Abstract
Background and Purpose
Diabetes is an independent risk factor for lacunar strokes. Few data are available regarding patient features, infarct location, and recurrent vascular events for diabetic patients with lacunar stroke.
Methods
We compared features at study entry and prognosis during 3.6 years of follow-up of diabetic vs. non-diabetic patients with recent lacunar stroke participating in the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial.
Results
Among the 3020 participants, the prevalence of diabetes was 37% with a mean duration of 11 years. Diabetes was independently associated with slightly younger age (63 years vs. 64 years, p<0·001), Hispanic ethnicity (36% vs. 28%, p<0·0001), ischemic heart disease (11% vs. 6%, p=0·002), and peripheral vascular disease (5% vs. 2%, p<0·001). Diabetic patients more frequently had intracranial stenosis ≥50% (p<0·001), infarcts involving the brainstem or cerebellum (p<0·001), and more extensive white matter abnormalities (p<0·001). Diabetic patients were almost twice as likely to have a recurrent stroke (HR 1·8; 95% CI 1·4–2·3), recurrent ischemic stroke (HR 1·8; 95% CI 1·4–2·4), disabling/fatal stroke (HR 1·8; 95% CI 1·2–2·9), myocardial infarction (HR 1·7; 95% CI 1·0–2·8) and death (HR 2·1 (95% CI 1·6–2·8) compared with non-diabetics.
Conclusions
Diabetic patients with lacunar stroke have a distinctive clinical profile that includes double the prevalence of systemic and intracranial atherosclerosis, preferential involvement of the posterior circulation, and a poor prognosis for recurrent stroke and death.
Keywords: stroke, diabetes, lacunar stroke, small artery disease
Background
Diabetes mellitus is an accepted independent risk factor for ischemic stroke, regardless of its mechanism. The prevalence of diabetes in stroke patients is between 10 and 20% and has been increasing over the last 20 years, probably in response to rising rates of overweight and obesity in the general population.1–3
A hospital-based case-control study of patients presenting with a first-ever lacunar stroke showed that the risk of lacunar stroke was 2 times higher in diabetic patients compared with age and sex- matched controls.4 In another case-control study, diabetes was associated with an increased prevalence of lacunar strokes compared with other ischemic stroke subtypes.5
Little is known about the clinical implications of diabetes in patients with lacunar strokes due to cerebral small artery disease. We hypothesized that diabetes would be an independent risk factor for the severity of cerebral small artery disease in patients with lacunar strokes. Here, we characterize the differences between diabetic and non-diabetic patients in a large, well-defined cohort of patients with lacunar strokes attributed to cerebral small vessel disease participating in an international clinical trial. The anatomic distribution of infarcts, ethnic differences, prognosis regarding major vascular events, and mechanisms underlying recurrent strokes are analyzed.
Methods
We included 3020 participants of the SPS3 trial carried-out in eight countries between 2001 and 2011. The rationale, design, and main results of SPS3 have been reported elsewhere.6 In brief, patients ≥30 years old with a recent, MRI-defined small subcortical ischemic stroke were included if there was no ipsilateral carotid stenosis >50% or a major cardioembolic source requiring anticoagulation. Qualifying stroke had to demonstrate at least one of the following four specific MRI criteria: i) diffusion-weighted imaging (DWI) lesion ≤20 mm in size at largest dimension (including rostro-caudal extent); ii) well delineated focal hyperintensity ≤20 mm in size at largest dimension (including rostro-caudal extent) on FLAIR or T2 and clearly corresponding to the clinical syndrome; iii) multiple hypointense lesions of size 3–15 mm at largest dimension (including rostro-caudal extent) only in the cerebral hemispheres on FLAIR or T1 in patients whose qualifying event is clinically hemispheric. If qualifying event was clinically brainstem or cerebellar, this criterion alone was not sufficient for study entry; iv) well delineated hypointense lesion ≤15 mm in size at largest dimension (including rostro-caudal extent) on FLAIR or T1 corresponding to the clinical syndrome. Patients with disabling strokes (modified Rankin scale ≥4) were excluded. Criteria for diabetes included one or more of a self-reported history of diabetes, prescribed antidiabetic medications, elevated glucose in medical records (>120 mg/dl), or diagnosis within 3 months of study entry.
White matter hyperintensities were evaluated visually on FLAIR images using the Age-Related White Matter Changes (AWRMC) scale (range 0–16) by readers unaware of clinical information.7 A priori, scores of 0–4 on the ARWMC scale were defined as none-mild disease, 5–8 moderate, and 9+ severe. A neuroradiologist (CB) who was unaware of clinical data recorded the number of lacunar strokes and graded the severity of stenosis. The topography of lacunes was categorized as anterior circulation (basal ganglia, internal capsule, corona radiata), thalamic and posterior circulation (brainstem and cerebellum).
Recurrent ischemic strokes were classified according to TOAST criteria; neuroimaging was available in 99% of the recurrent strokes.
We examined differences in participant features between diabetic and non-diabetic patients using two-sample t-tests and chi-square tests of association, as appropriate. Any factors found to differ between diabetic and non-diabetic patients in a univariate fashion were then included in a multivariable logistic regression model assessing associations with the likelihood of being diabetic at baseline. We then computed the rates of events for diabetic and non-diabetic patients, and determined whether they differed using a Cox proportional hazards model. Initial models were run without adjustment; then factors found to be related to diabetes in the multivariable logistic regression model were included in Cox models to determine whether observed differences in rates of events were still significant after accounting for factors known to be related to diabetes. We further examined subtypes of stroke, and whether they differed in frequency by diabetes status, among those who experienced an ischemic stroke. Significance was assessed at an alpha level of 0.05 for all tests.
Results
Baseline characteristics
Of 3020 participants, 37% (n=1106) were classified as diabetics, and in 71% the hemoglobin A1c exceeded 7% at study entry. Of those patients with diagnosis of diabetes at study entry (91%), the mean estimated duration of diabetes was 11 years. There were no significant differences in gender (63% male) or prevalence of hypertension between both groups. Hypertension was highly prevalent both in diabetic patients (92%) and in non-diabetic patients (80%) with lacunar strokes. (Table 1)
Table 1.
Demographic features according to diabetic status at study entry
| Non-Diabetic (n=1914) | Diabetic (n=1106) | p-value | p-value from Multivariable model* | |
|---|---|---|---|---|
|
| ||||
| Age, mean (yrs) | 63·8 (11·3) | 62·6 (9.7) | 0·002 | 0.0048 |
|
| ||||
| Male, % | 1204 (63%) | 698 (63%) | 0·91 | |
|
| ||||
| Race, % | <0·0001 | <0.0001 | ||
| White | 1049 (55%) | 486 (44%) | ||
| Hispanic | 539 (28%) | 399 (36%) | ||
| Black | 278 (15%) | 179 (16%) | ||
| Other | 48 (2·5%) | 42 (3·8%) | ||
|
| ||||
| Region, % | 0·78 | |||
| U.S. and Canada | 1249 (65%) | 711 (64%) | ||
| Latin America | 432 (23%) | 262 (24%) | ||
| Spain | 233 (12%) | 133 (12%) | ||
|
| ||||
| Hypertension†, % | 1694 (89%) | 1015 (92%) | 0·004 | 0.57 |
|
| ||||
| Average blood pressure @ entry‡ (mmHg) | 142 (19)/79 (11) | 144 (19)/ 77 (10) | 0·01/<0·0001 | |
|
| ||||
| Mean number of blood pressure meds @ entry | 1·6 (1·1) | 1·9 (1·3) | <0·0001 | <0.0001 |
|
| ||||
| Stage of hypertension § | <0·0001 | |||
| - normotensive | 67 (3·5%) | 46 (4·2%) | ||
| - stage II | 558 (29%) | 256 (23%) | ||
| - stage III | 754 (39%) | 412 (37%) | ||
| - stage IV | 535 (28%) | 392 (35%) | ||
|
| ||||
| Newly diagnosed diabetes || | ----- | 104 (9%) | ||
|
| ||||
| History of diabetes | ----- | 1002 (91%) | ||
|
| ||||
| Average duration in those with hx of diabetes (yrs) (N=943) | N/A | 11·2 (9·2) | N/A | |
|
| ||||
| Hemoglobin A1C >7%, % | ----- | 631 (71%) | ||
|
| ||||
| Ischemic heart disease, % | 107 (6%) | 118 (11%) | <0·0001 | 0.0022 |
|
| ||||
| Current tobacco smoker, % | 436 (23%) | 181 (16%) | <0·0001 | 0.0009 |
|
| ||||
| Regular alcohol use, % | 615 (32%) | 233 (21%) | <0·0001 | <0.0001 |
|
| ||||
| Prior symptomatic lacunar stroke, % | 172 (9%) | 136 (12%) | 0·004 | 0.87 |
|
| ||||
| Mean Mini-Mental Status score | 28·1 (2·3) | 27·8 (2·5) | 0·002 | 0.03 |
|
| ||||
| Antiplatelet therapy @ index stroke, % | 500 (26%) | 439 (40%) | <0·0001 | <0.0001 |
|
| ||||
| Statin use at entry, % | 1300 (68%) | 781 (71%) | 0·12 | |
|
| ||||
| Mean serum creatinine (mg/dl) | 0·96 (0·23) | 0·95 (0·28) | 0·25 | |
|
| ||||
| Mean eGFR (ml/min) | 79·5 (18·1) | 81·6 (20·1) | 0·004 | 0.0002 |
|
| ||||
| Peripheral vascular disease | 37 (2%) | 58 (5%) | <0·0001 | 0.0004 |
|
| ||||
| Multiple infarcts | 748 (41%) | 398 (37%) | 0·07 | |
Includes all factors that differ significantly between diabetics and non-diabetics in univariate analyses.
SPS3 criteria.
Average from first 2 SPS3 visits.
SPS3 criteria based on observed blood pressure and number of antihypertensive medications.
Diagnosis from randomization up to 3 months of follow up.
Diabetes was independently associated with younger age (63 years vs. 64 years, p<0·005), Hispanic ethnicity (36% vs. 28%, p<0·0001), ischemic heart disease (p=0·002), and peripheral vascular disease (p<0·0004) compared with non-diabetics (Table 1). Measured blood pressure at study entry was higher in diabetics vs. non-diabetic patients (systolic 144 mmHg vs. 142 mmHg, respectively) despite the use of significantly more antihypertensive medications by diabetic patients. (p<0·0001). (Table 1) Current tobacco smoking was significantly less frequent among diabetic (16%) vs. non-diabetic patients (23%) (p=0·0009). The mean serum creatinine levels were not different, but average estimated glomerular filtration rates were slightly (2·1 mL/min), but significantly, higher among diabetics vs. nondiabetics (p<0002). (Table 1)
Brain imaging from 3004 patients (>99% of participants) was available for central review. The location of the qualifying lacunar infarct was more often in the brainstem or cerebellum (i.e. posterior circulation) in diabetic (32%) vs. non-diabetic patients (22%) (p<0·0001), and intracranial arterial stenosis ≥50% was significantly more frequent (23% in diabetic, 14% non-diabetic patients (p<0·0001). (Table 2) The proportion of posterior circulation intracranial stenosis ≥50% was not significantly increased in diabetics (p =0.50). (Table Iin the online-only Data Supplement). Diabetic patients had more extensive white matter abnormalities on baseline imaging, but this was not significant after adjustment for other independent predictors. (p=0.11, Table 1).
Table 2.
Radiologic features according to diabetic status at study entry
| Non-diabetic | Diabetic | p-value | p-value from Multivariable model | |
|---|---|---|---|---|
|
| ||||
| Intracranial stenosis ≥50 % any artery, % (N=2908) | 255 (14%) | 248 (23%) | <0·0001 | <0.0001 |
|
| ||||
| Anatomic location of qualifying stroke | <0·0001 | <0.0001 | ||
| - basal ganglia/internal capsule | 552 (29%) | 289 (26%) | ||
| - corona radiata / centrum semiovale | 510 (27%) | 209 (19%) | ||
| - thalamus | 425 (22%) | 252 (23%) | ||
| - brainstem / cerebellum | 425 (22%) | 356 (32%) | ||
|
| ||||
| ARWMC score * mean (sd) | 0.017 | 0.11 | ||
| ARWMC group, % | ||||
| 0–4 | 920 (49%) | 570 (52%) | ||
| 5–8 | 518 (28%) | 318 (29%) | ||
| >9 | 439 (23%) | 207 (19%) | ||
ARWMC: Age-Related White Matter Changes (Wahlund L, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to mri and ct. Stroke 2001;32:1318–1322).
Major vascular events and mortality during follow-up
During a mean follow-up of 3·6 years, recurrent ischemic stroke occurred in 11.4% of diabetic vs. 5.9% of nondiabetic patients (p<0.0001). After adjustment for other predictors, the rates of recurrent stroke (HR 1·8; 95% CI 1·4–2·3), recurrent ischemic stroke (HR 1·8; 95 % CI 1·4–2·4), disabling/fatal recurrent stroke (HR 1·8; 95 % CI 1·2–2·9) and myocardial infarction (HR 1·7; 95 % CI 1·0–2·8) were almost two times higher in diabetic vs. non-diabetic patients.(Table 3) All-cause mortality was twice as frequent in diabetic patients (HR 2·1; 95% CI 1·6–2·8) as well as death due to vascular and uncertain etiologies (HR 1·8; 95% CI 1·1–3·0 and 3·9; 95% CI 2·1–7·0) vs. non-diabetic patients.(Table 3) There were no significant differences in the risk of non-vascular death (HR 1·6; CI 0.9–2.6) or major extracranial hemorrhages (HR 0·88; 95% CI 0·6–1·3) among diabetic patients.
Table 3.
Major outcomes during follow-up
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-diabetics (n=1914) | Diabetics (n=1106) | HR (95% CI) | p value | HR (95% CI) | p value | |||
| N | Rate | N | Rate | |||||
| All stroke (ischemic & hemorrhage) | 137 | 1·9 | 140 | 3·7 | 1·9 (1·5, 2·4) | <0·0001 | 1·8 (1·4, 2·3) | <0·0001 |
| Ischemic stroke* | 116 | 1·6 | 127 | 3·3 | 2·0 (1·6, 2·6) | <0·0001 | 1·8 (1·4, 2·4) | <0·0001 |
| Intracranial hemorrhage* | 21 | 0·29 | 13 | 0·34 | 1·2 (0·6, 2·3) | 0·65 | 1·4 (0·6, 2·9) | 0·41 |
| Disabling/fatal stroke† | 42 | 0·58 | 47 | 1·2 | 2·1 (1·4, 3·2) | 0·0006 | 1·8 (1·2, 2·9) | 0·010 |
| Myocardial infarct | 37 | 0·49 | 39 | 0·96 | 2·0 (1·3, 3·1) | 0·0032 | 1·7 (1·0, 2·8) | 0·046 |
| Other thromboembolism‡ | 21 | 0·28 | 15 | 0·36 | 1·3 (0·7, 2·5) | 0·42 | 1·2 (0·5, 2·4) | 0·71 |
| Major vascular events§ | 179 | 2·5 | 169 | 4·5 | 1·8 (1·4, 2·2) | <0·0001 | 1·7 (1·3, 2·1) | <0·0001 |
| Deaths (all) | 102 | 1·3 | 105 | 2·5 | 1·9 (1·5, 2·5) | <0·0001 | 2·1 (1·6, 2·8) | <0·0001 |
| -Vascular | 36 | 0·48 | 41 | 0·99 | 2·1 (1·3, 3·3) | 0·0012 | 1·8 (1·1, 3·0) | 0·018 |
| -Non-vascular | 46 | 0·61 | 29 | 0·70 | 1·2 (0·7, 1·9) | 0·51 | 1·6 (0·9, 2·6) | 0·089 |
| -Uncertain | 20 | 0·26 | 35 | 0·84 | 3·3 (1·9, 5·7) | <0·0001 | 3·9 (2·1, 7·0) | <0·0001 |
| Major extracranial hemorrhages | 91 | 1·2 | 42 | 1·0 | 0·8 (0·6, 1·2) | 0·30 | 0·9 (0·6, 1·3) | 0·51 |
HR = hazard ratio yr = year; CNS = central nervous system; CI = confidence interval; TIA = transient ischemic attack; pt-yr = patient-year.
Time to first event in the specific category; rates are annualized. Total patient-years of exposure for the primary outcome (all strokes) were 5026 for those assigned to aspirin alone and 5114 for those assigned to clopidogrel plus aspirin.
Two strokes without neuroimaging were adjudicated as probable ischemic and included with ischemic strokes.
Of 81 total disabling/fatal strokes, 16 were fatal strokes, 65 were disabling based on Rankin score. Another 14 were unable to be classified and were excluded from these analyses. For non-disabling strokes, patients were censored at the time of their primary event for these analyses.
Other thromboembolism included venous thromboembolism (18 with dual antiplatelet, 10 with aspirin) and peripheral arterial embolism (2 with dual antiplatelet, 1 with aspirin).
Stroke, myocardial infarct, or vascular death.
Etiological subtype of recurrent ischemic strokes and effect of trial interventions
Most recurrent ischemic strokes were lacunar and attributed to cerebral small artery disease, with no significant difference between diabetic vs. non-diabetic patients. (Table 4) The prescence/absence of anterior or posterior intracranial stenosis did not significantly increase the rate of recurent ischemic strokes in diabetics (p-value for interaction 0.72). (Table II in the online-only Data Supplement) The response to dual antiplatelet therapy vs. aspirin alone and to lower vs. higher target of systolic blood pressure on recurrent ischemic stroke did not differ between diabetic vs. nondiabetic participants. (Figure 1)
Table 4.
Recurrent ischemic strokes: Etiologic subtypes*
| Non-diabetics N (%) |
Diabetics N (%) |
p-value* | |
|---|---|---|---|
| All recurrent ischemic strokes† | 113 (5.9%) | 125 (11.4 %) | <0.0001 |
| Recurrent ischemic stroke subtypes‡ | |||
| Lacunar | 61 (54%) | 75 (60%) | 0.35 |
| Large artery atherosclerosis | 18 (16%) | 10 (8%) | 0.057 |
| - intracranial | 11 (61%) | 7 (70%) | 0.23 |
| - extracranial | 7 (39%) | 3 (30%) | 0.14 |
| Cardioembolism | 12 (11%) | 9 (7%) | 0.35 |
| Other specific cause | 3 (3%) | 6 (5%) | 0.38 |
| Unknown strokes | 19 (17%) | 25 (20%) | 0.53 |
p-value derived from the chi-square test.
Excludes two strokes without neuroimaging for which the determination was “probable ischemic”.
Based on classification by central adjudication committee.
Figure 1.
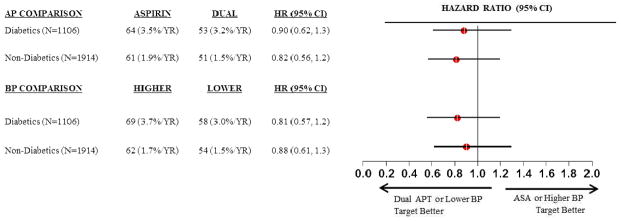
Effects of dual antiplatelet therapy and systolic blood pressure targets in diabetics vs. nondiabetics on ischemic strokes. AP = antiplatelet, BP = blood pressure, Dual = clopidogrel plus aspirin, ASA = acetyl salicylic acid (aspirin).
Discussion
This is the first large study to describe in detail the specific risk factors and prognosis of patients with lacunar stroke according to the presence of diabetes. Diabetic patients with lacunar strokes were slightly younger with nearly double the frequencies of intracranial arterial stenosis, ischemic heart disease and peripheral vascular disease, i.e. manifestations of systemic generalized atherosclerosis. Diabetes independently doubled the risks of recurrent stroke, recurrent lacunar stroke, myocardial infarction and death. The association between worse clinical outcomes and diabetic status after stroke has been reported previously. Meghberi et al. found that in a large European cohort, diabetic status was significantly associated with increased disability at 3 months after stroke.8
The excess risk of stroke in diabetics and particularly in women was also observed in a recent systematic review and meta-analysis 9
Lacunar strokes tend to “breed true”, with recurrent strokes in patients with lacunar strokes are likely to be lacunar. Atherosclerosis at the ostium of the perforating arteries is one of the mechanisms that leads to lacunar infarcts. Although diabetic patients in this cohort had significantly higher prevalence of intracranial stenosis, it is notable that the proportion of recurrent strokes that were classified as lacunar were similar for diabetics and non-diabetics, although the absolute rate of recurrent lacunar stroke was double among diabetics.
The qualifying lacunar stroke significantly more frequently involved the posterior circulation in diabetics vs. non-diabetics. This finding confirms other observational studies. 10
Diabetics were more likely to be Hispanics compared to other ethnic groups. This association as well as the increased prevalence of intracranial atherosclerosis in Hispanics has also been observed by others.11
It was previously reported that there were no significant differences in effects of the randomized interventions tested in the SPS3 trial (clopidogrel plus aspirin vs. aspirin, higher vs. lower systolic blood pressure targets) on all recurrent stroke between diabetic vs. non-diabetic patients.12–14 Here, novel additional data are presented restricted to recurrent ischemic stroke.(Figure 1) We hypothesized during the design of the trial that addition of clopidogrel might be particularly beneficial for prevention of recurrent ischemic stroke for relatively “aspirin-resistant” diabetic participants, but there was no support for this construct in the SPS3 results (p for interaction by diabetic status 0·98). It was additionally hypothesized that assignment to a lower target of systolic blood pressure would be particularly beneficial for prevention of recurrent stroke among diabetics with lacunar stroke, but this was not confirmed. (Figure 1)
Consequently, in the absence of differential effects of the randomized interventions according to the presence of diabetes, we hypothesize that intrinsic cerebral small artery disease remains the most likely etiology for recurrent stroke in both diabetics and non-diabetics with lacunar stroke. (Table 4)
Limitations include the absence of data about the control of diabetes during follow-up. We also acknowledge that the SPS 3 inclusion and exclusion criteria may produce selection bias in at least two ways. First, participants in clinical trials are nearly always healthier than in population-based studies. Second, SPS 3 excluded lacunar stroke patients with occlusive extracranial atherosclerosis and major cardioembolic sources in an effort to include, patients with relatively “pure” small vessel disease.
In conclusion, diabetic patients with lacunar strokes have distinctive risk factor profile and infarct location, have double the frequency of clinically-manifest atherosclerosis and carry a substantially worse prognosis compared with non-diabetic patients. The predominant recurrent stroke subtype was lacunar. While management implications specific to diabetic patients did not emerge from the SPS3 trial, diabetic patients with lacunar stroke represent a large high-risk subgroup of patients with cerebral small artery disease with distinctive clinical features that warrant further study.
Supplementary Material
Acknowledgments
Sources of Funding:
The sponsor of the study, National Institute of Neurological Disorders and Stroke (U01 NS38529-04A1), participated in study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Conflicts of Interest: None.
Clinical Trial Registration-URL:http://www.clinicaltrials.gov. Unique identifier: NCT00059306
Contributor Information
Santiago Palacio, Department of Neurology, University of Texas Health Science Center, San Antonio, Texas, USA
Leslie A. McClure, University of Alabama at Birmingham, Birmingham, Alabama, USA
Oscar R. Benavente, Department of Medicine (Neurology), University of British Columbia, Vancouver, British Columbia, Canada
Carlos Bazan, III, Department of Radiology, University of Texas Health Science Center, San Antonio, Texas, USA
Pablo Pergola, Department of Medicine, University of Texas Health Science Center, Renal Associates PA, San Antonio, Texas USA
Robert G. Hart, Professor of Medicine (Neurology) McMaster University / Population Health Research Institute Hamilton, Ontario, Canada
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet, 2011. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2011. [Accessed April 16, 2012]. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.Pdf. [Google Scholar]
- 2.Mokdad A, Ford E, Bowman B, Dietz W, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes and obesity-related health risk factors. JAMA. 2001;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.You R, McNeil J, O’Malley H, Davis S, Donnan G. Risk factors for lacunar infarction syndromes. Neurology. 1995;45:1483–1487. doi: 10.1212/wnl.45.8.1483. [DOI] [PubMed] [Google Scholar]
- 5.Tuttolomondo A, Pinto A, Salemi G, Di Raimondo D, Di Sciacca R, Fernandez P, et al. Diabetic and non-diabetic subjects with ischemic stroke: Differences, subtype distribution and outcome. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18:152–157. doi: 10.1016/j.numecd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Benavente O, White C, Pearce L, Pergola P, Roldan A, France Benavente M, et al. The secondary prevention of small subcortical strokes (SPS3) study. Int J Stroke. 2011;6:164–75. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahlund L, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to mri and ct. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 8.Megherbi S, Milan C, Minier D, Couvreur G, Osseby G, Tilling K, et al. Association between diabetes and stroke subtype on survival and functional outcome at 3 months after stroke. Stroke. 2003;34:688–694. doi: 10.1161/01.STR.0000057975.15221.40. [DOI] [PubMed] [Google Scholar]
- 9.Peters S, Huxley R, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775385 individuals and 12539 strokes. [published online ahead of print March 7] [Accessed April 23, 2014];Lancet. 2014 doi: 10.1016/S0140-6736(14)60040-4. http://dx.doi.org/10.1016/S0140-6736(14)60040-4. [DOI] [PubMed]
- 10.Ichikawa I, Kuriki A, Kinno R, Katoh H, Mukai M, Kawamura M, et al. Occurrence and Clinicotopographical Correlates of Brainstem Infarction in patients with Diabetes Mellitus. Journal of Stroke and Cerebrovascular Diseases. 2012;21:890–897. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Sacco R, Kargman D, Gu Q, Zamanillo M. Race-Ethnicity and Determinants of Intracranial Atherosclerotic Cerebral Infarction. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 12.The SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–25. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacova C, Pearce L, Costello R, Mc Clure L, Holliday S, Hart R, et al. Cognitive impairment in lacunar strokes. Ann Neurol. 2012;72:351–362. doi: 10.1002/ana.23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The SPS3 Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–15. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


