Abstract
Over the last several years there has been a growing interest in placebo, not only as an inert control in clinical trials, but also in the placebo effect as a group effect as well as a reaction in individual subjects. Methodological factors such as regression to the mean and natural history of the disease play a role in the evaluation of a possible placebo effect. In this report, we discuss several factors including Pavlovian conditioning, beliefs outcome, expectations, and other factors as potential mediators of the placebo response. Placebo effects are common in gastrointestinal diseases and there seems to be no clear difference between placebo effects in functional gastrointestinal diseases (functional dyspepsia and irritable bowel syndrome) and organic gastrointestinal disease (duodenal ulcer and inflammatory bowel disease).
Keywords: Placebo, Gastrointestinal disease, Regression to the mean, Natural history, Pavlovian conditioning, Outcome expectation, Beliefs
INTRODUCTION
A "placebo" is an inert or sham treatment used as a pharmacological control when assessing an active drug. This is a standard requirement in clinical trials designed to asses the effectiveness of drug treatment. However, it is widely believed and there is sufficient evidence that placebos can elicit therapeutic benefits even if the placebo has no intrinsic effect[1-5].
Over the last 50 years placebo effect has received a good deal of attention. While Beecher[6] in an early form of meta-analysis of 15 selected clinical trials, suggested an average of 35% placebo effect, a recent quantitative review[7] was unable to consistently find a strong placebo effect in the studies reviewed according to very restrictive methodological criteria. There was a small effect for subjective outcome measures but it was questioned whether this effect was clinically relevant. However, both studies can be criticized for methodological reasons. While Beecher[6] included studies without a non-treatment group, which basically does not allow any conclusions about a possible placebo effect. Hrobjartsson & Gotzsche[7] included a wide variety of diseases, some of which are not very likely to respond to placebo intervention. A revised version of the study by these authors[8], included more trials and revealed greater evidence for the existence of a placebo effect. Nonetheless, the insight remains that placebo effects are overall rather small and can vary extensively according to the diagnosis and method of placebo intervention. Thus, meta-analyses on placebo effects focussing only on one type or group of diagnoses is likely to be more informative about the circumstances under which placebo effects are likely to occur[4].
Perhaps the most powerful placebo effect can be elicited in patients suffering from chronic pain syndromes[9]. This is also true for experiments in healthy subjects when pain is used as an intervention[3]. Placebo analgesic effects are well established and have largely helped to understand the neurobiology of pain-associated placebo response[3,10-12]. In this context, the placebo effect has been shown to be stronger when complex[13] and invasive interventions[14] are used, and placebos given at a higher frequency appear to be more effective[15]. In addition to acute and chronic pain, placebo effect has been observed in patients with depression[16], Parkinson's disease[17], irritable bowel syndrome (IBS)[18,19], and several other clinical conditions.
In order to structure the discussion on "placebo", Hoffmann et al[1] suggested distinguishing placebo effect from placebo response. These authors proposed that the term placebo effect should be used to refer to an average improvement in a group of subjects who received placebo treatment, whereas the term placebo response should refer to a change in the individual in response to placebo treatment. Such a distinction is helpful in classifying the problems associated with the placebo effect, to identify effects which maybe mistaken as placebo effects, and to investigate the mechanisms underlying the true placebo response. Two major methodological biases may be mistaken for a true placebo response: the effect of natural history and regression to the mean.
NATURAL HISTORY AND REGRESSION TO THE MEAN
It can be assumed that a pathological condition, such as for example stomach pain, will peak at some point and then eventually subside as long as it does not follow a chronic course. An ineffective treatment will not influence the course of the disease, while an effective treatment will decrease the peak and most likely flatten the slope of the curve, such that the symptoms are either less intense and/or will subside earlier. A successful placebo will decrease the slope of the curve, maybe even to the extent an effective treatment would (in this case, the treatment would not be superior to placebo)[1]. However, only those studies that include a "non-treatment group" are able to separate natural history from a placebo effect. Thus, even in Beecher's[6] first systematic analysis of the placebo effect, some of the effects may also be attributed to other mechanisms such as natural history and regression to the mean[20].
Typically, subjects show individual variation of symptoms over time. These fluctuations tend to change towards the mean. The mechanism is similar to the law of initial values: subjects starting with a high value have a higher chance of decreasing values than subjects starting with low values. Hoffman et al[1] argue that patients have the highest chance of seeking treatment, when the symptom level is high, or at least close to maximal. This will most likely also be the point, where these individuals have the highest chance to be entered into a study. Thus, since their initial symptom level is high, they have a higher chance for decreased symptom levels in their second assessment, even though the decrease in symptoms is not exclusively attributable to treatment or placebo, but may as well be natural history or regression to the mean. Again, the only reliable way to control for both effects, besides blinding the study participants and the investigator, is an untreated control group. In their meta-analysis, Hrobjartsson & Gotzsche[7] compared only clinical trials including a no-treatment group. They concluded that often there was only a small difference between the placebo group and the untreated control group, with the exception of pain treatment, where the placebo effect was more substantial.
Regression to the mean is more pronounced in disease states, which have a cyclical course (e.g., IBS, depression). Thus, it can be expected, that the longer the trial lasts, the more pronounced the effect will be. Nonetheless, the average placebo response in patients with irritable bowel syndrome is about 40%, even if the study period is as long as one year[21]. Moreover, factors such as the methods used to measure symptoms (global symptom scores vs specific symptom scores including symptom severity), and the length of the interval to be rated by the patients retrospectively[22], also influence whether placebo effect can be separated from regression to the mean.
FACTORS INFLUENCING THE PLACEBO RESPONSE
It is usually very difficult to assess the placebo effect in clinical studies or by means of meta-analyses, since the aim of these studies is to asses the effectiveness of an active treatment and not the placebo. In most of these papers, the placebo response is rather an unwanted side effect (for methodological discussion see[23-25]). However, possible mediators of the placebo response such as the patient's expectations and classical conditioning, and less well evaluated factors such as beliefs, have been identified. These factors influence the "signal-to-noise ratio" which describes the ability of the patient or the doctor to identify a change in symptoms[5] (Figure 1).
Figure 1.
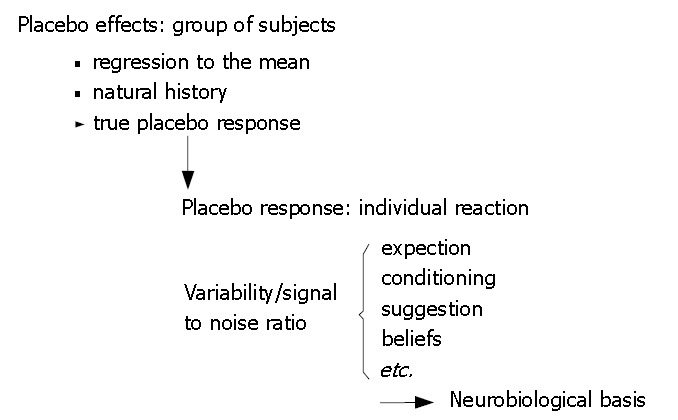
Characteristics of the placebo effect and possible confounding mechanisms compared to possible mechanisms of the placebo response[1,26].
One of the most plausible theories put forward to explain at least some of the placebo responses, the Pavlovian conditioning, is derived from learning theory: the response to an inert stimulus, a neutral stimulus in learning theory and the placebo in the healing context, is a consequence of its previous pairing with an active component that induced symptom improvement. As a consequence, the whole illness history of the patient will influence the response to placebo[26]: were pain killers always successful given as pills, a placebo pill will most likely be effective as well.
Pavlovian conditioning has been shown to influence immunological, endocrinal, intestinal, and other body functions[27]. Moreover, Benedetti et al[10] were able to show that Pavlovian conditioning in the pain context was able to override verbal placebo instructions. Presumably, Pavlovian conditioning to a placebo will induce a placebo response, which activates the same biological mechanism as the active drug. This mechanism has been investigated in detail for placebo analgesia. Placebo analgesia is mediated through the endogenous opiate system and can be blocked by the opiate antagonist naloxone, while naloxone alone does not induce an enhancement of pain in a non-treatment group[10,28-31]. Furthermore, in healthy subjects placebo analgesia activates the same cortical areas in experimental pain as opiate analgesia[11]. These cortical areas are also activated, when pain is anticipated[12].
Outcome expectations have been shown to induce placebo responses[19,32,33]. A recent experiment in patients with IBS showed that within 15 min after rectal lidocaine infusion, patients reported relief of both rectal pain as well as lower limb cutaneous hyperalgesia. The placebo (saline infusion) was also able to reduce the rectal and limb pain, though to a lesser extend[34]. When the placebo application was combined with verbal suggestion for pain relief in another study from the same group[35], the placebo response reached the same level of effectiveness as lidocaine.
Outcome expectations may also interact with Pavlovian conditioning. In a recent study by Benedetti et al[10], verbal instruction was as successful in inducing placebo analgesia by means of Pavlovian conditioning. However, this effect was not replicated in conditioned hormonal response. Conditioned suppression of cortisol secretion was able to override verbal instructions, but verbal instructions alone were not sufficient to override the conditioned hormonal response. These findings emphasize that placebo analgesia, a process associated with a high level of conscious symptom awareness, differs substantially from other placebo responses[2].
PLACEBO EFFECTS IN PATIENTS WITH FUNCTIONAL BOWEL DISORDERS
According to two recent meta-analyses and results of 45 published trials, the placebo response rate in functional dyspepsia varies between 6% and 72%[36,37]. The placebo response rate in IBS (based on 50 placebo controlled trials) ranged between 3% and 84%[38-42].
The reason for this wide range remains unclear, but the factors influencing the placebo response seem to be the duration of the study[43], the number of follow up visits during the study[44] and the number of patients included in the study[26]. Enck & Klosterhalfen[26] reanalysed the data compiled by Spiller[43] with respect to the association between the amplitude of the placebo effect and the study duration. They also included the only available 1 year study[21]. Even considering that only three studies lasted longer than three weeks, it appears that with longer treatment duration, the placebo response will stabilize at around 40%. Moreover, if the sample size is greater than 500 patients, the placebo effect tends to stabilize around 40%[26]. Considering the initial range (3%-84% for IBS and 6%-72% in functional dyspepsia), this finding supports the important role of "regression to mean" as a contributing factor to the placebo effect.
PLACEBO EFFECTS IN OTHER GASTROINTESTINAL DISORDERS
In patients with ulcerative colitis[45], the placebo effect was as high as 40% for subjective measures such as clinical benefit, > 30% for endoscopic remission, and around 25% for histological remission. However, the placebo effect was also strongly dependent on the number of study visits: 3 or less visits were less likely to induce large placebo response rates compared to 4 or more visits during the trial. Placebo effects for Crohn's disease were on average 18% for remission, however, there was a large inter-study variability, ranging between 0% and 50%[46]. Potent predictors of the placebo response were the number of study visits, study duration and severity of disease at entry.
In patients with duodenal ulcer, the placebo effect ranged between 0% and 100% (with an average of about 35%)[47]. In this condition, the placebo rate was dependent on dose frequency and was 6% to 8% higher when the medication was given four times a day compared to twice daily[13]. A high covariation between the response rates to the active drug and the placebo response rates within and across trials are indicative of a low verum effect and a high rate of "spontaneous healing"[47]. This finding is common in diseases with a high rate of spontaneous fluctuation and has also been reported for ulcer disease[48,49]. The correlation between the drug healing rate and the placebo healing rate based on data from 117 ulcer treatment trials was highly significant (r = 0.4). Moerman's[48] observations strongly support the argument for the inclusion of untreated control groups, if the true placebo response is to be separated from "natural history".
METHODOLOGICAL ASPECTS AND IMPLICATIONS FOR CLINICAL TRIALS
It is clear that "placebo" is not a homogeneous concept or phenomenon. Besides general methodological problems such as regression to the mean and natural history which maybe mistaken as a placebo effect, the placebo response itself and whether it occurs or not is strongly dependent on several factors such as the disease being assessed (e.g., pain associated symptoms are more susceptible to placebo), variables associated with the patient (e.g., outcome expectation and beliefs) and the doctor (e.g., outcome expectation and instruction), and the setting (e.g., Pavlovian conditioning). It is important to control for these variables when designing a clinical trial; however, in the clinical setting some effects may even be desired, and it maybe useful to consistently establish beliefs and outcome expectations to create the "healing setting" as powerful as possible.
In order to control for regression to the mean and natural history in a clinical trial, an untreated control group should be included whenever this is possible and ethically justified. If that is not possible, the minimum control should be a group treated with standard therapy[4]. A very useful design for the control of factors influencing the placebo response is the "balanced placebo design"50] (for detailed discussion see[4]). The original design includes a minimum of four groups, an active drug group and it's control, placebo, and two more groups, which are varied according to factors influencing the placebo response, such as beliefs induced by instructions (Table 1), doctors attention, study visits, medication regimen etc. Such a design if completed for an untreated control or standard medication group, allows conclusions about the amplitude of the placebo effect (placebo vs waiting/standard treatment) and the components influencing the placebo response (e.g., attention, beliefs, expectation).
Table 1.
An example of a possible "balanced placebo design" study
|
Information |
|||
| Drug | Placebo | ||
| Application | Drug | True positive | False negative |
| Placebo | False positive | True negative | |
One half of each group receiving either the drug or placebo in a blinded fashion is informed that they have received the drug, the other half is informed that they have received a placebo.
CONCLUSION
In general, placebo effects manifest themselves in subjective outcome measures[8]. However, even if subjective improvement is the most prominent change, other studies[45] show, that placebo benefits may be associated with improvements, although not to the same extent, in objective disease markers (endoscopic and histological abnormalities). If, in addition to the appropriate medical care, the patient feels good about the treatment, because he or she shares the same outcome expectations as their doctor, this should be a desirable benefit (for discussion of beneficial aspects of placebo in gastrointestinal disease see[51]).
Placebo effects in gastrointestinal diseases are as common as placebo effects in other diseases and constitute a significant confounding factor in the assessment of drug efficacy. However, since an appropriate control in clinical trials is possible, the focus of placebo research is shifting from being a nuisance factor in clinical trials towards studies designed for a better understanding of the factors influencing the placebo response in the individual and the neurobiological basis of the placebo response.
Footnotes
S- Editor Liu Y L- Editor Anand BS E- Editor Lu W
References
- 1.Hoffman GA, Harrington A, Fields HL. Pain and the placebo: what we have learned. Perspect Biol Med. 2005;48:248–265. doi: 10.1353/pbm.2005.0054. [DOI] [PubMed] [Google Scholar]
- 2.Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain. 2005;114:3–6. doi: 10.1016/j.pain.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci. 2005;6:545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- 4.Linde K. The specific placebo effect. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2006;49:729–735. doi: 10.1007/s00103-006-0002-z. [DOI] [PubMed] [Google Scholar]
- 5.Klosterhalfen S, Enck P. Psychobiology of the placebo response. Auton Neurosci. 2006;125:94–99. doi: 10.1016/j.autneu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 7.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 8.Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2004;(3):CD003974. doi: 10.1002/14651858.CD003974.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA. 1994;271:1609–1614. [PubMed] [Google Scholar]
- 10.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 12.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 13.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 14.de Craen AJ, Tijssen JG, de Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. 2000;247:183–188. doi: 10.1007/s004150050560. [DOI] [PubMed] [Google Scholar]
- 15.de Craen AJ, Moerman DE, Heisterkamp SH, Tytgat GN, Tijssen JG, Kleijnen J. Placebo effect in the treatment of duodenal ulcer. Br J Clin Pharmacol. 1999;48:853–860. doi: 10.1046/j.1365-2125.1999.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch I, Saphirstein G. Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prevention & Treatment (online Journal) Available from: http: //content.apa.org/journals/pre/1/1/2.
- 17.de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 18.Pitz M, Cheang M, Bernstein CN. Defining the predictors of the placebo response in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:237–247. doi: 10.1016/s1542-3565(04)00626-3. [DOI] [PubMed] [Google Scholar]
- 19.Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Kienle G, Kienle H. Plazeboeffekt und Plazebokonzept – eine kritische methodologische und konzeptionelle Analyse von Angaben zum Ausma des Plazeboeffekts. Forsch Komlementärmedizin. 1996;3:121–138. [Google Scholar]
- 21.Chey WD, Chey WY, Heath AT, Dukes GE, Carter EG, Northcutt A, Ameen VZ. Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2004;99:2195–2203. doi: 10.1111/j.1572-0241.2004.30509.x. [DOI] [PubMed] [Google Scholar]
- 22.Gordon S, Ameen V, Bagby B, Shahan B, Jhingran P, Carter E. Validation of irritable bowel syndrome Global Improvement Scale: an integrated symptom end point for assessing treatment efficacy. Dig Dis Sci. 2003;48:1317–1323. doi: 10.1023/a:1024159226274. [DOI] [PubMed] [Google Scholar]
- 23.Welge JA, Keck PE. Moderators of placebo response to antipsychotic treatment in patients with schizophrenia: a meta-regression. Psychopharmacology (Berl) 2003;166:1–10. doi: 10.1007/s00213-002-1299-4. [DOI] [PubMed] [Google Scholar]
- 24.Robertson C, Idris NR, Boyle P. Beyond classical meta-analysis: can inadequately reported studies be included? Drug Discov Today. 2004;9:924–931. doi: 10.1016/S1359-6446(04)03274-X. [DOI] [PubMed] [Google Scholar]
- 25.Moayyedi P. Meta-analysis: Can we mix apples and oranges? Am J Gastroenterol. 2004;99:2297–2301. doi: 10.1111/j.1572-0241.2004.40948.x. [DOI] [PubMed] [Google Scholar]
- 26.Enck P, Klosterhalfen S. The placebo response in functional bowel disorders: perspectives and putative mechanisms. Neurogastroenterol Motil. 2005;17:325–331. doi: 10.1111/j.1365-2982.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 27.Klosterhalfen S, Rüttgers A, Krumrey E, Otto B, Stockhorst U, Riepl RL, Probst T, Enck P. Pavlovian conditioning of taste aversion using a motion sickness paradigm. Psychosom Med. 2000;62:671–677. doi: 10.1097/00006842-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.ter Riet G, de Craen AJ, de Boer A, Kessels AG. Is placebo analgesia mediated by endogenous opioids? A systematic review. Pain. 1998;76:273–275. doi: 10.1016/S0304-3959(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 29.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205–215. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci. 1999;19:3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bausell RB, Lao L, Bergman S, Lee WL, Berman BM. Is acupuncture analgesia an expectancy effect? Preliminary evidence based on participants' perceived assignments in two placebo-controlled trials. Eval Health Prof. 2005;28:9–26. doi: 10.1177/0163278704273081. [DOI] [PubMed] [Google Scholar]
- 33.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you'll do? A systematic review of the evidence for a relation between patients' recovery expectations and health outcomes. CMAJ. 2001;165:174–179. [PMC free article] [PubMed] [Google Scholar]
- 34.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 35.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 36.Mearin F, Balboa A, Zárate N, Cucala M, Malagelada JR. Placebo in functional dyspepsia: symptomatic, gastrointestinal motor, and gastric sensorial responses. Am J Gastroenterol. 1999;94:116–125. doi: 10.1111/j.1572-0241.1999.00781.x. [DOI] [PubMed] [Google Scholar]
- 37.Allescher HD, Böckenhoff A, Knapp G, Wienbeck M, Hartung J. Treatment of non-ulcer dyspepsia: a meta-analysis of placebo-controlled prospective studies. Scand J Gastroenterol. 2001;36:934–941. doi: 10.1080/003655201750305440. [DOI] [PubMed] [Google Scholar]
- 38.Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:355–361. doi: 10.1046/j.1365-2036.2001.00937.x. [DOI] [PubMed] [Google Scholar]
- 39.Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15:79–86. doi: 10.1046/j.1365-2982.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 40.Jackson JL, O'Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 41.Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med. 2003;163:265–274. doi: 10.1001/archinte.163.3.265. [DOI] [PubMed] [Google Scholar]
- 42.Klein KB. Controlled treatment trials in the irritable bowel syndrome: a critique. Gastroenterology. 1988;95:232–241. doi: 10.1016/0016-5085(88)90319-8. [DOI] [PubMed] [Google Scholar]
- 43.Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S–97S. doi: 10.1016/s0002-9343(99)00086-8. [DOI] [PubMed] [Google Scholar]
- 44.Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–340. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 45.Ilnyckyj A, Shanahan F, Anton PA, Cheang M, Bernstein CN. Quantification of the placebo response in ulcerative colitis. Gastroenterology. 1997;112:1854–1858. doi: 10.1053/gast.1997.v112.pm9178676. [DOI] [PubMed] [Google Scholar]
- 46.Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology. 2004;126:1257–1269. doi: 10.1053/j.gastro.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 47.Moerman DE, Jonas WB. Deconstructing the placebo effect and finding the meaning response. Ann Intern Med. 2002;136:471–476. doi: 10.7326/0003-4819-136-6-200203190-00011. [DOI] [PubMed] [Google Scholar]
- 48.Moerman DE. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med Anthropol Q. 2000;14:51–72. doi: 10.1525/maq.2000.14.1.51. [DOI] [PubMed] [Google Scholar]
- 49.Weihrauch TR, Gauler TC. Placebo--efficacy and adverse effects in controlled clinical trials. Arzneimittelforschung. 1999;49:385–393. doi: 10.1055/s-0031-1300432. [DOI] [PubMed] [Google Scholar]
- 50.Ross S, Krugman AD, Lyerly SB, Clyde DJ. Drugs and placebos: a model design. Psycholog Reports. 1962;10:383–392. [Google Scholar]
- 51.Bernstein CN. The placebo effect for gastroenterology: tool or torment. Clin Gastroenterol Hepatol. 2006;4:1302–1308. doi: 10.1016/j.cgh.2006.09.003. [DOI] [PubMed] [Google Scholar]


