Abstract
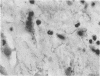
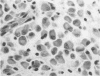
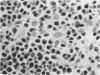
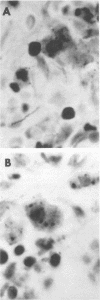
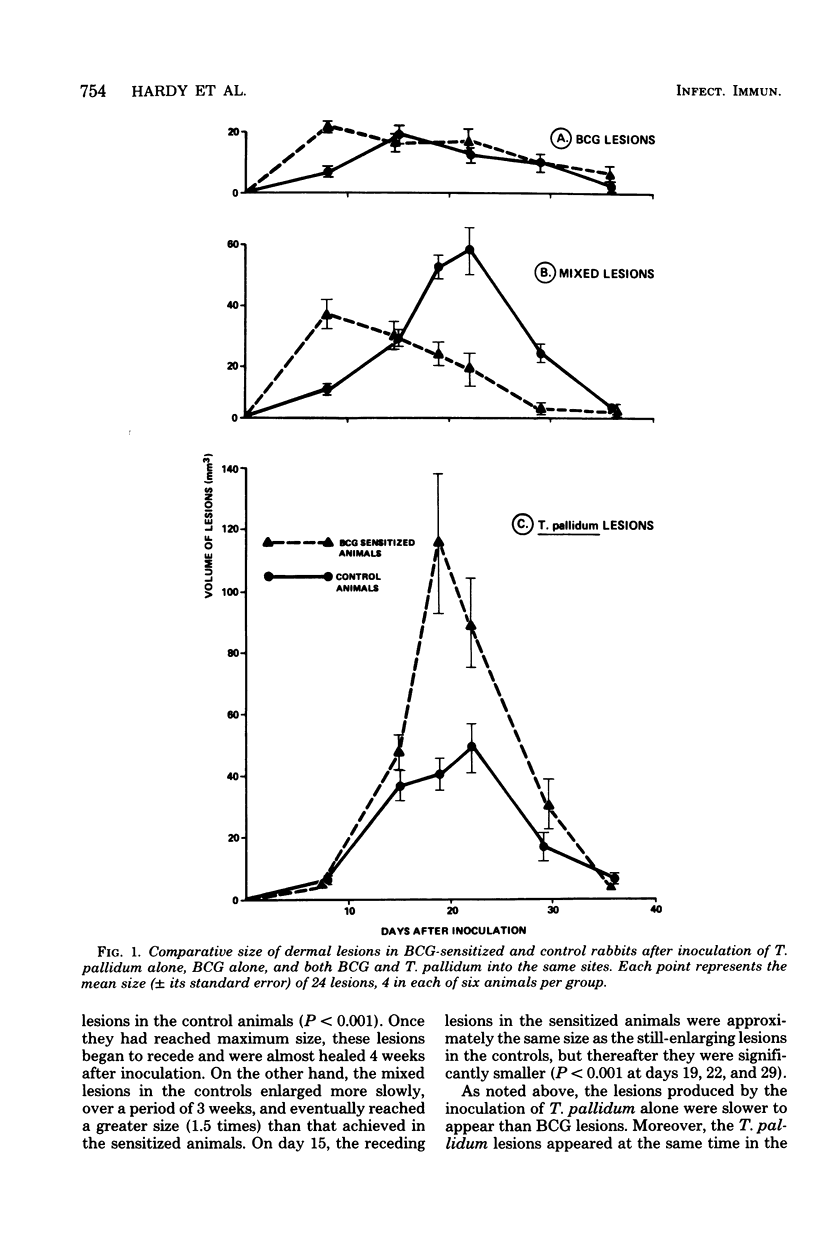
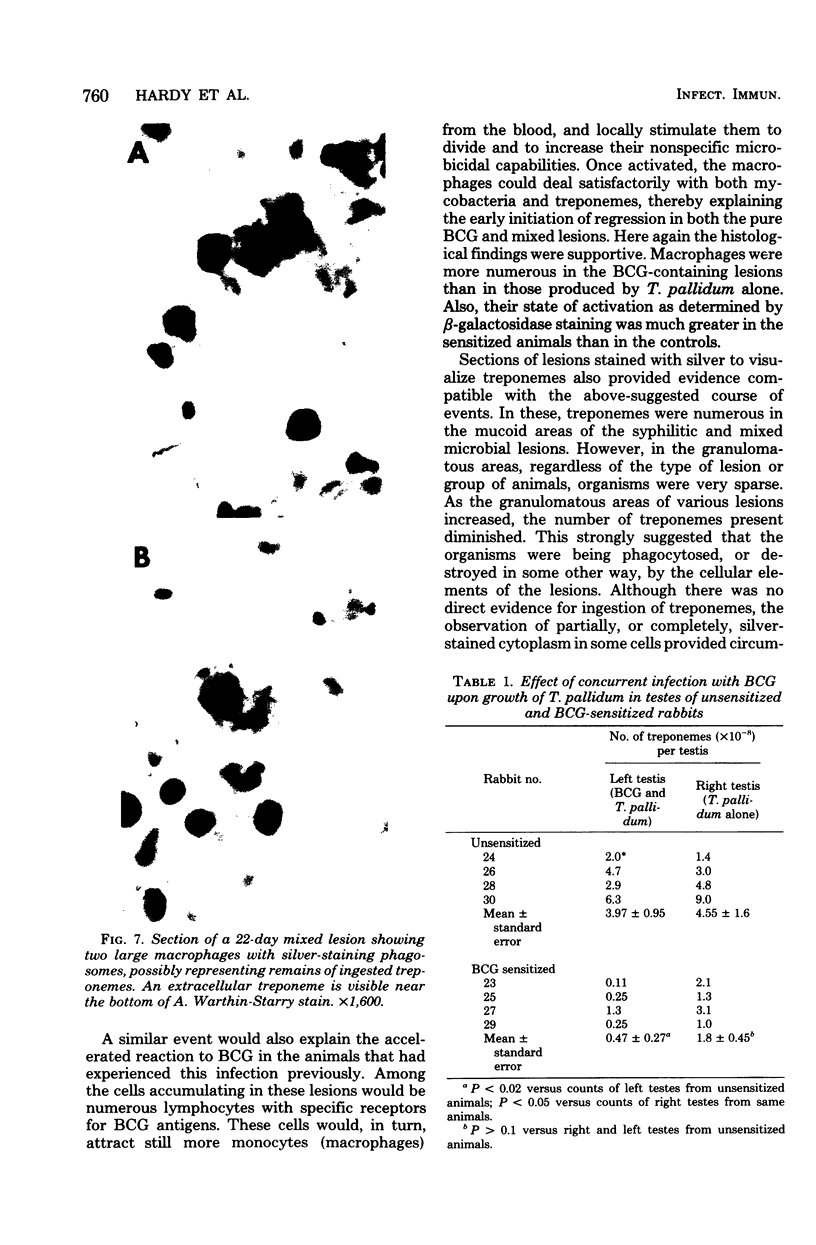
Paired groups of male rabbits were challenged with Treponema pallidum and Mycobacterium bovis BCG. One group had been sensitized to BCG by inoculation 3 weeks before challenge. All animals were challenged intradermally at multiple sites with T. pallidum alone, BCG alone, and both organisms into the same sites. The resulting lesions were followed clinically and histologically. BCG lesions enlarged more rapidly in sensitized rabbits, but they were otherwise no different from those in the controls. T. pallidum lesions enlarged and regressed simultaneously in both groups, but in the BCG-sensitized animals they became twice as large as those in the unsensitized rabbits. Mixed BCG-T. pallidum lesions showed the greatest differences in the two groups of animals. Like the pure BCG lesions, they enlarged more rapidly in the sensitized rabbits but began to recede after 1 week. The corresponding lesions in the controls enlarged more slowly and reached their maximum size after 3 weeks when the receding lesions in the sensitized animals were much smaller. The most marked histological-histochemical difference between the two groups of animals was in the number and activation of macrophages. These cells were more numerous in the mixed lesions of BCG-sensitized animals than in similar lesions of the controls and more activated as determined by beta-galactosidase staining. Although sparsely distributed, activated macrophages were more numerous in the pur T. pallidum lesions of sensitized animals than in those of control animals. Silver-stained sections revealed fewer treponemes in mixed lesions of sensitized animals than in the mixed lesions of control animals. Quantitation of treponemes in pure T. pallidum versus mixed lesions was determined in two groups of rabbits challenged intratesticularly. The total number of treponemes per testis in the mixed lesions of BCG-sensitized rabbits was significantly less than the number in the mixed lesions of control animals, and also less than the number in pure T. pallidum lesions of both groups of animals.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Dannenberg A. M., Jr, Sugimoto M., Tepper B. S. Histochemical studies relating the activation of macrophages to the intracellular destruction of tubercle bacilli. Am J Pathol. 1977 Mar;86(3):623–634. [PMC free article] [PubMed] [Google Scholar]
- Aronson J. D., Meranze D. R. The effect of syphilis on local tuberculous lesions in rabbits. Am J Pathol. 1938 Mar;14(2):163–176.3. [PMC free article] [PubMed] [Google Scholar]
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M., Knox J. M. Effect of sensitization with Propionibacterium acnes on the growth of Listeria monocytogenes and Treponema pallidum in rabbits. J Immunol. 1977 Jan;118(1):109–113. [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Brause B. D., Roberts R. B. Attachment of virulent Treponema pallidum to human mononuclear phagocytes. Br J Vener Dis. 1978 Aug;54(4):218–224. doi: 10.1136/sti.54.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Raffel S. Heterologous antigenic stimulation in induction of delayed hypersensitivity. J Immunol. 1977 Mar;118(3):874–880. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Meyer O. T., Esterly J. R., Kambara T. The local nature of immunity in tuberculosis, illustrated histochemically in dermal BCG lesions. J Immunol. 1968 May;100(5):931–941. [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. R., Johnson R. C. Effect of pretreatment with Mycobacterium bovis (strain BCG) and immune syphilitic serum on rabbit resistance to Treponema pallidum. Infect Immun. 1975 Nov;12(5):1029–1036. doi: 10.1128/iai.12.5.1029-1036.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLANDER D. H., TURNER T. B. The role of temperature in experimental treponemal infection. Am J Syph Gonorrhea Vener Dis. 1954 Nov;38(6):489–505. [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Kostiala A. A. Role of Lymphocytes in Cellular resistance in infection. Contemp Top Immunobiol. 1976;5:237–266. [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr The use of freeze-preserved treponemes in the Treponema pallidum immobilization test. Cryobiology. 1972 Oct;9(5):404–410. doi: 10.1016/0011-2240(72)90157-5. [DOI] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscopy of phagocytosis in syphilis and yaws. Br J Vener Dis. 1972 Aug;48(4):227–248. doi: 10.1136/sti.48.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine P. L., Weiser R. S., Klebanoff S. J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973 Nov;8(5):787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Bennedsen J., Larsen S. O., Riisgaard S., Spärck J. V. Correlation between in vivo and in vitro functional tests for activated macrophages. Infect Immun. 1979 Jan;23(1):34–40. doi: 10.1128/iai.23.1.34-40.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Musher D. M. Detection of nonspecific resistance to Listeria monocytogenes in rabbits infected with Treponema pallidum. Infect Immun. 1974 Apr;9(4):658–662. doi: 10.1128/iai.9.4.658-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R., Musher D., Jacobson K., Schwethelm P., Simmons C. Effect macrophage activation on infection with Treponema pallidum. Infect Immun. 1975 Sep;12(3):505–511. doi: 10.1128/iai.12.3.505-511.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. B., Hardy P. H., Jr, Newman B., Nell E. E. Effects of passive immunization on experimental syphilis in the rabbit. Johns Hopkins Med J. 1973 Nov;133(5):241–251. [PubMed] [Google Scholar]
- Weiser R. S., Erickson D., Perine P. L., Pearsall N. N. Immunity to syphilis: passive transfer in rabbits using serial doses of immune serum. Infect Immun. 1976 May;13(5):1402–1407. doi: 10.1128/iai.13.5.1402-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]