed at baseline, 14% developed incident depression while recurrence/persistence for those with depression at baseline was 66% (Nefs et al., 2012). It has been reported that as few as 40% of patients who have diabetes and depression remain depression free in the year following treatment, however, maintenance therapy using sertraline has been reported to prolong depression free intervals with improvements noted in HbA1C (Lustman et al., 2007). The need to study prevention of relapse in persons with depression who have diabetes using antidepressants, CBT, and collaborative care is important in order to prevent the downward spiral of emotions which contribute to poor diabetes self-care behaviors (Penckofer, Ferrans, Velsor-Friedrich, & Savoy, 2007). In addition, exploration of the most effective modes of treatment (individual, group, telephone, and web) is also needed (Mohr, Burns, Schueller, Clarke, & Klinkman, 2013).
Randomized clinical trials comparing the efficacy of CBT versus antidepressant medications on diabetes outcomes, both self-care and glycemic control, are needed. One such planned trial will compare antidepressant therapy (sertraline) to group CBT in 200 depressed adults with poorly controlled diabetes (type 1 and type 2; Petrak et al., 2013). Finally, trials to examine whether CBT, antidepressant medication, and collaborative care can improve subclinical depression and prevent the onset of major depression would also be significant since diabetes complications are greatest in those with comorbid depression. The development of novel interventions to target subclinical depression is timely (Pibernik-Okanovic, Ajdukovic, Lovrencic, & Hermanns, 2011). If we are able to prevent or delay the onset of depression, the benefit to the individual with T2DM, their families as well as society would be extraordinary.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR013906.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Sue Penckofer, Email: spencko@luc.edu, Professor and Faculty Scholar, School of Nursing, Loyola University Chicago, Building 125, Room 4529, Health Sciences Campus, 2160 S. First Avenue, Maywood, IL. USA.
Todd Doyle, Assistant Professor, Department of Psychiatry and Behavioral Neurosciences, Stritch School of Medicine, Loyola University Medical Center, Maywood, IL. USA.
Mary Byrn, Assistant Professor, Nursing Department, Saint Mary’s College, Notre Dame, IN. USA.
Patrick J. Lustman, Professor, Department of Psychiatry, School of Medicine, Washington University in St. Louis, St. Louis, MO. USA.
References
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analyses. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Balhara YPS, Verma R. Review Article: Management of depression in diabetes: A review of psycho-social interventions. Journal of Social Health and Diabetes. 2013;1:22–26. [Google Scholar]
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database of Systematic Reviews. 2012;12:CD008381. doi: 10.1002/14651858.CD008381.pub2. [DOI] [PubMed] [Google Scholar]
- Beck JS. Cognitive therapy Basics and beyond. New York, NY: The Guilford Press; 1995. [Google Scholar]
- Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: A randomized controlled trial. Annals of Family Medicine. 2012;10(1):15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;8(29) doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Reimer T, Opwis K. How do we learn in a negative mood? Effects of a negative mood on transfer and learning. Learning and Instruction. 2007;17(1):1–16. doi: 10.1016/j.learninstruc.2006.11.002. [DOI] [Google Scholar]
- Caplan S, Whittemore R. Barriers to treatment engagement for depression among Latinas. Issues in Mental Health Nursing. 2013;34(6):412–424. doi: 10.3109/01612840.2012.762958. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological basis of depression in adults with diabetes. Current Diabetes Report. 2010;10(6):396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- Chen HY, Ruppert K, Charron-Prochownik D, Noullet WV, Zgibor J. Effects of depression and antidepressant use on goal setting and barrier identification among patients with type 2 diabetes. The Diabetes Educator. 2011;37(3):370–380. doi: 10.1177/0145721711400662. [DOI] [PubMed] [Google Scholar]
- Chung H, Kim A, Neighbors CJ, Cummings J, Ricketts S, O’Grady MA, Raum D. Early experience of a pilot intervention for patients with depression and chronic medical illness in an urban ACO. General Hospital Psychiatry. 2013;35(5):468–471. doi: 10.1016/j.genhosppsych.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Ciechanowski P, Katon W, Russo J. Depression and diabetes: Impact of depressive symptoms on adherence, function, and costs. Archives of Internal Medicine. 2000;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- Cosgrove MP, Sargeant LA, Griffin SJ. Does depression increase the risk of developing type 2 diabetes? Occupational Medicine. 2008;58(1):7–14. doi: 10.1093/occmed/kqm105. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GC, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Medicine. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- de Groot M, Doyle T, Kushnick M, Shubrook J, Merril J, Rabideau E, Schwartz F. Can lifestyle interventions do more than reduce diabetes risk? Treating depression in adults with type 2 diabetes with exercise and cognitive behavioral therapy. Current Diabetes Report. 2012;12(2):157–166. doi: 10.1077/s11892-012-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Kushnick M, Doyle T, Merrill J, McGlynn M, Shubrook J, Schwartz F. Depression among adults with diabetes: Prevalence, impact and treatment options. Diabetes Spectrum. 2010;23(1):15–18. doi: 10.2337/diaspect.23.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakakos P, Pierce MB, Hardy R. Depressive symptoms and risk of type 2 diabetes in a national sample of middle-aged and older adults. Diabetes Care. 2010;33(4):792–797. doi: 10.2337/dc09-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TA, de Groot M, Harris T, Schwartz F, Strotmeyer ES, Johnson KC, Kanaya A. Diabetes, depressive symptoms, and inflammation in older adults: Results from the Health, Aging, and Body Composition Study. Journal of Psychosomatic Research. 2013;75(5):419–424. doi: 10.1016/j.jpscyores.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen E, Hollon SD. Cognitive behavioral therapy for mood disorders: Efficacy, moderators, and mediators. Psychiatry Clin North America. 2010;33(3):537–535. doi: 10.1016/j.psc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry D, Duran P, Bonds C, Lee M, Davidson MB. Effect of pharmacological treatment of depression on A1C and quality of life in low-income Hispanics and African Americans with diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Care. 2009;32(12):2156–2160. doi: 10.2337/dc09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede LE, Zheng D. Independent factors associated with major depressive disorder in a national sample of individuals with diabetes. Diabetes Care. 2003;26(1):104–111. doi: 10.2337/diacare.26.1.104. [DOI] [PubMed] [Google Scholar]
- Ell K, Katon W, Xie B, Lee PJ, Kapetanovic S, Guterman J, Chou CP. Collaborative care management of major depression among low-income, predominantly Hispanic subjects with diabetes. Diabetes Care. 2010;33(4):706–713. doi: 10.2337/dc09-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, Arean P, Mohr DC, Masharani U, Laurencin G. Clinical depression vs distress among patient with type 2 diabetes: Not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: A cumulative meta-analysis and review of longer term outcomes. Archives of Internal Medicine. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Kupperman E, Vileikyte L. Treating depression in diabetes: The case for integrative behavioral interventions. International Diabetes Monitor. 2010;22(6):253–260. doi: 10.1001/archinte.160.21.3278. [DOI] [Google Scholar]
- Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA. Depression and diabetes treatment nonadherence: A-meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icks A, Albers B, Haastert B, Pechlivanis S, Pundt N, Slomiany U, Moebus S. Risk for high depressive symptoms diagnosed and previously undetected diabetes: 5 year follow-up results of the Heinz Nixdorf Recall study. PLoS One. 2013;8(2):e56300. doi: 10.1371/journal.pone.0056300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior and Immunity. 2007;25(4):374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jamal M, Willem Van der Does AJ, Cuijpers P, Penninx BW. Association of smoking and nicotine dependence with severity and course of symptoms in patient with depressive or anxiety disorder. Drug & Alcohol Dependence. 2012;126:138–146. doi: 10.1016/j.drugalcdep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, Ismail K. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36(2):480–489. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, Unutzer J. Collaborative care models for depression: Time to move from evidence to practice. Archives of Internal Medicine. 2006;166(21):2304–2306. doi: 10.1001/archinte.166.21.2304. [DOI] [PubMed] [Google Scholar]
- Katon WJ. The comorbidity of diabetes mellitus and depression. American Journal of Medicine. 2008;121(11 Suppl 2):S8–15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Lin EHB, Von Korff M, Ciechanowski P, Ludman EJ, Young B, McCulloch D. Collaborative care for patients with depression and chronic illnesses. The New England Journal of Medicine. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ, Von Korff M, Lin EHB, Simon G, Ludman E, Russo J, Bush T. The pathways study: A randomized trial of collaborative care in patients with diabetes and depression. Archives of General Psychiatry. 2004;61(10):1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Twisk JWR, Beekman ATF, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- Lagomasino I, Stockdale S, Miranda J. Racial-ethnic composition of provider practices and disparities in treatment of depression and anxiety, 2003–2007. Psychiatric Services. 2011;62(9):1019–1025. doi: 10.1176/appi.ps.62.9.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Rutter C, Katon W, Heckbert S, Ciechanowski P, Oliver M, Von Korff M. Depression and advanced complications of diabetes: A prospective cohort study. Diabetes Care. 2010;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Katon W, Von Korff M, Rutter C, Simon G, Oliver M, Young B. Relationship of depression and diabetes, self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: A randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23(5):618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, McGill JB. Effects of nortriptyline on depression and glycemic control in diabetes: Results of a double-blind, placebo-controlled trial. Psychosomatic Medicine. 1997;59(3):241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith L, Freedand K, Kissel S, Clouse R. Cognitive behavior therapy for depression in type 2 diabetes mellitus: A randomized controlled trial. Annals of Internal Medicine. 1998;129(8):613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Williams MM, Sayuk GS, Nix BD, Clouse RE. Factors influencing glycemic control in type 2 diabetes during acute- and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care. 2007;30(3):459–466. doi: 10.2337/dc06-1769. [DOI] [PubMed] [Google Scholar]
- Ma Y, Balasubramanian R, Pagoto SL, Schneider KL, Culver AL, Olendzki B, Herbert JR. Elevated depressive symptoms, antidepressant use, and diabetes in a large multiethnic national sample of postmenopausal women. Diabetes Care. 2011;34:2390–2392. doi: 10.2337/dc11-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: Emerging findings. Psychosomatics. 2011;52(1):1–18. doi: 10.1016/j.psym.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albreicht S, Golden SH. Depression and type 2 diabetes over the lifespan. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Maletic V, Raison C. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Burns MN, Schueller SM, Clarke G, Klinkman M. Behavioral intervention technologies: Evidence review and recommendations for future research in mental health. General Hospital Psychiatry. 2013;35(4):332–338. doi: 10.1016/j.genhosppsych.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefs G, Pouwer F, Denollet J, Pop V. The course of depressive symptoms in primary care patients with type 2 diabetes: Results from the Diabetes, Depression, Type D Personality Zuidoost-Brabant (DiaDDZoB) Study. Diabetologia. 2012;55(3):608–616. doi: 10.1007/s00125-011-2411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, Pouwer F. Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia. 2010;53(12):2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Minority Health. Diabetes and Hispanic Americans. 2013a Retrieved from http://minorityhealth.hhs.gov/templates/content.aspx?lvl=3&lvlID=5&ID=3324.
- Office of Minority Health. Diabetes and African Americans. 2013b Retrieved from http://minorityhealth.hhs.gov/templates/content.aspx?lvl=3&lvlID=5&ID=3017.
- Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and pathophysiology and treatment of major depression. Brain, Behavior and Immunology. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paile-Hyvarinen M, Wahlbeck K, Eriksson JG. Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: A single-blind randomised placebo controlled trial. BMC Family Practice. 2003;4:7. doi: 10.1186/1471-2296-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paile-Hyvarinen M, Wahlbeck K, Eriksson JG. Quality of life and metabolic status in mildly depressed patients with type 2 diabetes treated with paroxetine: A double -blind randomised placebo controlled 6 month trial. BMC Family Practice. 2007;8:34. doi: 10.1186/1471-2296-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Hu FB. Bidirectional association between depression and type 2 diabetes in women. Archives of Internal Medicine. 2010;170(21):1884–1891. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, Rubin RR, Lucas M, Hu FB. Use of antidepressant medication and risk of type 2 diabetes: results from three cohorts of US adults. Diabetologia. 2012;55(1):63–72. doi: 10.1007/s00125-011-2268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Hong Y, Lee H, Ha E, Sung Y. Individuals with type 2 diabetes and depressive symptoms exhibited lower adherence with self-care. Journal of Clinical Epidemiology. 2004;57(9):978–984. doi: 10.1016/j.jclinepi.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Penckofer S, Ferrans C, Mumby P, Byrn M, Emanuele MA, Harrison PA, Lustman P. A psychoeducational intervention (SWEEP) for depressed women with diabetes. Annals of Behavioral Medicine. 2012;44(2):192–206. doi: 10.1007/s12160-012-9377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penckofer S, Ferrans C, Velsor-Friedrich B, Savoy S. The psychological impact of living with diabetes: Women’s day to day experiences. The Diabetes Educator. 2007;33(4):680–690. doi: 10.1177/0145721707304079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak F, Hautzinger M, Plack K, Kronfeld K, Ruckes C, Herpertz, Muller M. Cognitive behavioural therapy in elderly type 2 diabetes patients with minor depression or mild major depression: Study protocol of a randomized controlled trial (MIND_DIA) BMC Geriatrics. 2010;10(21) doi: 10.1186/1471-2318-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak F, Herpertz S. Treatment of depression in diabetes: an update. Curr Opin Psychiatry. 2009;22:211–217. doi: 10.1097/YCO.0b013e3283207b45.. [DOI] [PubMed] [Google Scholar]
- Petrak F, Herpertz S, Albus C, Hermanns N, Hiemke C, Hiller W, Muller M. Study protocol of the Diabetes and Depression Study (DAD): A multi-center randomized controlled trial to compare the efficacy of the diabetes-specific cognitive behavioral group therapy versus sertraline in patients with major depression and poorly controlled diabetes. BMC Psychiatry. 2013;13:206. doi: 10.1186/1471-244X-13-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibernik-Okanovic M, Ajdukovic D, Lovrencic MV, Hermanns N. Does treatment of subsyndromal depression improve depression and diabetes related outcomes: Protocol for a randomised controlled comparison of psycho-education, physical exercise and treatment as usual. Trials. 2011;12(17) doi: 10.1186/1745-6215-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Piette JD, Richardson C, Himle J, Duffy S, Torres T, Vogel M, Valenstein M. A randomized trial of telephone counselling plus walking for depressed diabetes patients. Med Care. 2011;49(7):641–648. doi: 10.1097/MLR.0b013e318215d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. TRENDS in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: A meta-analysis of longitudinal studies. Journal of Clinical Psychiatry. 2013;74(1):31–37. doi: 10.4088/JCP12r07922. [DOI] [PubMed] [Google Scholar]
- Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, For the Diabetes Prevention Program Research Group Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the Diabetes Prevention Program. Diabetes Care. 2008;31(3):420–426. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, Cagliero E. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2013 Oct 29; doi: 10.2337/dc13-0816. Advance online publication. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N, Atlantis E, Ismail K. A review of the association between depression and insulin resistance: Pitfalls of secondary analyses or a promising new approach to prevention of type 2 diabetes? Current Psychiatry Report. 2012;14:8–14. doi: 10.1007/s11920-011-0245-8. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults. Diabetes Care. 2000;23(10):1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine. 2008;358(3):252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Harpole L, IMPACT Investigators Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- van Bastelaar KMP, Pouwer F, Cuijpers P, Riper H, Snoek FJ. Web-based depression treatment for type 1 and type 2 diabetic patients. Diabetes Care. 2011;34(2):320–325. doi: 10.2337/dc10-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Feltz-Cornelis C, Nuyen J, Stoop C, Chan J, Jacobson AM, Katon W, Sartorius N. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: A systematic review and meta-analysis. General Hospital Psychiatry. 2010;32(4):380–395. doi: 10.1016/j.genhosppsych.2010.03.011. [DOI] [PubMed] [Google Scholar]
- van Dooren FEP, Nefs G, Schram MT, Verhey FRJ, Denollet J, Pouwer F. Depression and risk of mortality in people with diabetes mellitus: A systematic review and meta-analysis. PLoS ONE. 2013;8(3):e57058. doi: 10.1371/journal.pone.0057058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Allen NA, Swalley LM, Melkus GD, Whittemore R. Depression, depression treatment, and insulin sensitivity in adults at risk for type 2 diabetes. Diabetes Research & Clinical Practice. 2009;86(2):96–103. doi: 10.1016/j.diares.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Perkins D, Piette J, Lipton B, Aikens J. Racial difference in the discussion and treatment of depressive symptoms accompanying type 2 diabetes. Diabetes Research & Clinical Practice. 2009;86(2):111–116. doi: 10.1016/j.diabres.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Tennen H, Osborn C. Lifetime depression and diabetes self-management in women with type 2 diabetes: A case-control study. Diabetic Medicine. 2010;27(6):713–717. doi: 10.1111/j.1464-5491.2010.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlqvist ML, Lee M, Chuang S, Hsu C, Tsai H, Yu S, Chang HY. Increased risk of affective disorders in type 2 diabetes is minimized by sulfonylurea and metformin combination: A population-based cohort study. BMC Medicine. 2012;10(150) doi: 10.1186/1741-7015-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5(4):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Williams JW, Katon W, Lin EHB, Noel PH, Worchel J, Cornell J, Unutzer J. The effectiveness of depression care management on diabetes-related outcomes in older patients. Annals of Internal Medicine. 2004;140(12):1015–1024. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- Xue H. Paroxetine for depression in diabetes: A randomized controlled trial. Chinese Mental Health Journal. 2004;18:735–737. [Google Scholar]


