Abstract
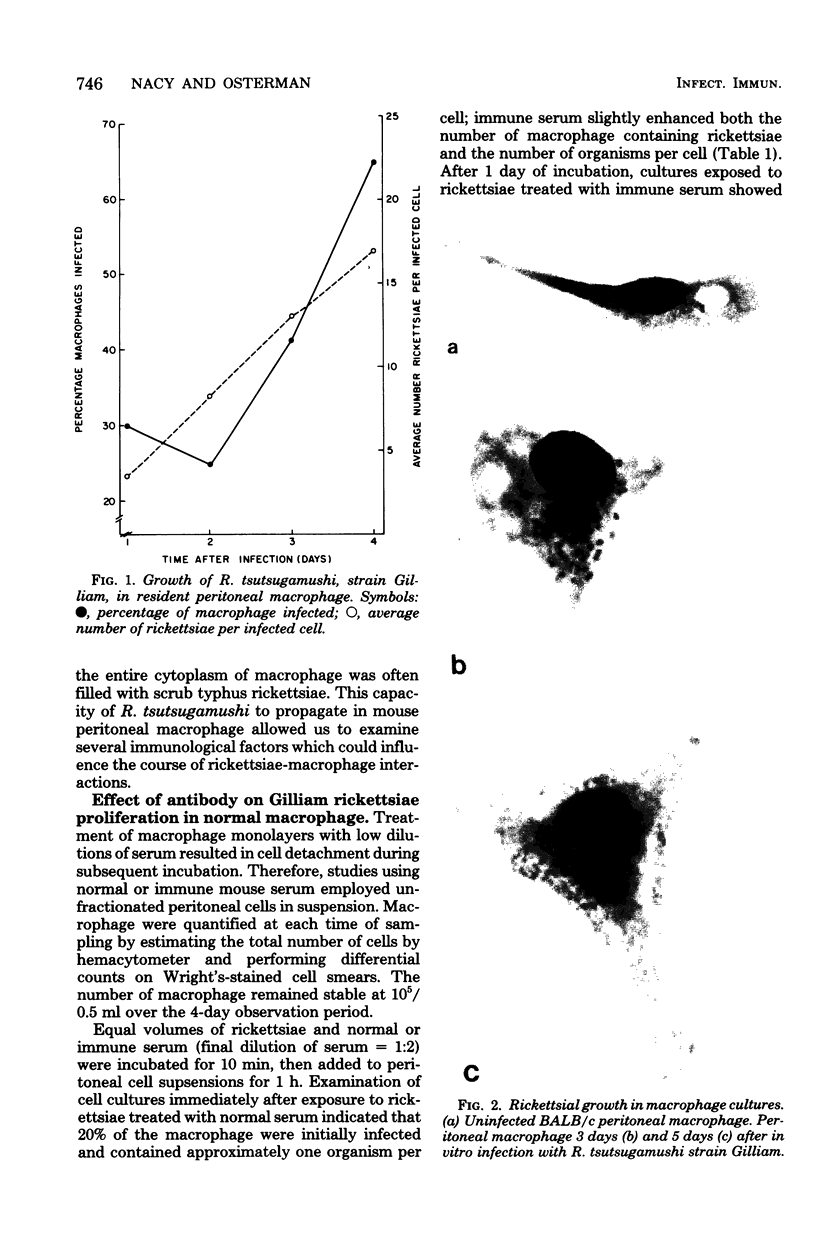
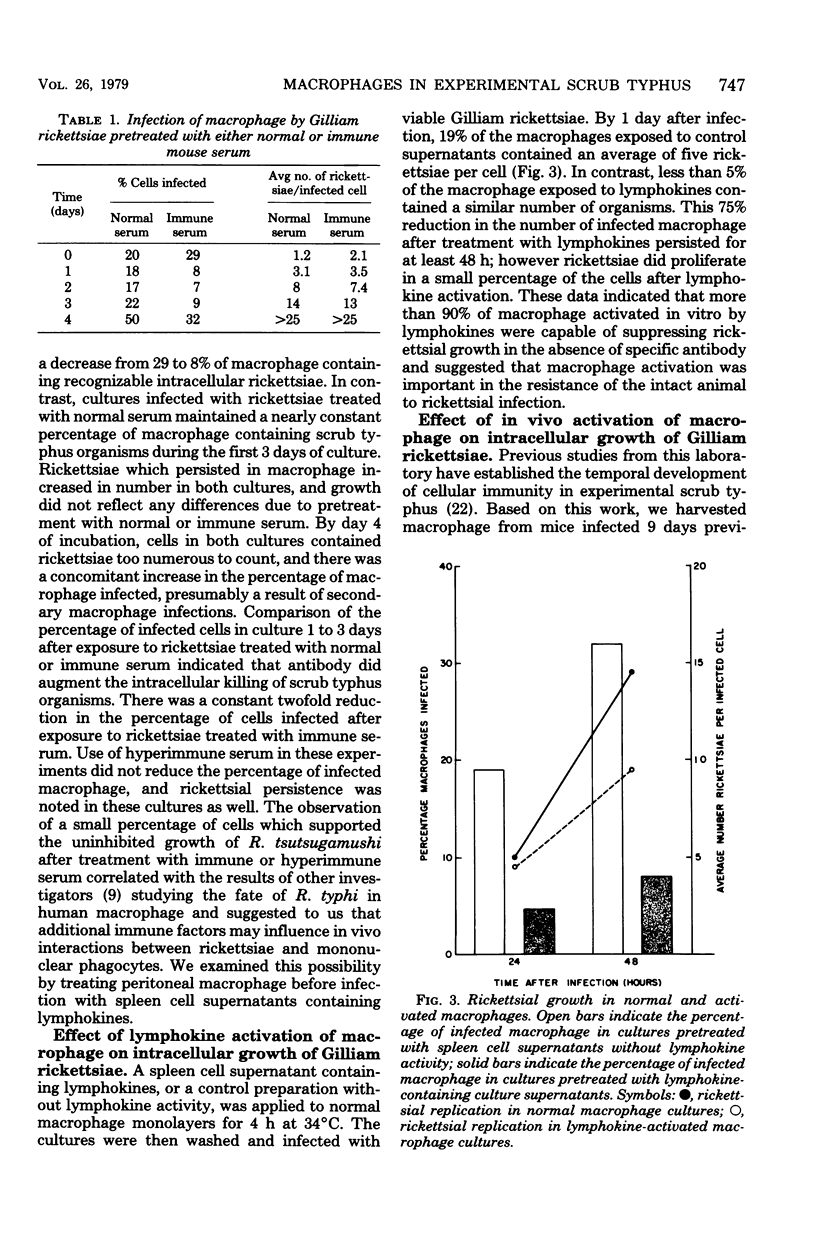
Resident peritoneal macrophage from BALB/c mice were infected in vitro with Rickettsia tsutsugamushi strain Gilliam, and rickettsial growth was estimated by microscopic examination of Giemsa-stained cells. Both number of infected macrophage per culture and number of intracellular rickettsiae per cell increased with time during culture. Treatment of rickettsiae with immune serum before infection macrophage cultures reduced the number of infected macrophage by 50%. Macrophage treated in vitro with lymphokines were able to suppress rickettsial growth in the absence of detectable antibody and exhibited a 75% reduction in infection compared with normal macrophage. We also obtained activated macrophage from immune mice and found that they were refractory to in vitro rickettsial infection. Macrophage populations activated in vitro or in vivo contained a small percentage of cells which supported unrestrained growth of rickettsiae. These data suggest that an early immunological event in experimental scrub typhus infection may be the development of activated macrophage capable of suppressing rickettsial proliferation before the appearance of circulating antibody.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOZEMAN F. M., ELISBERG B. L. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963 Mar;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- Barker L. F., Patt J. K., Hopps H. E. Titration and neutralization of Rickettsia tsutsugamushi in tissue culture. J Immunol. 1968 Apr;100(4):825–830. [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Shirai A., Hilderbrandt P. K., Osterman J. V. Host defenses in experimental scrub typhus: histopathological correlates. Infect Immun. 1976 Mar;13(3):861–875. doi: 10.1128/iai.13.3.861-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. P., Meltzer M. S., Zbar B. Tumor cytotoxicity in vitro by macrophages from mice infected with mycobacterium bovis strain BCG. J Natl Cancer Inst. 1974 Jun;52(6):1887–1895. doi: 10.1093/jnci/52.6.1887. [DOI] [PubMed] [Google Scholar]
- Downs C. M. Phagocytosis of coxiella burneti, phase I and phase II by peritoneal monocytes from normal and immune guinea pigs and mice. Zentralbl Bakteriol Orig. 1968 Apr;206(3):329–343. [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect Immun. 1973 Oct;8(4):631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs D. J., Jerrells T. R. In vitro evaluation of immunity to Coxiella burnetii. J Immunol. 1976 Sep;117(3):996–1003. [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Activation of guinea pig macrophages by Q fever rickettsiae. Cell Immunol. 1977 Jan;28(1):198–205. doi: 10.1016/s0008-8749(77)80020-8. [DOI] [PubMed] [Google Scholar]
- Kenyon R. H., McManus A. T. Rickettsial infectious antibody complexes: detection by antiglobulin plaque reduction technique. Infect Immun. 1974 May;9(5):966–968. doi: 10.1128/iai.9.5.966-968.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. A., Walker J. S. Interaction between Coxiella burnetii and guinea pig peritoneal macrophages. Infect Immun. 1976 Aug;14(2):416–421. doi: 10.1128/iai.14.2.416-421.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R. J., MACKANESS G. B. ELECTRON MICROSCOPICAL OBSERVATIONS ON THE PERITONEAL MACROPHAGES OF NORMAL MICE AND MICE IMMUNISED WITH LISTERIA MONOCYTOGENES. I. STRUCTURE OF NORMAL MACROPHAGES AND THE EARLY CYTOPLASMIC RESPONSE TO THE PRESENCE OF INGESTED BACTERIA. Br J Exp Pathol. 1963 Dec;44:601–607. [PMC free article] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: induction of tumoricidal macrophages by supernatants of PPD-stimulated Bacillus Calmette-Guérin-immune spleen cell cultures. J Immunol. 1977 Sep;119(3):889–896. [PubMed] [Google Scholar]
- SMADEL J. E., LEY H. L., Jr, DIERCKS R. H., CAMERON J. A. P. Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am J Hyg. 1952 Nov;56(3):294–302. doi: 10.1093/oxfordjournals.aje.a119553. [DOI] [PubMed] [Google Scholar]
- Schramek S. Deoxyribonucleic acid base composition of members of the typhus group of rickettsiae. Acta Virol. 1972 Sep;16(5):447–447. [PubMed] [Google Scholar]
- Sharma S. D., Middlebrook G. Antibacterial product of peritoneal exudate cell cultures from guinea pigs infected with mycobacteria, listeriae, and rickettsiae. Infect Immun. 1977 Mar;15(3):745–750. doi: 10.1128/iai.15.3.745-750.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Weiss E., Millar D. B., Bozeman F. M., Ormsbee R. A. DNA base composition of rickettsiae. Science. 1973 Apr 27;180(4084):415–417. doi: 10.1126/science.180.4084.415. [DOI] [PubMed] [Google Scholar]
- Walker W. S. Functional heterogeneity of macrophages: subclasses of peritoneal macrophages with different antigen-binding activities and immune complex receptors. Immunology. 1974 May;26(5):1025–1037. [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Sawyer S., Remington J. S. Role of activated macrophages in resistance of mice to infection with Trypanosoma cruzi. J Infect Dis. 1976 Dec;134(6):610–623. doi: 10.1093/infdis/134.6.610. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr DNA composition in Rickettsia mooseri by base analysis. Acta Virol. 1973 Sep;17(5):443–443. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Walsh W. T. Mechanisms of immunity in typhus infections. IV. Failure of chicken embryo cells in culture to restrict growth of antibody-sensitized Rickettsia prowazeki. Infect Immun. 1974 Mar;9(3):571–575. doi: 10.1128/iai.9.3.571-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



